Colour-tunable spiral photonic actuators†
Kwang-Un
Jeong
*ab,
Ji-Hyun
Jang
b,
Cheong Yang
Koh
b,
Matthew J.
Graham
c,
Kwang-Yong
Jin
a,
Soo-Jin
Park
ad,
Changwoon
Nah
a,
Myong-Hoon
Lee
a,
Stephen Z. D.
Cheng
c and
Edwin L.
Thomas
*b
aDepartment of Polymer-Nano Science and Technology, and Polymer Materials Fusion Research Centre, Chonbuk National University, Jeonju 561-756, Korea. E-mail: kujeong@chonbuk.ac.kr; Fax: +82 63 270 2341; Tel: +82 63 270 4633
bInstitute for Soldier Nanotechnologies and Department of Materials Science and Engineering, Massachusetts Institute of Technology, Cambridge, MA 02139, USA. E-mail: elt@mit.edu
cDepartment of Polymer Science, The University of Akron, Akron, OH 44325, USA
dDepartment of Textile Engineering, Chonbuk National University, Jeonju 561-756, Korea
First published on 4th March 2009
Abstract
Combining the multi-faceted environmental responsiveness of polymers with photonically active structures, we developed spiral photonic actuators which can reversibly change both shape and colour in response to the chemical environment.
Natural soft materials have inspired scientists and engineers to design, synthesize and fabricate intelligent soft materials and structures for various practical applications. Examples include photonic crystals from butterfly wings,1 actuators from muscles,2 and helical and spiral structures from proteins,3 which are of particular interests for the next generation of biological and electro-optical technologies.
One of the most important geometric structures in biological polymers such as proteins and deoxyribonucleic acid is the three-dimensional (3D) helical structure. In addition to biology, helical and spiral structures have been intensively studied and developed in electro-optical material science and technology.4 The simplest bio-mimetic actuator, which responds to environmental changes by converting energy to the mechanical deformation, is the 1D cantilever, which is a ubiquitous structure in the field of microelectromechanical systems. Among many materials, polymers have been key building blocks in the fabrication of actuator systems because of their ability to change shape and size in response to environmental changes such as ionic character, pH, temperature, and solvent.5 One of the most well known natural photonic crystals is the opal which has a periodic structure that can affect the propagation of electromagnetic waves in the same way as the periodic potential in a semiconductor crystal affects the motion of electrons, by defining allowed and forbidden electronic energy bands.6 The absence of allowed propagating modes for a range of wavelengths inside the structures gives rise to distinct optical phenomena such as inhibited spontaneous emission, and lossless reflection enabling low-loss-waveguiding.7 Recently, there has been significant effort put into creating colour-tunable photonic crystal sensors and actuators by changing either the periodic d-spacing of the structure or the dielectric constants of the materials.8
In this Communication, we demonstrate a method for fabricating reversible, colour-tunable, spiral, photonic actuators that respond to both hydrophilic and hydrophobic environments by changing shape through a symmetry breaking process and colour by a change in the peridicity of the photonic crystal structure. A schematic diagram of the fabrication of a reversible, colour-tunable, spiral, photonic actuator that undergoes simultaneous colour and shape changes is shown in Fig. 1a. In order to construct this device, colloidal silica spheres with diameters of 270 nm (purchased from Bangs Laboratories) were assembled, using Colvin's method several times, into thick near single crystalline opals with a face-centered cubic (FCC) structure9 (over 80% of the opal) on a fluorinated silane monolayer treated mold. Scanning electron microscope (SEM) images of the assembled silica colloidal FCC along the [111] and [01![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ] zones are shown in Fig. 1b and 1c, respectively. The reversible actuation of the device is induced by the different swelling ratios of the two strongly bonded layers in a solvent. A thermally curable poly(dimethylsiloxane) (PDMS) and a UV-curable polyurethane (PU)/2-hydroxyethyl methacrylate (HEMA) elastomeric precursor were selected as constituents of the bilayer because of their flexibility, optical transparency and dramatically different swelling responses to various solvents. The HEMA content in the hydrophilic PU/HEMA layer was optimized at 30 wt% by determining the glass transition temperature utilizing both differential scanning calorimetry and dynamic mechanical analysis as well as by the tensile properties of PU/HEMA layers at various compositions at room temperature (Fig. S1 and S2†). The next