Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Monitoring the influence of steam on highly-active rhodium catalyst during the combined reforming of biogas by transient and steady-state operando spectroscopic studies
Victoria
Garcilaso
,
Rubén
Blay-Roger
 ,
Miriam
González-Castaño
,
Luis F.
Bobadilla
,
Miguel A.
Centeno
,
Miriam
González-Castaño
,
Luis F.
Bobadilla
,
Miguel A.
Centeno
 and
José A.
Odriozola
and
José A.
Odriozola
 *
*
Departamento de Química Inorgánica and Instituto de Ciencia de Materiales de Sevilla, Centro Mixto Universidad de Sevilla – CSIC, Av. Américo Vespucio 49, 41092 Sevilla, Spain. E-mail: odrio@us.es
First published on 21st May 2024
Abstract
The impact derived from incorporating water into CH4/CO2 biogas stream for the generation of syngas was investigated over the Rh/MgAl2O4 catalyst using operando steady-state and transient DRIFT spectroscopy coupled with MS. The incorporation of steam resulted in improved CH4 conversion rates and attained syngas streams with higher H2/CO ratios. It was demonstrated that in the presence of steam, the generation of CHxO species through the reaction of CO* with active *OH species is favored at the metal support surface. Besides, the enhanced resistance delivered by water molecules towards deactivating the coking phenomena was associated with easier carbonaceous decomposition and the exposition of the very active Rh (100) surfaces for methane decomposition. The Rh/MgAl2O4 catalyst was demonstrated to be an effective catalyst for the production of H2-rich syngas streams. More importantly, the insights reported herein provide new evidences regarding the impact of steam on biogas reforming reactions.
1. Introduction
Over the last several years, the production of biogas from the anaerobic digestion of biomass wastes has attracted considerable interest as a renewable carbon source.1,2 As biogas is composed mainly of CH4 and CO2, the production of syngas by the reforming of biogas and the subsequent transformation into liquid fuels via Fischer–Tropsch synthesis (FTS) is the most suitable process and the most cost-effective from the industrial point of view.3,4 However, the chemical composition of raw biogas depends on the type of feedstock, design of the digester-fermenter and operating conditions.5–7 Typically, biogas contains 60–75 vol% of methane and 19–33 vol% of CO2 along with moisture besides other contaminants such as sulphur compounds, halogenated compounds, ammonia, organic silicon compounds (siloxanes), tars, and particulate matter.8–10 The most usual technologies applied to remove contaminants from raw biogas during the upgradation process include membrane technology, water scrubbing, pressure swing adsorption (PSA), cryogenic separation and biological techniques.11Apparently, the presence of steam in biogas is not a drawback but a major advantage for reforming processes.12 Although the formation of carbon is thermodynamically limited at typical reforming temperatures, the deposition of coke on the catalyst surface is unavoidable in the dry reforming reaction of methane.13 The formation of carbon can be minimized due to the presence of steam, which provides a higher oxidant level throughout the carbon gasification reaction. Unlike the dry reforming of model biogas, in which the syngas obtained is always present at a H2/CO ratio lower than one, the presence of water produces a syngas more flexible by adjusting the CO2/H2O ratio to match the desired value close to 2 required for the downstream production of the chemical value-added via Fischer–Tropsch synthesis.14 In this scenario, the combination of dry reforming of methane (DRM) and steam reforming of methane (SRM) has been explored extensively and it is known as the bi-reforming of methane15 or also the combined reforming of methane.16 This approach has attracted great interest from the industrial point of view and provides an opportunity for advanced research toward the commercialization of this technology.17,18
Catalysts used for combined reforming processes are usually based on nickel, but their stability is limited by the metal sintering and/or coke deposition processes.19,20 Stroud et al.21 proposed an Sn–Ni/Ce–Al catalyst able to enhance both methane conversion and catalytic stability in the presence of steam in the bi-reforming of methane compared to the same catalyst tested under dry-reforming conditions. On the other hand, MgO and Al2O3 have been probed as the most promising supports for the dry reforming of methane. It is well known that the increased basicity of the support favours the adsorption of CO2, facilitating the gasification of carbon to form CO, as Zhao et al.22 reported in a recent study. Herein, a bimetallic Ni–Co/MgAl2O4 catalyst showed a high stability over more than 30 h under reforming atmospheres without evidence of carbon deposits in the presence of H2O and CO2. In a microkinetic study, Geng et al.23 argued that oxygen atoms from steam promoted methane conversion by switching the reaction path from  + * →
+ * →  + H* to
+ H* to  + O* →
+ O* →  + OH* whilst restricting the generation of C* species, which constricted the generation of carbon deposits. With most of the studies focusing on Ni catalysts, the incorporation of adequate steam concentrations could increase the H2
+ OH* whilst restricting the generation of C* species, which constricted the generation of carbon deposits. With most of the studies focusing on Ni catalysts, the incorporation of adequate steam concentrations could increase the H2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO ratios and improve the catalyst stability by favouring the gasification of carbon deposits.24 In this sense, the larger OH concentrations over Ni catalysts generated in the presence of steam were proposed to enhance the gasification of carbon deposits generating CHO* species.25 Still, deactivation issues related to metal sintering and the partial oxidation of the active sites have also been reported.26,27
CO ratios and improve the catalyst stability by favouring the gasification of carbon deposits.24 In this sense, the larger OH concentrations over Ni catalysts generated in the presence of steam were proposed to enhance the gasification of carbon deposits generating CHO* species.25 Still, deactivation issues related to metal sintering and the partial oxidation of the active sites have also been reported.26,27
Although their limited availability and high cost restrain their use for large-scale applications, noble metals like Pt, Ru, Pd and Rh attain higher conversions and major coke resistance than transition metals.13 Among them, Rh was demonstrated to be the most active towards the conversion of methane when supported on CeO2/Al2O3.13 Over different supports and reaction conditions, Rh metal also exhibited the highest turnover frequency (TOF) compared to their homologues based on Ru and Pt.28 Maestri et al.29 proposed a consistent microkinetic model on Rh catalyst that pointed out the mechanistic analogies between steam and dry reforming. They proposed that methane consumption proceeds via decomposition and subsequent carbon oxidation by OH* following a sequential route (CH4 → C* → CO*), and the role of either CO2 or H2O is to provide the main oxidizer (OH*). Rostrup-Nielsen and Bak Hansen30 also observed that replacing steam by carbon dioxide hardly modified the reforming mechanism. Moreover, they demonstrated that Ru and Rh catalysts are highly stable for carbon-free operation, presenting high reforming rates combined with low coke formation rates.
