Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
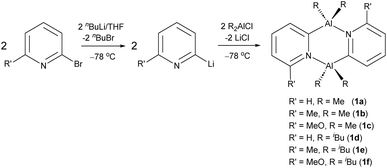
Oligomerisation and stereoselective polymerisation of alkenes and alkynes using pyridyl-based Al(III) catalysts
Dipanjana Choudhury†
 ,
Richard Danylyuk†,
Alexandros Terzopoulos
,
Richard Danylyuk†,
Alexandros Terzopoulos ,
Natalie S. Potter
,
Natalie S. Potter ,
Oren A. Scherman
,
Oren A. Scherman *,
Jonathan M. Goodman
*,
Jonathan M. Goodman * and
Dominic S. Wright
* and
Dominic S. Wright *
*
Yusuf Hamied Department of Chemistry, University of Cambridge, Lensfield Rd., Cambridge CB2 1EW, UK. E-mail: oas23@cam.ac.uk; jmg11@cam.ac.UK; dsw1000@cam.ac.uk
First published on 21st January 2026
Abstract
Dealkylation of the dimers [R2Al(2-py′)]2 (2-py′ = 6-substituted pyridyl, R = Me, iBu) with [Ph3C][B(C6F5)4] gives the putative cations [RAl(2-py′)2(µ-R)AlR]+ which can polymerise a range of alkenes with a high degree of stereoregularity (syndiotacticity in the case of polystyrene) and which cylotrimerise terminal alkynes to trisubstituted benzenes and a fulvene. This is the first report of stereoslective Al(III) polymerisation and of the cyclotrimerisation of alkynes by static catalysis using a main group metal.
Introduction
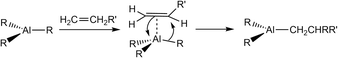
Sustainability is a central theme of concurrent chemistry, from alternative means of power generation and storage to chemical synthesis. In the area of catalysis, specifically, there is a major drive to move away from traditional, precious transition metal catalysts1,2 to more sustainable first-row transition metal and main group alternatives.3–8 Single-site aluminium catalysts have received significant attention within this context, as aluminium is the most abundant metal in the Earth's crust and offers associated cost advantages for industrial-scale applications over other main-group reagents.9–11 While research in this area is dominated by the search for redox catalysis using Al(I) (based on the Al(I)/Al(III) couple),12,13 the more mature area of static catalysis based on Al(III) (in which the metal oxidation state does not change) has already been successfully applied in a range of transition metal-mimetic reactions, including hydrogenation, dehydrocoupling, phosphination, hydrosilylation, hydroboration, as well as alkene, alkyne, diene and allylic ylide polymerisations.9,10,14–16From its earliest beginnings with reactions such as the carbalumination growth reaction (Scheme 1),13 the high Lewis acidity of Al(III) has made it especially useful in alkene and alkyne activation. In the absence of accessible d-orbitals for back-donation, alkene bonding to Al(III) primarily involves π-donation to the vacant p-orbital of the metal, reducing the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond strength and activating it to nucleophilic attack by another alkene molecule. A large range of catalytic homo- and co-polymerisations of olefins involving Al(III) has been reported, and these have been the subject of several reviews.10,17 However, there are no examples involving control of the tacticity of the polymer backbone. In the absence of stereo-control, atactic polymers are produced, with a random arrangement of the R-groups, while stereocontrol can produce either isotactic (R-groups with the same orientation along the polymer chain) or syndiotactic (R-groups alternating along the polymer backbone) polymers (Fig. 1). Controlling tacticity is important as it directly affects the thermal and mechanical properties of polymeric materials. Conventionally, Lewis acidic Zr(IV) catalysts have been used to control tacticity of polyolefins, with the local ligand symmetry around the metal (in addition to the primary interaction with the growing polymer) dictating the face of the alkene incorporated (si and/or re) during chain propagation.18,19
C bond strength and activating it to nucleophilic attack by another alkene molecule. A large range of catalytic homo- and co-polymerisations of olefins involving Al(III) has been reported, and these have been the subject of several reviews.10,17 However, there are no examples involving control of the tacticity of the polymer backbone. In the absence of stereo-control, atactic polymers are produced, with a random arrangement of the R-groups, while stereocontrol can produce either isotactic (R-groups with the same orientation along the polymer chain) or syndiotactic (R-groups alternating along the polymer backbone) polymers (Fig. 1). Controlling tacticity is important as it directly affects the thermal and mechanical properties of polymeric materials. Conventionally, Lewis acidic Zr(IV) catalysts have been used to control tacticity of polyolefins, with the local ligand symmetry around the metal (in addition to the primary interaction with the growing polymer) dictating the face of the alkene incorporated (si and/or re) during chain propagation.18,19
 | ||
| Scheme 1 The fundamental steps involved in the carbalumination growth reaction. Further 1,2-insertion of alkenes (giving longer chains) is possible but not shown. | ||
Surprisingly few studies of main group metal systems have involved tacticity control in alkene polymerisation, despite corresponding recent progress on aluminium-mediated cis/trans-stereoregular diene and allylic ylide polymerisations by Hadjichristidis et al.15,16 For polyolefins, based on the conventional ligand design of common Zr(IV) catalysts, Harder et al. explored the effects of ligand symmetry and steric bulk on the tacticity of polystyrene using a series of homo- and heteroleptic benzyl/fluorenyl calcium catalysts, in the best case achieving around 95% syndiotactic polystyrene.20–23 However, in the absence of significant Lewis acidity of the metal and due to the high polarity of Ca–C bonds, a living anionic polymerisation mechanism operates. Clearly, the greater covalent nature of Al–C bonds together with the high Lewis acidity of Al(III) should provide a much more rigid local ligand environment than s-block metals to support cationic stereoselective alkene insertion.
We recently proposed that C2-symmetric trans-[RAl(2-py′)2(µ-R)AlR]+ cations (2) (2-py′ = 6-substituted pyridyl, R = Me, iBu), generated by the dealkylation of the dimers trans-[R2Al(2-py′)]2, (1) might be useful in stereocontrolled alkene polymerisation, via a co-operative mechanism involving both Al(III) centres (Scheme 2).24 We show here that a high degree of syndiotacticity can indeed be obtained in the polymerisation of styrene using the readily prepared cations 2 (depending mainly on the Al-bonded R-group). These cations are also active in the catalytic cyclotrimerisation of terminal alkynes (phenyl and t-butyl acetylene) and can induce a high level of stereoregularity in the polymerisation of 1-hexene. To the best of our knowledge, this is the first report of stereocontrolled polymerisation of alkenes and the cyclotrimerisation of alkynes using an Al(III) catalyst.
