Open Access Article
Open Access ArticleSquaramide-based receptors in anion supramolecular chemistry: insights into anion binding, sensing, transport and extraction
Giacomo
Picci
*a,
Riccardo
Montis
 *b,
Vito
Lippolis
*b,
Vito
Lippolis
 a and
Claudia
Caltagirone
a and
Claudia
Caltagirone
 *a
*a
aDipartimento di Scienze Chimiche e Geologiche, Università degli Studi di Cagliari, S.S. 554 Bivio per Sestu, Monserrato (CA) 09042, Italy. E-mail: gpicci@unica.it; lippolis@unica.it; ccaltagirone@unica.it
bDepartment of Pure and Applied Science, University of Urbino, Via della Stazione 4, Urbino I-61029, Italy. E-mail: riccardo.montis@uniurb.it
First published on 11th March 2024
Abstract
Over the last 15 years, squaramide-based receptors have attracted the attention of supramolecular chemists working in the field of anion recognition. Herein, we highlight examples of squaramide-based receptors that are able to bind, sense, extract and transport anions.
Introduction
One of the main strategies adopted by supramolecular chemists to bind anions is the development of synthetic receptors that are able to interact with anionic guests via the formation of hydrogen bonds (H-bonds). In this regard, the use of urea, thiourea, amide, and sulphonamide functional groups has been widely explored since the early 90's. More recently, the squaramide moiety has been added to the toolbox of H-bonding groups for the development of receptors for anion binding. The first report on squaric acid derivatives dates back to 1966 when Cohen and Cohen described the synthesis of alcoxy, hydroxy and amino derivatives starting from 1,2-dihydroxycyclobutenedione.1 The success of the use of squaramides depends on some peculiar aspects related to the presence of a 4-membered ring in their molecular skeleton, such as its aromaticity according to the Hückel rule as the lone pair on the nitrogen atoms can delocalise on the cyclic structure and the aromatic character is increased when anion binding or deprotonation occurs as demonstrated by Frontera and Deya.2 The aromaticity is related to the Brønsted acidity of the NH bonds which is an important aspect when comparing this class of compounds with analogous croconamides and deltamides.3 Squaramides are also characterised by their ability to act both as H-bond donors (via the NH groups) and H-bond acceptors (via the carbonyl groups) and this aspect is particularly relevant for applications in organocatalysis.4 Several reports have demonstrated how the rigidity of the 4-membered ring, the directionality of the NH bonds, and the bond angles make squaramides better candidates as receptors for anion binding compared to analogous ureas.5,6For all these reasons, as well as for synthetic accessibility, squaramides have started to be widely used as receptors for anion binding, sensing, extraction, transport and, very recently, for the development of different anion-responsive materials. Some efforts have been directed towards exploring polymers,7,8 nanomaterials,9 and soft materials.10 As an example, Wezenberg and co-workers recently described that polymeric gels containing a squaramide crosslinker were able to swell and bend in the presence of different concentrations of various anions.11
Although the topic has already been the subject of previous reviews covering different aspects of application of squaramides in supramolecular chemistry,12–14 herein we will highlight examples of the use of squaramides for anion binding, extraction, sensing and transmembrane transport with the purpose of providing some inputs for the future of this very active field of research.
Anion binding
The first use of squaramide derivatives for carboxylate binding has been reported by Costa, Ballester and co-workers in 1998.15 In this seminal paper, they described the series of mono squaramides 1–4, symmetric and non-symmetric, bearing neutral or charged substituents (Fig. 1). When titrated with tetramethylammonium acetate in DMSO-d6 or in a mixture of DMSO-d6/10%D2O by means of 1H-NMR titrations, these receptors showed a moderate affinity for this anion forming adducts with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry with the highest association constant measured for squaramide 4 in DMSO-d6 (Kass = 14
1 stoichiometry with the highest association constant measured for squaramide 4 in DMSO-d6 (Kass = 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 ± 3200 M−1). As expected, the presence of the positively charged trimethylammonium group on one of the pendant arms of 4 improves the anion binding properties of this receptor.
200 ± 3200 M−1). As expected, the presence of the positively charged trimethylammonium group on one of the pendant arms of 4 improves the anion binding properties of this receptor.
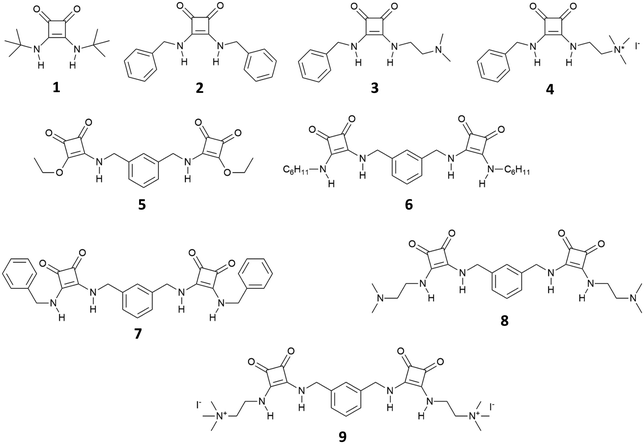 | ||
| Fig. 1 Receptors 1–9 developed by Costa, Ballester and co-workers for carboxylate and dicarboxylate binding. | ||
Authors showed that an efficient binding of dicarboxylates could be achieved with bis-squaramides 5–9 (Fig. 1). For example, squaramide 7 bearing two benzylic substituents showed association constants for the formation of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
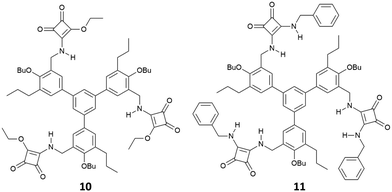
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 adduct with glutarate [as its tetrabutylammonium (TBA) salt] in DMSO-d6/10%D2O of 1400 ± 200 M−1 and in DMSO-d6/15%D2O of 150 ± 40 M−1 while in DMSO-d6 the association constant was too high to be measured by 1H-NMR spectroscopy (Kass > 104 M−1). The charged bis-squaramide 9 was able to bind glutarate in a very hydrophilic medium such as CD3CN/30%H2O with an association constant of 560 ± 50 M−1 demonstrating the efficiency of the squaramide NHs to act as H-bond donor groups even in a very competitive solvent medium. Finally, the authors reported the ability of receptors 10 and 11 characterised by a pseudo C3 symmetry to bind tricarboxylates (Fig. 2). Indeed, tris-squaramide 11 was able to bind trimesoate and cis-cyclohexentricarboxylate (both as their TBA salts) with association constants for the formation of the corresponding 1
1 adduct with glutarate [as its tetrabutylammonium (TBA) salt] in DMSO-d6/10%D2O of 1400 ± 200 M−1 and in DMSO-d6/15%D2O of 150 ± 40 M−1 while in DMSO-d6 the association constant was too high to be measured by 1H-NMR spectroscopy (Kass > 104 M−1). The charged bis-squaramide 9 was able to bind glutarate in a very hydrophilic medium such as CD3CN/30%H2O with an association constant of 560 ± 50 M−1 demonstrating the efficiency of the squaramide NHs to act as H-bond donor groups even in a very competitive solvent medium. Finally, the authors reported the ability of receptors 10 and 11 characterised by a pseudo C3 symmetry to bind tricarboxylates (Fig. 2). Indeed, tris-squaramide 11 was able to bind trimesoate and cis-cyclohexentricarboxylate (both as their TBA salts) with association constants for the formation of the corresponding 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 adducts in DMSO-d6/10%D2O of 3900 ± 400 M−1 and 7700 ± 1300 M−1, respectively.
1 adducts in DMSO-d6/10%D2O of 3900 ± 400 M−1 and 7700 ± 1300 M−1, respectively.
Costa's group also gave insight into the thermodynamic aspects behind the carboxylate binding by the mono and bis-squaramides described above, showing by ITC that in solvents such as CHCl3 or DMSO the formation of the adducts between squaramide derivatives (especially in the case of those bearing positively charged ammonium groups) and carboxylates anions are driven by the formation of H-bonds and electrostatic interactions, and the process is overall exothermic. In polar solvents, such as CH3OH, the interactions still occur but the process is endothermic and entropically driven by the reorganization of solvent molecules during the binding event.16
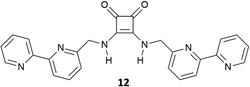
Al-Sayah and Branda have studied the carboxylate binding properties of squaramide 12 (Fig. 3) and compared them with those of the analogous thiourea.17 Upon 1H-NMR titration of 12 with TBAAcO in CD3CN/CDCl3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v), an association constant of 7390 M−1 was calculated for the formation of the 1
1 v/v), an association constant of 7390 M−1 was calculated for the formation of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 adduct, while a lower association constant (Kass = 5790 M−1) for the formation of the acetate adduct with the analogous thiourea was determined. Authors also performed calorimetric titration studies in CH3CN/CHCl3 (1
1 adduct, while a lower association constant (Kass = 5790 M−1) for the formation of the acetate adduct with the analogous thiourea was determined. Authors also performed calorimetric titration studies in CH3CN/CHCl3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) demonstrating that the binding event is both enthalpically and enthropically driven with a ΔH of −2209 cal mol−1 and a ΔS of 10.3 cal mol−1 K−1.
1 v/v) demonstrating that the binding event is both enthalpically and enthropically driven with a ΔH of −2209 cal mol−1 and a ΔS of 10.3 cal mol−1 K−1.
Very recently, Elmes and co-workers have reported on a family of symmetric squaramides (13–15) functionalised with naphthalimide groups differing for the length of the aliphatic chain between the squaramide core and the substituents (Fig. 4).18 As shown by the SEM image reported in Fig. 4(A), squaramide 13 undergoes molecular self-assembly/aggregation giving rise to some sort of pine cone structures. A similar behaviour was observed in the cases of 14 and 15. The aggregation behaviour was further confirmed by 1H-NMR studies in DMSO-d6 at variable temperatures (Fig. 4(B)) which demonstrated that at 298 K the signals assigned to the squaramide NHs and the naphthalimide CHs appeared broad while they became sharper and resolved well by increasing the temperature up to 358 K. The aggregation/disaggregation process was reversible as the peaks became broad again by cooling down back to 298 K. The three squaramide derivatives were able to selectively bind AcO− (as its TBA salt) in DMSO with low association constants (Kass = 422 ± 11.9% M−1 for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 adduct formation calculated with receptor 13 at 273 K). This is probably due to a competition between the self-aggregation of the free receptors and the formation of the adducts. The authors demonstrated that the length of the chain in the design of the receptors does not have any effect as they all showed a similar behaviour.
1 adduct formation calculated with receptor 13 at 273 K). This is probably due to a competition between the self-aggregation of the free receptors and the formation of the adducts. The authors demonstrated that the length of the chain in the design of the receptors does not have any effect as they all showed a similar behaviour.
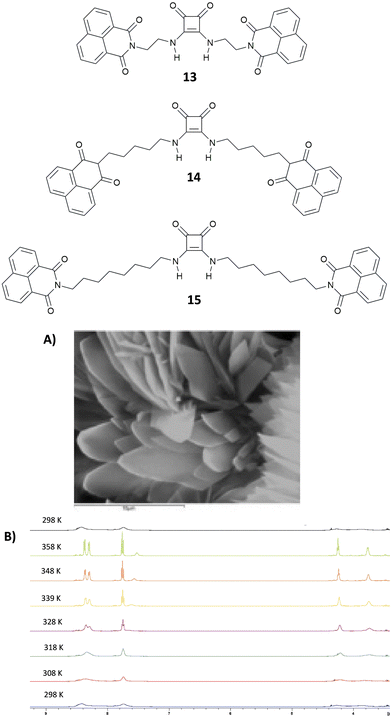 | ||
| Fig. 4 Squaramides 13–15 reported by Elmes and co-workers. SEM image of 13, and the scale bar is 10 μm (A). 1H-NMR spectrum of 13 (5.0 × 10−3 M) recorded at variable temperatures in DMSO-d6 (B). Reproduced with permission from ref. 18. Copyright Elsevier 2022. | ||
Recently, a series of squaramides able to bind non-steroidal anti-inflamatory drugs (NSAIDs) ketoprofen and naproxen as their sodium salts have been reported (Fig. 5).19,20 The macrocyclic systems 16 and 17 have been found to be able to bind ketoprofen in its anionic form in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry in CH3CN/DMSO solution (1
1 stoichiometry in CH3CN/DMSO solution (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) with association constants of Kass = 9.4 × 104 ± 0.6% and Kass = 8.3 × 104 M−1 ± 1%, respectively. Receptors 19–24 were employed successfully for developing ion selective electrodes (ISEs) able to discriminate ketoprofen and naproxen in their anionic forms in real samples.
1 v/v) with association constants of Kass = 9.4 × 104 ± 0.6% and Kass = 8.3 × 104 M−1 ± 1%, respectively. Receptors 19–24 were employed successfully for developing ion selective electrodes (ISEs) able to discriminate ketoprofen and naproxen in their anionic forms in real samples.
Few examples of squaramide receptors for selective phosphate binding have been reported in the literature, to the best of our knowledge. Elmes and co-workers have described two novel self-associating amphiphilic (SSA) squaramides 25 and 26 (Fig. 6), which are able to bind anions in DMSO, while in a more competitive solvent medium such as DMSO/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) the deprotonation event caused by H2PO4− (as its TBA salt) on receptor 16 is able to cause a colour change of the solution.21
1 v/v) the deprotonation event caused by H2PO4− (as its TBA salt) on receptor 16 is able to cause a colour change of the solution.21
A well-known strategy in anion supramolecular chemistry is the exploitation of vacant coordination positions on a metal centre to bind anions.22 This strategy was successfully used by Delgado and co-workers to bind phosphate anions in water. Squaramide 27, bearing dipicolylamine (dpa) and ethylpiperazine substituents, forms stable copper(II) complexes in water with different degrees of protonation depending on the pH. In particular, the binucluear complexes [Cu227H−i](4−i)+ in which the metal centres are in a distorted octahedral environment determined by the nitrogens of the dpa/ethylpiperazine moieties, a nitrogen atom and an oxygen atom of the squaramide unit (Fig. 7(A) for the [Cu227H−1]3+ calculated structure) and two H2O molecules. At physiological pH values, the dinuclear complexes [Cu227H−i](4−i)+ bind selectively ATP4− over ADP3−, AMP2−, aminoethylphosphate (Haep−) and phenylphosphate (PhPO42−), with significant interference of hydrogenpyrophosphate (HPPi3−) with very high association constants. DFT calculations, as shown in Fig. 7(B) and (C), revealed that in the presence of HPPi3− and ATP4−, the aqua ligands bound to the metal centres are replaced by the oxygen atoms of the anion guests and a further H-bond interaction between the hydroxyl group of the ribose unit and the squaramide oxygen atoms in the case of ATP4−, which stabilize the complexes.
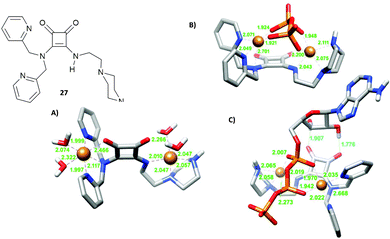 | ||
| Fig. 7 Squaramide 27 reported by Delgado and co-workers and its dinuclear copper(II) complex [Cu227H−1]3+ (A), complex [Cu227H−1(PPi)]− (B), complex [Cu227H−1(ATP)]− optimised by DFT calculations (C). Adapted with permission from ref. 22. Copyright RSC 2019. | ||
The cryptand-like receptor 28 (Fig. 8) was reported by Fusi, Micheloni and co-workers.23 It consists of two separate binding sites: a Me2[12]aneN4 polyaza macrocyclic unit suitable for cation binding bridged by two squaramide units suitable for anion binding.
