Synthesis, characterization, and electrogenerated chemiluminescence of phenyl-substituted, phenyl-annulated, and spirofluorenyl-bridged oligothiophenes
Abstract
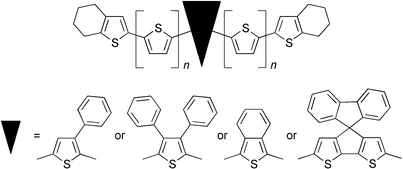
To overcome the insolubility of higher oligothiophenes and simultaneously to enhance their processability with respect to an application in molecularly doped organic light-emitting devices (OLEDs) we synthesized phenyl-substituted, phenyl-annulated, and spirofluorenyl-bridged oligothiophenes 1–5. Significantly improved solubilities in polar solvents of up to three orders of magnitude were found and their optical and electrochemical properties were investigated in solution. Reflecting small conformational changes and the almost complete electronic separation of the substituents, phenyl substitution and the introduction of a spiro core by bridging the central bithienyl unit only slightly affected optical and redox properties in comparison to the unmodified oligothiophenes (6–8). In contrast, the presence of an isothianaphthene (benzo[c]thiophene) unit in the oligomeric chain led to a distinct approximation of the frontier orbitals and consequently to a red-shift of both absorption and fluorescence. Finally, we demonstrated the applicability of some oligomers as dopants for OLEDs by electrogenerated chemiluminescence (ECL).

 Please wait while we load your content...
Please wait while we load your content...