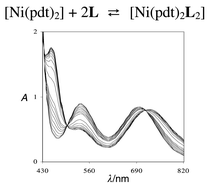
The reaction between trans-bis[O-methyl-(4-methoxyphenyl)phosphonodithioato]nickel (6) and pyridine (I), and o-, m-, and p-aminopyridines (II–IV) leads to the 1 ∶ 2 adducts 7–10. The corresponding formation constants have been determined by UV-visible spectroscopy. DFT calculations performed on the free I–IV ligands, and EHT-FMO calculations on their interactions with 6 predict an increase in the donor ability on passing from I to IV. The formation equilibrium constants follow the expected trend [log βeq
= 3.20, (7); 3.46, (9); 4.71 (10)], except for 8, which exhibits the lowest constant (log βeq
= 1.88), possibly due to steric effects. The adducts have also been obtained in the solid state, again with the exception of 8, and compounds 7 and 10 have been characterised by single crystal X-ray diffraction.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...