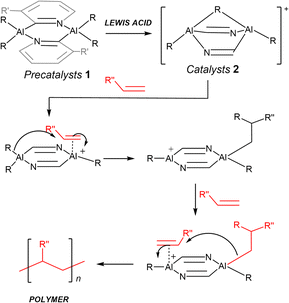 | ||
| Scheme 2 Proposed mechanism of polymerisation of terminal alkenes using the precatalyst 1. Note: the structure of the catalysts 2 is derived from DFT calculations reported by us earlier.24 | ||
Results and discussion
Generation of catalysts 2
In our previous report, we showed that Al(III) dimers of the type [R2Al(2-py′)]2 (2-py′ = 6-substituted pyridyl, R = Me, iBu) are readily prepared by the one-step reaction of dialkylaluminium chlorides with 2-lithiopyridines (2-Li-py′) (Scheme 3).24 It was found that installing Me-groups at the 6-position of the bridging pyridyl rings increases the thermodynamic stability of the desired trans isomers (the precatalysts used in the current study) on steric grounds.Our initial studies explored the demethylation of unsubstituted dimer [Me2Al(2-py)]2 (1a). NMR-scale reactions of 1 with B(C6F5)3 (BCF) in d6-benzene indicated incomplete reaction (1H NMR), with the formation of [(C6F5)3BMe]− and [(C6F5)2BMe] (11B NMR). Although encouraging in that demethylation of 1a had clearly occurred, the formation of [(C6F5)2BMe] indicates that the [(C6F5)3BMe]− anion is not stable in the presence of the cation. Similar observations had been made earlier by Smith et al. on demethylation of (TTP)-AlMe2 (TTP–H = 2-(p-tolylamino)-4-(p-tolylimino)-2-pentene) with BCF, with a C6F5 group from BCF being transferred to the Al(III) producing (TTP)AlMe(C6F5).25,26 Consequently, we turned to the use of [Ph3C][B(C6F5)4] which has previously been employed in the dealkylation of a range of Al(III) β-diketiminate complexes.27 As previously reported by us, 1H NMR studies of the dealkylation of 1a and 1c in d6-benzene or d8-toluene clearly showed the formation of Ph3CMe with no apparent decomposition of the [B(C6F5)4]− anion.24 This finding is also supported by in situ 11B and 19F NMR studies which only show the presence of the [B(C6F5)4]− anion and no B(C6F5)3 decomposition product in any of the Lewis-acid-activated precatalyst solutions. In the case of the iBu derivatives, dealkylation results in the formation of Ph3CH and the evolution of isobutene (for 1H NMR spectra, see SI Fig. S3 and S4).28 This Lewis acid was therefore used in all of the subsequent polymerisation and cyclotrimerisation studies.
Before moving on to the discussion of studies on alkene and alkyne activation, it is important to note that we have been unable to recrystallise any of the cationic catalysts 2 using a range of conditions (varying solvents, temperatures, and adding strong donors to stabilise the cationic Al(III) centres), with all attempts producing semisolids (even on removal of the reaction solvents under vacuum). As a result, the true nature of these species is not known at this stage. Our previously reported DFT calculations, however, suggest that dealkylation leads to alkyl-bridged arrangements [RAl(2-py′)2(µ-R)AlR]+ in model monomers (as depicted in Scheme 2).24 Importantly, the suggested mechanism of polymerisation involving co-operative transfer between the two bridged Al(III) centres of the cations 2 (see Mechanism of Polymerisation, later) accounts for the high degree of stereoselectivity observed. This is in stark contrast to control experiments using [Ph3C][B(C6F5)4] in the absence of the precatalyst dimers (see SI experimental and Fig. S5) and supports the suggested nature of the active catalysts.
Polymerisation of alkenes
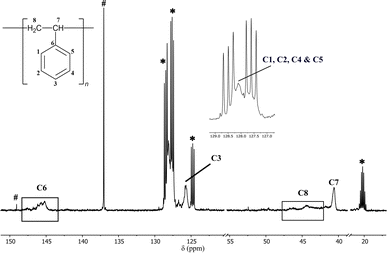 | ||
| Fig. 2 13C NMR (25 °C, d8-toluene, 126 MHz) spectrum of atactic polystyrene prepared using a 0.5 mol% precatalyst loading of 1a and [Ph3C][B(C6F5)4] at room temperature. The resonances are broad and overlapping and have been assigned based on existing literature.21,30,31 The signals marked as # can be best attributed to unreacted [Ph3C][B(C6F5)4], remnant toluene or potentially any unreacted styrene. *d8-toluene. | ||
Reasoning that the Al-bonded Me-groups of 1a may not provide sufficient steric influence over the cationic Al(III) reaction site, we turned to the more sterically hindered dimer [iBu2Al(6-Me-2py)]2 (1e). In 1e, the steric effects at the Al(III) and pyridyl rings are increased ([iBu2Al(2-py)]2 (1d) was not used because it is a semi-solid at room temperature). The reactions at −15 °C for 48 h using 0.5–10 mol% loadings of 1e and the initiator gave similar stretchable materials after quenching, removal of volatiles and washing with methanol. Very encouragingly, although the polystyrene produced in all of these reactions contain atactic components, they also contain syndiotactic components, as confirmed by 13C NMR (Fig. 3) and IR spectroscopy (Fig. 4). The ratio of atactic to syndiotactic components could not be determined accurately because of the overlapping of the characteristic resonances in the 1H NMR spectrum. However, analysis of the 13C NMR spectrum allowed a clearer indication of the tacticity.