step was to infiltrate the silica colloid with the refractive index matched hydrophobic PDMS precursor (n ≅ 1.43) and then to cure it at 65 °C for 12 h. The corresponding SEM [111] zone image of the PDMS infiltrated silica colloidal crystal is shown in Fig. 1d. Finally the hydrophilic layer is applied to the hydrophobic PDMS/silica photonic crystal to form the bilayer structure. Strong adhesion between the PDMS and PU70/HEMA30 layers with very different chemistries was achieved by creating a methacryloxypropyl-trimethoxysilane monolayer on the PDMS by the monolayer self-assembly on the oxygen plasma treated PDMS surface and then applying the hydrophilic PU70/HEMA30 layer. The methacryloxypropyl-trimethoxysilane monolayer serves as a strong adhesive between the two incompatible layers. In order to ensure an even sample thickness, parafilm and a glass plate were placed on top of the mold during the UV-curing process of the hydrophilic PU70/HEMA30 layer. A photograph of the final spiral photonic actuator device in the dry state is shown in Fig. 1e. More detailed fabrication procedures for the spiral photonic actuator are in the ESI.†
] zones are shown in Fig. 1b and 1c, respectively. The reversible actuation of the device is induced by the different swelling ratios of the two strongly bonded layers in a solvent. A thermally curable poly(dimethylsiloxane) (PDMS) and a UV-curable polyurethane (PU)/2-hydroxyethyl methacrylate (HEMA) elastomeric precursor were selected as constituents of the bilayer because of their flexibility, optical transparency and dramatically different swelling responses to various solvents. The HEMA content in the hydrophilic PU/HEMA layer was optimized at 30 wt% by determining the glass transition temperature utilizing both differential scanning calorimetry and dynamic mechanical analysis as well as by the tensile properties of PU/HEMA layers at various compositions at room temperature (Fig. S1 and S2†). The next step was to infiltrate the silica colloid with the refractive index matched hydrophobic PDMS precursor (n ≅ 1.43) and then to cure it at 65 °C for 12 h. The corresponding SEM [111] zone image of the PDMS infiltrated silica colloidal crystal is shown in Fig. 1d. Finally the hydrophilic layer is applied to the hydrophobic PDMS/silica photonic crystal to form the bilayer structure. Strong adhesion between the PDMS and PU70/HEMA30 layers with very different chemistries was achieved by creating a methacryloxypropyl-trimethoxysilane monolayer on the PDMS by the monolayer self-assembly on the oxygen plasma treated PDMS surface and then applying the hydrophilic PU70/HEMA30 layer. The methacryloxypropyl-trimethoxysilane monolayer serves as a strong adhesive between the two incompatible layers. In order to ensure an even sample thickness, parafilm and a glass plate were placed on top of the mold during the UV-curing process of the hydrophilic PU70/HEMA30 layer. A photograph of the final spiral photonic actuator device in the dry state is shown in Fig. 1e. More detailed fabrication procedures for the spiral photonic actuator are in the ESI.†
![(a) Schematic diagrams of the fabricated processes for the spiral photonic actuator. Scanning electron microscope (SEM) images of silica colloidal/air photonic crystal on (b) [111] and (c) [011̄] zones, respectably, and (d) of the PDMS-imbedded silica photonic crystal on [111] zone. (e) A photograph of the spiral photonic actuator.](/image/article/2009/JM/b822980p/b822980p-f1.gif) | ||
Fig. 1 (a) Schematic diagrams of the fabricated processes for the spiral photonic actuator. Scanning electron microscope (SEM) images of silica colloidal/air photonic crystal on (b) [111] and (c) [01![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ] zones, respectably, and (d) of the PDMS-imbedded silica photonic crystal on [111] zone. (e) A photograph of the spiral photonic actuator. ] zones, respectably, and (d) of the PDMS-imbedded silica photonic crystal on [111] zone. (e) A photograph of the spiral photonic actuator. | ||
In order to evaluate the photonic stop-bands of the photonic actuator, optical reflectivity was measured utilizing an optical microscope (Zeiss Axioscop) equipped with a fiber-optic spectrometer (Stellarnet EPP2000) and a near infrared spectrometer (6000i, Varian) using a silver-coated metallic mirror as a 100% reference. Fig. 2 shows the experimental reflectivity spectra from the [111] zone of a photonic actuator device in different chemical environments. The 270 nm silica colloid/air photonic crystals exhibited ∼60% reflectivity at a wavelength of 590 nm. Since the silica colloid and the PDMS are refractive index matched (n ≅ 1.43), there is no reflection peak and the sample appears transparent after infiltration of the PDMS into the silica photonic crystal (Fig. 1e and 2b).