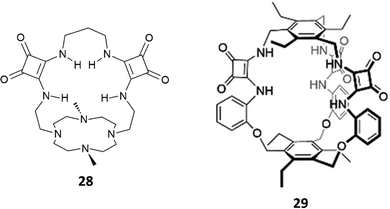 | ||
| Fig. 8 Cryptand-like and cage-like squaramide-containing receptors 28 and 29 for dihydrogenphosphate binding. | ||
Macrocycle 28 was found to be selective for H2PO4− over SO42− and H2P2O72− (the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kass values for SO42−, H2PO4−, and H2P2O72− are 2.96, 4.04, and 2.56, respectively) as determined by potentiometric titrations in 0.15 M NaClO4 aqueous solution. Potentiometric studies also highlighted that the selectivity for H2PO4− was maintained in the pH range of 4.0–10.5 with a peak of selectivity at pH 7.4.
Kass values for SO42−, H2PO4−, and H2P2O72− are 2.96, 4.04, and 2.56, respectively) as determined by potentiometric titrations in 0.15 M NaClO4 aqueous solution. Potentiometric studies also highlighted that the selectivity for H2PO4− was maintained in the pH range of 4.0–10.5 with a peak of selectivity at pH 7.4.
Very recently, Kim and Yang reported the excellent binding behaviour in solution and in the solid state of the cage-like squaramide receptor 29 (Fig. 8).24 The anion binding properties of 29 in the presence of a set of anions as their TBA salts (fluoride, chloride, bromide, iodide, hydrogensulfate, sulfate, hydrogenpyrophosphate, and dihydrogenphosphate or triethylammonium hydrogencarbonate) were studied in DMSO-d6/10%H2O by means of 1H-NMR titrations. A downfield shift of the signals attributed to the squaramide NHs was observed upon the addition of the anions. By fitting the titration data using a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding model, an association constant of Kass = (6.57 ± 1.12) × 102 M−1 was determined for the formation of the adduct of 29 with H2PO4−. The formation of the 1
1 binding model, an association constant of Kass = (6.57 ± 1.12) × 102 M−1 was determined for the formation of the adduct of 29 with H2PO4−. The formation of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 adduct was confirmed by a Job plot experiment in solution and by SCXRD analysis in the solid state. Single crystals were grown by slow evaporation of a CHCl3/CH3OH solution of 29 in the presence of an excess of TBAH2PO4. As shown in Fig. 9, the anion is bound to the squaramide NHs via four H-bonds. The anion also forms H-bonds with CH3OH molecules and the carbonyl groups of the squaramide moieties of an adjacent receptor molecule.
1 adduct was confirmed by a Job plot experiment in solution and by SCXRD analysis in the solid state. Single crystals were grown by slow evaporation of a CHCl3/CH3OH solution of 29 in the presence of an excess of TBAH2PO4. As shown in Fig. 9, the anion is bound to the squaramide NHs via four H-bonds. The anion also forms H-bonds with CH3OH molecules and the carbonyl groups of the squaramide moieties of an adjacent receptor molecule.
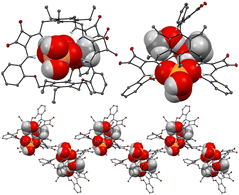 | ||
Fig. 9 Views of the single X-ray crystal structure of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 adduct of 29 with TBAH2PO4. Reproduced from ref. 24. Copyright RSC 2023. 1 adduct of 29 with TBAH2PO4. Reproduced from ref. 24. Copyright RSC 2023. | ||
One of the most targeted anions in the development of artificial receptors for anionic systems is sulfate, SO42−,25,26 which is involved in numerous biological, environmental, and chemical processes.27–29 This is not an easy task considering the tetrahedral shape of this anion, its charge density and high hydrophilicity (ΔGh = −1080 kJ mol−1),30 which require an accurate choice of building blocks, functional groups and related spatial disposition in the structure of the abiotic receptors in order to maximize non-covalent interactions via topological complementarity. In fact, SO42−, binding proteins (SBPs), which are involved in the high selective binding and active transport of this anion into bacteria cells, feature binding sites located in hydrophobic pockets far from the protein surfaces where the SO42− anion can be bound in a dehydrated form and completely shielded from the solvent.31 Furthermore, a precise arrangement in the binding sites of selected aminoacids with appropriately oriented H-bonding donor groups allows the exact encapsulation and efficient binding (an association constant of about 106 M−1 in H2O at pH 5–9)32 and recognition of the tetrahedral SO42− anion via the formation of seven H-bonds.
Inspired by nature, many researchers have proposed macrocyclic,33 interlocked,34 and tripodal35 scaffolds for selective SO42− receptors, featuring various H-bond donor binding sites, among which also squaramide moieties.
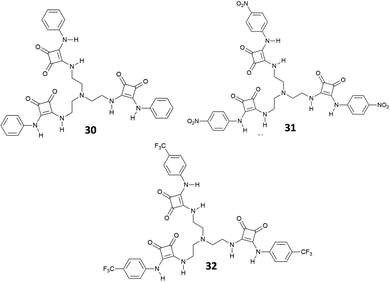
In 2013, considering the results achieved with the well-known tren-based tris-(thio)urea receptors for anion binding,36,37 Jiang, Lin and co-workers reported the tripodal receptors 30–32 (Fig. 10) and investigated by 1H-NMR their binding properties towards inorganic anions as TBA salts in DMSO-d6.38
The results indicated that receptors 30–32 show strong binding affinities or acid–base interactions with Cl−, SO42−, HSO4−, H2PO4−, and AcO− (Cl− and H2PO4− provoked deprotonation of 31 and 32) with a preference for SO42− [Kass: 4.75 (30), 4.95 (31), and 4.87 M−1 (32)] followed by the other anions in the order H2PO4− [Kass: 4.14 (30) M−1], HSO4− [Kass: 3.65 (30), 3.78 (31), and 3.65 M−1 (32)], AcO− [Kass: 2.82 M−1 (30)], and Cl− [Kass: 2.58 (30), 2.61 (31), and 2.65 M−1 (32)] and a fine modulation exerted by substituents present on the phenyl groups. In any case, these receptors showed a binding affinity for SO42− higher than those observed for urea-based tripodal systems.36,37 Interestingly, all evidences (including Job plots and DOSY NMR experiments) suggested the formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 adduct in solution between 30 and SO42−, despite in the solid state the 2
1 adduct in solution between 30 and SO42−, despite in the solid state the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 [30(SO4)]2(TBA)2 dimeric adduct was isolated and structurally characterized (Fig. 11). Each SO42− anion in the dimer is coordinated by the NH protons of the squaramide units from two arms of 30 and by the squaramide moiety belonging to a symmetry related receptor unit.
2 [30(SO4)]2(TBA)2 dimeric adduct was isolated and structurally characterized (Fig. 11). Each SO42− anion in the dimer is coordinated by the NH protons of the squaramide units from two arms of 30 and by the squaramide moiety belonging to a symmetry related receptor unit.
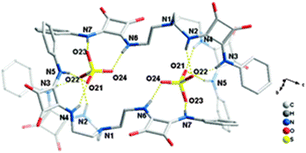 | ||
Fig. 11 Molecular structure of the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 adduct [[30(SO4)]2]2− in [30(SO4)]2(TBA)2. Reproduced with permission from ref. 38. Copyright RSC 2013. 2 adduct [[30(SO4)]2]2− in [30(SO4)]2(TBA)2. Reproduced with permission from ref. 38. Copyright RSC 2013. | ||
The same authors successfully employed these receptors as anionophores in the preparation of ISEs for sulfate recognition.39 In particular, the membrane prepared incorporating receptor 31 showed a Nernstian slope of −30.2 mV with a linear range from 1 μM to 100 mM sulfate concentration and was employed for the quantification of sulfate in cell lysates and drinking water. Jolliffe and co-workers have reported a series of neutral macrocyclic receptors of different ring size cavities, preorganization for SO42− binding, and solubility in aqueous media, featuring two or three squaramide moieties in their structures and two or three benzene (Fig. 12) or pyridine spacer units (Fig. 13).40,41
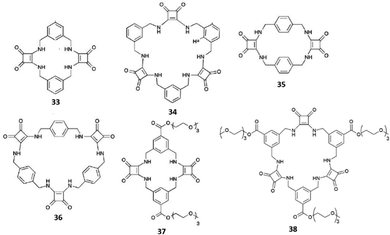 | ||
| Fig. 12 Cyclic squaramide-based receptors featuring aryl spacers and in the cases of 37 and 38 also triethylene glycol monomethyl pendants via ester linkages. | ||
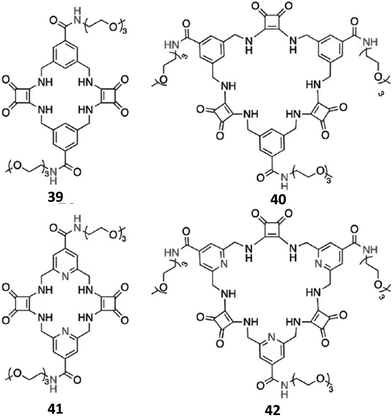 | ||
| Fig. 13 Cyclic squaramide-based receptors 39–42 featuring benzene or pyridine spacers and triethylene glycol monomethyl pendants via amide linkages. | ||
Receptors 33–36 contain alternating aryl and squaramide units (Fig. 12), and they do not feature triethyleneglycol monomethyl pendants via ester or amide linkages for 37–42. Unfortunately, despite clear evidences for the ability to bind strongly SO42−, due to the low solubility even in DMSO-d6, and the complexity of the 1H-NMR spectra, quantitative anion binding studies were not possible for 35 and 36.42 In the case of the more soluble 33 and 34, these studies were performed in DMSO-d6/0.5%H2O by titration with the TBA salts of the anions SO42−, H2PO4−, AcO−, and Cl−, for which preliminary results indicated a strong interaction with the two receptors in DMSO-d6 (in the presence of F−, deprotonation of the NH protons was observed for 33 and 34 in DMSO-d6).42 In this medium, both 33 and 34 were found to bind SO42− with association constants (Kass) higher than 104 M−1 for the formation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 adducts. However, significant differences were observed for the measured binding constants with the other anions with the highest selectivity for the SO42− observed in the case of 34 [Kass: >104 (SO42−), >104 (H2PO4−), 7530 (AcO−), and 28 M−1 (Cl−) for 33; >104 (SO42−), 409 (H2PO4−), 776 (AcO−), and 144 M−1 (Cl−) for 34].421H-NMR spectra indicated the involvement of both squaramide NH protons and aromatic CHs sitting between the two benzene ring substituents in SO42− and H2PO4− binding by both 33 and 34. For the other two anions, only the NH protons appeared to be involved in the binding. A clear picture of the binding mode of 33 with SO42− comes from the X-ray crystal structure of the complex [33(SO4)](TBA)2·2H2O.42 In the complex anion [33(SO4)]2−, the receptor adopts a bowl-like conformation, reminiscent of a calixarene in the cone conformation, with NH protons from both squaramide units and the two aromatic CH protons (one from each benzene ring) pointing towards the macrocyclic cavity (Fig. 14). In this conformation, the prevailing binding mode of the SO42− anion by the receptor involves the formation of NH⋯O H-bonds between each of the four amide protons and three of the anion oxygens as shown in Fig. 14(A) and (B). The free coordination hemisphere of SO42− is nestled among the butyl chains of the TBA+ counter cations. Interestingly, the optimized geometry of the anion [33(AcO)]− features the anion bound to the four NH protons, but the aromatic CH protons sitting between the two benzene ring substituents point away from the anion binding site and the conformation assumed by the receptor is reminiscent more of that observed for a 1,3-alternate calixarene.42
1 adducts. However, significant differences were observed for the measured binding constants with the other anions with the highest selectivity for the SO42− observed in the case of 34 [Kass: >104 (SO42−), >104 (H2PO4−), 7530 (AcO−), and 28 M−1 (Cl−) for 33; >104 (SO42−), 409 (H2PO4−), 776 (AcO−), and 144 M−1 (Cl−) for 34].421H-NMR spectra indicated the involvement of both squaramide NH protons and aromatic CHs sitting between the two benzene ring substituents in SO42− and H2PO4− binding by both 33 and 34. For the other two anions, only the NH protons appeared to be involved in the binding. A clear picture of the binding mode of 33 with SO42− comes from the X-ray crystal structure of the complex [33(SO4)](TBA)2·2H2O.42 In the complex anion [33(SO4)]2−, the receptor adopts a bowl-like conformation, reminiscent of a calixarene in the cone conformation, with NH protons from both squaramide units and the two aromatic CH protons (one from each benzene ring) pointing towards the macrocyclic cavity (Fig. 14). In this conformation, the prevailing binding mode of the SO42− anion by the receptor involves the formation of NH⋯O H-bonds between each of the four amide protons and three of the anion oxygens as shown in Fig. 14(A) and (B). The free coordination hemisphere of SO42− is nestled among the butyl chains of the TBA+ counter cations. Interestingly, the optimized geometry of the anion [33(AcO)]− features the anion bound to the four NH protons, but the aromatic CH protons sitting between the two benzene ring substituents point away from the anion binding site and the conformation assumed by the receptor is reminiscent more of that observed for a 1,3-alternate calixarene.42
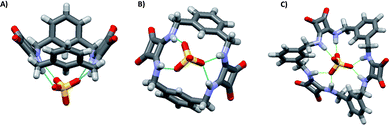 | ||
| Fig. 14 Two different views of the structure of the complex anion [33(SO4)]2− with the receptor in the dominant binding mode (A) and (B). The optimized model of the complex anion [34(SO4)]2− (C). Reproduced with permission from ref. 42. Copyright RSC 2016. | ||
The higher selectivity of 34 towards the SO42− anion is supported by the optimized structure of the complex anion [34(SO4)]2− (Fig. 14(C)). The SO42− anion sits inside the macrocyclic cavity with all four oxygens engaging H-bonds with the squaramide NH protons, all six of them being involved. Furthermore, all three benzene rings have one proton pointing towards the SO42− anion, thus suggesting the involvement of them in additional stabilizing interactions, which would contribute to the high affinity and selectivity of 34 for SO42−.
The introduction in 37 and 38 of triethylene glycol monomethyl pendants via ester linkages allowed anion binding in H2O/DMSO-d6 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v) to be studied by 1H-NMR. In this more competitive solvent mixture, a remarkable decrease of the association constant was observed for 37 [Kass: 1820 M−1 (SO42−)] compared to 33 [Kass: >104 M−1 (SO42−)] in the formation of the 1
2 v/v) to be studied by 1H-NMR. In this more competitive solvent mixture, a remarkable decrease of the association constant was observed for 37 [Kass: 1820 M−1 (SO42−)] compared to 33 [Kass: >104 M−1 (SO42−)] in the formation of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 adduct. However, receptor 38 still showed a high affinity for SO42− [Kass: >104 M−1]. This was attributed not only to the presence in 38 of an additional squaramide and aromatic CH binding sites, but mainly to the perfect match of the size of the anion to the cavity of the receptor, which allows the former to be protected from the interactions with solvent molecules in a sort of hydrophobic cavity. In H2O/DMSO-d6 (1
1 adduct. However, receptor 38 still showed a high affinity for SO42− [Kass: >104 M−1]. This was attributed not only to the presence in 38 of an additional squaramide and aromatic CH binding sites, but mainly to the perfect match of the size of the anion to the cavity of the receptor, which allows the former to be protected from the interactions with solvent molecules in a sort of hydrophobic cavity. In H2O/DMSO-d6 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v), 38 showed also great selectivity for SO42− in comparison with other tetrahedral anions such as phosphate species (Kass: 16 M−1 at pH 7 in the presence of both H2PO4− and HPO42−), SeO42− (Kass: 1900 M−1), CrO42− (Kass: 165 M−1), and ClO42− (Kass: too low to be determined). 1H-NMR titrations of 38 with SO42− and phosphate species in H2O/DMSO-d6 (1
2 v/v), 38 showed also great selectivity for SO42− in comparison with other tetrahedral anions such as phosphate species (Kass: 16 M−1 at pH 7 in the presence of both H2PO4− and HPO42−), SeO42− (Kass: 1900 M−1), CrO42− (Kass: 165 M−1), and ClO42− (Kass: too low to be determined). 1H-NMR titrations of 38 with SO42− and phosphate species in H2O/DMSO-d6 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v) were also performed at pH 9.1 (tris buffer) at which the predominant phosphate species is HPO42−; under these conditions, the Kass values observed for the two anions were 2045 and 13 M−1, respectively. A higher affinity of 38 for sulfate than other anions was also confirmed in aqueous mixtures [H2O/DMSO-d6 (1
2 v/v) were also performed at pH 9.1 (tris buffer) at which the predominant phosphate species is HPO42−; under these conditions, the Kass values observed for the two anions were 2045 and 13 M−1, respectively. A higher affinity of 38 for sulfate than other anions was also confirmed in aqueous mixtures [H2O/DMSO-d6 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v)] mimicking the anion composition of plasma (measurements made at pH 7.4) and nuclear waste (pH 3.2).