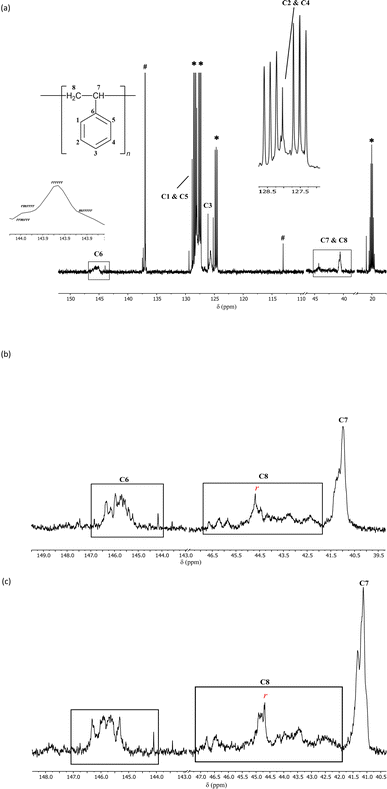 | ||
| Fig. 3 (a) 13C NMR (25 °C, d8-toluene, 126 MHz) spectrum of atactic + syndiotactic polystyrene prepared using 0.5 mol% catalytic loading of precatalyst 1e and [Ph3C][B(C6F5)4] at −15 °C depicting the signals for the aromatic ipso carbon and the methylene carbon. The broad signals for C6 and C8 represent the atactic component, while the sharper peaks represent the syndiotactic component. The sharp signal labelled with a red r marks the signal depicting a 100% probability of having r diads.21,32 The signals marked as # can be best attributed to unreacted [Ph3C][B(C6F5)4], remnant toluene or potentially any unreacted styrene. *d8-toluene. (b) 13C NMR (100 °C, d2-tetrachloroethane, 126 MHz, 1000 scans) showing the signals for the ipso carbon C6 and the methylene carbon C8. The sharp signal labelled with a red r marks the signal depicting a 100% probability of having r diads.21,32 (c) 13C NMR (100 °C, d4-o-dichlorobenzene, 126 MHz, 1000 scans) showing the signals for the ipso carbon C6 and the methylene carbon C8. The sharp signal labelled with a red r marks the signal depicting a 100% probability of having r diads.21,32 | ||
While the specific 13C NMR assignments for syndiotactic polystyrene have been debated, there is a wealth of literature on tacticity-based assignments.31,33–39 Here the initial tacticity-based assignments stated by Ishihara et al. were followed.30 For further confirmation, 13C NMR spectroscopy was conducted under the exact conditions outlined by Harder et al. (1000 scans at 100 °C in d2-tetrachloroethane and d4-o-dichlorobenzene; Fig. 3b and c, respectively).21 The presence of sharp peaks representing the aromatic ipso C (C6) in the δ 143.9–146 ppm range indicate the presence of a majority of r diads for up to heptad level assignments.21,38 For the methylene C (C8), sharp peaks at δ 44.29 ppm (Fig. 3a), 44.67 ppm (Fig. 3b) and 44.68 ppm (Fig. 3c) represent a polymeric chain with all r diads (from tetrad to hexad level).21,38 It can therefore be concluded that the use of 1e introduces a high level of regioregularity to the polymer chain, specifically one with a majority of r diads, thereby creating a large syndiotactic component (for further detailed discussion of assignments, see SI). Owing to the significantly lower molecular weight of these polymeric products and the low resolution of the NMR spectrum, assignments at levels higher than a diad were not attempted. Interestingly, for the 2.5 mol% loading of 1e, the 13C NMR spectrum of the polymeric product indicated an additional isotactic component, which appears further upfield of the syndiotactic and atactic resonances (ESI).40
Using [Me2Al(6-Me-2-py)]2 (1b), [Me2Al(6-MeO-2-py)]2 (1c) and [iBu2Al(6-MeO-2-py)]2 (1f) as precatalysts (0 °C, 1 h) gave only atactic polystyrene for 1b and 1c, while 1f gave a mixture of atactic and syndiotactic (sharp 13C NMR peaks denoting syndiotactic linkages). The presence of the syndiotactic component in 1f was also confirmed by IR spectroscopy (ESI).22,32,33 The slightly higher temperature (compared to the aforementioned −15 °C) was used to encourage a faster reaction and potentially higher molecular weight products.
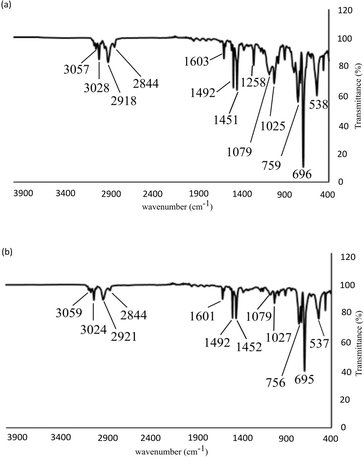
Fig. 4 compares the IR spectra of the polymeric products obtained using 1c (atactic) and 1e (syndiotactic). While the two polymers exhibit similar bands in their IR spectra for aliphatic and aromatic C–H and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching, and C–H bending vibrations, indicating a common polymeric backbone, most significantly, the weak band at 1258 cm−1 present in the polymer obtained from 1e is absent in that obtained from 1c. Ishihara et al. have reported that a peak at around 1220 cm−1 (close to that observed here) is diagnostic of syndiotactic polystyrene.30 The relative sharpness of the band at 538 cm−1 for the polystyrene obtained using 1e compared to that from 1c (537 cm−1) also confirms syndiotacticity as this peak is associated with the local trans–trans conformations. This conclusion is also supported by the observation of a sharper, more intense band at 1079 cm−1 for polystyrene obtained from 1e. The absence of the signature peaks for isotactic polystyrene (at 1364, 1314, 1297, and 1185 cm−1) discounts the presence of an isotactic component in either of the polymers obtained from 1c and 1e.41
C stretching, and C–H bending vibrations, indicating a common polymeric backbone, most significantly, the weak band at 1258 cm−1 present in the polymer obtained from 1e is absent in that obtained from 1c. Ishihara et al. have reported that a peak at around 1220 cm−1 (close to that observed here) is diagnostic of syndiotactic polystyrene.30 The relative sharpness of the band at 538 cm−1 for the polystyrene obtained using 1e compared to that from 1c (537 cm−1) also confirms syndiotacticity as this peak is associated with the local trans–trans conformations. This conclusion is also supported by the observation of a sharper, more intense band at 1079 cm−1 for polystyrene obtained from 1e. The absence of the signature peaks for isotactic polystyrene (at 1364, 1314, 1297, and 1185 cm−1) discounts the presence of an isotactic component in either of the polymers obtained from 1c and 1e.41
Differential scanning calorimetry (DSC) measurements were performed to analyse the thermal behaviour of the polystyrene samples. The DSC traces revealed a glass transition temperature (Tg) of 95.34 °C for the atactic polymeric product obtained from 1b and 105.12 °C for the syndiotactic product obtained using 1f (SI Fig. S23 and S24). These values agree with those reported for atactic and syndiotactic polystyrene, respectively, in the literature, complementing the NMR and IR characterisation.42,43
The results of the studies of 1a–c, 1e and 1f indicate that steric effects arising from the Al-bonded R-groups have the most significant result on stereoselectivity (rather than the R′-groups on the pyridyl rings). Turnover numbers (TONs) in the range 82–164 were determined for various precatalysts at different temperatures and loadings, illustrating that these reactions are indeed catalytic. These low values, however, suggest that the catalytic species themselves have a relatively short lifetime.
Table 1 shows the results of Gel Permeation Chromatography (GPC) studies of polystyrene formed using the range of precatalysts explored (see SI Fig. S8–S18 for GPC traces).
