 Open Access Article
Open Access ArticleFacile, fast and green synthesis of a highly porous calcium-syringate bioMOF with intriguing triple bioactivity†
Albert
Rosado
 *a,
Oriol
Vallcorba
*a,
Oriol
Vallcorba
 b,
Blanca
Vázquez-Lasa‡
cd,
Luís
García-Fernández
b,
Blanca
Vázquez-Lasa‡
cd,
Luís
García-Fernández
 cd,
Rosa Ana
Ramírez-Jiménez
cd,
María Rosa
Aguilar
cd,
Rosa Ana
Ramírez-Jiménez
cd,
María Rosa
Aguilar
 cd,
Ana M.
López-Periago
cd,
Ana M.
López-Periago
 a,
Concepción
Domingo
a,
Concepción
Domingo
 *a and
José A.
Ayllón
*a and
José A.
Ayllón
 *e
*e
aInstitut de Ciència de Materials de Barcelona (ICMAB), Consejo Superior de Investigaciones Científicas (CSIC), Campus UAB s/n, 08193 Bellaterra, Spain. E-mail: arosado@icmab.es; conchi@icmab.es
bALBA Synchrotron Light Source, 08290 Cerdanyoladel Vallés, Spain
cInstituto de Ciencia y Tecnología de Polímeros (ICTP), Consejo Superior de Investigaciones Científicas (CSIC), C/Juan de la Cierva, 3, 28006 Madrid, Spain
dNetworking Biomedical Research Centre in Bioengineering, Biomaterials and Nanomedicine (CIBER-BNN), Av. Mon-forte de Lemos, 3-5, 28029 Madrid, Spain
eDepartament de Química, Universitat Autònoma de Barcelona (UAB), Campus UAB s/n, 08193 Bellaterra, Spain. E-mail: JoseAntonio.Ayllon@uab.es
First published on 2nd March 2023
Abstract
A facile, fast and green strategy based on ethanol is utilized to prepare a new bioMOF, namely, CaSyr-1, with particular characteristics of full biocompatibility given by using just calcium and syringic acid, the latter being a phenolic natural product found in fruits and vegetables, permanent porosity with an outstanding surface area >1000 m2 g−1, and a micropore diameter of 1.4 nm close to mesopore values. Collectively, these data establish CaSyr-1 as one of the most porous bioMOFs reported to date, with high molecular adsorption capacity. The CaSyr-1 adsorptive behavior is revised here through the reversible adsorption of CO2 and the encapsulation of bioactive ingredients in the structure. Remarkably, CaSyr-1 enables the development of triple therapeutic entities, involving bioactive Ca2+, syringic acid and an impregnated drug.
1. Introduction
Metal organic frameworks (MOFs) are fascinating crystalline materials with remarkably high porosity and versatile chemistry.1 Biological MOFs, known as bioMOFs, are composed of biocompatible metals and organic biomolecules as linkers, and are suitable for applications as diverse as gas adsorption2 and medicine.3 The research on bioMOFs for biomedical applications, with particular interest in the development of drug delivery systems, is currently a hot topic in biomaterials science.4 For this purpose, bioMOFs can be used either as-synthesized or after encapsulation of specific active pharmaceutical products.5 Some interesting bioMOFs, constituted by non-toxic endogenous cations, e.g. Ca2+, Zn2+, Mg2+ or Fe2+/3+, and naturally occurring ligands have already been reported.6 Nevertheless, the integration of exclusively biocompatible units in the framework of a MOF is not a straightforward task, since most biomolecules possess low symmetry that prevents the formation of porous and stable networks, often requiring the use of (toxic) auxiliary ligands.7 Hence, the utilization of bioMOFs in the biomedical area has been limited by the difficulties found in preparing fully biocompatible compounds, considering not only the nature of the building units, but also the size of the particles as indicators of overall cytotoxicity.8 Regarding the latter, bioMOFs must be developed as nanoparticles, with a well-defined shape and narrow particle-size distribution, which are modulated by optimizing the experimental conditions during synthesis.9An important point to consider in the development of bioMOFs is the chosen synthetic route. A typical reaction path designed to develop MOFs relies on the use of high boiling point basic solvents, as those derived from formamide, under solvothermal conditions.10 This method is widely used because it provides crystals of high quality suitable for structural elucidation. However, the overall process generally involves harsh reaction conditions, long reaction/activation periods and large amounts of hazardous solvents that tend to get trapped in the internal cavities of the MOF, parameters that hinder not only their biomedical application, but also their scale-up production.11 Currently, more sustainable methods are under research, such as those based on the use of non-toxic solvents, e.g., water or ethanol, and applying easy scalable mechanochemical or spray-drying technologies.12
To advance the molecular design and synthetic aspects of bioMOFs, this work presents a facile, fast and green strategy for the preparation of a novel porous 3D framework, named here as CaSyr-1, composed of a calcium cation (Ca2+) as the metal node and a syringate dianion (Syr2−) as the organic linker (Fig. 1), with unique textural properties and intriguing sorption and biomedical applications.
The incorporation of Ca2+ into MOFs has recently gained significant attention, with more than 150 crystal structures already reported.13 This interest is attributed to multiple health benefits,14 together with the mostly unexplored unique properties that this cation can provide, which are different from the well-known features of the universally employed transition metals. Syringic acid (H2Syr) is a phenolic compound, often found in fruits and vegetables, with anti-oxidant, anti-inflammatory, anti-cancer and anti-hypertensive therapeutic properties.15 Contrary to Ca2+, the utilization of Syr2− in coordination chemistry has been scarce and absent in porous compounds.16
2. Results and discussion
2.1. Synthesis
Porous CaSyr-1 nanopowder was synthesized in this work with a large space–time yield following an eco-friendly route in the green solvent ethanol (EtOH).17 Remarkably, the material could be prepared in only a few min (i.e., 10 min) by simply mixing quantitative molar amounts of reactants, calcium acetylacetonate (Ca(acac)2) and H2Syr, in a reduced volume of the solvent (1 mmol of each reactant in 1 or 10 mL of EtOH, corresponding to reactants![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH with molar ratios of 1
EtOH with molar ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 and 1
8 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80, respectively) at room temperature. Powder X-ray diffraction (PXRD) indicates that the obtained product was in both cases the explored phase CaSyr-1 (Fig. 2a and S1† for 1
80, respectively) at room temperature. Powder X-ray diffraction (PXRD) indicates that the obtained product was in both cases the explored phase CaSyr-1 (Fig. 2a and S1† for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 and 1
80 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 molar ratios, respectively). However, narrow particle-size distributions, most appropriate for biomedical uses, were obtained in the system with an increase in the volume of EtOH (Fig. 2b, c and S2, S3†), which allows a better control over supersaturation and subsequent nucleation and crystal growth. CaSyr-1 was thus precipitated as isometric porous nanoparticles with a mean diameter of 64 ± 11 nm (Fig. 2b and c). According to dynamic light scattering (DLS) measurements, the mean hydrodynamic diameter in suspension was ca. 150 nm when suspended in EtOH (Fig. S4†). Moreover, the particle-size distribution was extremely narrow (100–200 nm), which indicates that CaSyr-1 exhibits adequate dimensional properties for biosystems designed for flowing in blood vessels.18 The synthetic protocol was optimized to reduce the cost and time of the process, but conserving the desired dimensional, morphological and textural properties of the end product.
8 molar ratios, respectively). However, narrow particle-size distributions, most appropriate for biomedical uses, were obtained in the system with an increase in the volume of EtOH (Fig. 2b, c and S2, S3†), which allows a better control over supersaturation and subsequent nucleation and crystal growth. CaSyr-1 was thus precipitated as isometric porous nanoparticles with a mean diameter of 64 ± 11 nm (Fig. 2b and c). According to dynamic light scattering (DLS) measurements, the mean hydrodynamic diameter in suspension was ca. 150 nm when suspended in EtOH (Fig. S4†). Moreover, the particle-size distribution was extremely narrow (100–200 nm), which indicates that CaSyr-1 exhibits adequate dimensional properties for biosystems designed for flowing in blood vessels.18 The synthetic protocol was optimized to reduce the cost and time of the process, but conserving the desired dimensional, morphological and textural properties of the end product.
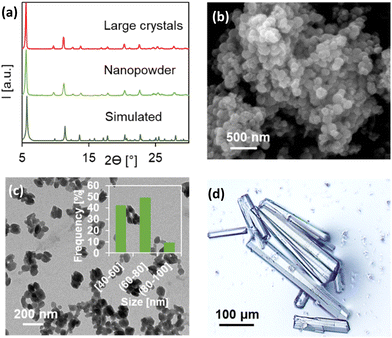 | ||
| Fig. 2 (a) PXRD pattern of CaSyr-1 species compared with the simulated, (b) SEM and (c) TEM with the particle size histogram of the nanopowder, and (d) an optical microscope image of large crystals. | ||
2.2. Crystal structure
In order to elucidate the crystalline structure of CaSyr-1, crystals of suitable quality (Fig. 2d) were prepared in dimethylformamide (DMF) at 393 K and characterized by synchrotron XRD. The resolved crystalline structure has the formula [Ca2(Syr)2(H2O)2]n⊂(solvent), which was confirmed by elemental analysis (Table S1†). A simulated PXRD pattern from the single crystal data matched those of both bulk large crystals precipitated in DMF and the nanopowder obtained in EtOH (Fig. 2a). The CaSyr-1 framework consists of interconnected dimeric units containing two crystallographically independent Ca2+ ions, two independent Syr2− linkers and two ancillary H2O ligands (Fig. 3a). A detailed description of the crystalline structure can be found in the ESI.† Briefly, regarding the calcium sphere, it is worth highlighting the double clamp-like coordination mode of Syr2− that is responsible for the connection between Ca1 and Ca2 (through two μ2-oxo bridges, O2 and O5) and, thus, for the formation of the dimer. This coordination mode is powered by the adjacent methoxy-phenolate-methoxy groups present in the ligand. Although isolated methoxy groups are extremely weak ligands, when positioned next to a coordinating group, such as phenolate, they tend to form this robust clamp-like coordination.19 With respect to syringate, the role of the dicarboxylate groups is also noteworthy in the extension of the structure (Fig. 3b), since they serve as bridges of two different dimeric units and promote the formation of the 3D porous framework (Fig. 3c–e). The resulting network displays two types of prisms, triangular and hexagonal (ratio 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), with a disposition in the space that resembles the Kagome lattice, frequently found in natural minerals, but also in many MOFs.20
1), with a disposition in the space that resembles the Kagome lattice, frequently found in natural minerals, but also in many MOFs.20
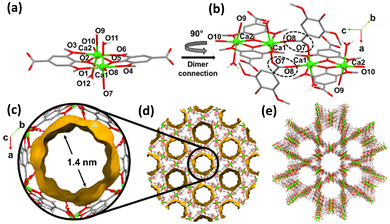 | ||
| Fig. 3 Structure of CaSyr-1: (a) dimeric unit, (b) connections through dicarboxylate bridges, (c) pore structure, (d) void arrangement, and (e) 3D framework. | ||
Void volume measurement using Mercury software (probe radius 1.2 Å) indicates that the porosity arises only from the hexagonal section, in the form of unidimensional channels with a diameter of 1.4 nm. Note that CaSyr-1 has the largest pore diameter value described for microporous bioMOFs, close to that of mesoporous bioMOFs.21 Additionally, a highly porous architecture of open pores is obtained, with 36 v% void space.
2.3. Porosity and thermal stability
CaSyr-1 porosity was experimentally confirmed by N2 physisorption at 77 K, giving an isotherm of type I (Fig. 4a). The high adsorption values at a very low relative pressures indicate microporosity (micropore volume of 0.32 cm3 g−1), which is in accordance with the size of the cavities observed in the resolved crystalline structure. N2 uptake at high relative pressures, accompanied by hysteresis, is assigned to interparticle mesoporous voids. A high value of an apparent BET surface area of 1080 m2 g−1 (Langmuir 1205 m2 g−1) was derived from the isotherm, which is exceptional in comparison with other reported bioMOFs that are considered totally biocompatible (Table 1), as CaSyr-1, not including either toxic auxiliary ligands in the framework and/or impregnated or coordinated harmful solvents in the pores. The measured surface area value for CaSyr-1 is just comparable with that reported for the γ-cyclodextrin macrocyclic CD-MOF-1. Regardless, CaSyr-1 exhibits a notably larger pore aperture diameter (1.4 nm) in comparison with CD-MOF-1 (0.42 and 0.78 nm), which would extend for the former, the scope of possible bioactive ingredients to be encapsulated.6a,c,22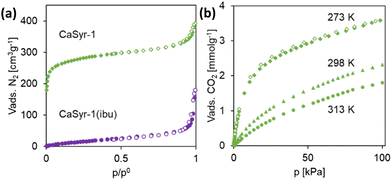 | ||
| Fig. 4 Adsorption behavior: (a) N2 isotherms at 77 K for pristine CaSyr-1 and impregnated CaSyr-1(ibu), and (b) CO2 isotherm at 273, 298 and 313 K of CaSyr-1. | ||
| BioMOF | Bioligand | Apparent BET surface area (m2 g−1) | Micropore volume (cm3 g−1) | Pore aperture diameter (nm) | Ref. |
|---|---|---|---|---|---|
| CaSyr-1 | Syringate | 1080 | 0.32 | 1.4 | This work |
| CD-MOF-1 | γ-Cyclodextrin | 1220 | 0.47 | 0.42/0.78 | 6a |
| sc-CCMOF-1 | Curcumine | 350 | 0.10 | N/A | 6c |
| [Cu2(μ3-ade)2(μ2-OOCCH3)2]·H2O | Adenine/acetate | 505 | 0.17 | N/A | 22a |
| {[Zn(FDC)]·H2O}n | 2,5-Furandicarboxylate | 780 | N/A | 0.63 | 22b |
| MOF-1201 | L-Lactate | 430 | 0.18 | 0.78 | 22c |
The lighter weight of Ca2+ with respect to transition metals offers gravimetric advantages for gas loading, which together with the large affinity of Ca2+ for CO2, makes Ca-based MOFs suitable materials for CO2 sorption. Uptakes in the order of 1–2 mmol g−1 (100 kPa and 273–298 K) are reported,13 which could only be increased by synthetizing mixed-metal MOFs.23 Remarkably, the monometallic CaSyr-1 bioMOF described in this work, with a CO2 sorption of 3.7, 2.4 and 1.8 mmol g−1 at 100 kPa and 273, 298 and 313 K (Fig. 4b), respectively, outperform the values previously described for Ca-based MOFs (Table S2†). Actually, the found value of CO2 sorption for CaSyr-1 is closer to that of basic adenine bioMOFs, specifically designed for this application,24 and the industrially used activated carbon or zeolite 13X (ca. 4 mmol g−1).25 From the data extracted at different adsorption temperatures, the CO2 enthalpy of adsorption (Qst) could be estimated, giving rise to a value of ca. 18 J mmol−1 (Fig. S7†) in the range of physisorption. The PXRD spectra of CaSyr-1 after these analyses indicate that CO2 was desorbed without any structural modification of the adsorbent. For environmental and process cost reasons, in the application of CO2 capture, the use of inexpensive renewable biolinkers for MOF design, such as that in CaSyr-1, is preferred vs. those derived from non-renewable petrochemical feedstocks.2
For adsorption-related applications, thermal stability plays an important role in ensuring the integrity of MOFs during interaction with an adsorbate and in the regeneration process of the material for further reuse.26 Here, the thermal stability of CaSyr-1 was evaluated through thermogravimetric analysis (TGA) under N2 flow (Fig. S8†). The weight decay at ca. 330 and 420 K is ascribed to the loss of EtOH and water molecules, respectively, both trapped in the cavities of the bioMOF during synthesis and manipulation, resulting in a total weight loss of about 20 wt%. The weight decrease does not involve any structural change in the framework of CaSyr-1, which was confirmed by a variable-temperature PXRD (VTPXRD) analysis, measured continuously from 298 to 500 K. Actually, no significant differences among the patterns recorded at the different temperatures were noticed (Fig. S9†). Hence, the removal of the solvent from the voids does not compromise the stability of the porous framework. The TGA suggests that CaSyr-1 is thermally stable up to ca. 600 K, the temperature at which the pyrolysis of the bioligand induces the degradation of the network. This notable thermal stability is usual in Ca-based MOFs containing O-donor linkers due to the ionic character of the formed Ca–O bonds.13
2.4. Biocompatibility and drug release
The focus on applications for the newly designed CaSyr-1 bioMOF is primarily directed to biomedicine. Although CaSyr-1 constituents are known to be non-toxic, it is of major importance to examine the bioMOF biocompatibility, since parameters such as constituents’ interactions, particle size or trapped solvent residue could influence the overall cytotoxicity, evaluated in this work through the half maximal inhibitory concentration (IC50). The IC50 for the bioMOF was calculated to be 9.6 mg cm−3, close to the 7.8 mg cm−3 found for pristine H2Syr (Fig. S10 and S11†) and far from the values considered cytotoxic,27 which situates CaSyr-1 as a biocompatible platform ready for biomedical use.The pristine bioMOF could be used to physiologically release Ca2+, essential for a healthy skeleton, and Syr2−, with a strong inhibition activity for COX-2 – recognized as the main anti-inflammatory target.28 To advance the therapeutic properties of CaSyr-1, this bioMOF can be loaded with a wide range of drugs, taking advantage of its high porosity and large pore diameter, to develop a triply bioactive CaSyr-1(drug) system, i.e., activity found in Ca2+, Syr2− and the impregnated drug (Fig. 5).
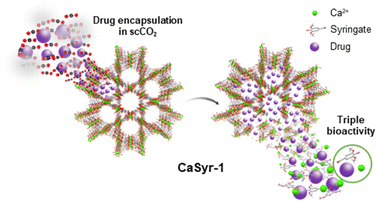 | ||
| Fig. 5 Schematic representation of drug encapsulation in CaSyr-1 and subsequent triply bioactive release. | ||
Therapeutic regimens based on the use of multicomponent drugs are an effective alternative to the one-target single molecular entities, since multiple targets can be involved in the treatment of a particular disease.29 Herein, this possibility was experimentally tested, demonstrating the easy impregnation of ibuprofen in CaSyr-1 using non-toxic supercritical CO2 (scCO2). In the impregnated sample, designated as CaSyr-1(ibu), ibuprofen was assumed to reside solely in the pores of the bioMOF, since no sign of drug recrystallization outside the nanoparticles was noticed in the SEM images (Fig. S12†) or in the PXRD pattern (Fig. S13†). For CaSyr-1(ibu), the adsorption branch of the N2 isotherm displayed a complete drop in gas adsorption at low relative pressures (Fig. 4a), which indicates that the micropores were already filled with the drug. The percentage of ibuprofen in CaSyr-1(ibu) was estimated by 1H nuclear magnetic resonance (1H-NMR) after sample treatment with hydrofluoric acid. The 1H-NMR spectrum shows a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of syringate
1 molar ratio of syringate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ibuprofen, which equates to a ca. 21 wt% ibuprofen loading (Fig. S14–S16†), similar to other reported values.30 By modifying the experimental conditions in the scCO2 approach, the ibuprofen loading can be controlled in the range ca. 10–25 wt%.
ibuprofen, which equates to a ca. 21 wt% ibuprofen loading (Fig. S14–S16†), similar to other reported values.30 By modifying the experimental conditions in the scCO2 approach, the ibuprofen loading can be controlled in the range ca. 10–25 wt%.
The multicomponent release of CaSyr-1(ibu) was studied here by suspending the impregnated bioMOF in a solution of PBS (buffer pH = 7.4) in D2O at 310 K for 1 day. During this period, several aliquots were collected at different times and measured by NMR (Fig. S17–S20†). Interestingly, in all the cases (from 10 min to 1 day), the signals assigned to both ibuprofen and H2Syr were visible, and the corresponding integration peaks ratio remained nearly constant (ca. 2.5–2.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, syringate
1, syringate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ibuprofen), with values consistent with the initial molar ratio in the solid form (3
ibuprofen), with values consistent with the initial molar ratio in the solid form (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). This indicates not only a fast delivery of the cargo, but also of CaSyr-1 building blocks, induced by the degradation of the framework under physiological conditions. For small hydrophobic drugs, such as ibuprofen, physiologically unstable MOFs have already been contemplated as potential delivery vehicles to increase their bioavailability.31 This favourable burst phenomenon is promoted by the release of the drug confined in the bioMOF in the molecular form, making it dissolve much more easily than crystalline drugs.
1). This indicates not only a fast delivery of the cargo, but also of CaSyr-1 building blocks, induced by the degradation of the framework under physiological conditions. For small hydrophobic drugs, such as ibuprofen, physiologically unstable MOFs have already been contemplated as potential delivery vehicles to increase their bioavailability.31 This favourable burst phenomenon is promoted by the release of the drug confined in the bioMOF in the molecular form, making it dissolve much more easily than crystalline drugs.
The IC50 of the impregnated sample was found to be 5 mg cm−3, almost half of the CaSyr-1 IC50 (Fig. S10†). The overall cytotoxicity increases due to the inherent mild toxicity of ibuprofen, with a calculated IC50 of 1.9 mg cm−3 (Fig. S11†). Ibuprofen, even as one of the most prescribed nonsteroidal anti-inflammatory drugs, must be administered with caution due to its negative secondary effects related to dosage.32 In the designed CaSyr-1(ibu), the merging of the COX-2 anti-inflammatory activity of syringic acid and that of ibuprofen is expected to result in a positive decrease in the required dosage of ibuprofen. Moreover, the use of ibuprofen is associated with increased blood pressure and must be excluded from patients diagnosed with hypertension, ca. 30% of the worldwide adult population.33 Quite the reverse, CaSyr-1 could help in decreasing blood pressure, since both calcium and syringic acid have demonstrated anti-hypertensive effects.34 Hence, the population targeted with the administration of ibuprofen could be increased by embedding hypertensive ibuprofen into the CaSyr-1 anti-hypertensive bioMOF.
3. Conclusions
Homogeneously sized nanoparticles of CaSyr-1 can be easily prepared from renewable biocompatible components in a sustainable strategy with a large space–time yield. These processing aspects, along with the displayed exceptionally high surface areas and pore sizes, make this material a promising candidate for adsorption. Two main application areas have been envisaged: the reversible adsorption of CO2 and the encapsulation of bioactive ingredients in the structure. It must be emphasized that CaSyr-1 enables the development of triply bioactive entities with promising activity as a multicomponent drug system.4. Experimental section
4.1. Materials
The employed reactants for the CaSyr-1 preparation were syringic acid (H2Syr 98%, abcr) and either calcium acetylacetonate (Ca(acac)2 98%, abcr) or calcium chloride (CaCl2 > 95%, Merck). The used solvents were absolute ethanol (EtOH, Scharlab) and dimethylformamide (DMF, abcr). For drug impregnation, the active compound was (S)-(+)-ibuprofen (ibu 99%, Sigma-Aldrich) dissolved in supercritical CO2 (scCO2), which was provided as compressed CO2, 99.95 wt%, by Carburos Metálicos S.A.4.2. Synthetic procedure
1 mL of EtOH. 1.0 mmol of Ca(acac)2 and 1.0 mmol of H2Syr were introduced into a vial containing 1 mL of EtOH. The attained molar ratio of reactants
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH was 1
EtOH was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8. The slurry was sonicated for 1 min and left to rest for 10 min at room temperature. The obtained crude product was washed three times with 10 mL of EtOH by vortex-centrifugation and dried under vacuum (yield = 82 wt%).
8. The slurry was sonicated for 1 min and left to rest for 10 min at room temperature. The obtained crude product was washed three times with 10 mL of EtOH by vortex-centrifugation and dried under vacuum (yield = 82 wt%).
10 mL of EtOH (the optimized protocol). 1.0 mmol of Ca(acac)2 and 1.0 mmol of H2Syr were separately introduced into two vials containing 5 mL of EtOH each and sonicated for 1 min, resulting in a suspension and a solution, respectively. The contents of both vials were then mixed, attaining a molar ratio of reactants
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH of 1
EtOH of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80, and left to rest for 10 min at room temperature. The obtained crude product was washed three times with 10 mL of EtOH by vortex-centrifugation and finally dried under vacuum (yield = 87 wt%).
80, and left to rest for 10 min at room temperature. The obtained crude product was washed three times with 10 mL of EtOH by vortex-centrifugation and finally dried under vacuum (yield = 87 wt%).
4.3. Characterization techniques
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) p) for a given adsorbed amount (n) as a function of the reciprocal temperature (1/T). The Qst at a specific adsorbate loading was then estimated using the Clausius–Clapeyron equation (eqn (1)):
p) for a given adsorbed amount (n) as a function of the reciprocal temperature (1/T). The Qst at a specific adsorbate loading was then estimated using the Clausius–Clapeyron equation (eqn (1)):Qst(n) = −R![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln(p2/p1)(T1T2/(T2 − T1)) ln(p2/p1)(T1T2/(T2 − T1)) | (1) |
4.4. Bioactive release and biocompatibility assays
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per cm3 and incubated at 310 K and 5 v% CO2. After 24 h, the medium was replaced by seriated dilutions of syringic acid in culture medium and ibuprofen in 1 v% DMSO added to the culture medium. The cells were incubated at 310 K and 5 v% CO2 for 24 h. MG63 cells in culture medium and 1 v% DMSO in culture medium were used as the control systems. After 24 h, the medium was replaced by the culture medium without phenol red (Sigma Aldrich).
000 cells per cm3 and incubated at 310 K and 5 v% CO2. After 24 h, the medium was replaced by seriated dilutions of syringic acid in culture medium and ibuprofen in 1 v% DMSO added to the culture medium. The cells were incubated at 310 K and 5 v% CO2 for 24 h. MG63 cells in culture medium and 1 v% DMSO in culture medium were used as the control systems. After 24 h, the medium was replaced by the culture medium without phenol red (Sigma Aldrich).
For CaSyr-1 and CaSyr-1(ibu), 200 μL of MG63 cells was cultured on transwell insets (collagen coated, cut-off = 0.4 μm, diameter = 6.5 mm, Costar) at 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per cm3. The insets were incubated in a 48-well plate with 400 μL of culture medium and at 310 K and 5 v% CO2. After 24 h, the medium of the inset was replaced by fresh medium, and the medium of the well plate was replaced by seriated dilutions of CaSyr-1 and CaSyr-1(ibu) in culture medium (starting at 25 mg cm−3). After 24 h, all the media were replaced by culture medium without phenol red.
000 cells per cm3. The insets were incubated in a 48-well plate with 400 μL of culture medium and at 310 K and 5 v% CO2. After 24 h, the medium of the inset was replaced by fresh medium, and the medium of the well plate was replaced by seriated dilutions of CaSyr-1 and CaSyr-1(ibu) in culture medium (starting at 25 mg cm−3). After 24 h, all the media were replaced by culture medium without phenol red.
| Cell viability (%) = 100 × (ODS − ODB)/(ODC − ODB) | (2) |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Spanish Ministry of Science and Innovation MICINN through the Severo Ochoa Program for Centers of Excellence (CEX2019–000917-S), by the Spanish National Plan of Research with projects PID2020-115631GB-I00 and PID2020-114086RB-100 and by CIBER (Spain) – Consorcio Centro de Investigación Biomédica en Red (Ref. CB06/01/0013), Instituto de Salud Carlos III, MICINN. This research work was performed in the framework of the Nanomedicine CSIC HUB (Ref. 202180E048). ALBA synchrotron is acknowledged for the provision of beam time. A. R. acknowledges the financial support of FPI 2019 grant. This work has been performed in the framework of the doctoral program “Chemistry” of the Universitat Autònoma de Barcelona by A. R.This work is dedicated to the memory of Blanca Vázquez-Lasa, who was a long-term collaborator of many of the authors. We feel grateful for having had the chance to interact with Blanca, a woman of great insight and sweetness. Though she did not witness the publication of this article, she motivated much of the work presented here, and accompanied it for as long as her health permitted. Both as scientists and as personal acquaintances, we miss Blanca greatly, and we would like to express our condolences to her family and friends.
References
- (a) S. T. Meek, J. A. Greathouse and M. D. Allendorf, Metal–organic frameworks: A Rapidly growing class of versatile nanoporous materials, Adv. Mater., 2011, 23, 249–267 CrossRef CAS PubMed; (b) H. C. Zhou, J. R. Long and O. M. Yaghi, Introduction to metal–organic frameworks, Chem. Rev., 2012, 112, 673–674 CrossRef CAS PubMed; (c) P. Horcajada, R. Gref, T. Baati, P. K. Allan, G. Maurin, P. Couvreur, G. Férey, R. E. Morris and C. Serre, Metal–organic frameworks in biomedicine, Chem. Rev., 2012, 112, 1232–1268 CrossRef CAS PubMed.
- A. Zulys, F. Yulia, N. Muhadzib and Nasruddin, Biological metal–organic frameworks (Bio-MOFs) for CO2 capture, Ind. Eng. Chem. Res., 2021, 60, 37–51 CrossRef CAS.
- A. C. McKinlay, R. E. Morris, P. Horcajada, G. Férey, R. Gref, P. Couvreur and C. Serre, BioMOFs: metal–organic frameworks for biological and medical applications, Angew. Chem., Int. Ed., 2010, 49, 6260–6266 CrossRef CAS PubMed.
- (a) I. Abánades Lázaro and R. S. Forgan, Application of zirconium MOFs in drug delivery and biomedicine, Coord. Chem. Rev., 2019, 380, 230–259 CrossRef; (b) W. Cai, J. Wang, C. Chu, W. Chen, C. Wu and G. Liu, Metal–organic framework-based stimuli-responsive systems for drug delivery, Adv. Sci., 2019, 6, 1801526 CrossRef PubMed; (c) A. Bieniek, A. P. Terzyk, M. Wiśniewski, K. Roszek, P. Kowalczyk, L. Sarkisov, S. Keskin and K. Kaneko, MOF materials as therapeutic agents, drug carriers, imaging agents and biosensors in cancer biomedicine: Recent advances and perspectives, Prog. Mater. Sci., 2021, 117, 100743 CrossRef CAS.
- S. Rojas, T. Devic and P. Horcajada, Metal organic frameworks based on bioactive components, J. Mater. Chem. B, 2017, 5, 2560–2573 RSC.
- (a) R. A. Smaldone, R. S. Forgan, H. Furukawa, J. J. Gassensmith, A. M. Z. Slawin, O. M. Yaghi and J. F. Stoddart, Metal–organic frameworks from edible natural products, Angew. Chem., Int. Ed., 2010, 49, 8630–8634 CrossRef CAS PubMed; (b) H. Su, F. Sun, J. Jia, H. He, A. Wang and G. Zhu, A highly porous medical metal–organic framework constructed from bioactive curcumin, Chem. Commun., 2015, 51, 5774–5777 RSC; (c) N. Portolés-Gil, A. Lanza, N. Aliaga-Alcalde, J. A. Ayllón, M. Gemmi, E. Mugnaioli, A. M. López-Periago and C. Domingo, Crystalline curcumin BioMOF obtained by precipitation in supercritical CO2 and structural determination by electron diffraction tomography, ACS Sustainable Chem. Eng., 2018, 6, 12309–12319 CrossRef; (d) S. Quaresma, V. André, A. M. M. Antunes, S. M. F. Vilela, G. Amariei, A. Arenas-Vivo, R. Rosal, P. Horcajada and M. T. Duarte, Novel antibacterial azelaic acid BioMOFs, Cryst. Growth Des., 2020, 20, 370–382 CrossRef CAS.
- (a) J. An, S. J. Geib and N. L. Rosi, Cation-triggered drug release from a porous zinc-adeninate metal–organic framework, J. Am. Chem. Soc., 2009, 131, 8376–8377 CrossRef CAS PubMed; (b) J. An, O. K. Farha, J. T. Hupp, E. Pohl, J. I. Yeh and N. L. Rosi, Metal-adeninate vertices for the construction of an exceptionally porous metal–organic framework, Nat. Commun., 2012, 3, 604 CrossRef PubMed.
- (a) M. Giménez-Marqués, T. Hidalgo, C. Serre and P. Horcajada, Nanostructured metal–organic frameworks and their bio-related applications, Coord. Chem. Rev., 2016, 307, 342–360 CrossRef; (b) C. Tamames-Tabar, D. Cunha, E. Imbuluzqueta, F. Ragon, C. Serre, M. J. Blanco-Prieto and P. Horcajada, Cytotoxicity of nanoscaled metal–organic frameworks, J. Mater. Chem. B, 2014, 2, 262–271 RSC.
- (a) P. Li, R. C. Klet, S. Y. Moon, T. C. Wang, P. Deria, A. W. Peters, B. M. Klahr, H. J. Park, S. S. Al-Juaid, J. T. Hupp and O. K. Farha, Synthesis of nanocrystals of Zr-based metal–organic frameworks with Csq-Net: Significant enhancement in the degradation of a nerve agent simulant, Chem. Commun., 2015, 51, 10925–10928 RSC; (b) C. R. Marshall, S. A. Staudhammer and C. K. Brozek, Size control over metal–organic framework porous nanocrystals, Chem. Sci., 2019, 10, 9396–9408 RSC; (c) M. Chang, T. Wei, D. Liu, J. X. Wang and J. F. Chen, A general strategy for instantaneous and continuous synthesis of ultrasmall metal–organic framework nanoparticles, Angew. Chem., Int. Ed., 2021, 60, 26390–26396 CrossRef CAS PubMed.
- K. Pobłocki, J. Drzeżdżon, B. Gawdzik and D. Jacewicz, Latest trends in the large-scale production of MOFs in accordance with the principles of green chemistry, Green Chem., 2022, 24, 9402–9427 RSC.
- X. Zhang, Z. Chen, X. Liu, S. L. Hanna, X. Wang, R. Taheri-Ledari, A. Maleki, P. Li and O. K. Farha, A historical overview of the activation and porosity of metal–organic frameworks, Chem. Soc. Rev., 2020, 49, 7406–7427 RSC.
- A. Carné-Sánchez, I. Imaz, M. Cano-Sarabia and D. Maspoch, Spray-drying strategy for synthesis of nanoscale metal–organic frameworks and their assembly into hollow superstructures, Nat. Chem., 2013, 5, 203–211 CrossRef PubMed.
- S. Xian, Y. Lin, H. Wang and J. Li, Calcium-based metal– organic frameworks and their potential applications, Small, 2021, 17, 2005165 CrossRef CAS PubMed.
- G. Cormick and J. M. Belizán, Calcium intake and health, Nutrients, 2019, 11, 1606 CrossRef CAS PubMed.
- (a) C. Srinivasulu, M. Ramgopal, G. Ramanjaneyulu, C. M. Anuradha and C. Suresh Kumar, Syringic acid (SA) – A review of its occurrence, biosynthesis, pharmacological and industrial importance, Biomed. Pharmacother., 2018, 108, 547–557 CrossRef CAS PubMed; (b) A. C. Mirza and S. S. Panchal, Safety evaluation of syringic acid: Subacute oral toxicity studies in Wistar rats, Heliyon, 2019, 5, e02129 CrossRef PubMed.
- (a) Z. Heren, H. Paşaoğlu, G. Kaştaş, L. Vurucu and O. Büyükgüngör, Synthesis, spectral and thermal properties, and crystal structure of bis(ethylenediamine) (aqua)copper(II) (bis)syringate ethylenediamine dihydrate [Cu(en)2(H2O)](Sy)2(en)(H2O)2, Z. Naturforsch., B: Chem. Sci., 2006, 61, 287–291 CrossRef CAS; (b) Z. Q. Huang, B. Wang, D. S. Pan, L. L. Zhou, Z. H. Guo and J. L. Song, Rational design of a N,S co-doped supermicroporous CoFe–organic framework platform for water oxidation, ChemSusChem, 2020, 13, 2564–2570 CrossRef CAS PubMed.
- K. Tekin, N. Hao, S. Karagoz and A. J. Ragauskas, Ethanol: A promising green solvent for the deconstruction of lignocellulose, ChemSusChem, 2018, 11, 3559–3575 CrossRef CAS PubMed.
- W. Bao, F. Tian, C. Lyu, B. Liu, B. Li, L. Zhang, X. Liu, F. Li, D. Li, X. Gao, S. Wang, W. Wei, X. Shi and Y. Li, Experimental and theoretical explorations of nanocarriers’ multistep delivery performance for rational design and anticancer prediction, Sci. Adv., 2021, 7, eaba2458 CrossRef CAS PubMed.
- (a) S. Mukherjee, J. Lu, G. Velmurugan, S. Singh, G. Rajaraman, J. Tang and S. K. Ghosh, Influence of tuned linker functionality on modulation of magnetic properties and relaxation dynamics in a family of six isotypic Ln2 (Ln = Dy and Gd) complexes, Inorg. Chem., 2016, 55, 11283–11298 CrossRef CAS PubMed; (b) T. J. Feuerstein, R. Müller, C. Barner-Kowollik and P. W. Roesky, Investigating the photochemistry of spiropyran metal complexes with online LEDNMR, Inorg. Chem., 2019, 58, 15479–15486 CrossRef CAS PubMed.
- (a) N. J. Ghimire and I. I. Mazin, Topology and correlations on the kagome lattice, Nat. Mater., 2020, 19, 137–138 CrossRef CAS PubMed; (b) J. E. Mondloch, W. Bury, D. Fairen-Jimenez, S. Kwon, E. J. Demarco, M. H. Weston, A. A. Sarjeant, S. T. Nguyen, P. C. Stair, R. Q. Snurr, O. K. Farha and J. T. Hupp, Vapor-phase metalation by atomic layer deposition in a metal–organic framework, J. Am. Chem. Soc., 2013, 135, 10294–10297 CrossRef CAS PubMed.
- (a) T. Li, M. T. Kozlowski, E. A. Doud, M. N. Blakely and N. L. Rosi, Stepwise ligand exchange for the preparation of a family of mesoporous MOFs, J. Am. Chem. Soc., 2013, 135, 11688–11691 CrossRef CAS PubMed; (b) K. Wang, D. Feng, T. F. Liu, J. Su, S. Yuan, Y. P. Chen, M. Bosch, X. Zou and H. C. Zhou, A series of highly stable mesoporous metalloporphyrin Fe-MOFs, J. Am. Chem. Soc., 2014, 136, 13983–13986 CrossRef CAS PubMed.
- (a) S. Pérez-Yañez, G. Beobide, O. Castillo, J. Cepeda, A. Luque, A. T. Aguayo and P. Román, Open-framework copper adeninate compounds with three-dimensional microchannels tailored by aliphatic monocarboxylic acids, Inorg. Chem., 2011, 50, 5330–5332 CrossRef PubMed; (b) D. Y. Ma, J. Xie, Z. Zhu, H. Huang, Y. Chen, R. Su and H. Zhu, Drug delivery and selective CO2 adsorption of a bio-based porous zinc-organic framework from 2,5-furandicarboxylate ligand, Inorg. Chem. Commun., 2017, 86, 128–132 CrossRef CAS; (c) J. Yang, C. A. Trickett, S. B. Alahmadi, A. S. Alshammari and O. M. Yaghi, Calcium L-lactate frameworks as naturally degradable carriers for pesticides, J. Am. Chem. Soc., 2017, 139, 8118–8121 CrossRef CAS PubMed.
- D. Saha, T. Maity and S. Koner, Metal–organic frameworks based on alkaline earth metals – Hydrothermal synthesis, X-ray structures, gas adsorption, and heterogeneously catalyzed hydrogenation reactions, Eur. J. Inorg. Chem., 2015, 2015, 1053–1064 CrossRef CAS.
- J. An, R. P. Fiorella, S. J. Geib and N. L. Rosi, Synthesis, structure, assembly, and modulation of the CO2 adsorption properties of a zinc–adeninate macrocycle, J. Am. Chem. Soc., 2009, 131, 8401–8403 CrossRef CAS PubMed.
- L. Hauchhum and P. Mahanta, Carbon dioxide adsorption on zeolites and activated carbon by pressure swing adsorption in a fixed bed, Int. J. Energy Environ. Eng., 2014, 5(4), 349–356 CrossRef CAS.
- H. U. Escobar-Hernandez, L. M. Pérez, P. Hu, F. A. Soto, M. I. Papadaki, H. C. Zhou and Q. Wang, Thermal stability of metal–organic frameworks (MOFs): Concept, determination, and model prediction using computational chemistry and machine learning, Ind. Eng. Chem. Res., 2022, 61, 5853–5862 CrossRef CAS.
- D. Campoccia, S. Ravaioli, S. Santi, V. Mariani, C. Santarcangelo, A. De Filippis, L. Montanaro, C. R. Arciola and M. Daglia, Exploring the anticancer effects of standardized extracts of poplar-type propolis: In vitro cytotoxicity toward cancer and normal cell lines, Biomed. Pharmacother., 2021, 141, 111895 CrossRef CAS PubMed.
- (a) R. Stanikunaite, S. I. Khan, J. M. Trappe and S. A. Ross, Cyclooxygenase-2 inhibitory and antioxidant compounds from the truffle Elaphomyces granulatus, Phytother. Res., 2009, 23, 575–578 CrossRef CAS PubMed; (b) M. H. Lee, H. Kang, K. Lee, G. Yang, I. Ham, Y. Bu, H. Kim and H. Y. Choi, The aerial part of Taraxacum coreanum extract has an anti-inflammatory effect on peritoneal macrophages in vitro and increases survival in a mouse model of septic shock, J. Ethnopharmacol., 2013, 146, 1–8 CrossRef CAS PubMed.
- C. T. Keith, A. A. Borisy and B. R. Stockwell, Multicomponent therapeutics for networked systems, Nat. Rev. Drug Discovery, 2005, 4, 71–78 CrossRef CAS PubMed.
- (a) P. Horcajada, C. Serre, G. Maurin, N. A. Ramsahye, F. Balas, M. Vallet-Regí, M. Sebban, F. Taulelle and G. Férey, Flexible porous metal–organic frameworks for a controlled drug delivery, J. Am. Chem. Soc., 2008, 130, 6774–6780 CrossRef CAS PubMed; (b) K. J. Hartlieb, D. P. Ferris, J. M. Holcroft, I. Kandela, C. L. Stern, M. S. Nassar, Y. Y. Botros and J. F. Stoddart, Encapsulation of ibuprofen in CD-MOF and related bioavailability studies, Mol. Pharm., 2017, 14, 1831–1839 CrossRef CAS PubMed.
- K. Suresh and A. J. Matzger, Enhanced drug delivery by dissolution of amorphous drug encapsulated in a water unstable metal–organic framework (MOF), Angew. Chem., Int. Ed., 2019, 58, 16790–16794 CrossRef CAS PubMed.
- S. Harirforoosh, W. Asghar and F. Jamali, Adverse effects of nonsteroidal antiinflammatory drugs: An update of gastrointestinal, cardiovascular and renal complications, J. Pharm. Pharm. Sci., 2013, 16, 821–847 Search PubMed.
- (a) K. T. Mills, A. Stefanescu and J. He, The global epidemiology of hypertension, Physiol. Behav., 2016, 176, 139–148 Search PubMed; (b) F. Ruschitzka, J. S. Borer, H. Krum, A. J. Flammer, N. D. Yeomans, P. Libby, T. F. Lüscher, D. H. Solomon, M. E. Husni, D. Y. Graham, D. A. Davey, L. M. Wisniewski, V. Menon, R. Fayyad, B. Beckerman, D. Iorga, A. M. Lincoff and S. E. Nissen, Differential blood pressure effects of ibuprofen, naproxen, and celecoxib in patients with arthritis: The PRECISION-ABPM (Prospective Randomized Evaluation of Celecoxib Integrated Safety Versus Ibuprofen or Naproxen Ambulatory Blood Pressure Measurement), Eur. Heart J., 2017, 38, 3282–3292 CrossRef CAS PubMed.
- (a) H. Nakamura, H. Tsujiguchi, A. Hara, Y. Kambayashi, S. Miyagi, T. T. T. Nguyen, K. Suzuki, Y. Tao, Y. Sakamoto, Y. Shimizu, N. Yamamoto and H. Nakamura, Dietary calcium intake and hypertension: Importance of serum concentrations of 25-hydroxyvitamin D., Nutrients, 2019, 11, 911 CrossRef CAS PubMed; (b) S. Kumar, P. Prahalathan and B. Raja, Syringic acid ameliorates L-NAME-induced hypertension by reducing oxidative stress, Naunyn-Schmiedebergs Arch. Pharmacol., 2012, 385, 1175–1184 CrossRef CAS PubMed.
- J. Juanhuix, F. Gil-Ortiz, G. Cuní, C. Colldelram, J. Nicolás, J. Lidón, E. Boter, C. Ruget, S. Ferrer and J. Benach, Developments in optics and performance at BL13-XALOC, the macromolecular crystallography beamline at the ALBA synchrotron, J. Synchrotron Radiat., 2014, 21, 679–689 CrossRef CAS PubMed.
- W. Kabsch, XDS, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2010, 66, 125–132 CrossRef CAS PubMed.
- G. M. Sheldrick, Crystal structure refinement with SHELXL, Acta Crystallogr., Sect. A: Found. Crystallogr., 2015, 71, 3–8 CrossRef PubMed.
- O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard and H. Puschmann, OLEX2: A complete structure solution, refinement and analysis program, J. Appl. Crystallogr., 2009, 42, 339–341 CrossRef CAS.
- International Standard ISO 10993-5. Third Edition 2009-06-01.
Footnotes |
| † Electronic supplementary information (ESI) available: Crystal structure description, additional figures (S1–S12) and tables (S1–S4). CCDC 2214086. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2qi02639b |
| ‡ This author is deceased. |
| This journal is © the Partner Organisations 2023 |