1 v/v)] mimicking the anion composition of plasma (measurements made at pH 7.4) and nuclear waste (pH 3.2).
In order to further increase the solubility in aqueous DMSO mixtures with a higher water content, and the SO42− binding affinity and selectivity over a wide pH range, Joliffe and co-workers also synthesised the receptors 39–42 (Fig. 13), which are structural analogues of 37 and 38, but feature amide linkages instead of ester ones for decorating the macrocyclic structures with the solubilizing triethyleneglycol chains, and pyridines instead of benzene spacers (41 and 42).40 As expected, receptors 40 and 42 (featuring three squaramide units in their structures) displayed higher affinities than analogues 39 and 41 (featuring two squaramide units in their structures), in both H2O/DMSO-d6 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 v/v) and H2O/DMSO-d6 (1
9 v/v) and H2O/DMSO-d6 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v) at pH 7, as already observed for 38 with respect to 37 (the low solubility of 41 in the latter solvent mixture prevented an evaluation of its SO42− binding affinity in this medium), with Kass values for the 1
2 v/v) at pH 7, as already observed for 38 with respect to 37 (the low solubility of 41 in the latter solvent mixture prevented an evaluation of its SO42− binding affinity in this medium), with Kass values for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 adduct formation higher than 104 M−1. While the replacement of ester linkages by amide ones resulted in a little impact on the SO42− binding ability, the replacement of benzene spacers with pyridine ones revealed to be a winning strategy. In fact, due the higher solubility of 40 and 42 in comparison to that of 34, 1H-NMR binding studies were performed for these two receptors also in DMSO-d6/H2O (1
1 adduct formation higher than 104 M−1. While the replacement of ester linkages by amide ones resulted in a little impact on the SO42− binding ability, the replacement of benzene spacers with pyridine ones revealed to be a winning strategy. In fact, due the higher solubility of 40 and 42 in comparison to that of 34, 1H-NMR binding studies were performed for these two receptors also in DMSO-d6/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) at pH 7. In this very competitive medium, 42 resulted to bind SO42− about three times more strongly than 40 (Kass: 4870 and 1340 M−1 for 42 and 40, respectively), and this selectivity was maintained even in DMSO-d6/H2O (1
1 v/v) at pH 7. In this very competitive medium, 42 resulted to bind SO42− about three times more strongly than 40 (Kass: 4870 and 1340 M−1 for 42 and 40, respectively), and this selectivity was maintained even in DMSO-d6/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v) (Kass: 480 and 130 for 42 and 40, respectively). In agreement with DFT calculations, this behaviour was attributed to a greater preorganization of 42 to SO42− binding due to intramolecular H-bonds between the pyridine N atoms and the NH protons with the free ligand that presents in its more stable conformation all squaramide protons already pointing towards the centre of the ring cavity. In contrast, 40 would undergo a significant conformational change for SO42− binding through all six NH protons (Fig. 15).
2 v/v) (Kass: 480 and 130 for 42 and 40, respectively). In agreement with DFT calculations, this behaviour was attributed to a greater preorganization of 42 to SO42− binding due to intramolecular H-bonds between the pyridine N atoms and the NH protons with the free ligand that presents in its more stable conformation all squaramide protons already pointing towards the centre of the ring cavity. In contrast, 40 would undergo a significant conformational change for SO42− binding through all six NH protons (Fig. 15).
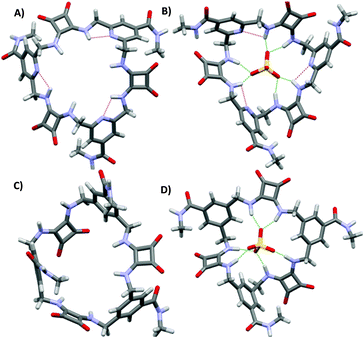 | ||
| Fig. 15 Optimized structures of 42 (A), 40 (C), [42(SO4)]2− (B), and [40(SO4)]2− (D). Reproduced with permission from ref. 41. Copyright RSC 2019. | ||
Significantly, in DMSO-d6/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v), binding of the anions AcO−, NO3−, Cl−, HCO3− and H2PO4− to 42 was negligible as no changes in the 1H-NMR spectrum of the receptor was observed upon addition of 5 equiv. of these anions. Furthermore, in this medium, 42 exhibited a binding selectivity for SO42− over SeO42− and CrO42− with a selectivity index for the latter (Kass(sulfate)/Kass(chromate) = 483) much better than that observed for 38 under the same experimental conditions. Compared to 38, the SO42− binding performances of 42 resulted significantly increased also in aqueous mixtures [DMSO-d6/H2O (1
1 v/v), binding of the anions AcO−, NO3−, Cl−, HCO3− and H2PO4− to 42 was negligible as no changes in the 1H-NMR spectrum of the receptor was observed upon addition of 5 equiv. of these anions. Furthermore, in this medium, 42 exhibited a binding selectivity for SO42− over SeO42− and CrO42− with a selectivity index for the latter (Kass(sulfate)/Kass(chromate) = 483) much better than that observed for 38 under the same experimental conditions. Compared to 38, the SO42− binding performances of 42 resulted significantly increased also in aqueous mixtures [DMSO-d6/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v)] mimicking the anion composition of plasma (measurements made at pH 7.4, Kass: 2490 M−1) and nuclear waste (pH 3.2, Kass: 4870 M−1) in the presence of competing anions, and under conditions of pH 14 (Kass: 610 M−1) in which the receptor resulted stable for 5 hours.
1 v/v)] mimicking the anion composition of plasma (measurements made at pH 7.4, Kass: 2490 M−1) and nuclear waste (pH 3.2, Kass: 4870 M−1) in the presence of competing anions, and under conditions of pH 14 (Kass: 610 M−1) in which the receptor resulted stable for 5 hours.
Neutral dipeptides 43 and 44 bearing squaramide functionalised side chains have been reported by Joliffe and co-workers as efficient SO42− receptors in mixtures of DMSO and H2O (Fig. 16).43
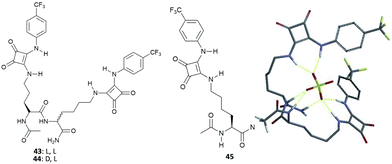 | ||
| Fig. 16 Molecular structures of dipeptides 43–45 featuring squaramide functionalized side chains and the modelled structure of the adduct [43(SO4)]2− showing the possible H-bond patterns responsible for the high binding affinity of 43 towards SO42− in very competitive solvent mixtures. Reproduced with permission from ref. 43. Copyright Wiley 2014. | ||
Due to the large association constant of these receptors with SO42− in DMSO-d6 containing 0.5% of water (v/v) (strong binding is suggested by 1H-NMR spectra over titration experiments, which could not be fitted to a suitable binding model, probably because of a two-stages binding process), titration experiments were repeated in DMSO-d6/20%H2O (v/v). In this more polar and competitive mixture, both 43 and 44 retained a high binding affinity for SO42− (Kass: >104 M−1, demonstrating the negligible effect of the different chirality of the two receptors), with the formation of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 adduct, and high selectivity over AcO− [Kass: 44.9 (43), 72.6 M−1 (44)] and BzO− [Kass: 17.1 (43), 29.7 M−1 (44)], the only anions that express detectable interactions with the two receptors in the same solvent mixture. Of note, the binding affinity for SO42− of 43 and 44 resulted significantly higher than those observed for the analogous systems featuring thiourea groups instead of squaramide moieties (Kass: 1282 and 775 M−1), for the modified amino acid 45 (Kass: 1116 M−1) (Fig. 16), and related amino acid-based squaramides.44 Moreover, Joliffe and co-workers observed that receptor structural analogues of 43 and 44, but featuring shorter peptide side chain lengths, increased length and flexibility of the peptide backbone, and the increased number of electron withdrawing or hydrophilic groups present on the squaramide phenyl substituents did not alter significantly the binding affinity and selectivity towards SO42− in DMSO-d6/20%H2O (v/v).45
1 adduct, and high selectivity over AcO− [Kass: 44.9 (43), 72.6 M−1 (44)] and BzO− [Kass: 17.1 (43), 29.7 M−1 (44)], the only anions that express detectable interactions with the two receptors in the same solvent mixture. Of note, the binding affinity for SO42− of 43 and 44 resulted significantly higher than those observed for the analogous systems featuring thiourea groups instead of squaramide moieties (Kass: 1282 and 775 M−1), for the modified amino acid 45 (Kass: 1116 M−1) (Fig. 16), and related amino acid-based squaramides.44 Moreover, Joliffe and co-workers observed that receptor structural analogues of 43 and 44, but featuring shorter peptide side chain lengths, increased length and flexibility of the peptide backbone, and the increased number of electron withdrawing or hydrophilic groups present on the squaramide phenyl substituents did not alter significantly the binding affinity and selectivity towards SO42− in DMSO-d6/20%H2O (v/v).45
To obtain insight into the possible binding mode of this class of receptors, the structure of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 [43(SO4)]2− adduct was modelled.43 The results of calculations show the SO42− anion wrapped by the receptor and forming seven H-bonds, of which four with the squaramide NH protons, two with the NH amide protons from the peptide backbone, and one with the carboxamide group (Fig. 16), in agreement with changes in the 1H-NMR spectra upon titration with SO42−.
1 [43(SO4)]2− adduct was modelled.43 The results of calculations show the SO42− anion wrapped by the receptor and forming seven H-bonds, of which four with the squaramide NH protons, two with the NH amide protons from the peptide backbone, and one with the carboxamide group (Fig. 16), in agreement with changes in the 1H-NMR spectra upon titration with SO42−.
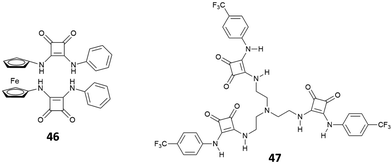
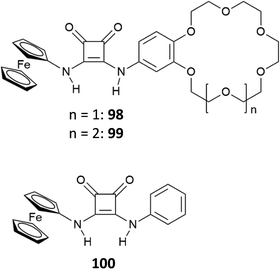
Recently, the bis[squaramido]ferrocene receptor 46 has been reported for SO42− binding and electrochemical recognition (Fig. 17).46
1H-NMR binding studies were performed initially in DMSO-d6 containing 1% of water by titrations of 46 with various anions as TBA salts. No changes in the 1H-NMR spectrum of 46 were observed upon addition of Cl−, HSO4−, and NO3−, while the more basic F− and AcO− caused NH deprotonation. SO42− and H2PO4− strongly interact with 46 with formation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 adducts (Kass: >104 M−1) (in contrast to the 1
1 adducts (Kass: >104 M−1) (in contrast to the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 adduct with H2PO4−, the 1
1 adduct with H2PO4−, the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 adduct with SO42− resulted quite stable even in the presence of a large excess this anion). On passing to a more competitive aqueous medium (DMSO containing 20% of H2O), it was possible by UV-Vis spectroscopy to quantify the Kass values for the 1
1 adduct with SO42− resulted quite stable even in the presence of a large excess this anion). On passing to a more competitive aqueous medium (DMSO containing 20% of H2O), it was possible by UV-Vis spectroscopy to quantify the Kass values for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 adducts of 46 with SO42− and H2PO4− (Kass: 3.1 × 105 and 1.4 × 104 M−1, respectively), which suggest a marked selectivity of the receptor for the former. Interestingly, the structural analogue of 46 featuring only one squaramide moiety linked to the ferrocene unit shows in DMSO-d6 containing 1% of H2O a much lower selectivity for SO42− with respect H2PO4−, and an association constant (measured by 1H-NMR titrations) for the formation of the 1
1 adducts of 46 with SO42− and H2PO4− (Kass: 3.1 × 105 and 1.4 × 104 M−1, respectively), which suggest a marked selectivity of the receptor for the former. Interestingly, the structural analogue of 46 featuring only one squaramide moiety linked to the ferrocene unit shows in DMSO-d6 containing 1% of H2O a much lower selectivity for SO42− with respect H2PO4−, and an association constant (measured by 1H-NMR titrations) for the formation of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 adduct with SO42− (Kass: 1.2 × 103 M−1) that is over two orders of magnitude lower than that measured for 46 in DMSO containing 20% of H2O, thus suggesting a higher efficiency of the scaffold having two stacked squaramide units in 46 for SO42− binding.
1 adduct with SO42− (Kass: 1.2 × 103 M−1) that is over two orders of magnitude lower than that measured for 46 in DMSO containing 20% of H2O, thus suggesting a higher efficiency of the scaffold having two stacked squaramide units in 46 for SO42− binding.
Chloride binding is of high interest due to the fact that the transport of this anion through channels is particularly relevant from a biological point of view (see the Anion transport section). Muthyala and co-workers have demonstrated that the insertion of carbonyl groups such as benzoyl groups on the squaramide skeleton facilitates chloride binding in polar solvents such as CH3CN by disrupting the intermolecular H-bonds between the carbonyl group of the substituents and the squaramide NHs which is instead favoured in apolar solvents such as CHCl3.47 The same authors have lately shown that these types of receptors can be used to detect chloride via excited state intramolecular proton transfer (see the Anion sensing section).
The tripodal receptor 47 (Fig. 17) was successfully employed for the development of ion selective electrodes (ISEs) to selectively sense chloride in sweat and blood samples.48 Interestingly, the membranes prepared incorporating 47 were found to have a high selectivity towards chloride over commonly found interferents such as salycilate (often found in patients treated with aspirin) and heparine (used as the anti-coagulant).