| Precatalysts 1 | Loading (mol%) | Temp. (oC) | Time (h) | Mn (g mol−1) | Mw (g mol−1) | Đ |
|---|---|---|---|---|---|---|
| a Mn = number average molecular weight, Mw = weight average molecular weight, Đ = dispersity determined by GPC (DMF, 0.1% LiBr, 40 °C) relative to polystyrene standards. | ||||||
| 1a | 0.5 | RT | 1 | 2191 | 6514 | 2.9 |
| 2.5 | RT | 1 | 1689 | 3595 | 2.1 | |
| 0.5 | −15 | 1 | 5103 | 8687 | 1.7 | |
| 2.5 | −15 | 1 | 5142 | 6030 | 1.2 | |
| 1b | 0.5 | 0 | 1 | 2118 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 740 740 |
5.0 |
| 1c | 0.5 | 0 | 1 | 3515 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 602 602 |
3.9 |
| 1e | 0.5 | −15 | 48 | 5123 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 376 376 |
2.0 |
| 2.5 | −15 | 48 | 1411 | 2959 | 2.1 | |
| 5.0 | −15 | 48 | 2045 | 5307 | 2.6 | |
| 10.0 | −15 | 1 | 2613 | 3195 | 1.2 | |
| 1f | 0.5 | 0 | 1 | 1961 | 5929 | 3.0 |
All the catalysts (1a–c, 1e and 1f) produce low molecular weight polystyrene (Mn < 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1), although it should be noted that the reaction conditions in Table 1 are not optimised. These molecular weights are significantly lower than obtained using either transition metal or calcium-based systems (ca. Mn = 105 g mol−1).29 Nonetheless, low molecular weight polystyrene has a number of important applications.40,44,45 Despite the limited data available so far, some overall conclusions can be made. Perhaps most significantly, it is clear from the data for 1a and 1e at −15 °C that the characteristics of the polymers produced (i.e., Mn, Mw, and Đ) depend on the specific catalyst employed, illustrating that they do not involve a common catalytic species. The trend in the data for 1a and 1e shows that highest molecular weights are generally achieved at lower catalyst loadings and lower temperatures. This is as expected since a smaller number of polymers are generated initially, in the presence of a large excess of monomers, along with greater control of the polymerisation process at lower temperature.
000 g mol−1), although it should be noted that the reaction conditions in Table 1 are not optimised. These molecular weights are significantly lower than obtained using either transition metal or calcium-based systems (ca. Mn = 105 g mol−1).29 Nonetheless, low molecular weight polystyrene has a number of important applications.40,44,45 Despite the limited data available so far, some overall conclusions can be made. Perhaps most significantly, it is clear from the data for 1a and 1e at −15 °C that the characteristics of the polymers produced (i.e., Mn, Mw, and Đ) depend on the specific catalyst employed, illustrating that they do not involve a common catalytic species. The trend in the data for 1a and 1e shows that highest molecular weights are generally achieved at lower catalyst loadings and lower temperatures. This is as expected since a smaller number of polymers are generated initially, in the presence of a large excess of monomers, along with greater control of the polymerisation process at lower temperature.
The 1H NMR spectra of the poly(1-hexene) products obtained using 1a and 1f reveal a level of stereoregularity mixed with atactic components (SI), which was supported by 13C NMR spectroscopy.47,48 The six expected resonances for the monomer fragment are observed in the 13C NMR spectrum of poly(1-hexene) produced using 1f, in addition to several other broad peaks. The sharpness of the six primary peaks indicates a level of stereoregularity and, based on the values reported in the literature, the peak at δ 34.46 ppm can be attributed to the rr triad, thereby indicating that the polymer is primarily syndiotactic.49 As was seen in the above studies of polystyrene, the smaller resonances adjacent to the primary peaks can be attributed to monomer misinsertions (highlighted in Fig. 5). These minor resonances suggest the incorporation of some carbon chains with different methylene-backbone and side-chain lengths (unlike the regular C4-chains expected for ‘standard’ poly(1-hexene) chain synthesised using the Ziegler–Natta process).
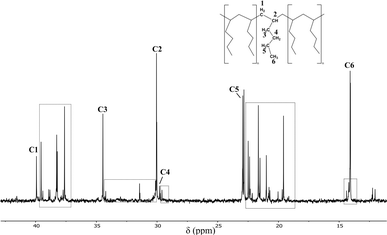 | ||
| Fig. 5 13C NMR (25 °C, d-chloroform, 126 MHz) spectrum of poly(1-hexene) prepared using a 10 mol% catalytic loading of 1f at room temperature. # vacuum grease. | ||
The 1H NMR spectra of polycyclohexene produced using 1a and 1f show the expected three resonances in the δ 0.9–2.5 ppm range for the CH2 units within the cyclohexane rings (some unreacted cyclohexene is also observed). Farona and Tsonis reported thirty resonances in the δ 13.9–35.9 ppm range in the 13C NMR spectrum of polycyclohexene, whereas we observe twenty-five clear signals.46 Thus, based on the NMR spectra it is clear that homopolymerisation has occurred in both reactions. IR spectroscopy also supports the formation of polycyclohexene using both precatalysts, based on the work of Farona and Tsonis; specifically, the observation of two characteristic bands at 906 and 890 cm−1 (lit. 890 and 850 cm−1; see SI Fig. S7for the IR spectrum).22 A similar conclusion to this previous report can be made here, that the polycyclohexene products are either made up of repeating 1,2-cylohexane units or a combination of 1,2-,1,3-, and/or 1,4-repeating units.46
GPC data (Table 2, SI Fig. S19–S22) show that similar, low molecular weight poly(1-hexene) and polycyclohexene are produced using 1a and 1f, which are highly monodisperse. Despite the low molecular weight of polycyclohexene obtained, this is on the higher side of what is previously reported in the literature. The results of DSC thermoanalytical measurements for the two polymers are included in Table 2 and the traces shown in the SI (Fig. S25–S28); although inconclusive with regards to observing side-chain crystallisation, the glass transition temperature obtained for poly(1-hexene), for which previous DSC data have been reported, matches the literature values.50,51
| Monomer | 1a | 1f | ||||||
|---|---|---|---|---|---|---|---|---|
| Mn (g mol−1) | Mw (g mol−1) | Đ | Tg (°C) | Mn (g mol−1) | Mw (g mol−1) | Đ | Tg (°C) | |
| a Mn = number average molecular weight, Mw = weight average molecular weight, Đ = dispersity determined by GPC (DMF, 0.1% LiBr, 40 °C) relative to polystyrene standards. Tg = glass transition temperature obtained using DSC. | ||||||||
| 1-Hexene | 4641 | 4899 | 1.1 | −57.28 (ref. 51) | 4518 | 4761 | 1.1 | −56.24 (ref. 50 and 51) |
| Cyclohexene | 4883 | 5078 | 1.0 | −50.62 | 5100 | 5380 | 1.0 | −49.28 |
Cyclotrimerisation of alkynes
The cyclotrimerisation of alkynes has long been the domain of transition metal catalysts52–56 and there has been surprising little work reported using non-d-block catalysts. The first examples of catalytic alkene trimerisation of this type were seen for terminal ethynyl ketones (RCOC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH) which trimerise to 1,3,5-benzenes in the presence of dimethylamine via enamine intermediates.57 In contrast, cyclotrimerisation reactions involving hexachlorodisilane (Cl6Si2) occur by a stepwise (silicon-centred) radical pathway, producing 1,3,5-benzenes.58 The most closely related system to transition metals involves the digermyne precatalyst Tbb–Ge
CH) which trimerise to 1,3,5-benzenes in the presence of dimethylamine via enamine intermediates.57 In contrast, cyclotrimerisation reactions involving hexachlorodisilane (Cl6Si2) occur by a stepwise (silicon-centred) radical pathway, producing 1,3,5-benzenes.58 The most closely related system to transition metals involves the digermyne precatalyst Tbb–Ge![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Ge–Tbb (Tbb = 2,6-{(Me3Si)2CH}2-C6H3) which cyclotrimerises terminal alkynes regioselectively to the 1,2,4-isomer and occurs by redox catalysis involving the Ge(II)/Ge(IV) couple.59
Ge–Tbb (Tbb = 2,6-{(Me3Si)2CH}2-C6H3) which cyclotrimerises terminal alkynes regioselectively to the 1,2,4-isomer and occurs by redox catalysis involving the Ge(II)/Ge(IV) couple.59
In our studies of the cyclotrimerisation of terminal alkynes, we again selected precatalysts 1a and 1f and focused initially on phenylacetylene. Cyclotrimerisation to mixtures of (minor) 1,3,5- and (major) 1,2,4-triphenylbenzene60 occurred readily using a range of precatalyst loadings (0.5–10 mol%), reaction times (1–24 h) and temperatures (from room temperature to 60 °C) in THF (Scheme 4a). Although the 1H NMR spectra (Fig. 6) show some distinct sets of resonances which are consistent with the presence of a combination of trimers in the above samples, these overlap extensively making it difficult to determine the relative amount of the major component. The relative intensities of the peaks, however, confirm that 1,2,4-triphenylbenzene is the major product in our case. Sub-stoichiometric TONs were calculated in the range 10–22 for these reactions.
