Abstract
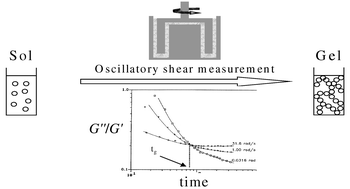
The gelation of silicon oxide and titanium oxide based materials obtained from the sol–gel process at room temperature was studied through rheological measurements. The determination of the gelation time tg was investigated by the variation of the storage and loss shear moduli as a function of time at several frequencies using Winter’s criterion. The effect of hydrolysis ratio on gelation was studied for both materials. It was found that the decrease of the gelation time as a function of hydrolysis molar ratio is well described by a power law with different exponent values for the two systems. Moreover a fractal dimension at the gelation time can be deduced from the rheological measurements due to the structural self-similarity of the sol–gel matrices. This fractal dimension df seems to be independent of the hydrolysis molar ratio for each system. The higher value of df for silicon oxide based gels shows that they possess a more dense structure than titanium oxide based gels.

 Please wait while we load your content...
Please wait while we load your content...