Three-dimensional ultrathin In2O3 nanosheets with morphology-enhanced activity for amine sensing†
Abstract
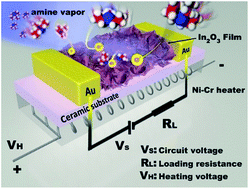
Ultrathin materials have a wide range of applications in catalysis and sensing owing to their very large surface to volume ratio and great amount of exposed active sites. Herein, we report the synthesis of three dimensional (3D) In2O3 materials with a high surface area composed of ultrathin nanosheets, ca. 2 nm, using indium glycerolate as the precursor. The structural evolution process of the indium glycerolate precursor was monitored by thermogravimetric analysis, infrared spectroscopy and transmission electron microscopy. The resulting In2O3 nanosheets show excellent amine sensing performance at room temperature because ultrathin nanosheets offer a large amount of active sites on the surface and the 3D structure adds an additional advantage of avoiding aggregation and facilitating the diffusion of the target gas. In addition, the gas sensing mechanism is also proposed in this study.

 Please wait while we load your content...
Please wait while we load your content...