The effects of distal ligand substitution on the copper(II)/bis-(2,6-dipyrazol-1-ylpyridine) centre†
Abstract
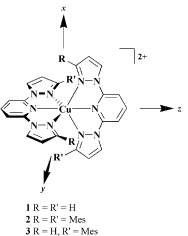
The complex [Cu(L4)2](BF4)2 (3; L4 = 2-[pyrazol-1-yl]-6-[3-{2,4,6-trimethylphenyl}pyrazol-1-yl]pyridine) has been synthesised. Complex 3 crystallises in two crystal forms from MeNO2/Et2O. The monoclinic α-form contains crystallographically ordered, pseudo-Jahn–Teller elongated {dy2 − z2}1 Cu(II) ions that are structurally very similar to [Cu(L2)2](BF4)2 (1; L2 = 2,6-dipyrazol-1-ylpyridine). The Cu ion in the orthorhombic β-polymorph at 180 K exhibits Cu–N bond lengths that are rather different from the α-form, and which could correspond to a disordered {dy2 − z2}1 Cu(II) centre; or, to a static, rhombically compressed ion with a {dz2}1 ground state. Variable temperature

 Please wait while we load your content...
Please wait while we load your content...