Synthesis of geminal bis(hydroxymethyl)pyrrolidine and pyrrolizidine imino sugars
Abstract
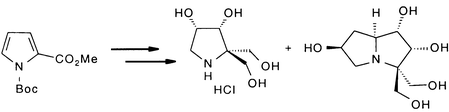
Reductive alkylation of N-Boc-pyrrolecarboxylate methyl ester 1 under Birch conditions with iodomethyl pivalate and subsequent reduction of the ester moieties provided the symmetric diol 3, a flexible building block for the synthesis of geminally disubstituted pyrrolidine imino sugars. This key intermediate was used for the first known synthesis of a 2,2-bis(hydroxymethyl)pyrrolidine imino sugar. In a similar manner a pyrrolizidine imino sugar was synthesised by oxidation of the deprotected pyrrolidine to the nitrone 10, which was subjected to a highly diastereoselective 1,3-dipolar cycloaddition with an allylic alcohol. Reductive cleavage of the N–O bond and recyclisation yielded the polyhydroxylated pyrrolizidine 13.

 Please wait while we load your content...
Please wait while we load your content...