Squaramide derivatives can be successfully employed for the development of interlocked structures such as rotaxanes49 which were used, for example, for sodium halide ion pair recognition.50 Very recently, Evans and co-workers have found that the addition of chloride to a solution of chiral 2-rotaxane 48 in CDCl3 caused the shuttling of the macrocycle from the squaramide station to the diphenyl stopper (Fig. 18) as demonstrated by 1H-NMR experiments with a measured association constant Kass of 1300 M−1.51
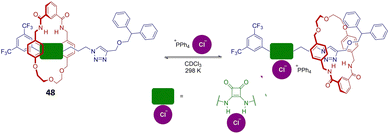 | ||
| Fig. 18 Scheme of the shuttling of the macrocycle in rotaxane 48 induced by the addition of chloride in CDCl3. Adapted with permission from ref. 51. Copyright Wiley 2023. | ||
Anion extraction
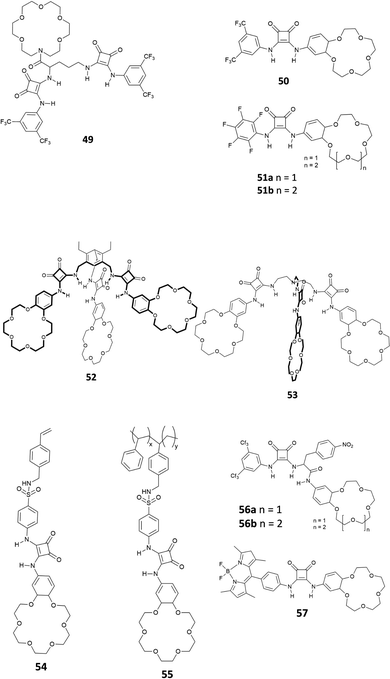
Recently, it has been discovered that squaramides can be used to develop efficient methods to extract cations, anions, and ion-pairs.Romański and coworkers have been very active in the field. They have developed various squaramide-based receptors (Fig. 19) that have been successfully used as extractants.52–60
As shown in Fig. 19, all the receptors developed in Romański's lab are characterised by the presence of at least one squaramide unit and one crown-ether unit, which are responsible for the binding of anions and cations, respectively. Indeed, receptors 49–57 are able to extract ion-pairs from water solution. As an example, receptor 50, presenting a squaramide unit non-symmetrically substituted with a 3,5-trifluoromethyl-phenyl moiety and a benzo-18-crown-6 unit, acts as an efficient extractant for potassium sulphate in liquid/liquid extraction (LLE) technology. It is well known that benzo-18-crown-6 is highly specific for potassium cation binding and this also improves the anion affinity (Kass = 2300 and 4300 M−1 for NaCl and KCl, respectively in CH3CN/5% H2O for the formation of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex, calculated from UV-Vis titrations). Interestingly, receptor 50 formed the 1
1 complex, calculated from UV-Vis titrations). Interestingly, receptor 50 formed the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 adducts with Cl−, I−, NO2− and NO3−, while in the case of SO42− a peculiar supramolecular assembly formed by the 4
1 adducts with Cl−, I−, NO2− and NO3−, while in the case of SO42− a peculiar supramolecular assembly formed by the 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 receptor/anion adduct was observed. The formation of the self-assembled structure was demonstrated by 1H-NMR titrations in CD3CN and DOSY-NMR (a decrease of 28% of the diffusion coefficient of the free receptor was measured), dynamic light scattering (DLS) experiments (an hydrodynamic diameter, dH, of 28 nm for the supramolecular assembly was measured) and single crystal X-ray diffraction analysis on the crystals obtained by slow diffusion of diethyl ether vapours into a methanol solution of receptor 50 in the presence of an excess of Na2SO4 (Fig. 20). The authors demonstrated for the first time the possibility to extract a highly hydrophilic anion such as SO42− from an aqueous solution of an alkaline earth salt to an organic phase. A 1H-NMR experiment was carried out by adding a 2 mM solution of 50 in CDCl3 aqueous solutions 50 mM of various anions (Cl−, Br−, NO3−, NO2−, H2PO4−, and SO42−) as their potassium salts. To confirm the importance of the counter cation, Na2SO4 was also tested. In all cases, except for K2SO4, the formation of insoluble solids was observed confirming the unique properties of receptor 50 to increase the solubility of the SO42− salt in organic solvents. Indeed, as shown in Fig. 20(B), a dramatic shift of the squaramide NH signals was observed when a solution of 50 was in contact with the aqueous solution of K2SO4. Back extraction experiments showed the ability of 50 to release the salt back to water.
1 receptor/anion adduct was observed. The formation of the self-assembled structure was demonstrated by 1H-NMR titrations in CD3CN and DOSY-NMR (a decrease of 28% of the diffusion coefficient of the free receptor was measured), dynamic light scattering (DLS) experiments (an hydrodynamic diameter, dH, of 28 nm for the supramolecular assembly was measured) and single crystal X-ray diffraction analysis on the crystals obtained by slow diffusion of diethyl ether vapours into a methanol solution of receptor 50 in the presence of an excess of Na2SO4 (Fig. 20). The authors demonstrated for the first time the possibility to extract a highly hydrophilic anion such as SO42− from an aqueous solution of an alkaline earth salt to an organic phase. A 1H-NMR experiment was carried out by adding a 2 mM solution of 50 in CDCl3 aqueous solutions 50 mM of various anions (Cl−, Br−, NO3−, NO2−, H2PO4−, and SO42−) as their potassium salts. To confirm the importance of the counter cation, Na2SO4 was also tested. In all cases, except for K2SO4, the formation of insoluble solids was observed confirming the unique properties of receptor 50 to increase the solubility of the SO42− salt in organic solvents. Indeed, as shown in Fig. 20(B), a dramatic shift of the squaramide NH signals was observed when a solution of 50 was in contact with the aqueous solution of K2SO4. Back extraction experiments showed the ability of 50 to release the salt back to water.
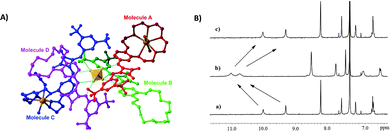 | ||
| Fig. 20 Crystal structure of the supramolecular assembly formed by Na2SO4 and receptor 50 (A). Changes in the chemical shift of selected protons of receptor 50 in CDCl3 before (a) and after (b) the addition of an aqueous solution of K2SO4 (B). The spectrum (c) is referred to a back extraction experiment and shows the reversibility of the process. Reproduced with permission from ref. 52. Copyright RSC 2019. | ||
Jolliffe and co-workers have also shown that squaramides can promote SO42− extraction. In this case, the macrocyclic receptor 58 was employed.40 LLE experiments were conducted by vigorously shaking a water solution containing TBA2SO4 with a CDCl3 solution containing receptor 58 and analysing the organic phase by 1H-NMR spectroscopy and it was demonstrated that one equivalent and even a substoichiometric concentration of SO42− could be extracted from the aqueous phase. However, in contrast to what was observed by Romański, the lipophilicity of the counter cation was crucial in this case as Na2SO4 could not be extracted. In a competitive experiment, it was found that a CDCl3 solution of receptor 58 could selectively extract SO42− from an aqueous solution containing both SO42− and NO3− and that a full recovery of SO42− could be obtained by precipitating it back in water in the form BaSO4 by using aqueous Ba(NO3)2 and forming the adduct 58·NO3−via an anion metathesis process. Starting from these results, a U-tube experiment was designed (Fig. 21) and the sulfate transport across a bulk chloroform membrane was demonstrated in a wide pH range (pH = 3.2–9.4). In this experiment, the SO42− extraction from the organic phase was monitored via ICP-MS using BaSO4 gravimetric analysis. SO42− transport was possible only in the presence of NO3− in the organic phase while a 2-fold increase in the transport rate was observed in the presence of BaCl2 in solution, which drove the equilibrium by the Le Chatelier principle.
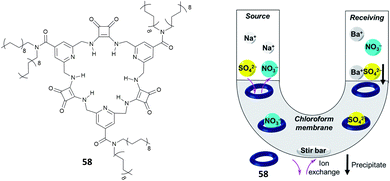 | ||
| Fig. 21 Structure of receptor 58 and scheme of the U-tube test to demonstrate sulfate transport across the chloroform membrane. Reproduced with permission from ref. 40. Copyright RSC 2020. | ||
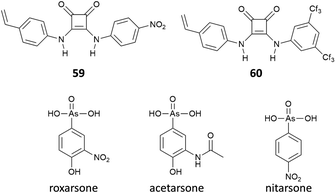
Interestingly, Maniesotis, Baggiani and co-workers showed that polymerisable squaramides 59 and 60 (already employed to prepare imprinting polymers)61 could be used for producing molecularly imprinted polymers (MIPs) for the solid state extraction of 4-hydroxy-3-nitrophenylarsonic acid (commonly known as roxarsone), an organoarsenic compound used in poultry production as a feed additive (Fig. 22).62 Monomers 59 and 60 showed good association constants for the formation of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 adducts with roxarsone as its TBA salt in the range of 103 M−1 in DMSO-d6 measured by 1H-NMR titrations. Imprinting polymers were prepared by mixing monomers and roxarsone salt in a 2
1 adducts with roxarsone as its TBA salt in the range of 103 M−1 in DMSO-d6 measured by 1H-NMR titrations. Imprinting polymers were prepared by mixing monomers and roxarsone salt in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio with the cross-linker dimethylacrylate (EDMA) and the free-radical initiator 2,2′-azobis(2,4-dimethyl)valeronitrile (ABDV). Imprinting polymers showed similar affinity towards roxarsone than the monomers but in the more competitive solvent methanol (Kass = 14.65 × 103 M−1 and 16.85 × 103 M−1, for the MIP prepared with 59 and 60, respectively). In terms of selectivity, a remarkable selectivity towards roxarsone when compared to its analogous acetarsone and nitarsone was observed for MIP based on squaramide 60.
1 ratio with the cross-linker dimethylacrylate (EDMA) and the free-radical initiator 2,2′-azobis(2,4-dimethyl)valeronitrile (ABDV). Imprinting polymers showed similar affinity towards roxarsone than the monomers but in the more competitive solvent methanol (Kass = 14.65 × 103 M−1 and 16.85 × 103 M−1, for the MIP prepared with 59 and 60, respectively). In terms of selectivity, a remarkable selectivity towards roxarsone when compared to its analogous acetarsone and nitarsone was observed for MIP based on squaramide 60.
This MIP was successfully used to establish a protocol for the solid state extraction of roxarsone by loading polypropylene solid-phase extraction cartridges with the MIP and then putting it in contact with an aqueous sample containing the target analyte. Cartridges were then washed, and the eluates were analysed by HPLC. With this method, it was possible to measure concentrations of roxarsone down to 1 μg mL−1 in water samples.
Very recently, Jolliffe and co-workers have shown a novel strategy for developing squaramide copolymers by combining together poly(ethylene glycol) methyl ether methacrylate (PEGMA) and [(tert-butoxycarbonyl)amino]ethyl methacrylamide (Boc-AEMA) in different ratios and functionalising the resulting polymer with squaramides bearing various substituents (3,5-(bis)-trifluoromethylphenyl and 2-fluornyl) after the removal of the Boc group. The polymers showed the ability to bind anions in DMSO with no selectivity and similarly to the corresponding molecular squaramides while no binding was observed in pure water.63
A fluorinated squaramide-based covalent organic framework (COF) (61, Fig. 23(A)) that is able to efficiently extract a broad-spectrum of per- and polyfluoroalkyl contaminants from water was described by Zheng, Ouyang and co-workers.64 COF 61 allows for multiple interactions with PFAS as it is able to act as the H-bond donor and H-bond acceptor and has fluorophilic moieties. The PFAS chosen for the extraction measurements comprise, among others, PFOA (perfluorooctanoic acid, in its anionic form), PFOS (perfluorooctane sulfonate), GenX (2,3,3,3- tetrafluoro-2-(1,1,2,2,3,3,3-heptafluoropropoxy)propanoic acid, in its anionic form), F-53B (chlorinated polyfluorinated ether sulfonate), the cationic PFOSAmS (perfluorooctaneamido quaternary ammonium salt), and the zwitterionic N-CMAmP-6:2FOSA (N-(carboxymethyl)-N,N-dimethyl-3- (((3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluorooctyl)sulfonyl)amino)1-propanaminium). Glass capillaries etched with HF were covered with COF 61 by dip-coating technology and immersed into a water solution spiked with PFAS. The extraction performance was analysed by measuring the areas of the mass spectra of the pollutants. As shown in Fig. 23(B), COF 61 showed a higher enrichment factor (that measures the overall success of the extraction) than other commonly used commercially available extractants such as PDMS (polydimethylsilossane), DVB (divnylbenzene), PA (polyacrylate), and carboxen.
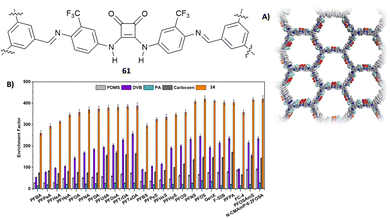 | ||
| Fig. 23 Structure of squaramide-based COF 61 and its structure obtained by PXRD (A). Enrichment factor for a family of PFAS for COF 61 and other commercially available extractants (B). Reproduced with permission from ref. 64. Copyright Wiley 2022. | ||
Anion sensing
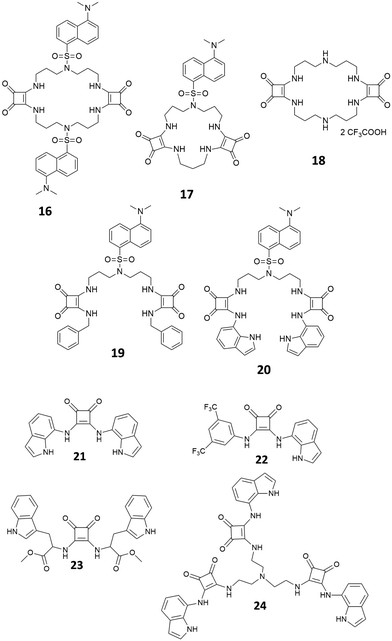
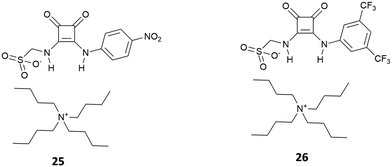
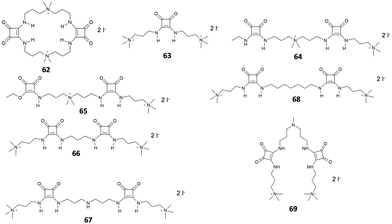
Since their introduction as receptors for the molecular recognition of anions,15 squaramide-based systems for anion binding and sensing represented an active field of research. Similarly, to the case of imidazole-, pyrrole-, urea- or amide-based systems, the design of squaramide-based molecular sensors has undergone significant developments, involving different approaches. Among these, one of the simplest involves the use of host–guest complexes between a receptor and a specific indicator, to be used in the so-called indicator displacement assays (IDAs).65 This strategy exploits the competition between the indicator and the target analyte in interacting with the receptor, producing a detectable response, such as changes in color or fluorescence emission, when the indicator is displaced from the receptor. Examples of this approach in squaramide-based systems were described in an early work by Costa et al., which focused on different families of squaramide-ammonium charge-assisted receptors (Fig. 24). Their design allows the combination of electrostatic interactions and hydrogen bonds to target oxyanions, such as sulfate and hydrogen phosphate, in aqueous media.66–68The anion binding ability of receptor 62 towards different anions was investigated by NMR and isothermal titration calorimetry (ITC).68 The results show a reduced binding ability of receptor 62 towards nitrates, halides and other monoanions, due to the selectivity promoted by the two tetra-alkylammonium groups. The authors suggested that the selectivity towards sulfates over other divalent anions might be due to constrains imposed by the squaramide functions, which favors interactions with tetrahedral sulfate anions. In the presence of fluorescein disodium salt, receptor 62 produced a quench of the fluorescein emission, as the consequence of a photo-induced electron transfer (PET) process from the squaramide donor to the excited singlet state of the fluorophore. Upon addition of sulfate anions, the PET is suppressed due to the complexation of the competing anion, restoring the fluorescence emission of the non-complexed fluorescein.
Receptors 63–68 were designed to work in a similar fashion.66 Different from receptor 62, this family was based on a linear molecular skeleton, showing subtle differences in the H-bonding capability and in the spacing between the charged sites, with distances between the electropositive sites that increase from receptor 63 to receptor 68. In this case, the sensing was achieved using a colorimetric ensemble, exploiting the acid–base equilibrium of the common pH indicator cresol red. When complexed by receptors 63–68, the indicator showed a purple coloration. This corresponds to the dianionic semiquinone form, which is preferentially complexed by receptors 63–68. The color turned to yellow upon addition of dianions such as HPO42− and SO42−. The selectivity of the ensembles was also investigated in the presence of potential competitive anions such as F−, Cl−, Br−, I−, CO32− and NO3− in EtOH/H2O mixtures (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v). The results showed a slight affinity towards sulfate over phosphates. This was particularly evident for receptor 68, which showed a relative sulfate/phosphate selectivity ratio of 2.28.
1 v/v). The results showed a slight affinity towards sulfate over phosphates. This was particularly evident for receptor 68, which showed a relative sulfate/phosphate selectivity ratio of 2.28.
The choice of the indicator can also affect the selectivity of the IDA towards specific anionic species. In a more recent article, Costa et al. described two IDAs based on receptor 69 complexed with cresol red and bromocresol green, respectively.67 In the presence of different anionic species in mixtures of EtOH/H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v), both IDAs produced a positive response only in the presence of phosphate and sulfate. In particular, phosphate and sulfate both produced a positive IDA response with cresol red, while bromocresol green proved to be selective only for the sulfate anion. On the basis of these results, the author implemented this approach using a UV-Vis microplate reader, enabling the parallel quantitative detection of these two anions by comparison with calibration curves.
1, v/v), both IDAs produced a positive response only in the presence of phosphate and sulfate. In particular, phosphate and sulfate both produced a positive IDA response with cresol red, while bromocresol green proved to be selective only for the sulfate anion. On the basis of these results, the author implemented this approach using a UV-Vis microplate reader, enabling the parallel quantitative detection of these two anions by comparison with calibration curves.
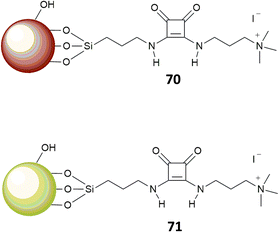
In a further development of this approach, Costa, Alarcon, Garcia-Espana et al.69 designed a squaramide based boehmite- (70) or silica-coated boehmite (71) IDAs, decorated with charged alkylammonium groups, able to complex bromocresol green (Fig. 25). The system works in a similar fashion as described above; however, different to the previous systems, this has the advantage to produce, among different anions, a selective response toward carboxylates and sulfates in pure water. The authors pointed that none of the previous systems were able to produce such a selective response in pure water and attributed this behavior to the presence of the nanoparticle substrate.
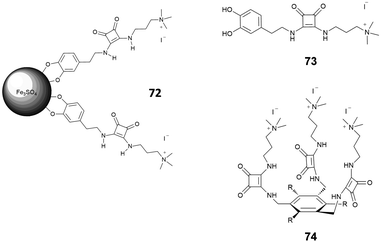
Morey et al.70 developed magnetic squaramide-coated Fe3O4 nanoparticles 72 for the detection of carboxylates, based on receptor 73 (Fig. 26). In this case, the nanoparticles were pretreated with a buffered (pH = 8) solution of fluorescein (2 × 10−5 M). This system was then tested with solutions of different anionic species (mono, di, tricarboxylates, F−, Cl−, Br−, NO3−, PO43− and SO42−), also in competing media such as water. The displacement of the fluorescein and the consequent increase of the emission at 490 nm was observed only in the case of mono and dicarboxylates. As pointed out by the authors, this system shows a selectivity analogous to that of more pre-organized receptors such as 74, previously published.71
A typical approach to the design of chemosensors, consists of a receptor covalently linked to a signaling unit, either directly or via a spacer. The interaction between the receptor unit and the analyte produces a specific response that depends, in the first place, to the intrinsic properties of the signaling unit. Accordingly, depending on the type of signaling unit, a chemosensor can be defined as fluorescent, colorimetric or redox. This strategy has been largely applied for designing chemosensors for different analyte sensing, including anions. Recently, it has also been applied to squaramide based systems.
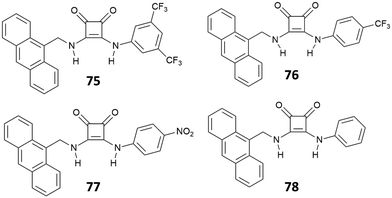
Jolliffe et al.72 described four chemosensors for anions (75–78), consisting of aryl substituted squaramides covalently bonded to a luminescent anthracene moiety (Fig. 27). The choice of different electron-withdrawing aryl substituents and different positions of substitution (3,5-CF3, 4-CF3, 4-NO2) allowed a modulation of the acidity/H-bonding ability of the squaramides. The sensing ability of the four receptors was investigated upon addition of different halides (F−, Cl−, I−, and Br−) and NO3− anions. The systems showed a selective response only upon the addition of Cl− anions, producing a quenching of the fluorescence. Specifically, receptors 75–77 exhibited a quench of the excimer emission at 530 nm, which was less evident on passing from 75 to 77, suggesting an influence of the substituents (i.e., type, position and number of substituents) in modulating the optical response.73,74 No particular changes in the fluorescence emission were observed for the less acidic receptor 78.
The response is attributed to the formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
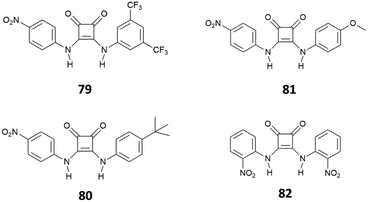
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex via H-bonding, as suggested by 1H-NMR and a solid-state analysis by single crystal X-ray diffraction. However, in the presence of a large excess of Cl−, receptors 75–77 showed an unusual example of deprotonation of a receptor by Cl−, as suggested by the disappearance of the 1H-NMR signals observed for the NH protons at high concentrations of this anion. The deprotonation was also evidenced by dramatic naked-eye color changes observed for 75–77, making these systems suitable also for colorimetric detection of Cl− anions via deprotonation of the squaramide NHs. Deprotonation induced by anions represents in fact another strategy for anion sensing and exploits the development of acidic receptors able to produce a change in color upon partial or complete deprotonation of the H-bond donor sites.75 This approach has been applied to several urea-based systems,76,77 and squaramide-based receptors have also been the subject of a number of recent studies.78–80 The first example of squaramide-based colorimetric receptors for anions (Fig. 28) has been developed by the Taylor group,81 which investigated the sensing ability of electron-deficient receptors 79–81 towards different anions and compared their behavior with that of N,N-bis(dinitrophenyl)urea (82).
1 complex via H-bonding, as suggested by 1H-NMR and a solid-state analysis by single crystal X-ray diffraction. However, in the presence of a large excess of Cl−, receptors 75–77 showed an unusual example of deprotonation of a receptor by Cl−, as suggested by the disappearance of the 1H-NMR signals observed for the NH protons at high concentrations of this anion. The deprotonation was also evidenced by dramatic naked-eye color changes observed for 75–77, making these systems suitable also for colorimetric detection of Cl− anions via deprotonation of the squaramide NHs. Deprotonation induced by anions represents in fact another strategy for anion sensing and exploits the development of acidic receptors able to produce a change in color upon partial or complete deprotonation of the H-bond donor sites.75 This approach has been applied to several urea-based systems,76,77 and squaramide-based receptors have also been the subject of a number of recent studies.78–80 The first example of squaramide-based colorimetric receptors for anions (Fig. 28) has been developed by the Taylor group,81 which investigated the sensing ability of electron-deficient receptors 79–81 towards different anions and compared their behavior with that of N,N-bis(dinitrophenyl)urea (82).
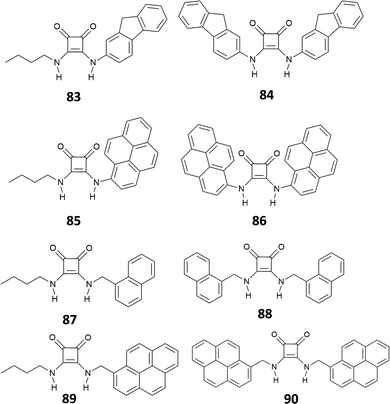
In a very recent work, Lane and Jolliffe82 described a case of colorimetric sensors as a part of a more general study, aimed at investigating the influence of the direct receptor–fluorophore conjugation in anion sensing. The authors compared the sensing ability of a family of squaramide-based chemosensors (83–90) functionalized with different fluorophores (Fig. 29), via both, a direct conjugation or a methylene spacer. The results suggest that direct conjugation has a strong effect in increasing the acidity of the NHs, probably due to the higher stabilization by electronic delocalization of the deprotonated species. This is reflected in the response of the conjugated receptors in the presence of anions. In particular, receptors 84–86 undergo deprotonation upon addition of 50 equiv. of different anions, accompanied by a color change, according to the basicity of the anions (yellow in the absence of anions, orange for the mono-deprotonated receptor and deep blue for complete deprotonation). Receptor 83 showed a different behavior. An investigation by UV-Vis spectroscopy showed a red shift in the maximum absorbance only in the presence of monovalent oxyanions, mainly H2PO4−, AcO−, consistent with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding model. Although this response was even more pronounced in the presence of SO42−, it was not possible identifying the binding model, probably due to complex equilibria involved. Receptors bearing a methylene spacer, 87–90, showed neither deprotonation nor changes in the UV-Vis spectra upon addition of anionic species. This was attributed to the methylene spacer that insulate the squaramide receptor from the substituents.
1 binding model. Although this response was even more pronounced in the presence of SO42−, it was not possible identifying the binding model, probably due to complex equilibria involved. Receptors bearing a methylene spacer, 87–90, showed neither deprotonation nor changes in the UV-Vis spectra upon addition of anionic species. This was attributed to the methylene spacer that insulate the squaramide receptor from the substituents.
While the fluorescence response of 83–86 in the presence of anions could not be investigated, due to the deprotonation or the shift of the absorbance maximum, 87–90 showed an interesting behavior. The emission spectra of 87 and 88 showed two bands at 325 nm and 495 nm, attributed to the monomer and the excimer, respectively. Dilution experiments suggested that the excimer is the result of intermolecular aggregation rather than intramolecular interactions promoted by a syn–syn conformation of the receptor. Upon addition of H2PO4−, AcO− and SO42−, 87 and 88 responded with a decrease of the emission intensity of the excimer band, with a more pronounced effect observed for 88 and, in general, a greater response in the presence of SO42− (Fig. 30).
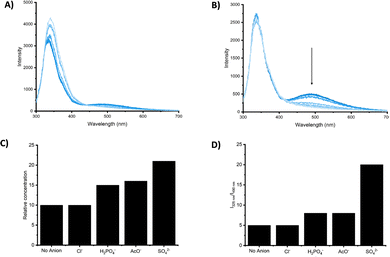 | ||
| Fig. 30 Emission spectra of 87 (20 μM) in DMSO (1% H2O) upon addition of TBA2SO4 (0 to 60 equiv.) (λex = 280 nm) at 298 K (A). Emission spectra 88 (20 μM) in DMSO/1%H2O upon addition of TBA2SO4 (0 to 80 equiv.) (λex = 280 nm) at 298 K (B). The ratios of I325/I490 for 87 (C) and 88 (D) in DMSO/1%H2O at 298 K in the presence of anion (10 equiv.). Adapted with permission from ref. 82. Copyright RSC 2023. | ||
Receptor 86 was also the object of a recent study by Caltagirone et al.83 The anion binding ability of the receptor towards a number of anions (F−, Cl−, Br−, I−, CN−, and BzO−) was investigated by 1H-NMR, UV-Vis and fluorescence spectroscopy in DMSO (DMSO-d6)/0.5%H2O. The results showed deprotonation in the presence of BzO−, CN− and F−, accompanied by a chromatic change of the solution, from colorless to yellow. Among the remaining halogenides, 86 showed a low affinity only upon addition of Cl−. This was explained considering that DMSO represents a competing molecule for the interaction with the squaramide, as suggested by the tendency of this receptor to form solvate phases in the solid state. In less competing solvents, such as CH3CN, 86 showed a higher affinity for Cl− anions. Investigation of the fluorescence response toward different anions suggested a scarce ability of 86 to operate as fluorescence molecular sensors for anion species. This was attributed to the steric hindrance in the receptor molecule, probably due to the direct conjugation between the pyrene moiety and the squaramide core. Previous studies84 suggest that increasing the flexibility of the system by adding an alkyl spacer might improve the response.
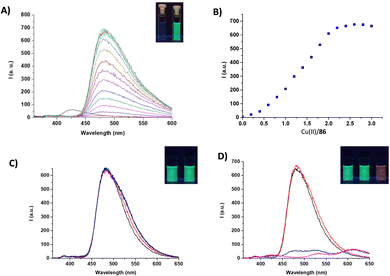
In this case, this was achieved using an alternative approach. Indeed, in the presence of copper(II) ions, 86 formed a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 metal-to-ligand complex, accompanied by a naked eye detectable fluorescence emission (Fig. 31). Among different cations, 86 selectively responded to copper(II), producing an emission band at 480 nm (λexc = 350 nm). Upon addition of anions, the 2
1 metal-to-ligand complex, accompanied by a naked eye detectable fluorescence emission (Fig. 31). Among different cations, 86 selectively responded to copper(II), producing an emission band at 480 nm (λexc = 350 nm). Upon addition of anions, the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 metal-to-ligand complex showed a variation of the fluorescence emission only in the presence of excesses of basic anions, resulting in a naked eye detectable change from green to a pale red (under a UV lamp). This was attributed to the deprotonation of the squaramide NHs of the metal complex, which caused the formation of a red-shifted emission band. The authors speculated that the 2
1 metal-to-ligand complex showed a variation of the fluorescence emission only in the presence of excesses of basic anions, resulting in a naked eye detectable change from green to a pale red (under a UV lamp). This was attributed to the deprotonation of the squaramide NHs of the metal complex, which caused the formation of a red-shifted emission band. The authors speculated that the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 metal complex could be used as a fluorescence chemodosimeter to recognize basic anions.
1 metal complex could be used as a fluorescence chemodosimeter to recognize basic anions.
 | ||
| Fig. 31 Emission spectra of 86 (1.0 × 10−5 M) in CH3CN upon addition of Cu(ClO4)2 hydrate (2.5 × 10−3 M), λexc = 350 nm (A) (inset: naked-eye colour change of 86 in the presence of 2 equiv. of Cu(ClO4)2 hydrate). The plot reporting the fluorescence emission intensity (480 nm) versus molar ratio relative to the titration of 86 with Cu(ClO4)2 hydrate (B). The emission spectra of the 86–Cu(II) complex (2.5 × 10−5 M) in the presence of increasing amounts of TBACl (2.5 × 10−3 M, λexc = 350 nm) (C) (inset: naked eye emission of 86 and upon addition of 10 equiv. TBACl). The emission spectra of the 86–Cu(II) complex (2.5 × 10−5 M) in the presence of increasing amounts of TBAOH (2.5 × 10−3 M, λexc = 350 nm) (D) (inset: naked eye emission of 86–Cu(II) complex, upon addition of 2 equiv. and 6 equiv. of TBAOH). Adapted with permission from ref. 83. Copyright MDPI 2021. | ||
The same authors recently described a family of macrocyclic and non-cyclic bisquaramide receptors (16–17 and 19–20 respectively), directly conjugated to a dansyl moiety.19 These receptors have been investigated as potential fluorescent probes for the recognition of salted forms of naproxene and chetoprofen (see the Anion binding section).
Another example of macrocyclic receptors was reported by Romański et al.,85 which developed a family comprising a simple squaramide based macrocyclic receptor for anions (91) and its ditopic analogues linked respectively to one (92–93) or two (94) benzo-18-crown-6 moieties (Fig. 32). In addition, in the case of 93, a fluorescent naphthalene moiety was also incorporated into the macrocyclic receptor, providing a platform suitable for ion pair sensing. The anion and ion pair ability of 91 and 92 were investigated by 1H-NMR, also comparing the influence of the cations in enhancing the binding ability.
The results suggest that, in general, anion binding occurs preferentially via hydrogen bonds involving the squaramide NHs rather than the amide donors. Among the anions tested, these receptors showed a higher affinity towards V-shaped and tetrahedral anions, such as benzoate and sulfates. In general, the ditopic receptors, due to the presence of the electron donating alkoxy groups, showed a weaker anion recognition with respect to monotopic 92; however, in the presence of cations such as Na+ and K+, an enhanced binding is observed. The optical response of 93 towards different anions was also characterized. 93 produced a selective optical response in the presence of sulfate anions which consists of a bathochromic shift from 291 nm to 296 nm in the electronic absorption spectrum. Furthermore, the emission spectra showed an increase of the fluorescence emission and a concomitant formation of a new band at 420 nm. 93 was also tested as an optical ion pair sensor, responding in the presence of potassium sulfate.
In a second article, Romański et al.86 described a family of luminescent squaramide-based receptors analogous to that reported by Elmes et al.72 Receptors 95–97 (Fig. 33) show an anthracene moiety as a fluorogenic unit. Furthermore, 95 and 96 bear a macrocyclic unit capable of complexing metal ions, making these systems suitable for ion pair detection (see the Anion extraction section). The presence of the metal ion in the macrocyclic cavity induced an enhancement of the affinity towards anions. Receptor 95 selectively extracts SO42− salts from aqueous and organic phases, providing quenching or an increase of the fluorescence, depending on the water content in the receptor solution.
A family of redox chemosensors (98–100) was recently developed by the same authors,87 using the same molecular skeleton proposed for 95 and 97, only differing for the replacement of the fluorescent anthracene moiety with a ferrocene redox signaling unit (Fig. 34). The family consists of a monotopic receptor 100, and two ditopic receptors 98 and 99, obtained by linking the squaramide receptor to 15-crown-5 ether and an 18-crown-6 ether, respectively. The binding ability of these systems towards cations and ion pairs was characterized via different techniques, including single crystal X-ray diffraction, spectrophotometric, spectroscopic, and electrochemical measurements. The choice of different cavity sizes was reflected in different selectivities towards cations. 98 moderately captured Na+ ions (Ksodium = 9400 M−1) while 99 showed strong binding with both Na+ (Ksodium = 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 M−1) and K+ (Kpotassium = 144
900 M−1) and K+ (Kpotassium = 144![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 M−1), though showed higher selectivity towards K+. The binding ability of 98–100 in the simultaneous presence of anions and cations showed a behavior consistent with that previously observed. Due to the absence of the electron donating alkoxy groups of the ditopic receptors 98 and 99, the monotopic receptor 100 showed the strongest anion binding ability. However, in the presence of Na+ or K+ cations, acetonitrile solutions of 98 and 99 showed an enhanced binding. This effect was attributed to the presence of the cations in the macrocylic cavity, which increased the hydrogen bond donor nature of the squaramide function, influencing the strength of the interactions with the anionic species. The sensing ability of 98–100 towards different anions was investigated in the presence or absence of Na+ or K+ cations by cyclic voltammetry measurements. The results showed that, upon the addition of different anions, the presence of Na+ or K+ cations induced a more significant response in oxidation potentials when compared to that measured in their absence. In particular, for 98, this effect was observed in the presence of Na+, while, for 99, with both Na+ and K+ cations.
200 M−1), though showed higher selectivity towards K+. The binding ability of 98–100 in the simultaneous presence of anions and cations showed a behavior consistent with that previously observed. Due to the absence of the electron donating alkoxy groups of the ditopic receptors 98 and 99, the monotopic receptor 100 showed the strongest anion binding ability. However, in the presence of Na+ or K+ cations, acetonitrile solutions of 98 and 99 showed an enhanced binding. This effect was attributed to the presence of the cations in the macrocylic cavity, which increased the hydrogen bond donor nature of the squaramide function, influencing the strength of the interactions with the anionic species. The sensing ability of 98–100 towards different anions was investigated in the presence or absence of Na+ or K+ cations by cyclic voltammetry measurements. The results showed that, upon the addition of different anions, the presence of Na+ or K+ cations induced a more significant response in oxidation potentials when compared to that measured in their absence. In particular, for 98, this effect was observed in the presence of Na+, while, for 99, with both Na+ and K+ cations.
A further example of redox sensors was recently reported by Jolliffe, Bowman-James and Adriaenssens46 which reported the binding and sensing ability of the bis[squaramido]ferrocene 46 (for a description of the anion binding properties of receptor 46 see the Anion binding Section). Receptor 46 showed an irreversible oxidation at −63 mV vs. Fc/Fc+ with the anodic current that was about 3 times higher than the cathodic one. Upon the addition of SO42− or H2PO4−, a two-wave behavior was observed (Fig. 35).
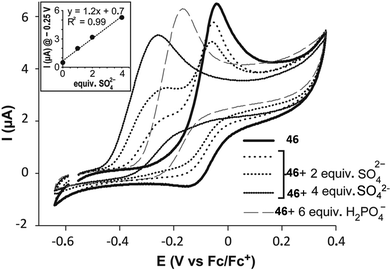 | ||
| Fig. 35 Cyclic voltammetry of 46 (0.25 × 10−3 M) in DMSO/20%H2O, after addition of 2, 4 equiv. of (TBA)2SO4 and 6 equiv. of TBAH2PO4 (0.1 M TBAClO4) at a scan rate of 100 mV s−1 and T of 80 °C. Inset: electrochemical response of 46 after addition of SO42− at −0.25 V vs. Fc/Fc+. Reproduced with permission from ref. 46. Copyright RSC 2022. | ||
In particular, the new wave corresponding to the oxidation of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 adduct of 46 with the SO42− anion resulted significantly shifted to more a negative potential (ΔE1/2 = 242 mV vs. free 46) as compared to the wave recorded for the adduct with H2PO4− (ΔE1/2 = 107 mV vs. free 46), in agreement with the higher affinity of 46 for SO42− than that for H2PO4−. Furthermore, for SO42−, the anodic current increased linearly when the concentration of the anion was increased up to 4 equiv. (Fig. 35).
1 adduct of 46 with the SO42− anion resulted significantly shifted to more a negative potential (ΔE1/2 = 242 mV vs. free 46) as compared to the wave recorded for the adduct with H2PO4− (ΔE1/2 = 107 mV vs. free 46), in agreement with the higher affinity of 46 for SO42− than that for H2PO4−. Furthermore, for SO42−, the anodic current increased linearly when the concentration of the anion was increased up to 4 equiv. (Fig. 35).
The recognition of guest species exploiting the variation of emission due to an excited-state intramolecular proton transfer (ESIPT) mechanism represents another approach for anion sensing. In an ESIPT process, a proton of the chromophore is transferred after photo-excitation from an HB-donor (generally amines or hydroxyl groups) to a nearby HB-acceptor (for example N, O and S) producing a fast enol–cheto photo-tautomerization.65 Therefore, molecules featuring ESIPT require the presence of an intramolecular hydrogen bond within the chromophore to allow the proton transfer. In general, ESIPT can produce variable luminescence phenomena (for example dual emission), large Stoke shifts and is very sensitive to the surrounding environment. These features can be conveniently exploited for sensing and imaging.
Muthyala et al. proposed a sensing approach based on the suppression of ESIPT,88 designing three squaramide-based receptors 101–103 (two ortho- and one meta-benzoyl squaramide derivatives) for the recognition of Cl− anions (Fig. 36). The results highlighted differences in the behaviour between ortho- and meta derivatives. Specifically, the two ortho-derivatives showed a solvent polarity-dependent emission, not observed for the meta-derivative, with a decrease of fluorescence emission by a factor 1000, passing from acetonitrile to the lower polar chloroform. Furthermore, upon the addition of different halides in acetonitrile, ortho-squaramides produced an enhancement of the emission intensity only in the presence of Cl− anions, while the meta-derivative showed a decrease of the emission irrespective of the anion added. The differences observed between ortho- and meta-substituted squaramides were ascribed to the ability of the formers to interact intramolecularly via hydrogen bonds between the N–H donors and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O acceptors. In the presence of polar solvents or anions, the intramolecular interaction was weakened or disrupted, suppressing the ESIPT and producing a variation of the fluorescence emission. In a more recent article, Muthyala et al.89 investigated in more detail the emission enhancement promoted by Cl− binding of ortho-substituted squaramides, investigating the three receptors 101 and 103–105 experimentally and computationally. The results suggest that this behaviour could ascribed to two degenerate excited states, resulting from two distinct competing charge transfer pathways. In the absence of anions, the emission is decreased due to the competition of these two pathways. The complexation of Cl− suppresses one of the two charge-transfer pathways, resulting in the increase of the emission.
O acceptors. In the presence of polar solvents or anions, the intramolecular interaction was weakened or disrupted, suppressing the ESIPT and producing a variation of the fluorescence emission. In a more recent article, Muthyala et al.89 investigated in more detail the emission enhancement promoted by Cl− binding of ortho-substituted squaramides, investigating the three receptors 101 and 103–105 experimentally and computationally. The results suggest that this behaviour could ascribed to two degenerate excited states, resulting from two distinct competing charge transfer pathways. In the absence of anions, the emission is decreased due to the competition of these two pathways. The complexation of Cl− suppresses one of the two charge-transfer pathways, resulting in the increase of the emission.
Due to their intrinsic features, such as the presence of strong hydrogen bond donors and the aromatic cyclobutenedione ring, squaramides tend to self-assemble via hydrogen bonds and π–π stacking. This ability has been exploited to design self-assembled systems able to respond to anion binding with changes in one of their properties, such as luminescence. There are different ways in which self-assembly or its perturbation can induce changes in the optical properties of the system. Examples of these are “aggregation-caused quenching” (ACQ), “aggregation-induced emission” (AIE) and “disaggregation-induced emission” (DIE).90,91 In this respect, Elmes focused both on simple molecules18,92 and more complex systems.21
In a recent work,92 the two chemosensors 106 and 107 (Fig. 37(A)), consisting of a squaramide-based receptor linked via a short spacer to a naphtalimide fluorogenic unit, were investigated as chemosensors, exploiting a disaggregation-induced emission (DIE) mechanism to recognize halides. Self-assembly of 106 and 107 was investigated in solution and the solid-state. Investigation by 1H-NMR at 298 K and 343 K showed a strong tendency to self-aggregate in aqueous solutions (Fig. 37(B)). Increasing the temperature resulted in disruption of the self-assembly of both 106 and 107. Attempts to crystallize the two receptors from DMSO and obtain some insights into the self-assembly in the solid state resulted in the formation of amorphous materials, featuring interesting morphologies (Fig. 37(C)). Characterization of 106 and 107 by UV-vis and fluorescence spectroscopy showed three absorption bands at 280 nm, 340 nm, and 445 nm and an emission band at approximately 525 nm. In particular, by varying temperature in DMSO/5%H2O, both 106 and 107 showed a dramatic enhancement of the fluorescence emission (220–300%) as the temperature increased (Fig. 37(D)). This was attributed to the disaggregation promoted by the increase of temperature, as also suggested by the results of the 1H-NMR variable temperature experiments (Fig. 37(B)). The anion binding ability of 106 and 107 was investigated in 0.5% aqueous DMSO-d6 by 1H-NMR, upon the addition of different anions (30 equiv.) as their TBA salts (AcO−, H2PO4−, SO42−, Br−, Cl−, F−, and I−). Anions such as AcO−, H2PO4−, SO42− and F− showed a complex behavior, leading to disappearance or either sharpening or broadening of the NH signals. In particular, in the presence of F−, both receptors underwent deprotonation, accompanied by a stark colour change of the solution, which turned from yellow to red. The other halides showed a significant downfield shift of the NH signals only in the presence of Cl− and Br−, suggesting interactions with these anions via hydrogen bonds. The results of the NMR titration fitted with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding model and produced association constants in the range of 100–500 M−1. Based on these results, the authors investigated whether interactions with different anions promoted disaggregation, resulting in an enhancement of the fluorescence emission by DIE. Bases such as AcO−, H2PO4−, SO42− and F− promoted a quenching of the emission band at 525 nm. This, at least in the case of F−, was attributed to the deprotonation. The addition Cl− and Br− anions induced a consistent enhancement of the fluorescence emission. In particular, Br− produced the best response, resulting in an enhancement of the fluorescence emission of about 500% for 106 and 600% for 107 (Fig. 37(E)).
1 binding model and produced association constants in the range of 100–500 M−1. Based on these results, the authors investigated whether interactions with different anions promoted disaggregation, resulting in an enhancement of the fluorescence emission by DIE. Bases such as AcO−, H2PO4−, SO42− and F− promoted a quenching of the emission band at 525 nm. This, at least in the case of F−, was attributed to the deprotonation. The addition Cl− and Br− anions induced a consistent enhancement of the fluorescence emission. In particular, Br− produced the best response, resulting in an enhancement of the fluorescence emission of about 500% for 106 and 600% for 107 (Fig. 37(E)).
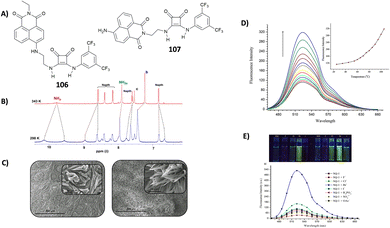 | ||
| Fig. 37 Receptors 106 and 107 (A). 1H-NMR spectra showing disaggregation phenomena when temperature is increased from 298 K (bottom) to 343 K (up) (B). Scanning electron microscopy (SEM) images of the solid-state self-assembly for 106 (left) and 107 (right) (C). Variable temperature fluorescence emission experiments (from 25° to 110 °C) in DMSO/5%H2O, reported for 107 (5.0 × 10−6 M) (D). Fluorescence response of 106 (5 μM) in DMSO/5%H2O upon addition of different anions (λexc 435 nm) (bottom), and fluorescence responses of 106 and 107 toward different halides in 5% aqueous DMSO as seen by the naked eye under a UV-vis lamp (top) (E). Adapted with permission from ref. 92. Copyright Frontiers 2019. | ||
Anion transport
Since Costa and co-workers introduced the evidence that squaramide-based compounds were able to bind marvellously both cations and anions, there has been an increasing interest in the exploiting their great anion binding properties, especially towards halogens such as chloride. On this basis, squaramide-based compounds represent excellent candidates as ionophores for the transmembrane transport of anion species such as chloride, bicarbonate, and sulphate.The first example of squaramide-based ionophores for this purpose was reported by Gale and co-workers, one of the pioneers in the use of supramolecular architectures for transmembrane anion transport.93 Squaramides 108–110 reported in Fig. 38, previously studied for their anion binding properties, were also tested for their capability to transport Cl− across the synthetic membranes in comparison with their analogous urea- and thiourea-derivatives A–F (Fig. 39).
 | ||
| Fig. 39 Ureas and thioureas used in anion transport studies in comparison with the analogous squaramides 108–114. | ||
To investigate their transport activity, antiport Cl−/NO3−, H+/Cl− or Na+/Cl− symport assays were conducted. The results reported in Fig. 40 demonstrate that squaramides 108 and 109 are able to transport Cl− out of liposomes much faster than that of the analogous thioureas and ureas.
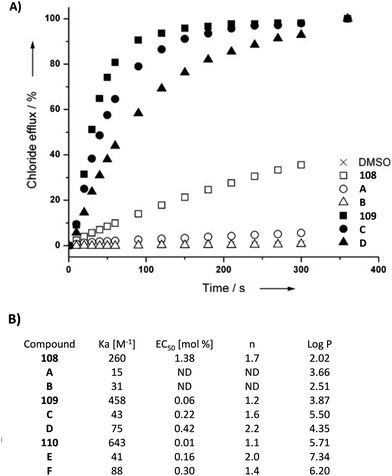 | ||
Fig. 40 Chloride efflux promoted by 108 and 109 and their analogous urea and thiourea (A–B and C–D for 108 and 109, respectively) at a concentration of 1 mol% (receptor to lipid) from unilamellar POPC vesicles loaded with 489 mM NaCl solution buffered to pH 7.2 with 5 mM sodium phosphate salts and dispersed in 489 mM NaNO3 solution buffered to pH 7.2 with 5 mM sodium phosphate salts (A). Summary of the chloride association constants (Kass) and anion transport data obtained through Hill analysis (EC50 and n), and calculated log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P of 108–110 and their analogous ureas and thioureas A–F (B). Reproduced with the permission from ref. 93. Copyright Wiley 2012 (Fig. 1). P of 108–110 and their analogous ureas and thioureas A–F (B). Reproduced with the permission from ref. 93. Copyright Wiley 2012 (Fig. 1). | ||
Nevertheless, similar results are obtained in the case of 110. Indeed, the values of the EC50 obtained through the Hill analysis suggested that 108–110 are more efficient with respect to the analogous ureas and thioureas, in particular 109 and 110 (EC50 of 109 is about 4-fold and 7-fold higher than that of C and D, respectively, while EC50 of 110 is about 16-fold and 30-fold higher than that of E and F, respectively).
Most likely, the explanation for the superior transport abilities of the squaramide systems 108–110 in comparison to their urea and thiourea analogues is the enhanced anion-binding properties of the former.
In a subsequent study, Gale and co-workers proposed another family of fluorinated squaramide-derivatives 115–119 (Fig. 41) able to induce caspase mediated cell death.94 In particular, also 109 was able to disrupt autophagy by HCl symport, while the apoptosis of the HeLa and A549 cancer cells was caused by the concomitant transport of Cl− and Na+.
Based on these results obtained on squaramides, Gale, Jolliffe and co-workers considered also the use of thiosquaramides 111–113 for anion binding and transport.95 The antiport Cl−/NO3− assay conducted on unilamellar POPC vesicles loaded with 489 mM NaCl solution buffered to pH 7.2 with 5 mM sodium phosphate salts and dispersed in 489 mM NaNO3 solution buffered to pH 7.2 with 5 mM sodium phosphate salts evidenced the scarce anion transport ability of 111–113 at this pH. However, the spectrophotometric determination of the pKa suggested that at pH 7.2 thiosquaramides considered are in their deprotonated form, thus characterized by a negative charge and not able to bind and transport chloride species across to the membrane (pKa = 7.3, 5.3, and 4.9 for 111, 112, and 113, respectively). The transport assay repeated at pH 4.0 of the vesicles internal and external buffered solutions, highlighted how under these experimental conditions the anion transport ability of thiosquaramides 111–112 switched on, due to the presence of the compounds in their neutral form, demonstrating a strong pH-dependence of their anion transport properties. This experimental evidence is further supported by the value of the EC50 calculated through the Hill analysis, which pointed out that oxosquaramides 108–110 are potentially potent anion transporters at both pH 7.2 and 4.0, the analogous thiosquaramide 111 is unable to do transport at pH 7.2 and can be switched on at pH 4.0, whereas the anion transport properties of 112 dramatically increased at lower pH values. It is worthy noting that 113 is unable to transport the anion species at pH 4.0 probably due to its too high lipophilicity (see Table 1).
Finally, the same group decided to investigate the anion binding and transport properties of a mixed oxothiosquaramide 114,96 based on the results previously obtained and reported above that allowed them to identify in the oxo- and thio-squaramide bearing the p-trifluoromethylphenyl group as the substituent in the symmetric structure the best anion transporters through synthetic membranes. Indeed, authors first reported the anion binding properties of 114 towards Cl− (as TBA salt) by means of 1H-NMR in DMSO-d6/0.5%H2O, which pointed out an association constant for the formation of the host–guest adduct similar to that calculated for the analogous oxosquaramide. Interestingly, the value of the pKa of 114 was found to be close to that calculated for the analogous thiosquaramide 112. For this reason, to investigate the anion transport properties of 114, a series of experiments were conducted at pH values of 7.2 and 4.0. The results indicated that, as already observed in the case of the analogous thiosquaramide, 114 possessed a higher anion transport ability at pH 4.0. In this case also, the value of the EC50 calculated through the Hill analysis of the data supported the experimental trend.
After the first results obtained on oxo-, thio-, and oxo-thiosquramides, the effect in Cl− binding and transport of the presence of additional H-bond donor groups in the molecular skeleton of the transporters was investigated.
Caltagirone and co-workers described the ability of bis-indolylsquaramide 21 (see the Anion binding section) to bind and transport chloride.84 It was demonstrated that 21 was able to bind chloride with 10-fold higher association constant than the analogous urea (Kass = 1200 and 128 M−1 in DMSO-d6/0.5%H2O for 21 and the analogous urea, respectively) and that it was able to efficiently transport this anion across the synthetic phospholipidic membrane with EC50 = 0.12 mol%. Starting from these results, the same group in collaboration with Quesada demonstrated that the combination of the indolyl and the 3,5-trifluoromethylphenyl substituents on the molecular skeleton of squaramide 120 (Fig. 42) allowed in obtaining the transport efficiency results similar to those obtained for the symmetric squaramide 110 in the Cl−/NO3− antiport assay, and slightly better in the Cl−/HCO3− exchange assay.97
The authors concluded that the simultaneous presence of the indolyl group and the electro-withdrawing 3,5-trifluoromethylphenyl group confers an ideal lipophilicity to the system. In the same study, Caltagirone and co-workers proposed the first simple numerical model to fit and analyse the experimental data of Cl− transport. Based on these preliminary results, Scorciapino and co-workers proposed an extended version of this theoretical model taking into account the impact of several important parameters on the transport efficiency of the anionophore, such as the equilibrium in the formation of the adduct, the balance between the affinity of the transporter towards chloride and the lipophilicity of the anionophore, and the effect of the diffusivity of the carrier and its complex through the membrane.98 It is worthy to highlight that in silico investigation and quantitative structure–activity relationship (QSAR) analysis were previously proposed to better understand and predict the anion transport properties of this type of simple squaramides.99,100
A similar non-symmetric squaramide 121 (Fig. 43) was reported by Gale and co-workers.101 This system was able to transport Cl− with an IC50 of 0.15 from a Cl−/NO3− exchange assay. In this case, the presence of the 1,8-naphthalimide fluorophore also allows compound 121 to be used for fluorescence imaging in cells, with high efficiency in staining of living A549 cells (Fig. 43).
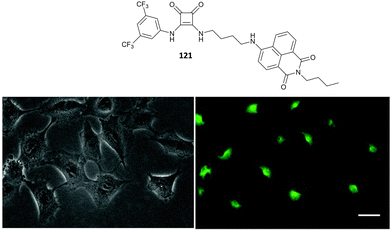 | ||
| Fig. 43 Bright field (left) and fluorescence images (right) of A549 cells after 24 hours incubation with 121 (1.0 μM). Adapted from ref. 101. Copyright RSC 2018. | ||
Davis, Gale and co-workers have highlighted that an appropriate balance between the affinity for Cl− and the transport efficiency should be considered when designing anionophores. Indeed, steroidal squaramides 122–127 express excellent chloride binding properties (Kass in the range 106–109 M−1 in water-saturated CHCl3) but negligible transport activity (Fig. 44 for Cl−/NO3− exchange in large unilammelar vesicles).102
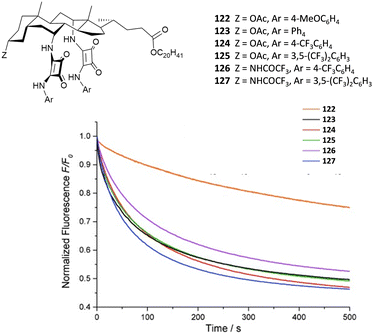 | ||
| Fig. 44 Steroidal squaramides 122–127 and lucigenin assay for the Cl−/NO3− exchange by 121–126. Reproduced with permission from ref. 102. Copyright Wiley 2015. | ||
Very recently, Elmes and co-workers have described the moderate transport properties of amidosquaramides which can be switched on at acidic pH as shown above for thiosquaramides.103
Valkenier and co-workers described a calix[6]arene functionalised with three squaramide groups 128 (Fig. 45) which is able to efficiently transport Cl−, NO3−, HCO3−, and AcO− as demonstrated by HPTS and lucigenin fluorescence assays. This represents a rare example of a tris-squaramide as an active anion transporter.104
Recently, Langton and co-workers have shown a very elegant example of a photo-switchable azobenzene family (129–133) functionalised with squaramide substituents which can switch ON and OFF their chloride transport ability (Fig. 46).105 Indeed, by using the HPTS-assay, the authors showed that the Z isomers were able to transport chloride with an EC50 8-fold higher than the E isomer (EC50 = 0.07 mol% and EC50 = 0.58 mol% for Z and E, respectively for compound 128). This difference was attributed to a change in the mobility in the membrane as well as to chloride encapsulation.
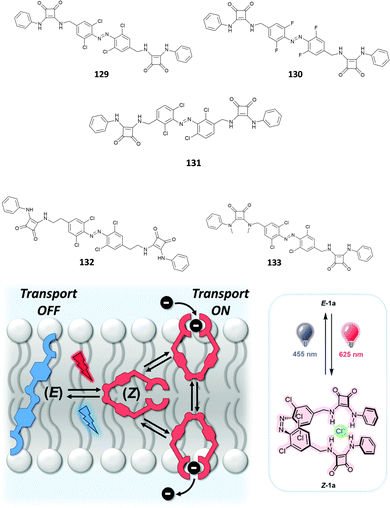 | ||
| Fig. 46 Photo-switchable squaramides 129–133 and cartoon illustrating their ON–OFF transport abilities. Reproduced with permission from ref. 105. Copyright RSC 2021. | ||
Another example of transport modulation by light was recently reported by Costa, Rotger and co-workers.106 As shown in Fig. 47(A), upon irradiation at 365 nm, squaramide 110 was photo-converted to maleic anhydride 110a which is not able to act as chloride transporter as demonstrated by the lucigenin assay. When large unilamellar POPC vesicles were irradiated in the presence of 110, a loss in the transport efficiency was observed during time due to the photo-conversion (Fig. 47(B)).
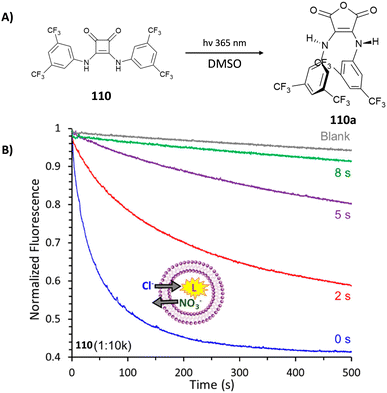 | ||
| Fig. 47 Photo-conversion of 110 to 110a upon irradiation in DMSO (A). Evidence of efficiency loss in Cl− transport upon different irradiation times studied by the lucigenin assay (B). Reproduced with permission from ref. 106. Copyright ACS 2023. | ||
Conclusions
In this review, we have highlighted the versatility of the squaramide H-bonding motif in the design of receptors for anions with different functions. The results reported in the literature so far corroborate the excellent properties of this class of compounds in the anion binding, sensing, and extraction, and in the transmembrane anion transport. While simple bis-substituted squaramides have been successfully employed for anion transmembrane transport, macrocyclic and acyclic systems featuring two or more squaramide moieties in their structures appear more versatile for anion binding, sensing, and extraction, in particular in the case of sulfate and phosphate tetrahedral anions. However, solubility issues have, in most of the cases, prevented the application of squaramide systems in real matrices. For this reason, particular attention in the future design of squaramide receptors should be given to the functionalisation with polar groups for increasing the solubility, possibly in water.In terms of fluorescence responses, the results reported in the literature suggest that the direct conjugation of fluorogenic fragments to the squaramide central core in most cases hampers the optical properties of the systems. On the other hand, the introduction of an alkyl or more flexible spacer seems to facilitate the fluorescence emission. It is interesting to note that, to the best of our knowledge, in terms of optical sensing, squaramides have never been employed in the development of arrays for anion recognition.
Moreover, the fundamental parameters such as the affinity for the anion species, binding modes, acid–base equilibrium, and lipophilicity of the system must be carefully balanced when designing an anionophore. On the other hand, the higher the affinity towards the anion species, the more efficient the anion binding and sensing properties of the receptor. Despite the low number of examples reported so far, squaramides have been demonstrated to be useful synthons in the construction of interlocked systems such as rotaxanes and their ability to be photoconverted or to be linked in photo-switchable molecules is opening novel strategies for the development of more sophisticated and stimuli-responsive materials. In particular, the possibility to act as both the H-bond acceptor and donor favours the self-assembly of squaramides to form complex architectures for the development of supramolecular gels which can be used for remediation or sensing applications or for the development of MOFs in the presence of metal ions for anion recognition.
We believe that in the next few years we will assist to an increasing interest towards this class of molecules.
Author contributions
All authors contributed equally to this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
R. M. acknowledges Ms E. Palazzetti for valuable advice in producing the graphical content. The authors thank the Italian Ministero dell’Università e della Ricerca (MIUR) (PRIN_PNRR P2022XHLTX), and NextGeneration EU, Return project (PE0000005 CUP:F53C22000730002) for financial support.Notes and references
- S. Cohen and S. G. Cohen, J. Am. Chem. Soc., 1966, 88, 1533–1536 CrossRef CAS.
- D. Quiñonero, C. Garau, A. Frontera, P. Ballester, A. Costa and P. M. Deyà, Chem. – Eur. J., 2002, 8, 433–438 CrossRef.
- V. E. Zwicker, K. K. Y. Yuen, D. G. Smith, J. Ho, L. Qin, P. Turner and K. A. Jolliffe, Chem. – Eur. J., 2018, 24, 1140–1150 CrossRef CAS PubMed.
- J. Alemán, A. Parra, H. Jiang and K. A. Jørgensen, Chem. – Eur. J., 2011, 17, 6890–6899 CrossRef PubMed.
- V. Amendola, G. Bergamaschi, M. Boiocchi, L. Fabbrizzi and M. Milani, Chem. – Eur. J., 2010, 16, 4368–4380 CrossRef CAS PubMed.
- V. Amendola, L. Fabbrizzi, L. Mosca and F.-P. Schmidtchen, Chem. – Eur. J., 2011, 17, 5972–5981 CrossRef CAS PubMed.
- P. Lach, A. Garcia-Cruz, F. Canfarotta, A. Groves, J. Kalecki, D. Korol, P. Borowicz, K. Nikiforow, M. Cieplak, W. Kutner, S. A. Piletsky and P. S. Sharma, Biosens. Bioelectron., 2023, 236, 115381 CrossRef CAS PubMed.
- A. Rostami, C. J. Wei, G. Guérin and M. S. Taylor, Angew. Chem., Int. Ed., 2011, 50, 2059–2062 CrossRef CAS PubMed.
- S.-J. Choi, B. Yoon, J. D. Ray, A. Netchaev, L. C. Moores and T. M. Swager, Adv. Funct. Mater., 2020, 30, 1907087 CrossRef CAS.
- D. Wu, R. Jiang, L. Luo, Z. He and J. You, Inorg. Chem. Front., 2016, 3, 1597–1603 RSC.
- S. Mommer and S. J. Wezenberg, ACS Appl. Mater. Interfaces, 2022, 14, 43711–43718 CrossRef CAS PubMed.
- X.-J. Cai, Z. Li and W.-H. Chen, Mini-Rev. Org. Chem., 2018, 15, 148–156 CrossRef CAS.
- R. Ian Storer, C. Aciro and L. H. Jones, Chem. Soc. Rev., 2011, 40, 2330–2346 RSC.
- L. A. Marchetti, L. K. Kumawat, N. Mao, J. C. Stephens and R. B. P. Elmes, Chem, 2019, 5, 1398–1485 CAS.
- R. Prohens, S. Tomàs, J. Morey, P. M. Deyà, P. Ballester and A. Costa, Tetrahedron Lett., 1998, 39, 1063–1066 CrossRef CAS.
- R. Prohens, M. C. Rotger, M. N. Piña, P. M. Deyà, J. Morey, P. Ballester and A. Costa, Tetrahedron Lett., 2001, 42, 4933–4936 CrossRef CAS.
- M. H. Al-Sayah and N. R. Branda, Thermochim. Acta, 2010, 503–504, 28–32 CrossRef CAS.
- A. Grundzi, S. A. Healy, O. Fenelon and R. B. P. Elmes, Res. Chem., 2022, 4, 100652 CAS.
- G. Picci, M. C. Aragoni, M. Arca, C. Caltagirone, M. Formica, V. Fusi, L. Giorgi, F. Ingargiola, V. Lippolis, E. Macedi, L. Mancini, L. Mummolo and L. Prodi, Org. Biomol. Chem., 2023, 21, 2968–2975 RSC.
- G. Picci, S. Farotto, J. Milia, C. Caltagirone, V. Lippolis, M. C. Aragoni, C. Di Natale, R. Paolesse and L. Lvova, ACS Sens., 2023, 8, 3225–3239 CrossRef CAS PubMed.
- L. K. Kumawat, C. Wynne, E. Cappello, P. Fisher, L. E. Brennan, A. Strofaldi, J. J. McManus, C. S. Hawes, K. A. Jolliffe, T. Gunnlaugsson and R. B. P. Elmes, ChemPlusChem, 2021, 86, 1058–1068 CrossRef CAS PubMed.
- C. V. Esteves, J. Costa, D. Esteban-Gómez, P. Lamosa, H. Bernard, C. Platas-Iglesias, R. Tripier and R. Delgado, Dalton Trans., 2019, 48, 10104–10115 RSC.
- G. Ambrosi, M. Formica, V. Fusi, L. Giorgi, E. Macedi, M. Micheloni, P. Paoli, R. Pontellini and P. Rossi, Chem. – Eur. J., 2011, 17, 1670–1682 CrossRef CAS.