Operando diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) has been widely employed as a powerful tool to elucidate the reaction mechanism and gain insights into the nature of surface intermediates. In a previous work,31 we studied the activity of the Rh/MgAl2O4 catalyst toward the biogas reforming reaction using transient and steady-state DRIFTS experiments, proposing a reaction mechanism in which the dissociative adsorption of CO2 takes place initially and the active oxygen species formed on the metallic sites facilitates C–H bond activation. Although numerous articles have been published on the dry reforming of methane reaction on different supported Rh catalysts, there is not a clear and unambiguous explanation of the real effect of steam on the nature of the reaction. The scarce studies focusing on the role of steam and OH surface groups on the reaction mechanism and constituted intermediates might be associated with the low concentration established under DRM atmospheres.32 Considering the impact of larger OH coverages generated in the presence of steam and the structure sensitivity character of reforming processes,33,34 establishing the role of steam for catalytic systems aside of Ni metal is extremely convenient. The present study contributes a new perspective to understand the effect of steam in the activation process of CO2 and CH4 during the reforming of biogas by means of transient experiments and DRIFTS coupled MS studies over the Rh/MgAl2O4 catalyst. The integration of both transient and steady-state operando experiments provides a comprehensive vision of the catalyst surface properties, and their correlation with catalytic performance is very useful for achieving the rational design of catalysts.
2. Experimental details
A catalyst based on 1 wt% of Rh dispersed on MgAl2O4 support was used in this study. The preparation method and characterization details of this catalyst have been described in a previous work.31 This catalyst was labelled as Rh/MgAl2O4.The biogas reforming reaction was carried out in a commercial computerized microactivity reference catalytic reactor from PID Eng & Tech employing a stainless-steel reactor with 9 mm internal diameter. A fixed-bed composed by 50 mg of catalyst diluted in SiC (both sieved in 100–200 μm) to ensure a good heat and mass transfer was placed at the centre of the reactor in direct contact with a thermocouple. Before catalyst testing, the sample was in situ reduced in a flow of 50% H2/N2 at 750 °C for 3 h (100 mL min−1). Then, 50 mL min−1 of nitrogen was used to purge the reactor system for 30 min before reaction experiments at 750 °C. The reaction was performed at 750 °C and 1 bar, maintaining a total flow rate of 100 mL min−1 with a weight hourly space velocity (WHSV) equal to 200 L h−1 g−1 and varying amounts of steam. Reactant gas mixtures consisting of CH4, CO2, and N2 with a CH4/CO2 molar ratio equal to 1.5 were introduced using mass-flow controllers from Bronkhorst. Variable amounts of H2O were introduced using a HPLC pump (Gilbson Inc series) and evaporated to reach concentrations of 8, 12 and 17 vol% of steam. In order to maintain the space velocity and the partial pressures of CO2 and CH4, the flow of nitrogen was adjusted as a function of the steam incorporated. The reforming tests were performed under isothermal conditions, and every concentration of steam was maintained for 2 h to guarantee steady-state conditions. The effluent gases were analysed on-line using a micro-gas chromatograph (Varian 4900 model) equipped with two columns: Poraplot Q and molecular sieve 5A. The conversions of methane and CO2 were calculated on the basis of the difference between the input and output of methane and CO2, respectively.
Operando DRIFTS-MS experiments were performed in a high temperature reaction chamber (Praying Mantis from Harrick) fitted with ZnSe windows and connected to a Thermo Nicolet Nexus FTIR spectrometer equipped with a liquid N2-cooled MCT detector. Spectra were obtained with a spectral resolution of 4 cm−1 and an average of 128 scans. The whole optical path was continuously purged with a flow of water vapour- and CO2-free nitrogen, and the background spectrum was collected using an aluminium reflective mirror. Typically, about 80 mg of sample was put in the DRIFTS cell to ensure that the gases pass through the catalyst packed-bed mimicking the plug-flow condition of the reaction. Prior to the measurements, the sample was activated in situ at 750 °C for 1 h in 50% H2/Ar at a total flow rate of 50 mL min−1 and then cooled down to the reaction temperature by purging with Ar. The spectrum of the activated catalyst was used as the reference. The dry biogas reforming reaction was performed by feeding into the cell a gas mixture of 10 vol% CO2 and 15 vol% CH4 balanced in argon with a total flow rate of 50 mL min−1. Analogously, the bi-reforming reaction of biogas was carried out using a mixture of 10 vol% of CO, 15 vol% CH4 and 10 vol% of H2O in argon with a total flow rate of 50 mL min−1. For it, an adequate amount of liquid water was fed continuously using a HPLC pump and vaporising the liquid in a homemade evaporator. In both the reactions, the temperature was increased from 550 °C to 750 °C with intervals of 50 °C, and each temperature was maintained for 30 min to achieve steady-state conditions. For the transient experiments, the feed composition was temporarily varied following two different sequences: 1) 10%CO2/Ar → Ar → 15%CH4/Ar, and 2) 10%CO2/10%H2O/Ar → 10%H2O/Ar → 15%CH4/10%H2O/Ar. The different mixtures were introduced under isothermal conditions at 550 °C every 20 min. A four-way valve was installed to switch the gas mixtures. The gas composition exiting the DRIFTS cell were sent to a mass spectrometer (PFEIFFER MS Vacuum Prisma Plus) using a 1/16-inch stainless steel capillary tubing heated at 150 °C for analysis to avoid condensation. The continuous data acquisition of MS was recorded using the Quadera software, following mainly the signals m/z = 2, 15, 18, 28 and 44.