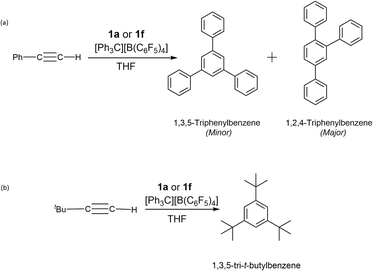 | ||
| Scheme 4 Products of the reactions of (a) phenylacetylene, and (b) t-butyl-acetylene with 1a or 1f/[Ph3C][B (C6F5)4]. | ||
Further studies show that t-butyl acetylene is also cyclotrimerised, this time into 1,3,6-tri-t-butylfulvene at 30 °C for 24 h (Scheme 4b).61–64 The exclusive formation of the latter was shown by IR spectroscopy, which matches the literature spectrum (ESI Fig. S6).64 The presence of 1,3,5-tri-t-butyl benzene was discounted on the basis of IR spectroscopy data since it is not consistent with this trimer.63
Lewis acidity. The Lewis acid strength of the cation [MeAl(2-py)2(µ-Me)AlMe]+ [derived from demethylation of [Me2Al(2-py)]2 (1a)] was initially determined by calculating its fluoride ion affinity (FIA).65 Carried out at the ωB97XD/6-311++G(d,p) level,66–71 the calculated enthalpy change for the reaction with CF3O− (SI), subsequently corrected using the known experimental value,65 gave a value of 711 kJ mol−1. Compared to 485 kJ mol−1 for AlCl3 and 637 kJ mol−1 for Ph3C+, this suggests that the capacity of the bridged cation to polymerise alkenes and trimerise phenylacetylene can be attributed to its exceptional Lewis acidity.
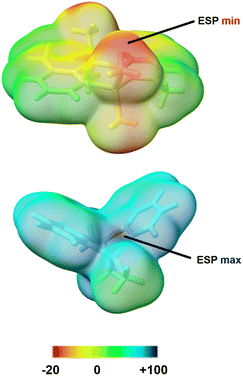
The local distribution of Lewis acidity and basicity in the cation versus the neutral precatalyst can be illustrated visually through molecular electrostatic potential (ESP) surface maps (shown for two representative species, precatalyst 1b and cation 2a in Fig. 7).72 The ESP for the two species was calculated at the ωB97X-D3/def2-TZVP level of theory,73–76 and the value of this rigorously defined physical measure can indicate what an incoming reactant ‘sees’ at the early stages of a covalent interaction.72 Notably, the minimum (negative) value is observed on the Lewis basic terminal methyl groups of the neutral precatalyst, consistent with their facile demethylation using Ph3C+ (Fig. 7, left). Conversely, the maximum (highly positive) value is found at the σ-hole site of Al(III) in the bridged cation, demonstrating the extraordinary Lewis acidity at that location.
Complementary to the ESP surface analysis, local nucleophilicity and electrophilicity can be probed using theory-derived measures such as the average local ionisation energy (ALIE) and the local electron attachment energy (LEAE), respectively.77–79 Their mappings on the surfaces of the two representative molecules (at the same level of theory as the ESP) lead to similar conclusions and highlight, in particular, the reactivity of the Al(III) σ-hole in the bridged cation (SI Fig. S29 and S30).80–82 The reactive nature of the activated alkyl-bridged cations, as illustrated by the FIA calculations and quantitative molecular surface analysis, presumably adds to the practical difficulty of isolating these species for crystallographic characterisation.
Mechanism of Polymerisation. DFT calculations were conducted at the ωB97XD/6-311++G(d,p)//B3LYP-D3/6-31G(d) level65 of theory to assess whether the initial mechanism of polymerisation we had proposed (see Scheme 2) is viable, using styrene as the monomer. Due to the large conformational space associated with torsional freedom in iBu groups, they were replaced by methyl groups to reduce the computational cost. Further, the substituents on the pyridine ring were replaced by an H-atom for initial studies into the mechanistic pathway since they were deemed to be unlikely to participate significantly. Initial calculations following a concerted carboalumination reaction showed that the barrier to this mechanism was too high (ca. 150 kJ mol−1). A transannular carboalumination also had an excessively high energy transition state (ΔG‡ = 150.2 kJ mol−1) (Fig. 8).
 | ||
| Fig. 8 Initially calculated transition state (ΔG‡ = 150.2 kJ mol−1) representing the “switching” of the monomer between the Al atoms. | ||
Polymer chain growth by dimer transfer was therefore considered as an alternative mechanism (Fig. 9a). Following activation of one monomeric unit by the catalyst, the π (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) bond of another styrene monomer attacks the Al-bonded alkene. The resulting benzylic carbocation 1-INT2 is now part of a 4-carbon tether that can access the axial methyl group on the second Al atom. This participates in a SE2 reaction that migrates the methyl group onto the tether and creates a new highly Lewis acidic site that can activate another styrene molecule. The cycle continues by further dimerisation of styrene at the Al centre, generating another tethered benzylic carbocation that participates in an SE2 reaction to elongate the growing chain by two monomeric styrene units.
C) bond of another styrene monomer attacks the Al-bonded alkene. The resulting benzylic carbocation 1-INT2 is now part of a 4-carbon tether that can access the axial methyl group on the second Al atom. This participates in a SE2 reaction that migrates the methyl group onto the tether and creates a new highly Lewis acidic site that can activate another styrene molecule. The cycle continues by further dimerisation of styrene at the Al centre, generating another tethered benzylic carbocation that participates in an SE2 reaction to elongate the growing chain by two monomeric styrene units.