- J. H. Yang and S. K. Kim, Chem. Commun., 2023, 59, 9988–9991 RSC.
- J. L. Sessler, Anion Receptor Chemistry, Royal Society of Chemistry, Cambridge, 2016 Search PubMed.
- I. Ravikumar and P. Ghosh, Chem. Soc. Rev., 2012, 41, 3077–3098 RSC.
- S. H. Murch, T. T. MacDonald, J. A. Walker-Smith, P. Lionetti, M. Levin and N. J. Klein, Lancet, 1993, 341, 711–714 CrossRef CAS PubMed.
- A. V. Nieuw Amerongen, J. G. M. Bolscher, E. Bloemena and E. C. I. Veerman, Biol. Chem., 1998, 379, 1–18 CAS.
- A. B. Olomu, C. R. Vickers, R. H. Waring, D. Clements, C. Babbs, T. W. Warnes and E. Elias, N. Engl. J. Med., 1988, 318, 1089–1092 CrossRef CAS PubMed.
- R. Custelcean and B. A. Moyer, Eur. J. Inorg. Chem., 2007, 1321–1340 CrossRef CAS.
- J. W. Pflugrath and F. A. Quiocho, Nature, 1985, 314, 257–260 CrossRef CAS PubMed.
- B. L. Jacobson and F. A. Quiocho, J. Mol. Biol., 1988, 204, 783–787 CrossRef CAS PubMed.
- Z. Rodriguez-Docampo, E. Eugenieva-Ilieva, C. Reyheller, A. M. Belenguer, S. Kubik and S. Otto, Chem. Commun., 2011, 47, 9798–9800 RSC.
- K. M. Mullen and P. D. Beer, Chem. Soc. Rev., 2009, 38, 1701–1713 RSC.
- R. Custelcean, Chem. Commun., 2013, 49, 2173–2182 RSC.
- A. Pramanik, B. Thompson, T. Hayes, K. Tucker, D. R. Powell, P. V. Bonnesen, E. D. Ellis, K. S. Lee, H. Yu and M. A. Hossain, Org. Biomol. Chem., 2011, 9, 4444–4447 RSC.
- C. Raposo, M. Almaraz, M. Martín, V. Weinrich, M. L. Mussóns, V. Alcázar, M. C. Caballero and J. R. Morán, Chem. Lett., 1995, 759–760 CrossRef CAS.
- C. Jin, M. Zhang, L. Wu, Y. Guan, Y. Pan, J. Jiang, C. Lin and L. Wang, Chem. Commun., 2013, 49, 2025–2027 RSC.
- Y. Liu, Y. Qin and D. Jiang, Analyst, 2015, 140, 5317–5323 RSC.
- L. Qin, S. J. N. Vervuurt, R. B. P. Elmes, S. N. Berry, N. Proschogo and K. A. Jolliffe, Chem. Sci., 2020, 11, 201–207 RSC.
- L. Qin, J. R. Wright, J. D. E. Lane, S. N. Berry, R. B. P. Elmes and K. A. Jolliffe, Chem. Commun., 2019, 55, 12312–12315 RSC.
- L. Qin, A. Hartley, P. Turner, R. B. P. Elmes and K. A. Jolliffe, Chem. Sci., 2016, 7, 4563–4572 RSC.
- R. B. P. Elmes, K. K. Y. Yuen and K. A. Jolliffe, Chem. – Eur. J., 2014, 20, 7373–7380 CrossRef CAS PubMed.
- R. B. P. Elmes and K. A. Jolliffe, Supramol. Chem., 2015, 27, 321–328 CrossRef CAS.
- N. A. Tzioumis, K. K. Y. Yuen and K. A. Jolliffe, Supramol. Chem., 2018, 30, 667–673 CrossRef CAS.
- J. D. E. Lane, W. J. H. Greenwood, V. W. Day, K. A. Jolliffe, K. Bowman-James and L. Adriaenssens, New J. Chem., 2022, 46, 18119–18123 RSC.
- V. Ramalingam, M. E. Domaradzki, S. Jang and R. S. Muthyala, Org. Lett., 2008, 10, 3315–3318 CrossRef CAS PubMed.
- L. Fan, T. Xu, J. Feng, Z. Ji, L. Li, X. Shi, C. Tian and Y. Qin, Electroanalysis, 2020, 32, 805–811 CrossRef CAS.
- J. Beswick, V. Blanco, G. De Bo, D. A. Leigh, U. Lewandowska, B. Lewandowski and K. Mishiro, Chem. Sci., 2015, 6, 140–143 RSC.
- A. Arun, A. Docker, H. Min Tay and P. D. Beer, Chem. – Eur. J., 2023, 29, e202301446 CrossRef CAS PubMed.
- R. L. Spicer, C. C. Shearman and N. H. Evans, Chem. – Eur. J., 2023, 29, e202203502 CrossRef CAS PubMed.
- D. Jagleniec, Ł. Dobrzycki, M. Karbarz and J. Romański, Chem. Sci., 2019, 10, 9542–9547 RSC.
- D. Jagleniec, S. Siennicka, Ł. Dobrzycki, M. Karbarz and J. Romański, Inorg. Chem., 2018, 57, 12941–12952 CrossRef CAS PubMed.
- D. Jagleniec, N. Walczak, Ł. Dobrzycki and J. Romański, Int. J. Mol. Sci., 2021, 22, 10754 CrossRef CAS PubMed.
- D. Jagleniec, M. Wilczek and J. Romański, Molecules, 2021, 26, 2751 CrossRef CAS PubMed.
- M. Zaleskaya, Ł. Dobrzycki and J. Romański, Int. J. Mol. Sci., 2020, 21, 9465 CrossRef CAS PubMed.
- M. Zaleskaya, M. Karbarz, M. Wilczek, Ł. Dobrzycki and J. Romański, Inorg. Chem., 2020, 59, 13749–13759 CrossRef CAS PubMed.
- M. Zaleskaya-Hernik, Ł. Dobrzycki and J. Romański, Int. J. Mol. Sci., 2023, 24, 8536 CrossRef CAS PubMed.
- M. Zaleskaya-Hernik, E. Megiel and J. Romański, J. Mol. Liq., 2022, 361, 119600 CrossRef CAS.
- S. Zdanowski, P. Piątek and J. Romański, New J. Chem., 2016, 40, 7190–7196 RSC.
- P. Manesiotis, A. Riley and B. Bollen, J. Mater. Chem. C, 2014, 2, 8990–8995 RSC.
- S. Cavalera, F. Di Nardo, G. Spano, L. Anfossi, P. Manesiotis and C. Baggiani, Anal. Methods, 2020, 12, 5729–5736 RSC.
- J. D. E. Lane, G. Shiels, P. Ramamurthi, M. Müllner and K. A. Jolliffe, Macromol. Rapid Commun., 2023, 2300406 CrossRef PubMed.
- J. Huang, Y. Shi, G.-z Huang, S. Huang, J. Zheng, J. Xu, F. Zhu and G. Ouyang, Angew. Chem., Int. Ed., 2022, 61, e202206749 CrossRef CAS PubMed.
- A. C. Sedgwick, J. T. Brewster, T. Wu, X. Feng, S. D. Bull, X. Qian, J. L. Sessler, T. D. James, E. V. Anslyn and X. Sun, Chem. Soc. Rev., 2021, 50, 9–38 RSC.
- M. Neus Piña, M. Carmen Rotger, A. Costa, P. Ballester and P. M. Deyà, Tetrahedron Lett., 2004, 45, 3749–3752 CrossRef.
- M. N. Piña, B. Soberats, C. Rotger, P. Ballester, P. M. Deyà and A. Costa, New J. Chem., 2008, 32, 1919–1923 RSC.
- R. Prohens, G. Martorell, P. Ballester and A. Costa, Chem. Commun., 2001, 1456–1457 RSC.
- E. Delgado-Pinar, C. Rotger, A. Costa, M. N. Piña, H. R. Jiménez, J. Alarcón and E. García-España, Chem. Commun., 2012, 48, 2609–2611 RSC.
- K. A. López, M. N. Piña and J. Morey, Sens. Actuators, B, 2013, 181, 267–273 CrossRef.
- A. Frontera, J. Morey, A. Oliver, M. N. Piña, D. Quiñonero, A. Costa, P. Ballester, P. M. Deyà and E. V. Anslyn, J. Org. Chem., 2006, 71, 7185–7195 CrossRef CAS PubMed.
- R. B. P. Elmes, P. Turner and K. A. Jolliffe, Org. Lett., 2013, 15, 5638–5641 CrossRef CAS PubMed.
- R. P. Orenha, V. B. da Silva, G. F. Caramori, F. S. de Souza Schneider, M. J. Piotrowski, J. Contreras-Garcia, C. Cardenas, M. Briese Gonçalves, F. Mendizabal and R. L. T. Parreira, New J. Chem., 2020, 44, 17831–17839 RSC.
- R. P. Orenha, V. B. da Silva, G. F. Caramori, M. J. Piotrowski, G. R. Nagurniak and R. L. T. Parreira, Phys. Chem. Chem. Phys., 2021, 23, 11455–11465 RSC.
- C. B. Black, B. Andrioletti, A. C. Try, C. Ruiperez and J. L. Sessler, J. Am. Chem. Soc., 1999, 121, 10438–10439 CrossRef CAS.
- M. Boiocchi, L. Del Boca, D. E. Gómez, L. Fabbrizzi, M. Licchelli and E. Monzani, J. Am. Chem. Soc., 2004, 126, 16507–16514 CrossRef CAS PubMed.
- T. Gunnlaugsson, M. Glynn, G. M. Tocci, P. E. Kruger and F. M. Pfeffer, Coord. Chem. Rev., 2006, 250, 3094–3117 CrossRef CAS.
- A. A. Abogunrin, S. A. Healy, O. Fenelon and R. B. P. Elmes, Chemistry, 2022, 4, 1288–1299 CrossRef CAS.
- C. Jin, M. Zhang, C. Deng, Y. Guan, J. Gong, D. Zhu, Y. Pan, J. Jiang and L. Wang, Tetrahedron Lett., 2013, 54, 796–801 CrossRef CAS.
- H. Niu, Q. Shu, S. Jin, B. Li, J. Zhu, L. Li and S. Chen, Spectrochim. Acta, Part A, 2016, 153, 194–198 CrossRef CAS PubMed.
- A. Rostami, A. Colin, X. Y. Li, M. G. Chudzinski, A. J. Lough and M. S. Taylor, J. Org. Chem., 2010, 75, 3983–3992 CrossRef CAS PubMed.
- J. D. E. Lane and K. A. Jolliffe, Org. Biomol. Chem., 2023, 21, 3226–3234 RSC.
- G. Picci, J. Milia, M. C. Aragoni, M. Arca, S. J. Coles, A. Garau, V. Lippolis, R. Montis, J. B. Orton and C. Caltagirone, Molecules, 2021, 26, 1301 CrossRef CAS PubMed.
- G. Picci, M. Kubicki, A. Garau, V. Lippolis, R. Mocci, A. Porcheddu, R. Quesada, P. C. Ricci, M. A. Scorciapino and C. Caltagirone, Chem. Commun., 2020, 56, 11066–11069 RSC.
- M. Zaleskaya, D. Jagleniec and J. Romański, Dalton Trans., 2021, 50, 3904–3915 RSC.
- M. Zaleskaya-Hernik, Ł. Dobrzycki, M. Karbarz and J. Romański, Int. J. Mol. Sci., 2021, 22, 13396 CrossRef CAS PubMed.
- M. Zaleskaya, D. Jagleniec, M. Karbarz, Ł. Dobrzycki and J. Romański, Inorg. Chem. Front., 2020, 7, 972–983 RSC.
- M. Porel, V. Ramalingam, M. E. Domaradzki, V. G. Young, V. Ramamurthy and R. S. Muthyala, Chem. Commun., 2013, 49, 1633–1635 RSC.
- A. Danao, V. Ramalingam, V. Ramamurthy and R. S. Muthyala, J. Photochem. Photobiol., A, 2017, 344, 108–113 CrossRef CAS.
- Y. Hong, J. W. Y. Lam and B. Z. Tang, Chem. Soc. Rev., 2011, 40, 5361–5388 RSC.
- D. Zhai, W. Xu, L. Zhang and Y.-T. Chang, Chem. Soc. Rev., 2014, 43, 2402–2411 RSC.
- L. K. Kumawat, A. A. Abogunrin, M. Kickham, J. Pardeshi, O. Fenelon, M. Schroeder and R. B. P. Elmes, Front. Chem., 2019, 7, 354 CrossRef CAS PubMed.
- N. Busschaert, I. L. Kirby, S. Young, S. J. Coles, P. N. Horton, M. E. Light and P. A. Gale, Angew. Chem., Int. Ed., 2012, 51, 4426–4430 CrossRef CAS PubMed.
- N. Busschaert, S.-H. Park, K.-H. Baek, Y. P. Choi, J. Park, E. N. W. Howe, J. R. Hiscock, L. E. Karagiannidis, I. Marques, V. Félix, W. Namkung, J. L. Sessler, P. A. Gale and I. Shin, Nat. Chem., 2017, 9, 667–675 CrossRef CAS PubMed.
- N. Busschaert, R. B. P. Elmes, D. D. Czech, X. Wu, I. L. Kirby, E. M. Peck, K. D. Hendzel, S. K. Shaw, B. Chan, B. D. Smith, K. A. Jolliffe and P. A. Gale, Chem. Sci., 2014, 5, 3617–3626 RSC.
- R. B. P. Elmes, N. Busschaert, D. D. Czech, P. A. Gale and K. A. Jolliffe, Chem. Commun., 2015, 51, 10107–10110 RSC.
- G. Picci, I. Carreira-Barral, D. Alonso-Carrillo, C. Busonera, J. Milia, R. Quesada and C. Caltagirone, Org. Biomol. Chem., 2022, 20, 7981–7986 RSC.
- M. A. Scorciapino, G. Picci, R. Quesada, V. Lippolis and C. Caltagirone, Membranes, 2022, 12, 292 CrossRef CAS PubMed.
- N. Busschaert, S. J. Bradberry, M. Wenzel, C. J. E. Haynes, J. R. Hiscock, I. L. Kirby, L. E. Karagiannidis, S. J. Moore, N. J. Wells, J. Herniman, G. J. Langley, P. N. Horton, M. E. Light, I. Marques, P. J. Costa, V. Félix, J. G. Frey and P. A. Gale, Chem. Sci., 2013, 4, 3036–3045 RSC.
- I. Marques, P. M. R. Costa, M. Q. Miranda, N. Busschaert, E. N. W. Howe, H. J. Clarke, C. J. E. Haynes, I. L. Kirby, A. M. Rodilla, R. Pérez-Tomás, P. A. Gale and V. Félix, Phys. Chem. Chem. Phys., 2018, 20, 20796–20811 RSC.
- X. Bao, X. Wu, S. N. Berry, E. N. W. Howe, Y.-T. Chang and P. A. Gale, Chem. Commun., 2018, 54, 1363–1366 RSC.
- S. J. Edwards, H. Valkenier, N. Busschaert, P. A. Gale and A. P. Davis, Angew. Chem., Int. Ed., 2015, 54, 4592–4596 CrossRef CAS PubMed.
- L. A. Marchetti, T. Krämer and R. B. P. Elmes, Org. Biomol. Chem., 2022, 20, 7056–7066 RSC.
- A. Singh, A. Torres-Huerta, T. Vanderlinden, N. Renier, L. Martínez-Crespo, N. Tumanov, J. Wouters, K. Bartik, I. Jabin and H. Valkenier, Chem. Commun., 2022, 58, 6255–6258 RSC.
- A. Kerckhoffs, Z. Bo, S. E. Penty, F. Duarte and M. J. Langton, Org. Biomol. Chem., 2021, 19, 9058–9067 RSC.
- M. Vega, L. Martínez-Crespo, M. Barceló-Oliver, C. Rotger and A. Costa, Org. Lett., 2023, 25, 3423–3428 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |