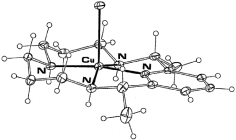
A low-temperature single-crystal X-ray structural determination of [CuCl(L1)]ClO4·H2O [where L1 = meso-2,12-dimethyl-3,7,11,17-tetraazabicyclo[11.3.1]heptadeca-1(17),13,15-triene] is reported. The coordination geometry of the complex cation approximates square pyramidal, with the chloro ligand occupying an apical position. The latter group is associated with an intermolecular hydrogen bonding network involving the uncoordinated water molecule and the perchlorate counter ion. Density functional theory (DFT) has been applied to modeling the structure of this complex, both in the presence and the absence of the intermolecular hydrogen-bonding network. In the absence of this network a good fit to the X-ray structural parameters was obtained except for the Cu–Cl bond length. For this bond the calculated value (2.346 Å) was significantly shorter than the X-ray value [2.541(2) Å] but, on incorporation of the hydrogen-bonded network, while the major portion of the computed structure remained almost identical to that obtained from the initial calculation, the Cu–Cl bond was now found to be longer at 2.572 Å, approximating the X-ray value quite closely. The study has been extended to similar/related systems for which structural data are available, confirming the wider applicability of this approach in modeling arrays of this type.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...