A reinvestigation of compound CpMo(PMe3)2(CH3)2: alkylation by single electron transfer and radical addition?
Abstract
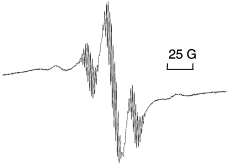
The synthesis of the half-sandwich molybdenum(III) diphosphine dimethyl complex CpMo(PMe3)2(CH3)2 has been reinvestigated. The compound was obtained from the corresponding dichloro complex CpMo(PMe3)2Cl2 and

 Please wait while we load your content...
Please wait while we load your content...