The electronic energy profile of the reaction (and the enthalpy profile) of the suggested mechanism has sufficiently low barriers to be feasible at room temperature (SI). However, significant loss in translational entropy during the polymerisation raises the free energy of intermediates, with each styrene association having an entropic cost of around 167 J K−1 mol−1 (50 kJ mol−1 of free energy at room temperature). A free-energy adjusted profile (Fig. 9b) has the largest barrier at 121 kJ mol−1 above the resting state catalyst. Despite this, styrene will be present in large excess, it is therefore possible that this pathway may still be operative given the enthalpic favourability.
We next explored the origin of the experimentally observed stereocontrol. The first stereocentre to be formed during the polymerisation of styrene occurs during the addition of the second styrene monomer. The relative stereochemistry between the two phenyl groups is then dictated by the geometry of the SE2 step. The energies of the diastereomeric transition states (1-TS3 and 1-TS3′) differ by 1.5 kJ mol−1 (Fig. 10) and the experimentally observed anti stereochemistry is favoured.
A model system was constructed to study the stereochemical outcome of the SE2 step, this time featuring the Me substituent in the 2-position of the pyridine. The (styrene, styrene) chain was replaced with an (ethene, styrene) chain to maintain the benzylic carbocation at the reacting centre. The other chain was constructed with a Me group capping the styrene unit in place of another styrene monomer. Calculations showed only conformers with the H atom projecting towards chain are sufficiently low energy (SI). This left four isomeric transition states to be considered (outlined in Fig. 11). For either absolute stereochemistry, the anti TS is favoured over the syn.
At 298 K, the energy of the syn structure 1-TS3b is 5.2 kJ mol−1 higher than that of the most stable anti arrangement 1-TS3a, which corresponds to a 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 preference for the anti-stereochemistry; this means that the anti relationship of the Ph groups will be favoured in the polymer chain, promoting the formation of the syndiotactic polymer as observed experimentally. The extent of the stereoselectivity is predicted to be higher in the isomeric 1-TS3a′.
1 preference for the anti-stereochemistry; this means that the anti relationship of the Ph groups will be favoured in the polymer chain, promoting the formation of the syndiotactic polymer as observed experimentally. The extent of the stereoselectivity is predicted to be higher in the isomeric 1-TS3a′.
The steric properties of the system can be examined further using topographic steric maps, which are commonly used to rationalise the behaviour of catalytic pockets.83,84 Two neutral systems (precatalysts 1c and 1d), representative of the start of the propagation step, were selected for their differing R and R′ groups (Fig. 12a). The examination of the steric profile on each aluminium (excluding the growing chain) reveals the local effect of the 2-pyridyl substituents (Fig. 12a, left), while the overall hindrance from the ring system is not highly variable—in line with our considerations above for the SE2 step. In contrast, the bridged cationic species (as exemplified by cation 2a, Fig. 12b), experiences a much more pronounced steric pressure from the ring system. Such an effect, especially along the direction of the approaching alkene, can have a dramatic impact in the energetics of the initiation step; previous studies have investigated the relation between the percentage of buried volume (Vbur) and Lewis acidity.85
Mechanism of cyclotrimerisation. The cyclotrimerisation of phenylacetylene into triphenyl-benzene was subsequently investigated (Fig. 13a). The rigidity of the sp2 carbon-containing tether would preclude the SE2 reaction suggested for styrene polymerisation, so the action of the complex as a Lewis acid on one Al site was considered, starting with the addition of the phenylacetylene to the rest state of the catalyst. This addition was found to be electronically barrierless.
 | ||
| Fig. 13 Proposed reaction mechanism and energy profile diagram of the calculated trimerisation reaction pathway (Gibbs free energies in kJ mol−1). | ||
Addition of a second alkyne gives diene 2-INT3 which then undergoes another barrierless addition (SI) to form 2-INT4. Cyclisation via a five-membered ring forms a benzylic carbene 2-INT5. Addition of the carbene to the π bond results in cyclopropane 2-INT6 which undergoes a ring-opening to give the final 1,2,4-subsituted aromatic product (Fig. 13). An alternative carbene addition to the Al-bonded C atom, on the pathway to the 1,3,5-product, resulted in a higher energy TS (ΔE = 4 kJ mol−1; SI), consistent with the experimental results. The carbene intermediate may also undergo a 1,2 H shift to form the fulvene product. It is likely that steric crowding makes this process dominant over the carbene addition when t-butylacetylene is the substrate used (SI).
Conclusions
In this study we have shown that dimers [R2Al(2-py′)]2 (1) are precatalysts for the polymerisation of alkenes and for the cyclotrimerisation of alkynes. Although only low molecular weight polymers are produced from alkenes, syndioselectivity is observed especially where sterically bulky Al-bonded groups are present. To the best of our knowledge this is the first alkene polymerisation catalyst based on Al(III) where stereoselectivity has been observed. In addition, the observed trimerisation of alkynes is not only a rare example involving non-transition element catalysis of this type but is also the first example involving aluminium (or any fully metallic main group element). DFT calculations trace this reactivity to the high Lewis acidity of the catalytically active cations, which is greater than that of Ph3C+. Theoretical studies also indicate that a co-operative polymerisation mechanism involving dimer transfer is viable, in which both Al(III) centres of the proposed catalytic intermediates are involved. This mechanism can also explain the syndioselectivity of the resulting polymers. A related mechanism also explains the cyclotrimerisation of alkynes.Above all, this work shows the potential promise of static catalysis based on Al(III) in a broad range of organic transformations. On this basis, we are expanding our studies to explore other transition metal-mimetic systems based on aluminium.
Author contributions
DC did all of the synthetic work, collated experimental data, and supplied samples for GPC analysis. RD performed DFT calculations pertaining to the mechanism. AT performed NMR measurements and the calculations for the qualitative molecular surface analyses and steric maps. NSP did the GPC measurements. OAS, JMG and DSW supervised the project. DSW and JMG devised the idea. All authors contributed to the writing of the initial manuscript, which was revised by DC, AT and DSW. All authors read and approved the final version.Conflicts of interest
There are no conflicts to declare.Data availability
Data is available in the supplementary information (SI) that accompanies this paper. DFT output files are available at https://doi.org/10.17863/CAM.121268. The supplementary information includes the synthetic procedures, IR and NMR spectra, GPC and DSC data, and full details of DFT calculations; see DOI: https://doi.org/10.1039/d5sc07624b.Acknowledgements
DC would like to acknowledge the FfWG Foundation Grant for their support with living expenses. AT would like to thank the Cambridge Commonwealth, European and International Trust for a Cambridge International Scholarship. NSP would like to thank Churchill College and the Cambridge Trust for the Gulbenkian Yuval Cambridge International Scholarship. RD would like to acknowledge funding from an EPSRC DTP studentship (ref. EP/W524633/1) and a University of Cambridge Vice-Chancellor's Award. This work was performed using resources provided by the Cambridge Service for Data Driven Discovery (CSD3) operated by the University of Cambridge Research Computing Service (https://www.csd3.cam.ac.uk), provided by Dell EMC and Intel using Tier-2 funding from the Engineering and Physical Sciences Research Council (capital grant EP/T022159/1), and DiRAC funding from the Science and Technology Facilities Council (https://www.dirac.ac.uk). We would also like to thank Robert Cornell of the Thermal Characterisation Facility at the Department of Materials Science & Metallurgy, University of Cambridge, for help with the DSC measurements and data analysis. Lastly, we thank Dr Yuanyuan Wei for helpful discussions regarding the DSC data.Notes and references
- J. P. Collman, L. S. Hegedus, J. R. Norton and R. C. Finke, Principles and applications of organotransition metal chemistry, University Science Books, California, 1987 Search PubMed.
