Research progress of stimuli-responsive ZnO-based nanomaterials in biomedical applications
Zhenzhen
Weng
a,
Yingying
Xu
b,
Jie
Gao
c and
Xiaolei
Wang
 *ac
*ac
aSchool of Chemistry and Chemical Engineering, Nanchang University, Nanchang, Jiangxi 330088, P. R. China. E-mail: wangxiaolei@ncu.edu.cn
bThe Affiliated Stomatological Hospital, Nanchang University, Nanchang, Jiangxi 330006, P. R. China
cThe National Engineering Research Center for Bioengineering Drugs and the Technologies, Institute of Translational Medicine, Nanchang University, Nanchang, Jiangxi 330088, P. R. China
First published on 26th October 2022
Abstract
Zinc oxide nanoparticles (ZnO NPs), an attractive oxide semiconductor material, are widely used in the biomedical field due to their good biosafety and economy. The proposal of stimuli-responsive materials provides a new way for the further application of single ZnO-based nanomaterials that cannot meet the ever-changing requirements. In this review, the emerging advances in exogenous stimuli (light, ultrasound, mechanical force, etc.) and endogenous stimuli (pH, enzymes, etc.) responsive systems of ZnO-based nanomaterials in biomedical applications are highlighted. First, the basic characteristics, response mechanisms and construction principles of single-stimulus-responsive ZnO-based nanomaterials, as well as their recent advances in tissue repair, medical devices and theranostics are summarized. Subsequently, the design method of multi-stimuli-responsive ZnO-based biomaterials is discussed, and the application advantages of multi-functional responsive systems are explained by analyzing typical cases of biomedical applications of multi-responsive strategies. Finally, we discuss the prospects for the design and development of stimuli-responsive ZnO-based biomaterials for biomedical applications and point out their advantages as well as the places that need to be further improved. The current review may provide a useful reference for researchers interested in constructing such abundant, inexpensive and widely applicable multi-stimuli-responsive materials.
1. Introduction
Zinc oxide (ZnO), a common wide band gap (∼3.37 eV) oxide semiconductor, is widely used in the textile industry,1 sensors,2 supercapacitors3 and catalysis4 owing to its easy synthesis, low cost and unique physicochemical properties. Zinc is also an essential trace element in body tissues, which actively participates in the metabolic process of proteins, nucleic acids, lipids, etc., thus playing an indispensable role in health maintenance.5 Consequently, it is crucial to develop and explore the specific roles of ZnO in the biomedical field, which serves as a novel zinc delivery agent. At present, as one of the safe drugs approved by the Food and Drug Administration (FDA),6 biocompatible ZnO has received increasing attention in various directions of biomedical research, including anti-inflammatory,7 antibacterial,8 anticancer,9 drug delivery,10 bioimaging,11 gene therapy12 and tissue regeneration.13,14With the deepening of research, the single biomaterial treatment modality based on ZnO-based nanomaterials has been unable to satisfy the requirements of biomedical applications. In view of this, researchers began to construct a series of stimuli-responsive systems around ZnO-based nanomaterials according to the characteristics and difficulties of disease therapy. Stimuli-responsive materials are a class of smart materials that can respond to exogenous and endogenous stimuli, in which exogenous stimuli consist of light, ultrasound, magnetic fields, electric fields, pressure, etc., while endogenous stimuli include temperature, humidity and so on.15 This kind of smart material is sensitive to external environmental signals or pathological abnormalities, which can convert external stimuli into changes in its own dynamic structure or shape through various ways, thereby obtaining other unique physical or chemical properties, and exhibiting corresponding specific functions.16 Recently, the system constructed by combining stimuli-responsive materials with extraordinary triggers has effectively achieved on-demand switching of treatment modes and reconciled the conflict between biocompatibility and therapy, which has shown great application potential in the fields of antibacterial, drug delivery, medical diagnosis and tissue engineering.17,18 Nevertheless, although some stimuli-responsive ZnO-based materials have been reviewed,19,20 they are mainly concentrated in a single stimulus or application, and few studies have systematically summarized or classified this material. Furthermore, there are fewer reviews on stimulus-responsive ZnO-based nanomaterials extensively studied for biomedical applications according to various stimulus sources.
This review focuses on the varying stimuli-responsive strategies of ZnO-based biomaterials, including exogenous stimuli (light, ultrasound, mechanical force, etc.) and endogenous stimuli (pH, enzymes, etc.) responsive strategies, and showcases the recent classical biomedical applications of ZnO-based biomaterials, such as antibacterial, anticancer, drug delivery and so on (Fig. 1). Firstly, the basic characteristics, response mechanisms and design principles of ZnO-based nanomaterials in response to a single stimulus are expounded, and a series of biomedical applications of such intelligent responsive systems in tissue repair, medical devices and therapeutic diagnostics are revealed. In addition, the constructions of multiple stimuli-responsive systems designed around ZnO-based nanomaterials are summarized, including two external stimuli-responsive strategies, as well as internal and external stimuli combined responsive strategies, while the biomedical applications of typical multi-responsive systems are enumerated. Finally, we also discuss the current challenges, improvement strategies and future prospects of stimuli-responsive ZnO-based biomaterials. The knowledge involved in this review may be conducive to constructing futuristic multifunctional biomaterials and designing corresponding intelligent responsive systems to meet the needs of a variety of disease diagnosis and treatment.
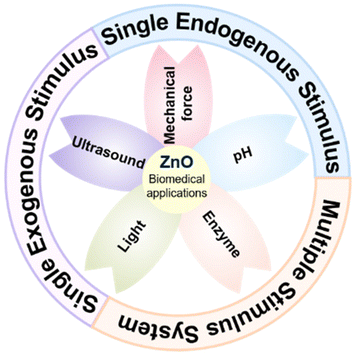 | ||
| Fig. 1 A basic classification of major stimulus responsive systems associated with ZnO-based nanomaterials in biomedical applications. | ||
2. Design and biomedical applications of single exogenous stimuli-responsive ZnO-based materials
ZnO-based materials play an important role as chemical sensitizers, which can intelligently respond to the corresponding changes in the external environment when exposed to exogenous stimuli, thus exerting specific functions in biomedical application research.21 Exogenous stimuli, including light, ultrasound, electricity, magnetic field and mechanical force, can supply certain external energy, thereout constructing a series of non-intrusive therapeutic methods, such as photodynamic therapy (PDT), photothermal therapy (PTT) and sonodynamic therapy (SDT).22 In the research progress of biomedical applications, the treatment strategy triggered by external energy has attracted much attention due to its weak invasiveness, high controllability, low damage and appreciable curative effect. Latterly, researchers have constructed diverse exogenous stimuli-responsive systems based on various ZnO-based nanomaterials and applied them to the fields of anticancer,23 antimicrobial,24 biosensors25 and tooth bleaching.262.1 Light-triggered therapy
Light, as an engaging external stimulus, is equivalent to a non-invasive, temporally or spatially controllable switch trigger, which is not limited by factors such as ionic strength, temperature and pH, playing a vital role in the design of personalized intelligent treatment platforms.27 In consequence, light-responsive biomaterials are favored in the research of different stimuli-responsive smart materials, which are widely used in various domains of biomedicine, including biosensing, drug delivery, fluorescence imaging, tissue repair and cancer therapy.28–30 After the photosensitizer contained in the photoresponsive material is excited by light, the optical signal is converted into a physicochemical signal through the photochemical reaction process, so that the photosensitive material acquires homologous physical and chemical properties.31 Among them, ZnO nanoparticles (ZnO NPs), the third generation of photosensitizers,32 are important semiconductors with fascinating light response characteristics, which lay the foundation for designing safe and controllable therapeutic (or diagnostic) systems for many biomedical applications.Based on this, many studies have reported effective means to improve the defect that ZnO can only utilize radiation in the near UV region (Table 1). For example, only the surface chemical processing of ZnO is carried out to narrow the band gap of ZnO by introducing defects.40 In addition, it also involves reducing the band gap by doping (non-metal, metal elements, etc.),41–43 recombination (metal, non-metal, etc.),44–46 hybridization (MOFs, UCNPs, etc.),47,48 coupling narrow band gap materials49 and developing oxygen vacancies,50 thereby broadening the light absorption range.51–54 Among them, Bahadur et al.55 triumphantly synthesized copper (Cu)-substituted ZnO nanoassemblies (Cu-ZnO NAs) excited by visible light in 2017. It was found that the substitution of Cu ions could remarkably improve the light absorption properties, charge separation efficiency and reactive oxygen species (ROS) levels, which in turn enhanced the photosensitivity for sustained antibacterial and anticancer activity under dark and visible-light irradiation conditions. At the same time, it was demonstrated that Cu–ZnO NAs had a higher surface area to offer a mass of active sites, which facilitated the reinforcement of the photodynamic effect (Fig. 2A). In addition, Lai et al.48 recently modified a zinc-based zeolitic-imidazolate framework (ZIF-8) on the surface of ZnO to form a core–shell structure (ZnO@ZIF-8) by traditional precipitation and hydrothermal methods. Compared with pure ZnO, the photocatalytic inactivation efficiency of ZnO@ZIF-8 under visible light excitation was markedly improved, which achieved coordinated antimicrobial effects with peroxymonosulfate (PMS). Subsequent analysis of the mechanism of photocatalytic performance enhancement suggested that the core–shell structure of ZnO@ZIF-8 enabled the photogenerated holes to have better charge separation efficiency and stronger oxidation ability, further strengthening its bacterial inactivation effect (Fig. 2B). A great deal of research indicated that compared with traditional antibiotics, visible light-responsive photocatalytic materials could eliminate infection and inflammation in a short time, with better controllability as well as less adverse reactions, which were less likely to induce drug resistance.41 In other words, the development of photocatalytic antibacterial materials is an effective strategy to decrease the misuse of antibiotics.
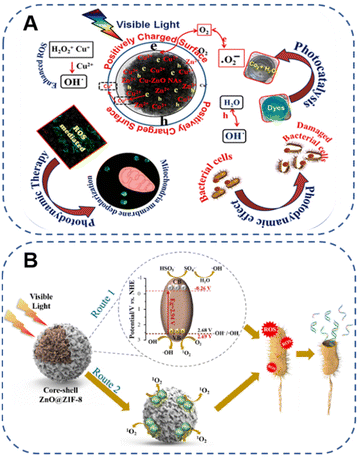 | ||
| Fig. 2 (A) A new strategy to induce a photodynamic effect utilizing Cu–ZnO NAs under visible light excitation. Adapted with permission from ref. 55, copyright 2017, American Chemical Society. (B) Schematic diagram of the bacterial inactivation mechanism of PMS/ZnO@ZIF-8 with visible light irradiation. Adapted with permission from ref. 48, copyright 2022, Elsevier. | ||
| Materials | Band gap | Light source | Mechanism | Ref. |
|---|---|---|---|---|
| ZnO NPs | 3.28 eV | UV (368–378 nm) | Increase in crystallinity after high temperature calcination | 51 |
| NC-ZnO | 3.12 eV | Visible light (>400 nm) | Doping nitrogen-doped carbon (NC) derived from ZIF-8 suppresses the recombination of e− and h+ | 41 |
| m-ZnO | 3.05 eV | Visible light (400, 555 and 665 nm) | Enhancement of oxygen vacancies | 50 |
| Tourmaline@ZnO | 2.96–3.05 eV | Visible light (>400 nm) | Limiting the recombination rate of electron–hole pairs and improving the charge transfer efficiency at the interface | 52 |
| N-ZnO | 2.99–3.04 eV | Visible light (≥400 nm) | Generation of new electronic states located between the valence and conduction bands due to the introduction of N atoms | 42 |
| ZnO@ZIF-8 | 2.94 eV | Visible light (≥400 nm) | Higher charge separation efficiency | 48 |
| g-C3N4/ZnO | 2.82 eV | Visible light (400–800 nm) | Facilitating the separation of photogenerated e− and h+ | 43 |
| p-Cu2O/n-ZnO | 2.11–2.14 eV | Visible light (>400 nm) | Promoting the efficient separation of photogenic carriers | 53 |
| ZnO@CdS | 1.67 eV | Sunlight | Synergistic effect and shift of transmittance edges to lower regions | 46 |
| Zn2GeO4 | 1.25 eV | Air mass 1.5 (300–680 nm) | The interaction between O 2p and Ge 3d states near the Fermi level | 54 |
In view of this, a series of novel porous ZnO composites with high specific surface area and narrow band gap (the narrowest band gap was 1.9 eV) were prepared by a simple microwave-induced method, and the corresponding material formative mechanism was discussed for the first time.26 Because of the introduction of carbon, the synthesized ZnO composites could be excited by long-wavelength yellow light instead of harmful short-wave radiation, which exhibited efficient photocatalysis under the irradiation of yellow light, thereby radically improving the biosafety of ZnO photocatalytic applications. In the follow-up research works, aiming at a narrow band gap and high biosafety yellow light responsiveness, a variety of ZnO-based nanomaterials were systematically constructed, and their antibacterial applications in implant infection,56 tissue reconstruction,57 dental protective agent58 and hearing protection59 were explored respectively. Additionally, terbium doped ZnO (ZT, 2.4 eV) with green light response performance was also developed, which was modified with polydopamine coating and loaded with nitric oxide (NO) precursor drug N,N′-Di-sec-butyl-N,N′-dinitroso-1,4-phenylenediamine (BNN6) to prepare ZT@P@B nanoparticles for the modification of contact lenses.60 Related experiments testified that the ROS formed by ZT with the assistance of green light could offer a satisfactory therapeutic window, fully exerting the photodynamic bactericidal effect. Of particular importance, under the irradiation of 808 nm near-infrared (NIR), the photothermal reaction mediated by polydopamine could achieve in situ release of NO, further enhancing the antibacterial properties while regulating the expression of related inflammatory factors, which was propitious to the improvement of bacterial keratitis. In conclusion, visible light driven ZnO-based photosensitizers are recognized for their potential usage in various biomedical applications. It is worth noting that the light source mentioned for the PDT of ZnO-based nanomaterials is not only restricted to visible light, but the research on ZnO-PDT treatment systems mediated by infrared light is also thriving. Taking the research work of Wu's group as an example,61 they prepared an antimicrobial dressing with bacterial capture performance and short-term high antibiosis activity, namely the AgNPs/N-CD@ZnO xerogel. Encouragingly, its excellent antimicrobial activity was dependent on 808 nm NIR mediated PDT of ZnO NPs. In this study, N-CD@ZnO nanomaterials were synthesized by doping N-doped carbon dots (N-CDs), and it was found that after ZnO NPs were decorated by N-CD, the photodynamic response region was transferred from the original UV region to the 808 nm NIR region by up-conversion technology, indicating that N-CD@ZnO was a reliable upconversion material. As mentioned above, many researchers have reported visible/NIR mediated PDT of ZnO-based nanomaterials for antimicrobial infection, inflammation regulation and antitumor therapy.62–64
Moreover, PTT is an effective and hopeful antibacterial means. For eradication of pathogenic bacterial infections, a novel NIR-triggered antibacterial platform based on a thermoresponsive polymer (TRB-ZnO@G) of MOF-derived 2D-carbon nanosheets (2D-CN) was reported in 2019.68 The results indicated that the system possessed flexible 2D nanostructures, high photothermal activity, sustained zinc ions (Zn2+) release and phase-size switching capability, which facilitated local clearance of multiple bacteria and enhanced anti-infective therapy (Fig. 3A). Recently, Wang et al.69 deposited a photothermal multifunctional composite coating, namely Ti-PDA/BP/ZnO, on a titanium (Ti) substrate to design an anti-biofilm phototherapy platform with high thermal support for antimicrobial capability. The designed antimicrobial coating not only displayed good photothermal conversion ability under 808 nm NIR irradiation, but also increased the susceptibility of bacteria to Zn2+ to obtain dramatic bactericidal performance, while accelerating the destruction of Staphylococcus aureus and Escherichia coli biofilms (Fig. 3B). Wherefore, the NIR triggered ZnO-PTT synergistic antibacterial strategy offered a conducive idea for in situ anti-biofilm adhesion of medical implant coatings, which also opened up new possibilities for the prevention and treatment of clinical implant infections.
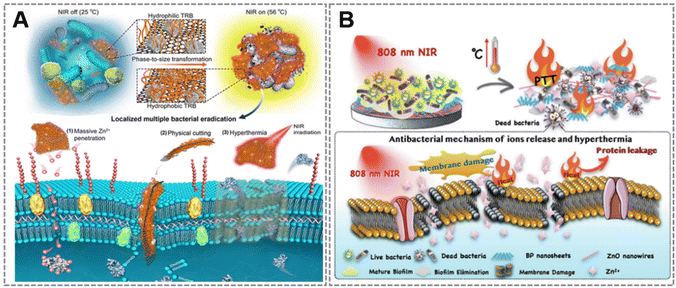 | ||
| Fig. 3 (A) Sustained and topical triple antibacterial activity of the proposed TRB-ZnO@G, including chemical, mechanical and photothermal sterilization. Adapted with permission from ref. 68, copyright 2019, American Chemical Society. (B) Schematic illustration of the NIR triggered synergistic antibacterial activity of Ti-PDA/BP/ZnO coatings. Adapted with permission from ref. 69, copyright 2022, Elsevier. | ||
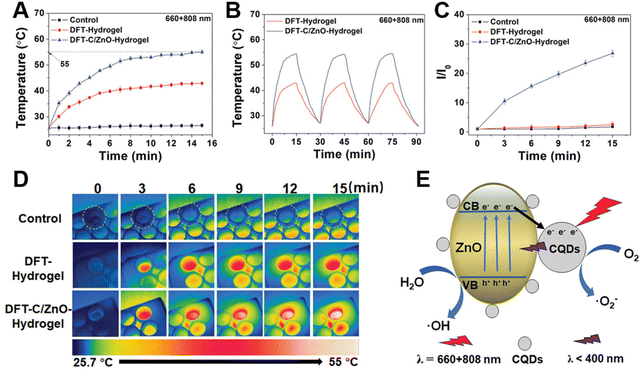 | ||
| Fig. 4 Photothermal and photodynamic properties of hydrogels. (A) Photothermal curves of the hydrogel under 15 min light irradiation (660 nm and 808 nm). (B) Temperature rising and cooling curves of the hydrogel with 660 nm red light and 808 nm NIR irradiation. (C) Real-time infrared thermal images of different hydrogels immersed in PBS aqueous solution under light illumination at 660 nm and 808 nm. (D) Fluorescence intensity (FI) at 525 nm after immersion in fluorescent dye solution and irradiation with light. I0: FI of the initial value, I: FI of different times. (E) Schematic diagram of the photothermal and photodynamic performance process of hydrogels under mixed light. Adapted with permission from ref. 74, copyright 2022, Wiley. | ||
Although PDT and PTT based on ZnO nanomaterials have been widely used in various aspects of the biomedical field (Table 2), there are still potential limitations to be broken through in existing phototherapy strategies. In the case of PDT, the photosensitizer absorbs light energy and transfers it to oxygen, which leads to the generation of energy-state transitions, thereby increasing the production of ROS such as singlet oxygen (1O2).75 In addition to light sources and photosensitizers, oxygen is an indispensable part of the PDT process. However, hypoxic conditions, such as in tumor site76 and bacterial infection microenvironments,77 severely inhibit the efficacy of PDT. In order to alleviate the hypoxia, a number of oxygen-producing materials (nanozymes, hydrogel, microalgae, etc.) have been developed, but they are inactive due to the acidic environment of the lesions.78 Besides, the mitochondrial respiration of related cells is relatively active, which consumes a part of the oxygen, further increasing the difficulty of improving the hypoxic environment.79 Hence, it is necessary to optimize the existing oxygen supply materials or develop new photosensitizers from the perspective of continuous oxygen production and inhibition of oxygen consumption.
| Materials | Light source | Phototherapy strategy | Application field | Ref. |
|---|---|---|---|---|
| LCL/ZnO | Chemiluminescence of luminol (∼660 nm) | PDT | Antitumor | 64 |
| CEP-Ag@AgCl/ZnO | Visible light (>420 nm) | PDT | Antibacterial | 63 |
| ZnO/BiOBr | Visible light (>400 nm) | PDT | Antibacterial | 62 |
| ZnO/UCNPs | 808 nm NIR | PDT | Antibacterial | 47 |
| AgNPs/N-CD@ZnO | 808 nm NIR | PDT | Bacteria-infected wounds | 61 |
| t-ZnO | 527 nm green light | PDT | Wound healing | 40 |
| DOX-FA-PEG-ZnO NS | 808 nm NIR | PTT | Anti-breast cancer | 67 |
| ZnO@PDA-DOX/DZ | 808 nm NIR | PTT | Antitumor | 66 |
| Ti-PDA/BP/ZnO | 808 nm NIR | PTT | Implant infections | 69 |
| TRB-ZnO@G | 808 nm NIR | PTT | Anti-infective | 68 |
| RP/ZnO | Solar light | PDT/PTT | Antibacterial | 73 |
| DFT-C/ZnO-hydrogel | 660 nm red light and 808 nm NIR | PDT/PTT | Wound healing | 74 |
| Ti-ZnO@Col-I | 583 nm yellow light and 808 nm NIR | PDT/PTT | Peri-implant diseases | 56 |
2.2 Ultrasound-triggered therapy
Despite the advantages of light as described above, its poor penetration into tissue hinders further applications. On the contrary, ultrasound with high tissue penetration overcomes the critical problem of penetration depth limitation, making it possible to monitor and treat deep diseases.80 Studies have shown that ZnO NPs are ideal biocompatible ultrasound contrast agents. For instance, a novel type of implantable passive sensor for mechanical properties of biological tissues was constructed by embedding ZnO NPs into polydimethylacrylamide nanocomposite hydrogels, which was able to operate with large linear ultrasonic sensitivity and cover the strain monitoring of soft tissue and hard tissue, realizing remote assessment of organ deformation after implantation.81 Furthermore, ultrasound has the advantages of clinical safety, non-invasiveness as well as high selectivity, and the customized diagnosis and treatment platform constructed by ultrasound shows gigantic application potential in the fields of drug delivery, biological imaging and disease treatment.82 The treatment method using the synergistic effect of ultrasound and a sonosensitizer is called SDT.83 The major principle is to utilize low-intensity (0.5–4.0 W cm−2) ultrasound to irradiate the lesion location of the enriched sonosensitizer, which can in situ activate the sonosensitizer to generate toxic ROS, including superoxide anions (˙O2−), hydroxyl radicals (˙OH) and 1O2, so as to enable site-specific disease treatment. So far, correlation research studies have shown that the possible mechanism of SDT to produce ROS is mainly the ultrasonic cavitation effect.84 The cavitation effects induced by ultrasound include stable cavitation generated by the continuous oscillation of microbubbles, as well as inertial cavitation caused by rapid growth and rupture of microbubbles, which is closely related to the generation of ROS. Inertial cavitation can induce hydrothermal dissociation to generate ROS, namely ˙OH. Additionally, the sonoluminescence phenomenon invoked by cavitation is to excite the electron orbital of the sonosensitizer by transferring energy, and promote the production of ROS when the excited electrons return to the ground state, while the increase of the ROS concentration in the body depends on the structure of the sonosensitizer.85Compared with conventional therapies, SDT, a hopeful non-invasive treatment technique, is implemented to target precisely the lesion location through the selection and modification of sonosensitizers, which leads to apoptosis of pathological cells, as well as less damage to normal cells and tissues.86 It is noteworthy that the selection of sonosensitizers is one of the key links in achieving effective SDT. The ideal sonosensitizer should have good biosafety, precise aggregation targeting, high stability in specific environments and strong sensitivity to ultrasound. Currently, the commonly used sonosensitizers are mostly organic sonosensitizers,87,88 such as hematoporphyrin, phthalocyanine, DOX, etc., most of which have poor biocompatibility, low water solubility, weak chemical stability, and short blood circulation time which cannot effectively accumulate at the disease site, making SDT ineffective. In view of this, researchers have successively synthesized inorganic nanomaterials89 with the characteristics of sonosensitizers, which contain the advantages of distinctive energy level structure, good chemical stability and high concentration at the lesion site compared with organic sonosensitizers, making them widely used in SDT. Among them, inorganic semiconductor sonosensitizers represented by ZnO NPs90,91 and titanium dioxide nanoparticles92,93 have received extensive attention. In the early years, Webster et al.94 demonstrated that ZnO NPs exhibited superior antibacterial properties against Staphylococcus aureus, which further enhanced its bactericidal activity in the presence of ultrasound, expecting to be used for the anti-infection of medical devices. Similarly, Bahadur et al.95 selected ultrasound as an in vitro stimulus source for smart drug carriers based on porous ZnO NPs. They verified that ultrasound could cause extreme changes in the temperature and pressure of the interface between the anticancer drug DOX as well as ZnO NPs through the cavitation mechanism within a short period of time, which facilitated drug detachment from the carrier, thereby reinforcing controlled and targeted on-demand drug delivery. Unfortunately, pure wide-bandgap ZnO NPs as inorganic sonosensitizers suffer from low yields of ROS (e.g., 1O2 and ˙OH) under ultrasound activation due to the fast recombination rate of electrons (e−) and holes (h+),96 so the therapeutic effect is not evident. Furthermore, a low specific surface area is also a significant factor limiting ultrasonic sensitivity and leading to poor acoustic dynamics, because a high specific surface area is conducive to enhancing the ultrasonic cavitation effect to release higher energy.97 In consequence, how to design an ideal ZnO-based nanomaterial sonosensitizer with biocompatibility and dramatic sonodynamic therapeutic efficiency has become a challenging research topic.
Based on the above problems, researchers have adopted effective strategies such as biochar loading,98 metal doping99 and defect engineering100 to tune the electronic structure, band gap, specific surface area as well as the catalytic performance of ZnO NPs. For instance, Shim et al.101 reported a new means to construct piezoelectric nanohybrids (Au@P-ZnO NRs) for chemical piezoelectric catalytic tumor therapy by incorporating gold nanoparticles (Au NPs) to heighten the piezoelectric catalytic activity of ZnO NRs and loading the pro-oxidant anticancer drug piperlongumine. The finding manifested that the deposition of Au NPs could significantly increase the piezoelectric catalysis of ZnO NRs, further enhancing ROS formation (Fig. 5A). Furthermore, in vitro and in vivo experimental results demonstrated the feasibility of piezoelectric nanohybrids (Au@P-ZnO NRs) to realize ultrasound-triggered piezoelectric catalytic cancer therapy combined with cancer-specific chemotherapy. In an outstanding work, Professor Zhang's group100 improved the performance of ZnO-SDT by adjusting electron transport through defect engineering, synthesizing a novel sonosensitizer, defect-rich gadolinium (Gd) doped ZnO nanospheres (D-ZnOx:Gd), which overcame the difficulty of low quantum yield of ROS produced by pure ZnO NPs. Compared with the defect-free ZnO NPs, the abundant oxygen-deficient sites of D-ZnOx:Gd could supply electron capture sites, which was beneficial to boost the separation of e− and h+ from the energy band structure after ultrasonic irradiation, thus evidently strengthening the sonodynamic effect. Meanwhile, the defect-rich D-ZnOx:Gd more easily adsorbed oxygen and water molecules, which greatly enhanced the ability to generate ROS (Fig. 5B). The results certificated that the defect-rich D-ZnOx:Gd sonosensitizer obtained by defect engineering could produce a large amount of ROS regardless of tissue depth, thereby achieving high ultrasound-mediated killing efficiency of breast cancer cells. The ultrasonic responsive system of ZnO-based nanomaterials is favored by biomedical experts due to its clinical safety, non-invasiveness and high tissue penetration, while its defects to be improved also attract much attention. Likewise, SDT has analogous problems with PDT, where oxygen is an essential element of the SDT process. As such, how to build ZnO-based sonosensitizers that can generate oxygen deserves further exploration by researchers.
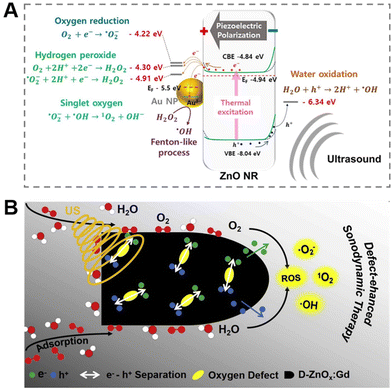 | ||
| Fig. 5 (A) Piezoelectric catalytic mechanism of Au@P-ZnO NRs. Adapted with permission from ref. 101, copyright 2022, Elsevier. (B) Mechanism of ROS generation from defect-rich D-ZnOx:Gd. Adapted with permission from ref. 100, copyright 2020, Elsevier. | ||
2.3 Mechanical force-triggered therapy
Mechanical force102 is very common in biological systems and exists extensively in diverse forms of intrinsic involuntary or voluntary stimulation, including heartbeat, lung dilation, chewing, etc. Conversely, appropriate mechanical stimuli can also participate in biological activities, such as angiogenesis and bone regeneration, affecting multiple biomechanical processes.103 Therefore, it is crucial to design and construct a mechanoresponsive nanoplatform for diagnosis and treatment. Piezoelectric materials,104 a class of intelligent substances with piezoelectric properties, can convert mechanical energy into electrical energy under mechanical stimulation. Currently, a variety of piezoelectric materials have been explored for biomedical research, including piezoelectric inorganics, piezoelectric polymers and hybrid piezoelectric materials.105,106 Among them, ZnO is a typical piezoelectric inorganic material, usually with extremely high piezoelectric coefficient and good mechanical properties, showing excellent application potential in the field of electroactive tissue repair.107 Based on this, related researchers have prepared a series of therapeutic platforms endowed with mechanical response functions based on ZnO NPs, comprising tissue engineering scaffolds,108,109 composite material coatings,110 piezoelectric dermal patches,111etc. Their effects on angiogenesis, wound healing and bone tissue formation were also explored.Numerous studies have indicated that electrical stimulation generated by piezoelectric materials can modulate the activity of associated cells such as cardiomyocytes, osteoblasts and neural cells, while affecting cellular processes including proliferation, migration as well as differentiation.112,113 For example, Murillo et al.114 chose a two-dimensional ZnO nanosheet network as a piezoelectric nanogenerator, which could respond to the intrinsic force generated by cells and induce local electric field stimulation, thereby promoting the proliferation and differentiation of macrophages and human osteosarcoma cells (Saos-2). On this basis, researchers modified a ZnO nanosheet coating on the surface of a Ti-based alloy (Ti45Zr15Pd30Si5Nb5) to induce electrical self-stimulation of osteoblasts, while avoiding the use of silicon.110 Besides, in terms of nerve repair, Zheng et al.113 loaded ZnO into polycaprolactone (PCL), a commonly used material for nerve conduit manufacturing, and fabricated ZnO/PCL piezoelectric nanogenerator scaffolds by means of 3D injectable multilayer biofabrication technology. Subsequently, the effect of the ZnO/PCL scaffold on the viability of Schwann cells was investigated, which suggested that the ZnO/PCL scaffold had low toxicity similar to the PCL scaffold (Fig. 6A), and the oxidative stress produced by the ZnO/PCL scaffold was also comparable to that on the PCL scaffold (Fig. 6B and C). Next, the morphology and adhesion of Schwann cells on the two scaffolds were also observed, which concluded that the electrical signals generated by the ZnO-loaded scaffolds stimulated protrusion extension and increased cell adhesion to the material interface (Fig. 6D–I). Finally, it was verified that physical exercise was selected as a simulation of external mechanical signals to trigger the ZnO/PCL scaffold to produce a bioelectric environment, when rats exercised on a treadmill. The unique approach not only speeded up nerve conduction, but also promoted axonal myelination, ultimately restoring motor function by repairing endplate muscles. In conclusion, the mechano-informative biomimetic piezoelectric scaffold designed by the authors provided a new perspective for nerve repair and might have great application potential in the neural engineering of regenerative medicine.
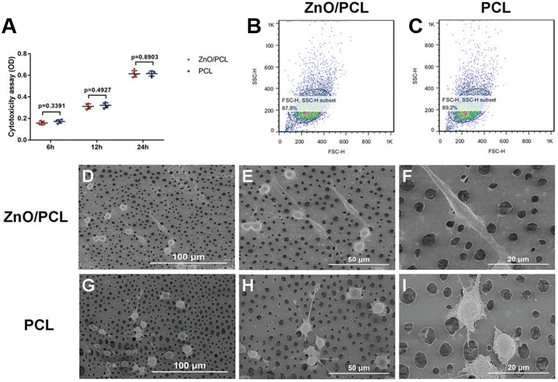 | ||
| Fig. 6 (A) Cytotoxicity assay of Schwann cells seeded on 10% ZnO/PCL and PCL scaffolds. (B and C) Oxidative stress in Schwann cells on ZnO/PCL and PCL scaffolds. (D–F) Schwann cell morphology on ZnO/PCL scaffolds. (G–I) Schwann cells morphology on PCL scaffolds. Adapted with permission from ref. 113, copyright 2020, Wiley. | ||
Moreover, the piezoelectric effect endows ZnO-based nanomaterials with more promising antibacterial and healing properties.115 To accomplish spontaneous wound healing, Kim et al.111 developed a ZnO nanorod piezoelectric patch (PZP) based on bidirectional growth by using the characteristic. It was found that piezoelectric materials could generate endogenous electric fields even under small mechanical deformation, avoiding the utilization of auxiliary external electrical equipment to induce endogenous electric fields. In vitro and in vivo experiments testified that the piezoelectric patch application supported an endogenous skin regeneration process on account of electrical stimulation, which accelerated wound healing by regulating inflammatory responses, promoting cellular regenerative activity, quickening neovascularization and enhancing tissue remodeling. For the treatment of infected wounds, Fan et al.116 reported a novel bi-piezoresponse hydrogel scaffold, namely ZnO nanoparticle-modified polyvinylidene fluoride (PVDF)/sodium alginate (SA) piezoelectric hydrogel scaffold (ZPFSA), which rationally designed bi-piezoresponse properties including vertical and horizontal orientations, thus ensuring sustainable bioelectrical stimulation signals. The resulting bioelectric environment provided favorable conditions for activities related to wound healing, such as bacterial eradication, cell migration, collagen remodeling and vascularization. In summary, the unique work not only expanded the application of piezoelectric materials in wound dressings, but also offered an originative option for promoting rapid healing of infected wounds and preventing scarring.
It is worth mentioning that the source of mechanical stimulation of piezoelectric materials can also be provided by self-driving bacteria. Bdellovibrio,117 a natural predator with activity, can move rapidly (160 m s−1) by rotating unipolar flagella and hit its “prey”, as well as killing the “prey” after entering its peripheral cytoplasm. Inspired by this, Professor Zhang and his collaborators118 proposed a material-assisted microorganism (MAMO) strategy that introduced ZnO nanorods on the surface of predatory Bdellovibrio with high-speed collision capability to obtain ZnO-modified Bdellovibrio, namely ZnO@Bdello. The modified Bdellovibrio moved rapidly and collided with the biofilm, and the generated mechanical pressure induced ZnO polarization and produced a large amount of ROS, both of which had a synergistic antibacterial effect on the bacteria of the biofilm. Subsequently, the resistance of the system to biofilms and bacteria was verified in complex biofilm models including rat and rabbit periodontitis models. It was also found that ZnO@Bdello could not only destroy biofilms, but also reduce the abundance of periodontal pathogens to inhibit periodontitis without producing detectable pathogen resistance and side effects (Fig. 7). In addition to formulating the aforementioned disease treatment strategies, ZnO-based piezoelectric nanomaterials have also been generally applied to the construction of electronic skins, wearable devices and other applications related to disease diagnosis as well as health care, resulting in considerable progress.119–121
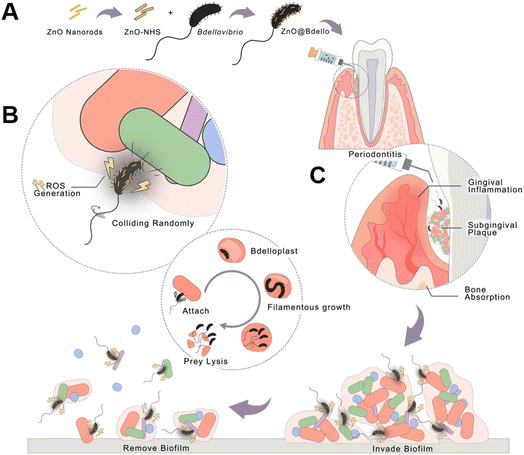 | ||
| Fig. 7 Schematic illustration of ZnO@Bdello removal of the dental plaque biofilm. (A) Preparation of ZnO@Bdello. (B) The predation process of ZnO@Bdello. (C) In periodontitis, ZnO@Bdello invaded and removed subgingival biofilm, thereby inhibiting gingival inflammation and alveolar bone resorption. Adapted with permission from ref. 118, copyright 2022, Elsevier. | ||
2.4 Other exogenous stimuli-triggered therapy
The microwave response behavior of ZnO NPs is another fundamental property for their biomedical applications due to their high exciton binding energy (∼60 meV) and wide bandgap (∼3.37 eV), which can absorb microwave energy and convert it into thermal energy, forming a local thermal effect.122,123 Simultaneously, microwave irradiation is also an external stimulus with excellent penetrating performance and good controllability, while furnishing an excellent thermal effect.124,125 Hence, microwaves have become one of the potential stimulus sources for ZnO-based intelligent materials. Wu et al.126 synthesized mesoporous core–shell nanomaterials (Fe3O4@mZnO) by homogeneous precipitation, a highly drug-loaded drug carrier with microwave-responsive and magnetic response properties, which was expected to be used for targeted delivery of drugs in combination with chemotherapy and microwave hyperthermia system construction. Furthermore, the magnetic response is also one of the stimulus sources of the ZnO-based responsive system, which has the special functions of controlling transmission, including telemanipulation and magnetic resonance imaging.127 As previously mentioned, the magnetically responsive ZnO-based smart system enables on-demand and site-specific drug delivery.126 In another study, Cauda et al.128 suggested an approach to reinforce ZnO performance through iron doping to design a multifunctional theranostic nanoplatform for cancer cell therapy, namely Fe:ZnO NPs. In this system, Fe:ZnO NPs had biological imaging potential, which offered a new avenue for the development of intelligent disease diagnosis systems.3. Design and biomedical applications of single endogenous stimuli-responsive ZnO-based materials
Endogenous signals can be highly disordered under pathological conditions, providing more possibilities for the construction of intelligent nanoplatforms for endogenous stimulus responses, which can be used to perform accurate and effective treatment by distinguishing healthy sites from diseased sites.129 In addition to the previously reviewed combination of ZnO-based materials with exogenous stimuli such as light, ultrasound and mechanical force for the diagnosis and treatment of diseases, including cancer, inflammation and microbial infections, researchers have designed a series of endogenous stimuli-responsive ZnO-based materials.130,131 The material can specifically respond to endogenous signals related to the microenvironment of the lesion site, such as pH, enzymes and glutathione, so as to ameliorate the defects and efficacy of existing therapies in biomedical applications containing drug delivery, disease diagnosis and disease treatment, which is expected to develop personalized treatment systems.3.1 pH-triggered therapy
The pH is one of the biological parameters usually altered in characteristic disease states, such as tumors and microbial infections (acidic pH), so it is essential to develop a pH-responsive platform based on bioresponsive materials.132 The design of pH responsive materials should satisfy the following requirements, that is, bioresponsive materials can dynamically change their structural properties, including swelling, contraction, dissociation and degradation, in the context of a pathological environment with low pH, thereby implementing particular bioactive molecule delivery and disease treatment.133 ZnO NPs are pH-sensitive materials that are only stable under normal physiological conditions (pH 7.4), while in acidic conditions (pH < 5.5), the nanostructures incline to dissolve rapidly and release Zn2+, which can be used for antiseptic and anticancer purposes.134 Meantime, ZnO NPs with pH-triggered characteristics have also become potential candidates for convenient smart nanocarriers due to their simple design, tunable size and low toxicity, which can not only quickly achieve specific biodegradation, but also selectively release drugs. In 2010, ZnO NPs were first reported as a pH-responsive drug delivery system.95,135 Since then, the class of materials has been rapidly developed and widely used in the delivery of various disease therapeutic drugs (Table 3). Other studies showed that after ZnO NPs dissolved in response to physiological and pathological pH signals, the generated Zn2+ exhibited obvious cytotoxic effects on cancer cells.136| Drug-delivery systems | Drug | Cell type | Highlight | Ref. |
|---|---|---|---|---|
| DOX-loaded ZnO NSs | DOX | HepG-2, HeLa | Biodegradable, tumor accumulation, ultrasmall particles | 149 |
| HA-ZnO-PEG | DOX | A549 | CD44-targetable, pH-responsive | 143 |
| PEG-cZnO-DOX | DOX | A549, MCF7 | Fully biodegradable, tumor-specific accumulation, synergistic antitumor | 148 |
| ZnO/DOX | DOX | MDA-MB-231, HeLa, MES-SA/Dx5, NCI/ADR-RES | Specific targeting, multi-faceted anti-cancer | 150 |
| ZnO-DOX@HAase/PEG-PAH-DMMA | DOX | 4T1 | Enhanced tumor penetration and proximity effect | 151 |
| ZnO-Gd-DOX | DOX | BxPC-3 | Fully biodegradable, bioimaging capabilities, no obvious side effects | 146 |
| ZnO@MSNs-DOX | DOX | HeLa | Prevents the cytotoxic drug from premature release | 137 |
| ZnO@MSN-K10 | DOX | HepG2 | Dual pH-sensitive, escape from the endosomes | 138 |
| ZnO@P (CBMA-co-DMAEMA) | DOX | HepG2 | Enhances water stability, increases blood circulation time, and promotes endocytosis | 145 |
| 5-FU@ZIF-90@ZnO | 5-FU | HeLa | High treatment efficiency, low drug toxicity, renal pathway excretion | 140 |
| ZnO-gated MCN | MIT | A549 | Double amide bond covalently linked, high biocompatibility | 139 |
| ZnO-PBA-curcumin | Curcumin | MCF-7 | Specific targeted delivery, tumor accumulation | 142 |
ZnO quantum dots (ZnO QDs) are a popular drug carrier due to their small particle size and easy phagocytosis by cells.137 ZnO QDs are mostly used as nanocarriers to convey antitumor drugs, such as DOX,138 mitoxantrone hydrochloride,139 5-fluorouracil (5-FU)140 and curcumin,141,142 explaining that the pH-triggered intelligent drug-releasing anti-cancer system can further enhance the efficacy of cancer treatment through synergistic anti-tumor of Zn2+ and drugs. Zhu's team137 and Feng's team138 constructed a pH-responsive ZnO@MSN drug delivery system (DDS) loaded with DOX based on acid-decomposable ZnO QDs and mesoporous silica nanoparticles (MSN), respectively. In their systems, ZnO QDs acted as gatekeepers to block the nanopores of MSN, enabling “zero premature” drug release in the physiological environment. Finally, the effective combination of ZnO QDs that released Zn2+ triggered by acidic pH, and the anti-tumor drug DOX achieved a synergistic anti-tumor effect, raising the therapeutic index while reducing side effects. Regrettably, whether mesoporous silica can be biodegraded and excreted remains to be discussed.143 Mesoporous carbon nanoparticles (MCN) have similar structural characteristics and properties to mesoporous silica, which show lower cytotoxicity than mesoporous silica, promising to replace mesoporous silica for the construction of drug delivery systems. Based on this, Du et al.139 reported an MCN-based drug delivery system, the gated MCN drug delivery system (ZnO-gated MCN), in which ZnO QDs were also selected as the gatekeepers of the system. The surface of the carboxylated MCN was covalently connected to the carboxylated ZnO QDs through double amide bonds, which could not only improve the drug loading capacity, but also minimize the premature release of the drug. After loading the hydrophilic antitumor drug mitoxantrone hydrochloride (MIT-HCL), it exhibited about 65% high cytotoxicity to A549 cells even at a drug dose of 0.066 ng per cell, implying that the system had a prospective application in tumor treatment.
ZnO QDs, however, are prone to agglomeration, and the strong adsorption of mesoporous materials on drugs leads to incomplete drug release, which is where further improvements are needed for the type of drug delivery system.144 In view of this, researchers successively exploited many modification methods of bare ZnO QDs, such as amino modification,143 zwitterionic polymer P (CBMA-co-DMAEMA) modification,145 gadolinium ion (Gd3+) doping,146etc., where the modified ZnO QDs were used as nanocarriers for carrying drugs.147 Kong et al.146 designed a novel pH-sensitive multifunctional composite nanoplatform by introducing polymer-coated ZnO QDs to compound gadolinium ions and adsorbing DOX, carrying out three functions of small animal fluorescence labeling, magnetic resonance imaging and drug release for tumor treatment, which was one of the representative works of diagnosis and treatment integration. In another study, it was also found that the construction of cluster-like ZnO QDs could improve their stability. For instance, an investigation by Lin et al.148 demonstrated an instant pH-responsive drug-loaded nanocluster (PEG-cZnO-DOX) based on ZnO QDs, which was composed of small single ZnO QDs capped by cross-linking dicarboxyl-terminated polyethylene glycol (PEG), further loading with a large amount of DOX through complexation and covalent interaction. When placed in tumor cell endosomes, PEG-cZnO-DOX specifically responded to low pH, and the carrier was completely dissolved while causing the metal-drug complex to be decomposed, thus realizing complete biodegradation and efficient drug release of the system. More importantly, the pH-responsive PEG-cZnO QDs cluster system with a size of about 70–80 nm could perform tumor homing and accumulation while circulating in the blood through enhanced permeability and retention effect, acting as a synergistic anti-tumor effect.
Besides, some studies have reported that appropriately sized ZnO NPs140,141 or ZnO nanospheres (ZnO NSs) assembled from ultra-small particles149 are also ideal pH-responsive drug carriers. As confirmed by Wang et al.,149 the as-synthesized ZnO NSs assembled by ultra-small particles, with excellent properties such as being size-tunable, monodisperse and biodegradable, were used for DOX charging. The structure of the DOX-loaded ZnO NSs nanoplatform was destroyed in a weakly acidic environment, while releasing drugs and ultra-small nanoparticles to meet the requirements of pH-stimulated drug release, which was beneficial to biodegradation. In vitro cell experiments made clear that the system effectively reduced the cell viability of human hepatocellular carcinomas (HepG-2) and HeLa cells, showing a dose-dependent and time-dependent effect. Finally, in vivo experiments bore out that the DOX-loaded ZnO NSs system displayed significant anti-tumor effects, with an average inhibition rate of 79% relative to the control group, and no obvious side effects (Fig. 8). Related researchers have proposed that ZnO NPs can be a nanocarrier as well as the multi-target therapeutic agent.150,151 Zhu's group150 demonstrated for the first time that ZnO NPs could efficaciously target a variety of cells, including cancer cells, cancer stem cells and macrophages. It was mainly reflected in the fact that ZnO/Dox could be effectively internalized by both drug-sensitive and multidrug resistant cancer cells and penetrate more efficiently through three-dimensional (3D) cancer cell spheroids compared with free Dox. Moreover, ZnO NPs could efficaciously downregulate the key surface marker CD44 of cancer stem cells and decrease the stemness of cancer stem cells, thus improving the sensitivity of Dox therapy, inhibiting the adhesion and migration of cancer cells, and preventing the formation of tumors (3D cancer cell spheres). In addition, as immunomodulators, ZnO NPs could protect macrophages from Dox-induced toxicity and promote the Dox-induced macrophages to polarize toward an M1-like phenotype. Synchronously, ZnO NPs perform multiple key functions, such as inhibiting cancer proliferation, sensitizing drug-resistant cancers, preventing cancer recurrence and metastasis, as well as resuscitating cancer immune surveillance. Additionally, some functionalization strategies have been proved for the modification of ZnO NPs. For example, the researchers explored the pH response of phenylboronic acid (PBA)-functionalized ZnO NPs (∼40 nm in diameter) for tumor tissue-specific delivery of curcumin (ZnO-PBA-curcumin).142 PBA conjugation was found to facilitate the targeted delivery of curcumin, and the anticancer efficacy of the system could be attributed to the differential oxidative stress-inducing characteristics of curcumin and Zn2+, which afforded insights into the PBA-functionalized tumor cell targeting mechanism. Furthermore, Wang et al.152 assembled a delicate self-catabolizing multifunctional DNAzyme nanosponge using ZnO NPs as stimulus-responsive DNAzyme cofactor precursors for programmable drug delivery and efficient gene silencing.
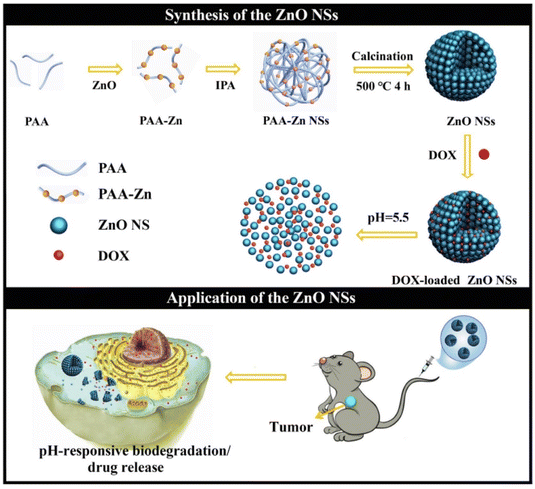 | ||
| Fig. 8 Tunable synthesis of pH-responsive biodegradable ZnO NSs assembled by ultra-small particles for cancer chemotherapy. Adapted with permission from ref. 149, copyright 2019, Elsevier. | ||
Besides constructing intelligent drug-loading systems, pH-responsive ZnO-based nanomaterials have also been applied to antibacterial, tissue regeneration and pneumonia treatment.153,154 For example, Zhao et al.153 developed pH-responsive Ber@ZnO-Z nanospheres, in which ZnO NSs and ZIF-8 acted as core–shell and shell (ZnO-Z), respectively, further encapsulating the fungicide berberine (Ber) in the core–shell ZnO-Z carrier. In this work, the authors systematically explored the pH sensitivity of the Ber@ZnO-Z nanoplatform. The schematic diagram of the pH-responsive release mechanism of Ber@ZnO-Z (Fig. 9A) and the SEM images (Fig. 9B) manifested that when it was placed in an acidic environment, the shell ZIF-8 gradually collapsed, exposing the ZnO NSs and releasing the sterilization agent Ber. Whereafter, the ZnO NSs were further completely dissolved or transformed into smaller ZnO NPs, which lowered the pH of the solution. Moreover, the UV-visible (UV-vis) spectra (Fig. 9C) proved the characteristic peaks of ZnO NSs, ZnO-Z as well as Ber@ZnO-Z. The result pointed out the difference that Ber@ZnO-Z could decompose carriers with the addition of HCl, and the characteristic absorption band of Ber appeared, which was caused by the strong UV absorption capacity of ZnO, indicating that Ber@ZnO-Z with pH response characteristics could be used as a controlled release carrier to transfer active ingredients. Based on the principle, the pH-triggered Ber release behavior was explored, when the system was exposed to PBS buffer (pH 5.0, 6.5 and 8.0) at different times (Fig. 9D). The results signified that the structure of the ZIF-8 shell was destroyed under the action of acid, resulting in the release of Ber, whose release rate was highly pH-dependent, thereby achieving pH-triggered controlled release of the fungicide. Similarly, Ber@ZnO-Z also exhibited pH-responsive release behavior in acidic soil (Fig. 9E).
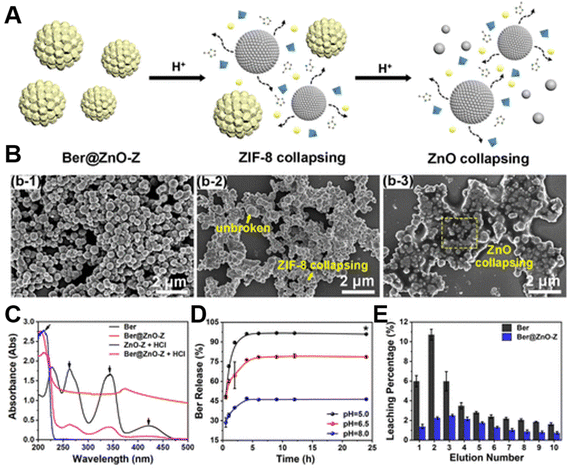 | ||
| Fig. 9 (A) pH-responsive release mechanism of Ber@ZnO-Z. (B) SEM images of Ber@ZnO-Z at different pH values (8.0/6.5/5.0). (C) UV-vis spectroscopic analysis. (D) Cumulative release of Ber from Ber@ZnO-Z in PBS buffer at pH 5.0, 6.5 or 8.0. (E) Release of Ber and Ber@ZnO-Z in acidic soil. Adapted with permission from ref. 153, copyright 2022, American Chemical Society. | ||
3.2 Enzyme-triggered therapy
Enzymes, a class of protein-structured biocatalysts that are ubiquitous in living organisms, regulate many biological processes.155,156 When the body undergoes changes including enzyme deficiency, activity reduction or overexpression, abnormal catalytic reactions are often induced, which in turn lead to the occurrence and development of metabolic disorders and even diseases. Therefore, enzymes can be used as markers for disease detection and treatment, because of their high selectivity and specificity, which are also important internal stimuli for constructing responsive therapeutic systems. In view of this, researchers proposed to develop a pH/enzyme-responsive drug delivery system based on porous silica nanospheres (pSiO2 NSs) and ZnO QDs, namely ZnO/HA gated pSiO2/PCA-Glu NSs, in which ZnO QDs and hyaluronic acid (HA) acted as pH/enzyme-responsive gatekeepers for drug release, respectively.157 Notably, HA could be particularly degraded by hyaluronidase (Hyal-1) overexpressed in the tumor microenvironment, which was beneficial to reduce premature drug release. Furthermore, Lai et al.158 employed a multi-stage enzyme-responsive drug delivery program to design ZnO NPs hydrogel sheets that could efficiently treat corneal abrasion. The constructed functional nanocarriers were classified into three types according to their degradation responses to intraocular enzymes, including rapidly degrading dipalmitoyl phosphatidylcholine liposomes (DPPC), moderately degrading (low cross-linking) and slowly degrading (highly cross-linked) HA nanocarriers. Subsequently, a rabbit corneal abrasion model was built to verify the therapeutic effect of the hydrogel sheet, which suggested that the system could release the corresponding drugs (epigallocatechin gallate, β-1,3-glucan and SB431542) in three stages, achieving many functions such as early anti-inflammatory, mid-stage healing and late prevention of scar formation. All in all, the therapeutic hydrogel material could accommodate a variety of drug molecules, opening up a new avenue for the treatment of complex ocular diseases. Another study found that the designed porous ZnO–Co3O4 nanocages had inherent peroxidase-like activity, which could rapidly and specifically detect amyloid-β peptide, one of the main markers of early onset of Alzheimer's disease (AD).1593.3 Other endogenous signals-triggered therapy
In addition to the above findings, the endogenous signaling molecule hydrogen sulfide (H2S) is a key mediator of various physiological functions and pathological processes.160 For instance, the excessive H2S produced by colorectal cancer becomes a high potential target for disease treatment,161 favoring the construction of endogenous gas signal activation nanoplatform. As is known to all, ZnO NPs are gas-sensing materials that have attracted much attention, and new gas sensors derived from them can be used for in vitro real-time detection of dangerous gases such as H2S162 and ammonia,163 with low cost, weak toxicity, good responsiveness as well as easy structure control. In theory, ZnO NPs can also specifically respond to these endogenous gas signals, testifying promising potential in disease diagnosis and treatment.164 Recent work by Zhou's group165 validated this point, developing an oral bite pad based on the photoluminescent properties of ZnO QDs, which offered a method for accurately locating obvious and hidden dental lesions by detecting the local release of volatile sulfide. Herein, a ZnO-polydimethylsiloxane composite (ZnO-PDMS) was used to fabricate wearable fluorescent dental braces, which specifically responded to sulfides (mainly H2S), resulting in fluorescence quenching, thereby realizing the visual and precise positioning of the patient's dental lesions and achieving the purpose of diagnosing the lesions (Fig. 10A and B). Additionally, Zhou et al.166 reported an H2S-responsive nanoplatform based on ZnO-coated virus-like silica (VZnO) nanoparticles, which reduced intracellular glutathione levels by reducing H2S concentration, further contributing to ferroptosis in colorectal cancer cells and enabling efficient treatment of colorectal cancer (Fig. 10C).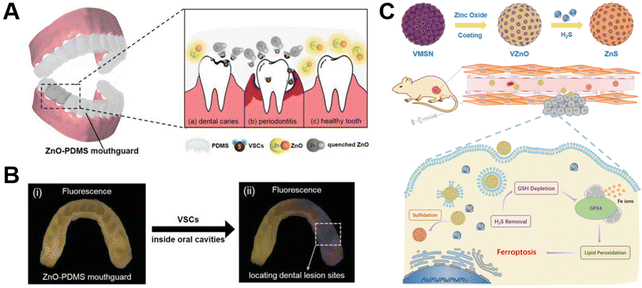 | ||
| Fig. 10 (A) Schematic illustration of the ZnO-PDMS mouthguard and its response to sulfide, resulting from (a) caries, (b) periodontitis, and (c) healthy teeth. (B) Fluorescence images of the ZnO-PDMS mouthguard used to observe dental lesions in vivo: (i) before and (ii) after wearing by carious patients, to locate the lesions. Adapted with permission from ref. 165, copyright 2020, Wiley. (C) A schematic diagram of VZnO synthesis routes and H2S removal for colorectal cancer treatment. Adapted with permission from ref. 166, copyright 2021, Springer. | ||
Likewise, exosomal concentrations and exosome proteins are considered promising cancer biomarkers.167,168 Qu et al.169 synthesized a high surface area and strong affinity bayberry-like magnetic beads around ZnO nanowires for capturing exosomes. The authors successfully designed a fluorescence detection method for the simultaneous detection of exosome concentration and cancer-related exosomal proteins, possessing the characteristics of high sensitivity, low detection limit and good accuracy, which provided important reference value for the development of new biosensors for exosome analysis and intelligent disease diagnosis. In addition, the chemical gradient generated by the lesion can act as a driving force for self-driven reactive substances. Some researchers chose ZnO nanorods and sulfonated polystyrene microspheres to design an artificial swarm intelligence based on ion exchange reaction, which could be transmitted by the interaction between active particles to achieve long-range collective migration and aggregation under local gradient excitation.170 Given their maneuverability and microscopic size, these artificial active material nanorobots were expected to be applied in biomedicine.171
4. Design and biomedical applications of multiple stimuli-responsive ZnO-based materials
Compared with single stimulus responsive materials, multi-stimuli-responsive materials have continued to be developed due to their versatility, and the corresponding multi-response synergistic therapy has yielded encouraging results.172,173 As mentioned above, Wu et al.74 reported DFT-C/ZnO-hydrogels that could simultaneously respond to light sources at two wavelengths (660 nm and 808 nm). The system played a photocatalytic antibacterial role under 660 nm red light irradiation and switched to a photothermal sterilization function under 808 nm NIR stimulation, which provided a strong guarantee for the healing of infected wounds. What's more, some researchers combined light and ultrasound as a stimulus source, and found that ZnO nanorods’ light/piezoelectric double catalytic effect is higher than a single catalytic effect.174 This was mainly attributed to the fact that the piezoelectric potential formed during the piezoelectric process helped to separate the photogenerated electron–hole pairs, thereby achieving a synergistic effect of photocatalysis and piezoelectric catalysis. In another study, Huo et al.175 confirmed that cobalt (Co) foil modified with a new ZIF-67/ZnO hybrid coating achieved photoelectrocatalytic sterilization after exposure to visible light and potential stimulation.In addition to combining the two extrinsic stimulus responsive strategies as described above, researchers have also attempted to combine the exogenous stimulus responsive strategy with the endogenous stimulus responsive strategy, thus achieving satisfactory results. For example, endogenous stimulation pH and exogenous stimulation light are simultaneously used as triggering mechanisms to construct a multifunctional dual stimulation responsive system for drug delivery, tumor treatment and wound healing.176,177 Among them, Hahn et al.136 incorporated acid-sensitive ZnO NPs into liposomes and loaded daunorubicin to construct a dual-responsive system of UV combined with pH, realizing the chemical photodynamic anticancer effect under the mode of acid-controlled drug release. Furthermore, Yang et al.178 put forward a pH-responsive nanoplatform for 808 nm NIR mediated photodynamic therapy combined with chemotherapy, namely the α-NaYbF4:Tm@CaF2:Nd@ZnO-PAA-DOX nanoplatform. On the one hand, the polyacrylic acid (PAA) coating could cause its own disintegration in response to acidity, which in turn led to the efficient release of the loaded drug DOX at the tumor site. On the other hand, upconverting nanoparticles converted 808 nm NIR to UV (349 and 363 nm), excited the emergence of hole–electron pairs in ZnO, and then generated enough ˙OH to kill tumor cells, while reducing the dependence on oxygen. It was worth mentioning that pH-triggered photothermal therapy systems also displayed excellent antitumor efficacy, such as the ZnO@polydopamine nanocomposites (ZnO@PDA-DOX/DNAzymes) designed by Zhou et al.66 for chemical/gene/photothermal therapy. Similarly, the multi-mode combined treatment strategy of pH-responsive drug release combined with photodynamic/photothermal therapy based on ZnO-based nanomaterials is also suitable for the treatment of infected wounds, which solves the problems of poor treatment effect on wound healing by traditional single treatment methods.179,180
The multiple stimuli-responsive system centered on ZnO-based nanomaterials provides the possibility for targeted, on-demand, precise and efficient treatment of diseases, revealing broad application prospects in the field of biomedicine (Table 4). The continuous development of the multi-response collaborative treatment platform can not only reduce the excessive dependence on a single stimulus, but also increase the sensitivity and compliance of the platform when treating different patients.181,182 Although the multi-responsive strategy developed around ZnO has made gratifying achievements, there are still some problems to be solved in future development and application. First of all, the current multi-stimuli responsive ZnO-based materials combine different strategies, and the explanation of the basic mechanism of complementary and synergistic effects of specific strategies is missing, which should be fully considered in future work. Secondly, ZnO-based biomaterials generally contain a variety of components and complex structures, as well as the related manufacturing processes are cumbersome and need to be further facilitated. Finally, the existing multi-stimuli responsive ZnO system is still focused on basic research, and its clinical transformation remains to be realized.
| Nanoplatform | Responsive strategy | Application field | Findings | Ref. |
|---|---|---|---|---|
| UZNP-PAA-DOX | 808 nm NIR and pH | Antitumor | The system realized the targeted delivery of drugs and multi-strategy anti-tumor effect | 178 |
| ZnO@PDA | 808 nm NIR and pH | Antitumor | Chemical/gene/PTT had a highly effective anti-tumor effect | 66 |
| Ti-ZnO@Col-I | 583 nm yellow light and 808 nm NIR | Peri-implant diseases | Dual light-responsive coatings provided customized treatments for peri-implantitis | 56 |
| PDA-rGO-ZnO | Electricity and 583 nm yellow light | Wound healing | Photoelectric dual stimulation accelerated wound repair | 57 |
| Ag/Ag@AgCl/ZnO | Visible light and pH | Wound healing | This hybrid system could boost the immune system and speed wound healing | 179 |
| ZnO QDs@GO-CS | 808 nm NIR and pH | Wound healing | The platform combined photothermal and chemokinetic effects for antibacterial and pro-healing functions | 180 |
5. Conclusion and outlook
This paper systematically summarizes the classification of ZnO nanomaterial based intelligent responsive systems in recent years, and reviews the application of related stimulus responsive systems in the biomedical field. Compared with single ZnO-based biomaterials, the application of stimuli-responsive systems has incomparable advantages, including safety, efficiency, maneuverability and multi-functionality, which makes it possible to formulate personalized treatment strategies.Stimuli-responsive materials are a class of materials that convert external stimuli into changes in their own dynamic structures or shapes through multiple approaches, thereby obtaining other peculiar physicochemical properties. According to the source of stimulation, stimuli-responsive ZnO-based materials can be divided into two categories, namely, responding to exogenous stimuli (light, ultrasound, mechanical force, etc.) and endogenous stimuli (pH, enzymes, gas signal molecules, etc.) of smart materials. Here, we first focus on the basic structure, response characteristics and application advantages of ZnO-based materials responsive to exogenous stimuli such as light, ultrasound and mechanical force. Related responsive systems have been commonly used in various aspects of the biomedical field. Second, the construction of endogenous stimulus responsive systems about ZnO-based nanomaterials is discussed. Endogenous response ZnO-based materials can respond to endogenous signals related to the presence or high degree of imbalance in the lesion location, including pH, enzymes, H2S gas signaling molecules, etc., further achieving active on-demand administration and efficient and accurate treatment, thus effectively reducing the impact of additional human intervention. In addition, a multi-stimuli-responsive ZnO-based biomaterial system can be designed to integrate various responsive materials on demand to flexibly implement various required diagnostic and therapeutic functions. Stimuli-responsive ZnO-based nanomaterials are widely utilized in the field of biomedicine, and play a role in improving the defects of existing therapies and the curative effect in drug delivery, disease diagnosis and disease treatment, showing potential to realize intelligent and personalized medicine.
Notably, the development and clinical application of stimuli-responsive ZnO-based biomaterials are still challenging. First, the commonly used preparations in multiple stimulus responsive systems are mainly multi-component materials, which have problems such as complex synthesis procedures and poor stability of synthetic materials. Moreover, multiple stimulus responsive systems often involve multiple stimulus sources, increasing the difficulty of operation. Most importantly, the long-term biocompatibility and in vivo metabolism of existing ZnO NP formulations are unclear, which limits further applications of the material. In future research, the physicochemical properties of ZnO NPs can be further optimized based on these contents, and a more practical, more stable, more convenient, safer and simpler stimulus-responsive system can be constructed. At present, such a multi-functional intelligent responsive therapy system is still in the laboratory research stage, and it also faces huge challenges on the way out of the laboratory into clinical practice. The construction of a multi-functional intelligent response platform based on ZnO is inseparable from the research and communication of multidisciplinary staff, which is also conducive to the clinical application of stimulus response system.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was funded by the National Natural Science Foundation of China (No. 31860263 to Xiaolei Wang), the Key Youth Project of Jiangxi Province (20202ACB216002 to Xiaolei Wang), the Key Research and Development Project of Jiangxi Province (20212BBG73004 to Xiaolei Wang), and the Graduate Innovation Special Fund Project of Jiangxi Province (YC2022-B005 to Zhenzhen Weng).References
- G. Schmidl, G. Jia, A. Gawlik, G. Andrä, K. Richter and J. Plentz, Mater. Today Energy, 2021, 21, 100811 CrossRef CAS.
- J. Xu, H. He, X. Jian, K. Qu, J. Xu, C. Li, Z. Gao and Y. Song, Anal. Chem., 2021, 93, 9286–9295 CrossRef CAS.
- F. Fu, D. Yang, H. Wang, Y. Qian, F. Yuan, J. Zhong and X. Qiu, ACS Sustainable Chem. Eng., 2019, 7, 16419–16427 CrossRef CAS.
- M. Zabilskiy, V. Sushkevich, M. A. Newton, F. Krumeich, M. Nachtegaal and J. A. V. Bokhoven, Angew. Chem., Int. Ed., 2021, 60, 17053–17059 CrossRef CAS PubMed.
- J. A. Ruszkiewicz, A. Pinkas, B. Ferrer, T. V. Peres, A. Tsatsakis and M. Aschner, Toxicol. Rep., 2017, 4, 245–259 CrossRef CAS PubMed.
- C. Hanley, J. Layne, A. Punnoose, K. Reddy, I. Coombs, A. Coombs, K. Feris and D. Wingett, Nanotechnology, 2008, 19, 295103 CrossRef.
- X. Wu, S. Liu, H. Zhu, Z. Ma, X. Dai and W. Liu, Adv. Sci., 2022, 9, 2103982 CrossRef CAS PubMed.
- Y. Deng, C. Yang, Y. Zhu, W. Liu, H. Li, L. Wang, W. Chen, Z. Wang and L. Wang, Nano Lett., 2022, 22, 2702–2711 CrossRef CAS PubMed.
- P. Sadhukhan, M. Kundu, S. Chatterjee, N. Ghosh, P. Manna, J. Das and P. C. Sil, Mater. Sci. Eng., C, 2019, 100, 129–140 CrossRef CAS.
- P. Sathishkumar, Z. Li, R. Govindan, R. Jayakumar, C. Wang and F. Gu, Appl. Surf. Sci., 2021, 536, 147741 CrossRef CAS.
- H. Hong, J. Shi, Y. Yang, Y. Zhang, J. Engle, R. Nickles, X. Wang and W. Cai, Nano Lett., 2011, 11, 3744–3750 CrossRef CAS.
- K. Vimala and K. Soundarapandian, Ann. Oncol., 2016, 27, ix38–ix39 CrossRef.
- A. Khan, K. Huang, M. Khalaji, F. Yu, X. Xie, T. Zhu, Y. Morsi, J. Zhao and X. Mo, Bioact. Mater., 2021, 6, 2783–2800 CrossRef CAS PubMed.
- F. Deng, Z. Bu, H. Hu, X. Huang, Z. Liu and C. Ning, Appl. Mater. Today, 2022, 27, 101433 CrossRef.
- X. Wang, M. Shan, S. Zhang, X. Chen, W. Liu, J. Chen and X. Liu, Adv. Sci., 2022, 9, 2104843 CrossRef CAS.
- S. S. Said, S. Campbell and T. Hoare, Chem. Mater., 2019, 31, 4971–4989 CrossRef CAS.
- P. Lavrador, M. Esteves, V. Gaspar and J. Mano, Adv. Funct. Mater., 2021, 31, 2005941 CrossRef CAS.
- Z. Fu, L. Ouyang, R. Xu, Y. Yang and W. Sun, Mater. Today, 2022, 52, 112–132 CrossRef CAS.
- B. Ortiz-Casas, A. Galdámez-Martínez, J. Gutiérrez-Flores, A. Ibañez, P. Panda, G. Santana, H. A. D. L. Vega, M. Suar, C. Rodelo, A. Kaushik, Y. Mishra and A. Dutt, Mater. Today, 2021, 50, 533–569 CrossRef CAS.
- H. Fatima, Z. Jin, Z. Shao and X. Chen, J. Colloid Interface Sci., 2022, 621, 440–463 CrossRef CAS PubMed.
- D. Wang, X. Yang, H. Yu, J. Gu, D. Qi, J. Yao and Q. Ni, J. Hazard. Mater., 2020, 383, 121123 CrossRef CAS PubMed.
- S. Chen, Z. Huang, M. Yuan, G. Huang, H. Guo, G. Meng, Z. Feng and P. Zhang, J. Mater. Sci. Technol., 2022, 125, 67–80 CrossRef.
- Y. Li, Y. Li, Y. Bai, R. Wang, L. Lin and Y. Sun, RSC Adv., 2020, 10, 38416–38423 RSC.
- P. Sivakumar, M. Lee, Y. Kim and M. Shim, J. Mater. Chem. B, 2018, 6, 4852–4871 RSC.
- N. Chauhan, S. Gupta, D. Avasthi, R. Adelung, Y. Mishra and U. Jain, ACS Appl. Mater. Interfaces, 2018, 10, 30631–30639 CrossRef CAS.
- X. Miao, F. Yu, K. Liu, Z. Lv, J. Deng, T. Wu, X. Cheng, W. Zhang, X. Cheng and X. Wang, Bioact. Mater., 2022, 7, 181–191 CrossRef CAS.
- S. Cao, Y. Xia, J. Shao, B. Guo, Y. Dong, I. Pijpers, Z. Zhong, F. Meng, L. K. E. A. Abdelmohsen, D. Williams and J. Hest, Angew. Chem., Int. Ed., 2021, 60, 17629–17637 CrossRef CAS PubMed.
- C. Chu, J. Yu, E. Ren, S. Ou, Y. Zhang, Y. Wu, H. Wu, Y. Zhang, J. Zhu, Q. Dai, X. Wang, Q. Zhao, W. Li, Z. Liu, X. Chen and G. Liu, Adv. Sci., 2020, 7, 2000346 CrossRef CAS.
- X. Ge, H. Cui, J. Kong, S. Lu, R. Zhan, J. Gao, Y. Xu, S. Lin, K. Meng, L. Zu, S. Guo and L. Zheng, Adv. Mater., 2020, 32, 2000037 CrossRef CAS PubMed.
- M. Zhu, H. Zhang, G. Ran, Y. Yao, Z. Yang, Y. Ning, Y. Yu, R. Zhang, X. Peng, J. Wu, Z. Jiang, W. Zhang, B. Wang, S. Gao and J. Zhang, Angew. Chem., Int. Ed., 2022, 61, e202204330 CAS.
- H. P. Lee and A. K. Gaharwar, Adv. Sci., 2020, 7, 2000863 CrossRef CAS PubMed.
- Z. Youssef, R. Vanderesse, L. Colombeau, F. Baros, T. Roques-Carmes, C. Frochot, H. Wahab, J. Toufaily, T. Hamieh, S. Acherar and A. Gazzali, Cancer Nanotechnol., 2017, 8, 1–62 CrossRef.
- S. Hackenberg, A. Scherzed, M. Kessler, K. Froelich, C. Ginzkey, C. Koehler, M. Burghartz, R. Hagen and N. Kleinsasser, Int. J. Oncol., 2010, 37, 1583–1590 CAS.
- S. Noimark, J. Weiner, N. Noor, E. Allan, C. Williams, M. Shaffer and I. Parkin, Adv. Funct. Mater., 2015, 25, 1367–1373 CrossRef CAS.
- A. Ancona, B. Dumontel, N. Garino, B. Demarco, D. Chatzitheodoridou, W. Fazzini, H. Engelke and V. Cauda, Nanomaterials, 2018, 8, 143 CrossRef.
- X. Wang, S. Xu, E. Chalmers, X. Chen, Y. Liu and X. Liu, ACS Appl. Mater. Interfaces, 2022, 14, 10769–10781 CrossRef CAS.
- A. Holmes, Z. Song, H. Moghimi and M. Roberts, ACS Nano, 2016, 10, 1810–1819 CrossRef CAS.
- A. Ginzburg, R. S. Blackburn, C. Santillan, L. Truong, R. Tanguay and J. Hutchison, Photochem. Photobiol. Sci., 2021, 20, 1273–1285 CrossRef CAS.
- M. Kciuk, B. Marciniak, M. Mojzych and R. Kontek, Int. J. Mol. Sci., 2020, 21, 7264 CrossRef CAS.
- L. Siebert, E. Luna-Cerón, L. García-River, J. S. Oh, J. Jang, D. Rosas-Gómez, M. Pérez-Gómez, G. Maschkowitz, H. Fickenscher, D. Oceguera-Cuevas, C. Holguín-León, B. Byambaa, M. Hussain, E. Enciso-Martínez, M. Cho, Y. Lee, N. Sobahi, A. Hasan, D. Orgill, Y. Mishr, R. Adelung, E. Lee and S. Shin, Adv. Funct. Mater., 2021, 31, 2007555 CrossRef CAS.
- C. Shuai, X. Yuan, Y. Shuai, G. Qian, J. Yao, W. Xu, S. Peng and W. Yang, Mater. Today Nano, 2022, 18, 100210 CrossRef CAS.
- H. Fu, Z. Feng, S. Liu, P. Wang, C. Zhao and C. Wang, Chin. Chem. Lett., 2022 DOI:10.1016/j.cclet.2022.04.023.
- X. Guo, J. Duan, W. Wang and Z. Zhang, Fuel, 2020, 280, 118544 CrossRef CAS.
- Y. Dong, Z. Liu, Y. Ning, S. Armes and D. Li, Chem. Mater., 2022, 34, 3357–3364 CrossRef CAS.
- W. Jiang, J. Low, K. Mao, D. Duan, S. Chen, W. Liu, C. Pao, J. Ma, S. Sang, C. Shu, X. Zhan, Z. Qi, H. Zhang, Z. Liu, X. Wu, R. Long, L. Song and Y. Xiong, J. Am. Chem. Soc., 2021, 143, 269–278 CrossRef CAS PubMed.
- M. Rani, J. Yadav, K. Shu and U. Shanker, J. Colloid Interface Sci., 2021, 601, 689–703 CrossRef CAS PubMed.
- Y. Zhao, L. Xu, L. Tan, D. Li and R. Qiao, Mater. Lett., 2021, 293, 129719 CrossRef CAS.
- Y. Jiang, Z. Xiong, J. Huang, F. Yan, G. Yao and B. Lai, Chin. Chem. Lett., 2022, 33, 415–423 CrossRef CAS.
- P. Sánchez-Cid, C. Jaramillo-Páez, J. A. Navío, A. N. Martín-Gómez and M. C. Hidalgo, J. Photochem. Photobiol., A, 2019, 369, 119–132 CrossRef.
- S. Ansari, M. Khan, S. Kalathil, A. Nisar, J. Lee and M. Cho, Nanoscale, 2013, 5, 9238–9246 RSC.
- M. Arakha, J. Roy, P. S. Nayak, B. Mallick and S. Jha, Free Radicals Biol. Med., 2017, 110, 42–53 CrossRef CAS.
- L. Sun, Y. Guo, Y. Hu, S. Pan and Z. Jiao, Sens. Actuators, B, 2021, 337, 129793 CrossRef CAS.
- X. Yu, H. Chen, Q. Ji, Y. Chen, Y. Wei, N. Zhao and B. Yao, Chemosphere, 2021, 267, 129285 CrossRef CAS PubMed.
- C. Zhang, Y. Yi, H. Yang, Z. Yi, X. Chen, Z. Zhou, Y. Yi, H. Li, J. Chen and C. Liu, Appl. Mater. Today, 2022, 28, 101531 CrossRef.
- J. Gupta and D. Bahadur, ACS Sustainable Chem. Eng., 2017, 5, 8702–8709 CrossRef CAS.
- S. Zhao, Y. Xu, W. Xu, Z. Weng, F. Cao, X. Wan, T. Cui, Y. Yu, L. Liao and X. Wang, ACS Appl. Mater. Interfaces, 2020, 12, 30044–30051 CrossRef CAS.
- Z. Weng, F. Yu, Q. Leng, S. Zhao, Y. Xu, W. Zhang, Z. Zhu, J. Ye, Q. Wei and X. Wang, Mater. Sci. Eng., C, 2021, 124, 112066 CrossRef CAS PubMed.
- S. Zhao, X. Yang, Y. Xu, Z. Weng, L. Liao and X. Wang, Nano Res., 2022, 15, 5245–5255 CrossRef CAS.
- H. Cheng, H. Liu, Z. Liu, Z. Xu, X. Liu, S. Jia, C. He, S. Liu, J. Zhang and X. Wang, Nano Res., 2022, 15, 6297–6305 CrossRef.
- Y. Sun, W. Zhang, M. Wang, H. Liu, Q. Li, J. Luo, M. Zhao, S. Liu and X. Wang, Nano Res., 2022 DOI:10.1007/s12274-022-4744-1.
- B. Huang, X. Liu, Z. Li, Y. Zheng, K. W. K. Yeung, Z. Cui, Y. Liang, S. Zhu and S. Wu, Chem. Eng. J., 2021, 414, 128805 CrossRef CAS.
- H. Yang, Q. Zhang, Y. Chen, Y. Huang, F. Yang and Z. Lu, Carbohydr. Polym., 2018, 201, 162–171 CrossRef CAS.
- N. Yu, H. Peng, L. Qiu, R. Wang, C. Jiang, T. Cai, Y. Sun, Y. Li and H. Xiong, Int. J. Biol. Macromol., 2019, 141, 207–217 CrossRef CAS PubMed.
- S. Zhang, Y. Li, Z. Li, G. Wang, A. Liao, J. Wang, H. Li, Z. Gu, B. Y. Cheng and X. Zhang, Small, 2022, 18, 2200038 CrossRef CAS PubMed.
- M. Zhang, W. Wang, M. Mohammadniaei, T. Zheng, Q. Zhang, J. Ashley, S. Liu, Y. Sun and B. Tang, Adv. Mater., 2021, 33, 2008802 CrossRef CAS PubMed.
- M. Liu, Y. Peng, Y. Nie, P. Liu, S. Hu, J. Ding and W. Zhou, Acta Biomater., 2020, 110, 242–253 CrossRef CAS PubMed.
- K. Vimala, K. Shanthi, S. Sundarraj and S. Kannan, J. Colloid Interface Sci., 2017, 488, 92–108 CrossRef CAS PubMed.
- X. Fan, F. Yang, J. Huang, Y. Yang, C. Nie, W. Zhao, L. Ma, C. Cheng, C. Zhao and R. Haag, Nano Lett., 2019, 19, 5885–5896 CrossRef CAS.
- J. Fang, Y. Wan, Y. Sun, X. Sun, M. Qi, S. Cheng, C. Li, Y. Zhou, L. Xu, B. Dong and L. Wang, Chem. Eng. J., 2022, 435, 134935 CrossRef CAS.
- J. Zou, Z. Yin, P. Wang, D. Chen, J. Shao, Q. Zhang, L. Sun, W. Huang and X. Dong, Chem. Sci., 2018, 9, 2188–2194 RSC.
- C. Mao, Y. Xiang, X. Liu, Y. Zheng, K. Yeung, Z. Cui, X. Yang, Z. Li, Y. Liang, S. Zhu and S. Wu, ACS Appl. Mater. Interfaces, 2019, 11, 17902–17914 CrossRef CAS.
- Q. Lei, D. He, L. Ding, F. Kong, P. He, J. Huang, J. Guo, C. J. Brinker, G. Luo, W. Zhu and Y. Yu, Adv. Funct. Mater., 2022, 32, 2113269 CrossRef CAS.
- J. Li, X. Liu, L. Tan, Y. Liang, Z. Cui, X. Yang, S. Zhu, Z. Li, Y. Zheng, K. Yeung, X. Wang and S. Wu, Small Methods, 2019, 3, 1900048 CrossRef.
- Y. Xiang, C. Mao, X. Liu, Z. Cui, D. Jing, X. Yang, Y. Liang, Z. Li, S. Zhu, Y. Zheng, K. Yeung, D. Zheng, X. Wang and S. Wu, Small, 2019, 15, 1900322 CrossRef PubMed.
- Q. Yao, J. Fan, S. Long, X. Zhao, H. Li, J. Du, K. Shao and X. Peng, Chem, 2022, 8, 197–209 CAS.
- W. Chen, C. He, N. Qiao, Z. Guo, S. Hu, Y. Song, H. Wang, Z. Zhang, B. Ke and X. Sun, Biomaterials, 2022, 286, 121582 CrossRef CAS PubMed.
- W. Xiu, L. Wan, K. Yang, X. Li, L. Yuwen, H. Dong, Y. Mou, D. Yang and L. Wang, Nat. Commun., 2022, 13, 3875 CrossRef CAS PubMed.
- W. Huang, F. Wang, A. Shen, L. Zhang, X. Nie, Z. Zhang, G. Chen, L. Xia, L. Wang, S. Ding, Q. Meng, W. Zhang, C. Hong and Y. You, Mater. Horiz., 2021, 8, 645–645 RSC.
- F. Dong, Q. Jiang, L. Li, T. Liu, S. Zuo, L. Gao, M. Fang, Y. Gao, B. Sun, C. Luo, Z. He and J. Sun, Nano Res., 2022, 15, 3422–3433 CrossRef CAS.
- Y. Chen, M. Du, Z. Yuan, Z. Chen and F. Yan, Nat. Commun., 2022, 13, 4468 CrossRef CAS PubMed.
- H. Jiang, N. M. Carter, A. Zareei, S. Nejati, J. Waimin, S. Chittiboyina, E. Niedert, T. Soleimani, S. Lelièvre, C. Goergen and R. Rahim, ACS Appl. Bio Mater., 2020, 3, 4012–4024 CrossRef CAS.
- K. Bian, W. Yang, Y. Xu, W. Zeng, H. Wang, H. Liang, T. Cui, Z. Wang and B. Zhang, Small, 2022, 18, 2202921 CrossRef CAS PubMed.
- X. Wang, M. Wu, H. Li, J. Jiang, S. Zhou, W. Chen, C. Xie, X. Zhen and X. Jiang, Adv. Sci., 2022, 9, 2104125 CrossRef CAS PubMed.
- G. Wang, C. Zhang, Y. Jiang, Y. Song, J. Chen, Y. Sun, Q. Li, Z. Zhou, Y. Shen and P. Huang, Adv. Funct. Mater., 2021, 31, 202102786 Search PubMed.
- A. Athanassiadis, Z. Ma, N. Moreno-Gomez, K. Melde, E. Choi, R. Goyal and P. Fischer, Chem. Rev., 2022, 122, 5165–5208 CrossRef CAS PubMed.
- Z. Cao, G. Yuan, L. Zeng, L. Bai, X. Liu, M. Wu, R. Sun, Z. Chen, Y. Jiang, Q. Gao, Y. Chen, Y. Zhang, Y. Pan and J. Wang, ACS Nano, 2022, 16, 10608–10622 CrossRef CAS PubMed.
- D. Wu, J. Li, S. Xu, Q. Xie, Y. Pan, X. Liu, R. Ma, H. Zheng, M. Gao, W. Wang, J. Li, X. