Secondary mould metabolites. Part 59.1Sesquiterpene illudanes: semi-synthesis of new illudins, structures and biological activity
Abstract
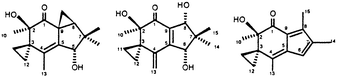
Owing to their unacceptable toxicity natural illudins are of limited interest as antitumor agents. In an attempt to exploit their cytotoxic potential several derivatives have been synthesized to obtain compounds with an improved therapeutic index. In this paper we describe some

 Please wait while we load your content...
Please wait while we load your content...