Abstract
The synthesis and transition temperatures are reported for several 2-(4-alkyl- and 4-alkoxy-phenyl)-5-cyano-1-benzofurans, 2-(4′-alkylbiphenyl-4-yl)-5-cyano- and 5-(4′-alkylbiphenyl-4-yl)-2-cyano-1-benzofurans, and for compounds with other combinations of terminal alkyl and cyano groups in 2,5-disubstituted-1-benzofurans containing two phenyl units; some isolated examples of related cyclohexane systems are also presented. The mesogenic behaviour of these compounds and several intermediates (e.g. amides, acids and esters) is discussed and the transition temperatures are rationalised on the following basis (a) 1-benzofuran is a superior core unit to benzene, (b) 2,5-disubstitution in 1-benzofuran gives a bent core which adversely affects mesogenicity, to an extent which depends on its position in the core, (c) antiparallel associations in terminal cyano compounds can eliminate the disadvantage of a bent core structure, (d) 2-aryl-1-benzofurans have negligible inter-annular twist but 5-aryl-1-benzofurans have similar inter-annular twist to that in biphenyls.
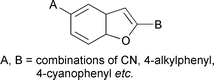
- This article is part of the themed collection: Molecular topology in liquid crystals: Materials Discussion 4

 Please wait while we load your content...
Please wait while we load your content...