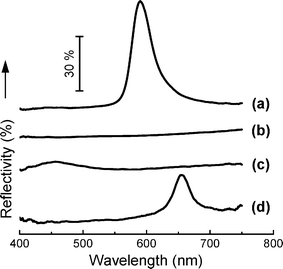 | ||
| Fig. 2 Experimental reflectivity spectra of the fabricated photonic crystals: (a) silica, (b) PDMS/silica, (c) PDMS/silica in hexane, and (d) PDMS/silica in acetic acid. | ||
The swelling of the PDMS in the appropriate solvent leads to a volume change in the photonic crystal and thus a red shift in the transmission notch. This swollen silica colloid/PDMS photonic crystal exhibited a reflection peak with its maximum intensity (∼20%) centered at 659 nm in acetic acid and reflected a broad, low intensity peak (∼7%) at 459 nm in hexane, (Fig. 2). It should be noted that the reason for the weak tranmission notches stems from the poly-crystallinity of the opal in the silica-PDMS layer as experimentally observed upon infiltration in addition to defects in the colloidal template. Using averaged swelling data (Fig. 3), we calculate the reflection peaks and d-spacings of the (110), (200), and (120) planes: 801 nm (d-spacing = 277 nm), 567 nm (d-spacing = 196 nm) and 507 nm (d-spacing = 175 nm), respectively. These calculated results match the light blue (Fig. 4a), yellow-green (Fig. 4b) and pink (Fig. 4c) colours seen at different viewing angles when the samples are back-lit in acetic acid. The functionality and sensitivity of the spiral photonic actuator was demonstrated by exposing the bilayer composite to hydrophilic acetic acid (Fig. 4a–4c), hydrophobic hexane (Fig. 4d–4f) and ethyl acetate (Fig. S5†) solvents. Kinetic swelling data for different solvents with respect to time is presented in Fig. 3. The maximum swelling ratio is defined as Vmax/V0, where Vmax is the maximum expanded volume at swelling time, t = tmax and V0, is the initial volume at t = t0. When the switch is swollen in the hydrophilic acetic acid solvent, Vmax/V0 = 1.10 for hydrophobic PDMS layer and Vmax/V0 = 2.05 for hydrophilic PU70/HEMA30. The asymmetric volume change results in a shape change and the swelling of the PDMS leads to an expansion of the photonic crystal structure and a refractive index change resulting in a colour change. The spiral wrapped around itself 3 to 4 times at this swelling ratio and switch length. The increased refractive index contrast enabled the structure to reflect light with the wavelength of the notch dependent on the optical thickness of the expanded lattice. In acetic acid, a red right-handed spiral forms as shown in Fig. 4a. The determination of handedness is discussed in the supplementary information (Fig. S4†). The silica colloid/PDMS photonic crystal actuator returned to a transparent planar structure when the acetic acid completely evaporated. This colour-tunable photonic actuator is fully reversible up to more than 5 times without any optical and mechanical property changes.
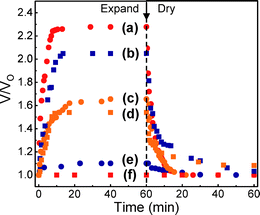 | ||
| Fig. 3 The expanded volume ratio (V/V0) with respect to swelling time (a) PDMS in hexane, (b) PU70/HEMA30 in acetic acid, (c) PDMS in ethyl acetate, (d) PU70/HEMA30 in ethyl acetate, (e) PDMS in acetic acid, and (f) PU70/HEMA30 in hexane. | ||
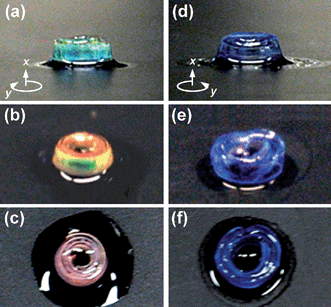 | ||
| Fig. 4 A right-handed spiral photonic actuator in acetic acid: (a) side view, (b) 45°-tilted view, and (c) top view, respectively. A left-handed spiral photonic actuator in hexane: (d) side view, (e) 45°-tilted view, and (f) top view, respectively. The geometric description of the sample is identical to that of Fig. 1a. | ||
When the colourless photonic crystal switch is swollen in hydrophobic hexane solvent, a bluish left-handed spiral structure is formed as shown in Fig. 4d–4f. The Vmax/V0 of the hydrophobic PDMS layer in hydrophobic hexane is 2.28, while the Vmax/V0 of hydrophilic PU70/HEMA30 in hexane is 1.00. In this case, the photonic crystal structure in PDMS is also extensively expanded resulting in a loss of long-range spatial correlation between the silica spheres thereby suppressing Bragg diffraction. Consequently, the blue colour is caused by the scattering of uncorrelated dielectric particles. This explains the small broad reflectivity peak and the independence of colour on viewing angle (Fig. 4d–4f). When the hexane is completely evaporated, the silica colloid/PDMS PC actuator becomes a transparent planar structure. This suggests that the loss of correlation is due to the swelling gradient which is not random, local, and irreversible swelling. This indicates that the colour functionality of the photonic crystal switch can operate by two separate reversible mechanisms depending on the swelling differential and the placement of the opal in the bilayer.