- J. Hartwig, Organotransition metal chemistry: from bonding to catalysis, University Science Books, Sausalito, CA, 2010 Search PubMed.
- P. P. Power, Nature, 2010, 463, 171–177 CrossRef CAS PubMed.
- C. Weetman, Chem. Eur J., 2021, 27, 1941–1954 CrossRef CAS PubMed.
- C. Weetman, in Encyclopedia of inorganic and bioinorganic chemistry, Wiley, 2021, pp. 1–27 Search PubMed.
- D. Franz and S. Inoue, Chem. Eur J., 2019, 25, 2898–2926 CrossRef CAS PubMed.
- C. Weetman and S. Inoue, ChemCatChem, 2018, 10, 4213–4228 CrossRef CAS.
- B. Qin and U. Schneider, in Catalysis Series No. 40: Catalysis with Earth-Abundant Elements, ed. U. Schneider and S. Thomas, RSC, 2021, pp. 261–283 Search PubMed.
- C. Ni, X. Ma, Z. Yang and H. W. Roesky, Eur. J. Inorg. Chem., 2022, 2022, 1–16 CrossRef.
- D. Choudhury and D. S. Wright, Eur. J. Inorg. Chem., 2025, 28, e202400638 CrossRef CAS.
- M. C. Baier, M. A. Zuideveld and S. Mecking, Angew. Chem., Int. Ed., 2014, 53, 9722–9744 CrossRef CAS PubMed.
- K. Hobson, C. J. Carmalt and C. Bakewell, Chem. Sci., 2020, 11, 6942–6956 Search PubMed.
- M. Pramanik, M. G. Guerzoni, E. Richards and R. L. Melen, Angew. Chem., Int. Ed., 2023, 63, e202316461 Search PubMed.
- I. Göttker-Schnetmann, P. Kenyon and S. Mecking, Angew. Chem., Int. Ed., 2019, 58, 17777–17781 CrossRef PubMed.
- P. Liu, X. Yang, H. Li, S. Zhang, Y. Hu, G. Zhou and N. Hadjichristidis, Angew. Chem., Int. Ed., 2024, 63, e202317494 Search PubMed.
- T. Zhao, M. Liao, Y. Hu, G. Zhou, P. Liu and N. Hadjichristidis, Macromolecules, 2024, 57, 6344–6353 CrossRef CAS.
- E. Fazekas, P. A. Lowy, M. Abdul Rahman, A. Lykkeberg, Y. Zhou, R. Chambenahalli and J. A. Garden, Chem. Soc. Rev., 2022, 51, 8793–8814 Search PubMed.
- K. Ziegler, E. Holzkamp, H. Breil and H. Martin, Angew. Chem., 1955, 67, 541–547 CrossRef CAS.
- G. Natta, P. Pino, P. Corradini, F. Danusso, E. Mantica, G. Mazzanti and G. Moraglio, J. Am. Chem. Soc., 1955, 77, 1708–1710 CrossRef CAS.
- S. Harder, F. Feil and A. Weeber, Organometallics, 2001, 20, 1044–1046 CrossRef CAS.
- F. Feil and S. Harder, Macromolecules, 2003, 36, 3446–3448 CrossRef.
- S. Harder and F. Feil, Organometallics, 2002, 21, 2268–2274 CrossRef CAS.
- S. Harder, F. Feil and K. Knoll, Angew. Chem., Int. Ed., 2001, 40, 4261–4264 CrossRef CAS PubMed.
- D. Choudhury, C.-C. Lam, N. Farag, J. Slaughter, A. Bond, J. M. Goodman and D. Wright, Chem. Eur J., 2024, 30, e202303872 CrossRef CAS PubMed.
- C. E. Radzewich, I. A. Guzei and R. F. Jordan, J. Am. Chem. Soc., 1999, 121, 8673–8674 CrossRef CAS.
- B. Qian, D. L. Ward and M. R. Smith, Organometallics, 1998, 17, 3070–3076 Search PubMed.
- T. E. Stennett, J. Pahl, H. S. Zijlstra, F. W. Seidel and S. Harder, Organometallics, 2016, 35, 207–217 CrossRef CAS.
- P. Mehrkhodavandi and R. R. Schrock, J. Am. Chem. Soc., 2001, 123, 10746–10747 CrossRef CAS PubMed.
- D. F.-J. Piesik, K. Häbe and S. Harder, Eur. J. Inorg. Chem., 2007, 2007, 5652–5661 CrossRef.
- N. Ishihara, T. Seimiya, M. Kuramoto and M. Uoi, Macromolecules, 1986, 19, 2464–2465 CrossRef CAS.
- J. Wm. Wackerly and J. F. Dunne, J. Chem. Educ., 2017, 94, 1790–1793 CrossRef CAS.
- J. Li, C. Zhao, J. Liu, H. Huang, F. Wang, X. Xu and C. Cui, Inorg. Chem., 2016, 55, 9105–9111 CrossRef CAS PubMed.
- J. Xu, J. Ouyang, Z. Fan, D. Chen, L. Feng and Y. Yang, Polym. J., 1998, 30, 720–722 CrossRef CAS.
- S. Suparno, J. Lacoste, S. Raynal, J. F. Regnier, F. Schué, R. Sempere and J. Sledz, Polym. J., 1980, 12, 861–865 CrossRef CAS.
- A. Karam, J. Pastran, E. Casas and B. Mendez, Polym. Bull., 2005, 55, 11–17 CrossRef CAS.
- R. Bahulekar, R. S. Ghadage, S. Ponrathnam and N. R. Ayyangar, Eur. Polym. J., 1990, 26, 721–725 CrossRef CAS.
- Y. Inoue and T. Konno, Polym. J., 1976, 8, 457–465 CrossRef CAS.
- H. N. Cheng and G. H. Lee, Int. J. Polym. Anal. Charact., 1996, 2, 439–455 CrossRef CAS.
- L. F. Johnson, L. F. Heatley and F. A. Bovey, Macromolecules, 1970, 3, 175–177 CrossRef CAS.
- V. Marcon and N. F. A. van der Vegt, Soft Matter, 2014, 10, 9059–9064 Search PubMed.
- H. Tadokoro, Y. Nishiyama, S. Nozakura and S. Murahashi, Bull. Chem. Soc. Jpn., 1961, 34, 381–391 CrossRef CAS.
- S. N. Goyanes, J. Appl. Polym. Sci., 2000, 75, 865–873 Search PubMed.
- A. Yoshioka and K. Tashiro, Macromolecules, 2004, 37, 467–472 Search PubMed.
- H. J. M. A. Mieras and C. F. H. van Rijn, Nature, 1969, 224, 165–166 CrossRef CAS.
- S. Ma, C. Con, M. Yavuz and B. Cui, Nanoscale Res. Lett., 2011, 6, 446 CrossRef PubMed.
- M. F. Farona and C. Tsonis, J. Chem. Soc. Chem. Commun., 1977, 363–364 Search PubMed.
- M. C. Murray and M. C. Baird, Can. J. Chem., 2001, 79, 1012–1018 Search PubMed.
- E. Y. Tshuva, I. Goldberg and M. Kol, J. Am. Chem. Soc., 2000, 122, 10706–10707 Search PubMed.
- T. Asanuma, Y. Nishimori, M. Ito, N. Uchikawa and T. Shiomura, Polym. Bull., 1991, 25, 567–570 CrossRef CAS.
- I. V. Vasilenko and S. V. Kostjuk, Polym. Bull., 2006, 57, 129–138 CrossRef CAS.
- F. Liu, H. Gao, Z. Hu, H. Hu, F. Zhu and Q. Wu, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 3859–3866 CrossRef CAS.
- M. Marigo, D. Millos, N. Marsich and E. Farnetti, J. Mol. Catal. Chem., 2003, 206, 319–329 Search PubMed.
- K. Yang, P. Wang, Z.-Y. Sun, M. Guo, W. Zhao, X. Tang and G. Wang, Org. Lett., 2021, 23, 3933–3938 CrossRef CAS PubMed.
- J. H. Li and Y. X. Xie, Synth. Commun., 2004, 34, 1737–1743 CrossRef CAS.
- Y. Huang, G. Huang, Y. Liu, J. K. Yu and Y. Tsai, Angew. Chem., 2017, 129, 15629–15633 CrossRef.
- Y. Xu, Y. Pan, Q. Wu, H. Wang and P. Liu, J. Org. Chem., 2011, 76, 8472–8476 CrossRef CAS PubMed.
- J. Yang and J. G. Verkade, Organometallics, 2000, 19, 893–900 CrossRef CAS.
- Z. Zhu, J. Wang, Z. Zhang, X. Xiang and X. Zhou, Organometallics, 2007, 26, 2499–2500 Search PubMed.
- T. Sugahara, J. D. Guo, T. Sasamori, S. Nagase and N. Tokitoh, Angew. Chem., Int. Ed., 2018, 57, 3499–3503 CrossRef CAS PubMed.
- C. Xi, Z. Sun and Y. Liu, Dalton Trans., 2013, 42, 13327–13330 RSC.
- J. Li, H. Jiang and M. Chen, J. Org. Chem., 2001, 66, 3627–3629 CrossRef CAS PubMed.
- E. S. Johnson, G. J. Balaich, P. E. Fanwick and I. P. Rothwell, J. Am. Chem. Soc., 1997, 119, 11086–11087 CrossRef CAS.
- J. A. Murphy, J. Garnier, S. R. Park, F. Schoenebeck, S. Zhou and A. T. Turner, Org. Lett., 2008, 10, 1227–1230 Search PubMed.
- I. E. Den Besten, L. Kaplan and K. E. Wilzbach, J. Am. Chem. Soc., 1968, 90, 5868–5872 CrossRef CAS.
- K. O. Christe, D. A. Dixon, D. McLemore, W. W. Wilson, J. A. Sheehy and J. A. Boatz, J. Fluor. Chem., 2000, 101, 151–153 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman, and D. J. Fox, Gaussian 16, Revision B.01, Gaussian, Inc., Wallingford CT, 2016 Search PubMed.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS.
- A. D. Becke, Phys. Rev. A:At., Mol., Opt. Phys., 1988, 38, 3098–3100 CrossRef CAS PubMed.
- C. Lee, W. Yang and R. G. Parr, Phys. Rev. B:Condens. Matter Mater. Phys., 1988, 37, 785–789 CrossRef CAS PubMed.
- J.-D. Chai and M. Head-Gordon, Phys. Chem. Chem. Phys., 2008, 10, 6615–6620 Search PubMed.
- S. Grimme, S. Ehrlich and L. Goerigk, J. Comput. Chem., 2011, 32, 1456–1465 CrossRef CAS PubMed.
- J. S. Murray and P. Politzer, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2011, 1, 153–163 CAS.
- Y.-S. Lin, G.-D. Li, S.-P. Mao and J.-D. Chai, J. Chem. Theory Comput., 2013, 9, 263–272 CrossRef CAS PubMed.
- F. Weigend and R. Ahlrichs, Phys. Chem. Chem. Phys., 2005, 7, 3297–3305 RSC.
- S. Grimme, J. Antony, S. Ehrlich and H. Krieg, J. Chem. Phys., 2010, 132, 154104 CrossRef PubMed.
- F. Neese, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2025, 15, e70019 Search PubMed.
- P. Politzer, J. S. Murray and M. C. Concha, Int. J. Quantum Chem., 2002, 88, 19–27 CrossRef CAS.
- P. Politzer, J. S. Murray and F. A. Bulat, J. Mol. Model., 2010, 16, 1731–1742 CrossRef CAS PubMed.
- T. Brinck, P. Carlqvist and J. H. Stenlid, J. Phys. Chem. A, 2016, 120, 10023–10032 Search PubMed.
- T. Lu and F. Chen, J. Comput. Chem., 2012, 33, 580–592 CrossRef CAS PubMed.
- T. Lu, J. Chem. Phys., 2024, 161, 082503 CrossRef CAS PubMed.
- T. Lu and F. Chen, J. Mol. Graph. Model., 2012, 38, 314–323 CrossRef CAS PubMed.
- L. Falivene, R. Credendino, A. Poater, A. Petta, L. Serra, R. Oliva, V. Scarano and L. Cavallo, Organometallics, 2016, 35, 2286–2293 CrossRef CAS.
- L. Falivene, Z. Cao, A. Petta, L. Serra, A. Poater, R. Oliva, V. Scarano and L. Cavallo, Nat. Chem., 2019, 11, 872–879 CrossRef CAS PubMed.
- L. Zapf, M. Riethmann, S. A. Föhrenbacher, M. Finze and U. Radius, Chem. Sci., 2023, 14, 2275–2288 RSC.
Footnote |
| † Both authors should be considered first author of this paper. |
| This journal is © The Royal Society of Chemistry 2026 |