3. Results and discussion
3.1. Effect of steam in the biogas reforming reaction
The effect of different steam concentrations in the biogas reforming reaction over Rh/MgAl2O4 catalyst was performed under isothermal conditions at 750 °C, feeding a CH4/CO2 molar ratio of 1.5. Fig. 1a shows that the presence of steam promotes the methane conversion but has a contrary effect on CO2 conversion. The general reaction of bi-reforming of methane can be represented by a combination of the steam reforming and dry reforming reactions, as shown in the following equations.| Dry reforming of methane: CH4 + CO2 → 2CO + 2H2 | (1) |
| Steam reforming of methane: CH4 + 2H2O → CO + 3H2 | (2) |
| Bi-reforming of methane: 3CH4 + CO2 + 2H2O → 4CO + 8H2 | (3) |
Additionally, a variety of different parallel reactions also can occur, including the following.35
| Water gas shift reaction (WGS): CO + H2O ↔ CO2 + H2 | (4) |
| Methane decomposition: CH4 ↔ C + 2H2 | (5) |
| Boudouard reaction: CO2 + C ↔ 2CO | (6) |
| Beggs reaction: C + H2O ↔ H2 + CO | (7) |
| CO2 methanation: CO2 + 4H2 → CH4 + 2H2O | (8) |
| CO methanation: CO + 3H2 → CH4 + H2O | (9) |
The enhancement of the CH4 conversion with the amount of water in the feed is mainly due to the participation of the steam reforming reaction (eqn (2)), whereas the lower CO2 conversion is related to the CO2 produced by the WGS reaction (eqn (4)). An increase in the concentration of water favours the WGS reaction against the dry reforming of methane, resulting in a negative CO2 balance, as predicted the by thermodynamics.15,21
Furthermore, Fig. 1b shows that the H2/CO molar ratio increases from 1.1 to 1.7 by increasing the vol% of water, which is strictly related to the production of hydrogen and the consumption of CO by the WGS reaction and the gasification of carbonaceous deposits (eqn (7)). In a collaboration work of our group, Stroud et al.21 reported a similar H2/CO ratio of 1.6 for a feed of CH4/CO2/H2O = 1/1/1 (33 vol% of H2O) at 700 °C on a bimetallic Ni–Sn supported catalyst. Moreover, it was demonstrated that the introduction of water in the bi-reforming reaction favours the gasification of carbonaceous species deposited on the catalyst surface. Roh et al.36 found an optimal CO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O
H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH4 feed ratio to obtain the syngas with a H2/CO molar ratio of 2 suitable for Fischer–Tropsch synthesis. The optimal concentration of water reported by these authors was 25 vol% of steam for a CH4/CO2 ratio of 2.5. In another previous work, we found that an H2/CO ratio close to 2 can be achieved using a feed of 28 vol% of water and a CH4/CO2 ratio of 2.5 over a Ni–Ru-based catalyst. In the current work, the reaction was performed using a typical biogas composition with a CH4/CO2 ratio of 1.5 and concentrations of water below 20 vol%. Nevertheless, the tendency observed for the H2/CO ratio with the water concentration (Fig. 1b) suggests that water concentration of about 8 to 18 vol% should result in industrially relevant H2/CO molar ratios.
CH4 feed ratio to obtain the syngas with a H2/CO molar ratio of 2 suitable for Fischer–Tropsch synthesis. The optimal concentration of water reported by these authors was 25 vol% of steam for a CH4/CO2 ratio of 2.5. In another previous work, we found that an H2/CO ratio close to 2 can be achieved using a feed of 28 vol% of water and a CH4/CO2 ratio of 2.5 over a Ni–Ru-based catalyst. In the current work, the reaction was performed using a typical biogas composition with a CH4/CO2 ratio of 1.5 and concentrations of water below 20 vol%. Nevertheless, the tendency observed for the H2/CO ratio with the water concentration (Fig. 1b) suggests that water concentration of about 8 to 18 vol% should result in industrially relevant H2/CO molar ratios.