In order to demonstrate the functionality and sensitivity of the switch to hydrophobicity, ethyl acetate was selected as a solvent (Fig. S5†). The Vmax/V0 of the hydrophobic PDMS layer in ethyl acetate is 1.65, and the Vmax/V0 of hydrophilic PU70/HEMA30 in ethyl acetate is 1.55. In this case the PDMS layer is significantly swollen but the small swelling differential translates into a smaller curvature. The result is a left-handed spiral structure where the reflected colours change with viewing angle like in acetic acid. In addition, kinetic swelling experiments as shown in Fig. 3 indicate that each solvent can completely swell the polymers within minutes. This is sufficiently fast for the desired switching speeds needed for practical application. Faster speeds could be achieved by using materials or solvents with a greater swelling differential or thinner samples.
In summary, we show that reversibly colour-tunable spiral photonic actuators with both dimensional and optical functionalities that respond to environmental chemistry can be constructed. When the transparent photonic actuator is swollen in hydrophilic acetic acid, right-handed spirals that exhibit angularly dependent colours from Bragg diffraction are formed. However, when the transparent photonic actuator is swollen in hydrophobic hexane solvent, a left-handed spiral acuator with an angularly independent bluish colour is formed. After deswelling, the spiral photonic actuators returned back to the transparent planar state. These colour-tunable, reversible spiral photonic switches can be useful as mechanical actuators and electrical devices as well as optical components.
Acknowledgements
This work was mainly supported by KRF-2007-331-D00119, Korea, Institute for Soldier Nanotechnologies of the U.S. Army Research Office (W911NF-07-D-0004), NSF CMS-0556211, DMR-0804449, DMR-0516602 and the Collaborative Center for Polymer Photonics, AFOSR.References
- (a) P. Vukusic, J. R. Sambles and C. R. Lawrence, Nature, 2000, 404, 457 CrossRef CAS; (b) M. Maldovan and E. L. Thomas, Nature Mater., 2004, 3, 593 CrossRef CAS; (c) N. V. Dziomkina and G. J. Vancso, Soft Matter, 2005, 1, 265 RSC; (d) A. Yethiraj, Soft Matter, 2007, 3, 1099 RSC; (e) I. Musevic and M. Skarabot, Soft Matter, 2008, 4, 195 RSC.
- (a) S. V. Ahir and E. M. Terentjev, Nature Mater., 2005, 4, 491 CrossRef CAS; (b) V. H. Ebron, J. W. Yang, D. J. Seyer, M. E. Kozlov, J. Y. Oh, H. Xie, J. Razal, L. J. Hall, J. P. Ferraris, A. G. MacDiarmid and R. H. Baughman, Science, 2006, 311, 1580 CrossRef CAS; (c) R. D. Rey, Soft Matter, 2007, 3, 1349 RSC; (d) S. Haider, S. Y. Park and S. H. Lee, Soft Matter, 2008, 4, 485 RSC; (e) P. D. Thornton, R. J. Mart, S. J. Webb and R. V. Ulijn, Soft Matter, 2008, 4, 821 RSC.
- (a) S. F. Mason, Nature, 1984, 311, 19 CAS; (b) C. Y. Li, S. Z. D. Cheng, J. J. Ge, F. Bai, J. Z. Zhang, I. K. Mann, F. W. Harris, L.-C. Chien, D. Yan, T. He and B. Lotz, Phys. Rev. Lett., 1999, 83, 4558 CrossRef CAS; (c) C. Y. Li, S. Z. D. Cheng, X. Weng, J. J. Ge, F. Bai, J. Z. Zhang, B. H. Calhoun, F. W. Harris, L.-C. Chien and B. Lotz, J. Am. Chem. Soc., 2001, 123, 2462 CrossRef CAS; (d) H. Shen, K.-U. Jeong, H. Xiong, M. J. Graham, S. Leng, J. X. Zheng, H. Huang, M. Guo, F. W. Harris and S. Z. D. Cheng, Soft Matter, 2006, 2, 232 RSC; (e) R. J. Mart, R. D. Osborne, M. M. Stevens and R. V. Ulijn, Soft Matter, 2006, 2, 822 RSC; (f) J. L. Gornall and .E M. Terentjev, Soft Matter, 2008, 4, 544 RSC; (g) G. Zubay, Biochemistry, Macmillan Publishing Company, New York, 2nd edn, 1988, pp. 845–1151 Search PubMed.
- (a) R. Purrello, Nature Mater., 2003, 2, 216 CrossRef CAS; (b) K.-U. Jeong, S. Jin, J. J. Ge, B. S. Knapp, M. J. Graham, J. Ruan, M. Guo, H. Xiong, F. W. Harris and S. Z. D. Cheng, Chem. Mater., 2005, 17, 2852 CrossRef CAS; (c) K.-U. Jeong, B. S. Knapp, J. J. Ge, S. Jin, M. J. Graham, H. Xiong, F. W. Harris and S. Z. D. Cheng, Macromolecules, 2005, 38, 8333 CrossRef CAS; (d) K.-U. Jeong, B. S. Knapp, J. J. Ge, M. J. Graham, Y. Tu, S. Leng, H. Xiong, F. W. Harris and S. Z. D. Cheng, Polymer, 2006, 47, 3351 CrossRef CAS; (e) K.-U. Jeong, D.-K. Yang, M. J. Graham, S. W. Kuo, B. S. Knapp, F. W. Harris and S. Z. D. Cheng, Adv. Mater., 2006, 18, 3229 CrossRef CAS; (f) K.-U. Jeong, B. S. Knapp, J. J. Ge, S. Jin, M. J. Graham, F. W. Harris and S. Z. D. Cheng, Chem. Mater., 2006, 18, 680 CrossRef CAS; (g) D.-K. Yang, K.-U. Jeong and S. Z. D. Cheng, J. Phys. Chem. B, 2008, 112, 1358 CrossRef CAS.
- (a) R. D. Kamien, Science, 2007, 315, 1083 CrossRef CAS; (b) Y. Klein, E. Efrati and E. Sharon, Science, 2007, 315, 1116 CrossRef CAS; (c) Y. Osada, H. Okuzaki and H. Hori, Nature, 1992, 355, 242 CrossRef CAS; (d) E. Smela, Adv. Mater., 2003, 15, 481 CrossRef CAS; (e) S. J. Kim, G. M. Spinks, S. Prosser, P. G. Whitten, G. G. Wallace and S. I. Kim, Nature Mater., 2006, 5, 48 CrossRef CAS.
- J. Y. Huang, X. D. Wang and Z. L. Wang, Nano Lett., 2006, 6, 2325 CrossRef CAS.
- J. D. Jonannopoulos, R. D. Meade, J. M. Winn, Photonic Crystals, Princeton University, 1995 Search PubMed.
- (a) J. H. Holtz and S. A. Asher, Nature, 1997, 389, 829 CrossRef CAS; (b) J. M. Weissman, H. B. Sunkara, A. S. Tse and S. A. Asher, Science, 1996, 274, 959 CrossRef CAS; (c) M. Fialkowski, A. Bitner and B. A. Grzybowski, Nature Mater., 2005, 4, 93 CrossRef CAS.
- (a) P. Jiang, J. F. Bertone, K. S. Hwang and V. L. Colvin, Chem. Mater., 1999, 11, 2132 CrossRef CAS; (b) P. Jiang, K. S. Hwang, D. M. Mittleman, J. F. Bertone and V. L. Colvin, J. Am. Chem. Soc., 1999, 121, 11630 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed descriptions of materials, equipment, and sample preparation as well as the fabrication procedure and definition of handedness of spiral photonic actuator. See DOI: 10.1039/b822980p |
| This journal is © The Royal Society of Chemistry 2009 |