3.2. Operando DRIFTS-MS studies
Operando DRIFTS-MS studies were performed to better understand the influence of steam and the intermediate species formed during the dry reforming and bi-reforming of biogas reaction over Rh/MgAl2O4 catalyst. A comprehensive vision of the reaction mechanism provides a useful guidance to design an optimal catalyst in terms of the activity, selectivity, and stability. | ||
| Fig. 2 Evolution of the DRIFT spectra with the temperature in the 4000–3100 and 2100–1300 cm−1 regions during the reduction pretreatment at 750 °C for 1 h in 50%H2/Ar over the Rh catalyst. | ||
Heating up the sample in the reduction flow, the physisorbed water disappeared completely at temperatures near 140 °C and on increasing the temperature above 450 °C, one band at 3720 cm−1 appeared, which is ascribable to “free” hydroxyls, disclosing the complete dehydration of the surface by the elimination of the broad band centred at ca. 3500 cm−1 associated with the H-bonded hydroxyls of weakly adsorbed water.39 The evolution of the bands assigned to carbonate species during the pretreatment suggests the different thermal stability and reactivity towards hydrogen. It is noteworthy that as the temperature increases above 200 °C, an alteration of the carbonate features is observed, and new bands appeared at 2010 and 1915 cm−1, which can be assigned to Rh-carbonyl species with different coordination geometries, respectively.40 It is well known that the thermal decomposition and/or reduction of carbonate species takes place in the presence of hydrogen over noble metals, leading to the formation of Rh-carbonyl intermediate species and CO gas at temperatures of 200–300 °C.41 Because Rh is an optimal active metal for the methanation reaction, the dissociation of CO on Rh particles to form “C” species and their subsequent hydrogenation to form methane gas should not be ruled out.42 In this sense, the disappearance of Rh-carbonyl species as the temperature is increased above 300 °C suggests the possible methanation of adsorbed CO. Nevertheless, the low concentration of methane formed is too low to be detected using an IR spectrometer. When the temperature reached 750 °C, all carbonate species were removed, indicating that the pretreatment is effective to obtain a clean surface. We can conclude that apparently the catalyst surface only contains “free” hydroxyls and Rh metallic particles highly dispersed after reduction pretreatment for 1 h at 750 °C.
As shown Fig. 3a, after introducing the reacting gas mixture of CO2 and CH4 free of water at 550 °C, a prominent peak at 2360 cm−1 associated with the asymmetric stretching (νas) of CO2 gas and two bands at 3016 and 1303 cm−1 associated with the rotational fine structure of methane gas appear.43 The bands appearing at 2030–2008 cm−1 and 1920–1860 cm−1 indicate the formation of linearly adsorbed CO and bridged CO species on Rh sites, suggesting that CO2 is dissociated into CO and “O” species.40 With increasing temperature, the appearance of the characteristic P branch between 2140 and 2200 cm−1 of the gaseous CO vibrational-rotational spectrum is more appreciable, which indicates the release of CO gas.43 Additionally, two complex pair of bands centred at 1595 and 1360 cm−1 were identified at lower temperatures. As the temperature increases, these two bands evolve together, giving a set of bands at 1630, 1535, 1377 and 1330 cm−1 at 750 °C. These latter bands are associated with the presence of residual carbonaceous species adsorbed on the support surface.44,45 Regarding the assignment of the bands at 1595 and 1360 cm−1, some authors have assigned the band at 1595 cm−1 to the νas(OCO) vibrational mode of the formate species. Nevertheless, the two characteristic bands of the formate species at 1395 and 1375 cm−1 ascribed to CH deformation and νs(OCO), respectively, were absent in our experimental data (Fig. 3). Additionally, a band at 2900–2800 cm−1 ascribed to the ν(C–H) stretching of formate should appear in the spectra. Although this region is overlapped with the rotational fine structure of gaseous methane, the band related to the C–H stretching of formate is hardly observable. Therefore, we cannot attribute these bands to formate species. It is more reasonable to assume that the bands at 1595–1360 cm−1 are attributable to monodentate carbonates with Δν3 of 100–200 cm−1 formed on the support's basic sites.46,47 These species are thermally labile carbonates, which are decomposed or reduced as the temperature increases.
In the presence of water (Fig. 3b), the bands associated with gaseous CO2 and CH4 were also observed, although important differences were noticed in the adsorbed species. In the 1700–1200 cm−1 spectral region, it is worth noticing that the bands at 1595 and 1360 cm−1 vanished rapidly with the temperature, whereas the bands at 1630, 1535, 1377 and 1330 cm−1 were hardly observed. This clearly reveals that steam favours the gasification/decomposition of carbonaceous species. On the other hand, the presence of CO carbonyl adsorbed species over Rh0 sites characterized by the bands at 2040–2028 cm−1 and 1920–1860 cm−1, assigned to CO adsorbed linearly and multi-bonded carbonyl species, respectively, was also observed.40,48 Thus, CO2 dissociation also takes place under bi-reforming conditions. Nevertheless, the infrared spectra associated with the carbonyl species were clearly affected by the reaction atmosphere.
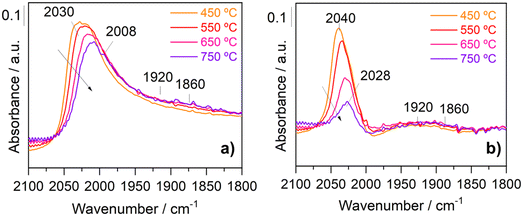
The differences in the adsorbed CO species can provide information about the species and sites responsible for the activation of CO2 and CH4 in the presence and absence of steam. In order to assess the influence of the steam on the CO adsorbed species on Rh in detail, Fig. 4 focuses on the different evolutions noticed on increasing the reaction temperature for the 2100–1800 cm−1 region. It can be noticed that the increase in the temperature from 450 °C to 750 °C caused a slight decrease in the intensity of the CO adsorption peaks associated with linearly adsorbed CO with a displacement to lower wavenumbers from 2030 to 2008 cm−1 in the absence of water (Fig. 4a), while in presence of steam, the equivalent bands were progressively removed with the temperature and displaced from 2048 to 2028 cm−1 (Fig. 4b). The vibrational frequency depends on the CO coverage, and the higher frequency observed at high CO coverages is due to the dipole–dipole coupling between the CO adsorbed molecules on neighbouring sites. In the presence of steam, dipole–dipole coupling accounts for the redshift observed for the carbonyl bands due to the lower CO coverage on Rh particles when the temperature is increased. Nevertheless, the displacement of carbonyl frequencies observed under dry conditions (Fig. 4a) cannot be related to dipole–dipole coupling and CO coverage phenomena. Simultaneously, the band at 2030 cm−1 shifts to 2008 cm−1 and preserves its intensity, meaning that the electronic density on the rhodium sites increases under these conditions. Accordingly, the formation of carbon species on the surface must be expected, suggesting that CO adsorbs on Rh sites close to carbonaceous deposits that increase the back-donation on the metal surface.38,49–51 This observation is coherent considering that the absence of water led to the covering of Rh particles by carbonaceous deposits, whereas the water participates in the carbon gasification, cleaning the Rh surface sites. On the other side, it is also noticed from Fig. 5a and b that no shift of the bands attributed to bridge-bonded CO on Rh (1860 and 1920 cm−1) was observed with the increase in the reaction temperature. The band at 1860 cm−1 has been ascribed to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 CO–Rh species while the peak at 1920 cm−1 is related to a 3
2 CO–Rh species while the peak at 1920 cm−1 is related to a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 stoichiometry.52 These stoichiometries depend on the type of the rhodium environment and clearly reveal that the presence of steam is affecting the coordination of Rh sites. According to Lavalley et al.,53 these two bands are certainly related to CO adsorption on two rhodium faces such as Rh (100) and Rh (111). The minor presence of the band at 1920 cm−1 under dry conditions may be tentatively explained by the blocking of bridged adsorption sites on the corresponding rhodium face. Wang et al.54 studied CH4 dehydrogenation on Rh (111), Rh (110) and Rh (100) surfaces using density functional theory (DFT) calculations. They found that CH4 dehydrogenation on the Rh (100) surface is more favourable in comparison with that on Rh (111) and Rh (110) surfaces. On this basis, carbonaceous deposits formed in the absence of steam could preferentially suppress CO-bridged adsorption on Rh (100) surface sites.
2 stoichiometry.52 These stoichiometries depend on the type of the rhodium environment and clearly reveal that the presence of steam is affecting the coordination of Rh sites. According to Lavalley et al.,53 these two bands are certainly related to CO adsorption on two rhodium faces such as Rh (100) and Rh (111). The minor presence of the band at 1920 cm−1 under dry conditions may be tentatively explained by the blocking of bridged adsorption sites on the corresponding rhodium face. Wang et al.54 studied CH4 dehydrogenation on Rh (111), Rh (110) and Rh (100) surfaces using density functional theory (DFT) calculations. They found that CH4 dehydrogenation on the Rh (100) surface is more favourable in comparison with that on Rh (111) and Rh (110) surfaces. On this basis, carbonaceous deposits formed in the absence of steam could preferentially suppress CO-bridged adsorption on Rh (100) surface sites.
 | ||
| Fig. 4 Evolution of the DRIFT spectra in the 2100–1800 cm−1 region with the temperature for the reduced sample under the reaction conditions in the absence of water (a) and presence of water (b). | ||
Regarding the evolution of the gas-phase analysed on-line by MS in the absence and presence of steam (Fig. 5a and b), substantial formation of H2 and CO was observed at temperatures above 450 °C during the dry reforming and the bi-reforming of biogas reaction on the Rh catalyst. As the temperature increased, the conversion of methane is higher and the signal of H2 (m/z = 2) is increased. In the absence of water at 750 °C, the significant decrease of methane conversion and H2 concentration was associated with catalyst deactivation due to the formation of carbon deposits or CO species strongly bonded to Rh particles. In agreement with the catalytic activity results, operating in the presence of water resulted in higher H2 concentration and H2/CO ratios close to 2. Remarkably, no signs of catalyst deactivation were noticed in the presence of water, and fairly stable hydrogen production for the entire temperature window was obtained.
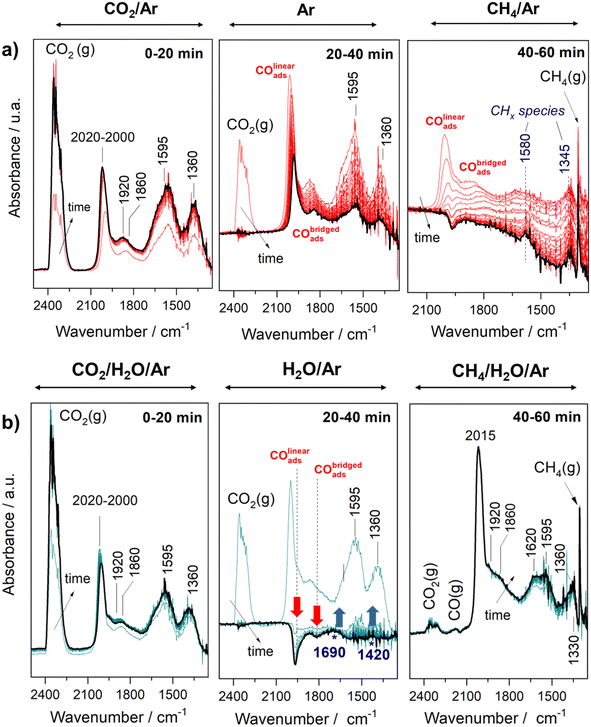
Fig. 6a and b display the evolution of the IR spectra sequentially recorded by switching the three stream effluents every 20 min in dry and wet conditions, respectively. As can be noticed, under CO2 stream, similar features related to carbonyl adsorbed species and carbonates were observed independently of the presence of water. The presence of both linear (2020–2000 cm−1) and bridged (1920–1860 cm−1) carbonyls under both stream conditions indicates that CO2 is dissociated on the metallic sites and that water plays no a significant role in the direct dissociation of CO2. Upon switching the CO2 stream to a purge stream, significant differences were observed in the absence and presence of water. As shown in Fig. 6a, the IR bands attributed to the carbonate species disappeared almost completely, whereas the intensities of the bands attributed to the adsorbed carbonyl species decreased only partially during the Ar purging step. Meanwhile, Fig. 6b reveals that in the presence of steam, the metallic carbonyls and carbonates disappeared very fast. Moreover, it is remarkable to notice the negative evolution of carbonyls and the simultaneous emergence of two bands at 1690 and 1420 cm−1. These two bands are assigned to CO stretching and CH bending in the CHxO species.55–57 These findings clearly reveal that CHxO species are reaction intermediates favored in the presence of steam. We can assume that the generation of CHxO species occurs through the reaction of CO* with the active *OH species formed likely at the metal support interface. Subsequently, by switching the gas feed from purge to CH4 stream, the impact of steam is even more evident. From Fig. 6a, it is evident that the bands related to carbonyls and carbonates completely disappear whereas residual bands at 1580 and 1345 cm−1 are developed. These features are ascribed to carbonaceous species.58,59 The observed negative band of carbonyl could be related to the transformation of linearly adsorbed CO species into –COH adsorbed species. Therefore, these observations clearly reveal that the catalyst's surface is a covering of accumulated carbon formed by methane dissociation in the absence of steam. In contrast, one can see in Fig. 6b that with the incorporation of CH4/H2O/Ar steam, CO2 and CO gases were released. Furthermore, bands ascribed to carbonyl species adsorbed over Rh metal sites and bands at 1620, 1595, 1360 and 1330 cm−1 ascribed to carbonates with different coordination geometry were also detected. The formation of CO and CO2 indicate that gasification and WGS reactions are happening on the catalyst surface.
Finally, Fig. 7a and b display the evolution of gaseous products monitored by on-line mass spectrometry (MS). Ion currents were measured for the m/z values of the H2O, CO2 CH4, H2 and CO and H2O. Firstly, it must be noticed that a large amount and stable production of hydrogen is achieved in the CH4/H2O/Ar steam, whereas in the absence of steam, the production of hydrogen decays rapidly due to the accumulation of carbonaceous deposits, in line with the above discussion.
3.3. Mechanistic insights
The observed findings enable rationalizing the impact of steam on the reaction pathway proposed for the Rh/MgAl2O4 catalyst in the absence and presence of water (Fig. 8a and b). Under both reaction conditions, CO2 and CH4 were dissociatively adsorbed over the metal surface. The resulting CHx species react, either over the metal surface or at the metal support interface, with close active O* atoms, producing CHxO species, which decompose to CO and H2. Still, remarkable differences concerning the type of surface species developed on the surface and Rh–CO interactions were observed. In our previous work, we found that the dissociative adsorption of CO2 over Rh metal surfaces was associated with the partial oxidation and re-dispersion of Rh atoms.31 The lower-coordinated Rh cluster were proposed to promote the generation of stable bridged carbonyls through the decomposition of unstable geminal carbonyls. In fact, it is generally accepted that the nature and geometry of the active metal determines the dissociation of CH4 and the overall catalyst performance. Herein, we observed that bridged carbonyl bands were developed over two different Rh surface adsorption sites. In this sense, the disappearance of stable bridged carbonyl might undermine the catalyst performance by blocking the Rh active sites through the generation of carbon species via Boudouard's and methane cracking reactions. The catalysts' deactivation derived from the coke deposits generated over the Rh surface in the absence of water agrees with the hydrogen drop observed in the MS analysis at higher temperatures (Fig. 5a).In the presence of steam, the absence of carbonaceous deposits observed on the catalysts surface and the greater CH4 conversion rates suggest that water molecules facilitate the decomposition of reaction intermediates into H2 and CO. From the operando DRIFT-MS results, the hydroxylated surface could improve the reaction of the CO* species with the nearby OH species, generating CHxO species that subsequently decomposes into the reaction products. Besides, the presence of water should also promote the forward WGS reaction, removing CO species from the metal surface, thereby hindering coke generation via the Boudouard reaction. Remarkably, the incorporation of water molecules also constricted the blocking of Rh active sites. In this sense, steam could promote the exposition of Rh surface active for methane decomposition to some extent and inhibit the accumulation of stable carbonaceous species over highly dispersed Rh sites. In all, the incorporation of water molecules enhances the performance of the Rh/MgAl2O4 catalyst by promoting the generation of CHxO species, enhancing the decomposition of carbonaceous species and inhibiting the accumulation of carbon deposits over the active Rh metal surfaces.
4. Concluding remarks
This work analyses the benefits attained by incorporating water into DRM stream over the Rh/MgAl2O4 catalyst by combining steady-state and transient operando DRIFT coupled with MS spectrometry. The catalytic activity results evidenced the generation of syngas with higher H2/CO ratios because of the promotion of CH4 conversion and lower CO2 conversions due to the impact of the forward WGS reaction. The incorporation of water affected the constituted Rh nanoparticles and reaction intermediates both in nature and concentration. Hence, the hydroxylated surface attained in the presence of water enhanced the reactivity of CHx species with the active O* species, thereby enhancing the generation of CHxO species, the proposed major reaction intermediate towards H2 production. Moreover, the presence of steam promoted the decomposition of carbonaceous species and constricted the coking phenomena over the metal surface, thus enhancing the catalyst lifetime.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Dra. Miriam González acknowledges to the EMERGIA program of the Junta de Andalucía by the EMC21_00052 contract. The authors acknowledge financial support from Spanish Ministry of Science through the projects NICER-BIOFUELS (PLEC2021-008086) financed by Next Generation European Funds and SMART-FTS (PID2021-126876OB-I00).References
- P. Verma and S. K. Samanta, in Proceedings of the First International Conference on Recent Advances in Bioenergy Research, 2016, pp. 227–243 Search PubMed.
- M. González-Castaño, M. H. Kour, J. González-Arias, F. M. Baena-Moreno and H. Arellano-Garcia, J. Environ. Manage., 2021, 300, 1–8 CrossRef PubMed.
- L. Yang, X. Ge, C. Wan, F. Yu and Y. Li, Renewable Sustainable Energy Rev., 2014, 40, 1133–1152 CrossRef CAS.
- M. Tabatabaei, M. Aghbashlo, E. Valijanian, H. Kazemi Shariat Panahi, A.-S. Nizami, H. Ghanavati, A. Sulaiman, S. Mirmohamadsadeghi and K. Karimi, Renewable Energy, 2020, 146, 1392–1407 CrossRef CAS.
- P. Ferreira-Aparicio, M. J. Benito and J. L. Sanz, Catal. Rev.: Sci. Eng., 2005, 47, 491–588 CrossRef CAS.
- S. Rasi, J. Lehtinen and J. Rintala, Renewable Energy, 2010, 35, 2666–2673 CrossRef CAS.
- M. Gustafsson and N. Svensson, J. Cleaner Prod., 2021, 278, 123535 CrossRef CAS.
- P. S. Roy, J. Song, K. Kim, C. S. Park and A. S. K. Raju, J. CO2 Util., 2018, 25, 275–282 CrossRef.
- I. Ullah Khan, M. Hafiz Dzarfan Othman, H. Hashim, T. Matsuura, A. F. Ismail, M. Rezaei-DashtArzhandi and I. Wan Azelee, Energy Convers. Manage., 2017, 150, 277–294 CrossRef CAS.
- P. Tarifa, T. R. Reina, M. González-Castaño and H. Arellano-García, Energy Fuels, 2022, 36(15), 8267–8273 CrossRef CAS PubMed.
- S. Sarker, J. J. Lamb, D. R. Hjelme and K. M. Lien, Fuel, 2018, 226, 686–697 CrossRef CAS.
- J. He, N. Han, M. Xia, T. Sun and H. Ghaebi, Int. J. Hydrogen Energy, 2023, 48, 21161–21175 CrossRef CAS.
- H. Zhang, Z. Sun and Y. H. Hu, Renewable Sustainable Energy Rev., 2021, 149, 111330 CrossRef CAS.
- A. M. Álvarez, M. Á. Centeno and J. A. Odriozola, Top. Catal., 2016, 59, 303–313 CrossRef.
- N. Kumar, M. Shojaee and J. J. Spivey, Curr. Opin. Chem. Eng., 2015, 9, 8–15 CrossRef.
- M. M. Danilova, Z. A. Fedorova, V. A. Kuzmin, V. I. Zaikovskii, A. V. Porsin and T. A. Krieger, Catal. Sci. Technol., 2015, 5, 2761–2768 RSC.
- L. Kaiwen, Y. Bin and Z. Tao, Energy Sources, Part B, 2018, 13, 109–115 CrossRef.
- R. Ma, B. Xu and X. Zhang, Catal. Today, 2019, 338, 18–30 CrossRef CAS.
- K. Jabbour, N. El Hassan, A. Davidson, S. Casale and P. Massiani, Catal. Sci. Technol., 2016, 6, 4616–4631 RSC.
- S. Arora and R. Prasad, RSC Adv., 2016, 6, 108668–108688 RSC.
- T. Stroud, T. J. Smith, E. Le Saché, J. L. Santos, M. A. Centeno, H. Arellano-Garcia, J. A. Odriozola and T. R. Reina, Appl. Catal., B, 2018, 224, 125–135 CrossRef CAS.
- Z. Zhao, P. Ren, W. Li and B. Miao, Int. J. Hydrogen Energy, 2016, 1–12 Search PubMed.
- Z. Geng, J. Gao, H. Dong and S. Wang, React. Kinet., Mech. Catal., 2022, 135, 705–721 CrossRef CAS.
- W. J. Jang, D. W. Jeong, J. O. Shim, H. M. Kim, H. S. Roh, I. H. Son and S. J. Lee, Appl. Energy, 2016, 173, 80–91 CrossRef CAS.
- B. A. V. Santos, J. M. Loureiro, A. M. Ribeiro, A. E. Rodrigues and A. F. Cunha, Can. J. Chem. Eng., 2015, 93, 510–526 CrossRef CAS.
- F. B. Noronha, M. C. Durão, M. S. Batista and L. G. Appel, Catal. Today, 2003, 85, 13–21 CrossRef CAS.
- A. Brush, E. J. Evans, G. M. Mullen, K. Jarvis and C. B. Mullins, Fuel Process. Technol., 2016, 153, 111–120 CrossRef CAS.
- V. A. Kondratenko, U. Karimova, A. A. Kasimov and E. V. Kondratenko, Appl. Catal., A, 2021, 619, 118143 CrossRef CAS.
- M. Maestri, D. G. Vlachos, A. Beretta, G. Groppi and E. Tronconi, J. Catal., 2008, 259, 211–222 CrossRef CAS.
- J. R. Rostrup-Nielsen and J. H. Bak Hansen, J. Catal., 1993, 144, 38–49 CrossRef CAS.
- L. F. Bobadilla, V. Garcilaso, M. A. Centeno and J. A. Odriozola, ChemSusChem, 2017, 10, 1193–1201 CrossRef CAS PubMed.
- K. Jabbour, J. Energy Chem., 2020, 48, 54–91 CrossRef.
- S. Yu, Y. Hu, H. Cui, Z. Cheng and Z. Zhou, Chem. Eng. Sci., 2021, 232, 116379 CrossRef CAS.
- M. Alabdullah, M. Ibrahim, D. Dhawale, J. A. Bau, A. Harale, S. Katikaneni and J. Gascon, ChemCatChem, 2021, 13, 2879–2886 CrossRef CAS.
- S. Singh, M. B. Bahari, B. Abdullah, P. T. T. Phuong, Q. D. Truong, D. V. N. Vo and A. A. Adesina, Int. J. Hydrogen Energy, 2018, 43, 17230–17243 CrossRef CAS.
- K.-J. Kim, K. Y. Kim, G. B. Rhim, M. H. Youn, Y.-L. Lee, D. H. Chun and H.-S. Roh, Chem. Eng. J., 2023, 468, 143632 CrossRef CAS.
- C. Morterra, C. Emanuel, G. Cerrato and G. Magnacca, J. Chem. Soc., Faraday Trans., 1992, 88, 339–348 RSC.
- F. Romero-Sarria, S. Garcia-Dali, S. Palma, E. M. Jimenez-Barrera, L. Oliviero, P. Bazin and J. A. Odriozola, Surf. Sci., 2016, 648, 84–91 CrossRef CAS.
- C. Morterra, G. Ghiotti, F. Boccuzi and S. Coluccia, J. Catal., 1978, 51, 299–313 CrossRef CAS.
- E. Finocchio, G. Busca, P. Forzatti, G. Groppi and A. Beretta, Langmuir, 2007, 23, 10419–10428 CrossRef CAS PubMed.
- R. Büchel, A. Baiker and S. E. Pratsinis, Appl. Catal., A, 2014, 477, 93–101 CrossRef.
- A. Beuls, C. Swalus, M. Jacquemin, G. Heyen, A. Karelovic and P. Ruiz, Appl. Catal., B, 2012, 113–114, 2–10 CrossRef CAS.
- F. Azzolina-Jury and F. Thibault-Starzyk, Top. Catal., 2017, 60, 1709–1721 CrossRef CAS.
- Z. Ferencz, K. Baán, A. Oszkó, Z. Kónya, T. Kecskés and A. Erdohelyi, Catal. Today, 2014, 228, 123–130 CrossRef CAS.
- R. Castillo, E. Dominguez Garcia, J. L. Santos, M. A. Centeno, F. Romero Sarria, M. Daturi and J. A. Odriozola, Catal. Today, 2020, 356, 390–398 CrossRef CAS.
- J. I. Di Cosimo, V. K. Díez, M. Xu, E. Iglesia and C. R. Apesteguía, J. Catal., 1998, 178, 499–510 CrossRef CAS.
- G. Busca and V. Lorenzelli, Mater. Chem., 1982, 7, 89–126 CrossRef CAS.
- D. Lorito, P. Fongarland and Y. Schuurman, Ind. Eng. Chem. Res., 2021, 60, 6698–6705 CrossRef CAS.
- K. Hadjiivanov, J.-C. Lavalley, J. Lamotte, F. Maugé, J. Saint-Just and M. Che, J. Catal., 1998, 176, 415–425 CrossRef CAS.
- E. Jiménez-Barrera, P. Bazin, C. Lopez-Cartes, F. Romero-Sarria, M. Daturi and J. A. Odriozola, Appl. Catal., B, 2018, 237, 986–995 CrossRef.
- F. Solymosi, J. Catal., 1982, 75, 78–93 CrossRef CAS.
- P. B. Rasband and W. C. Hecker, J. Catal., 1993, 139, 551–560 CrossRef CAS.
- J. C. Lavalley, J. Saussey, J. Lamotte, R. Breault, J. P. Hindermann and A. Kiennemann, J. Phys. Chem., 1990, 94, 5941–5947 CrossRef CAS.
- B. Wang, L. Song and R. Zhang, Appl. Surf. Sci., 2012, 258, 3714–3722 CrossRef CAS.
- A. M. Hernández-Giménez, J. Ruiz-Martínez, B. Puértolas, J. Pérez-Ramírez, P. C. A. Bruijnincx and B. M. Weckhuysen, Top. Catal., 2017, 60, 1522–1536 CrossRef.
- F. Romero-Sarria, L. F. Bobadilla, E. M. Jiménez Barrera and J. A. Odriozola, Appl. Catal., B, 2020, 272, 119032 CrossRef CAS.
- L. Azancot, L. F. Bobadilla, M. A. Centeno and J. A. Odriozola, Fuel, 2022, 320, 123843 CrossRef CAS.
- Q. Qian, J. Ruiz-Martínez, M. Mokhtar, A. M. Asiri, S. A. Al-Thabaiti, S. N. Basahel, H. E. van der Bij, J. Kornatowski and B. M. Weckhuysen, Chem. – Eur. J., 2013, 19, 11204–11215 CrossRef CAS PubMed.
- Q. Qian, J. Ruiz-Martínez, M. Mokhtar, A. M. Asiri, S. A. Al-Thabaiti, S. N. Basahel and B. M. Weckhuysen, Catal. Today, 2014, 226, 14–24 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |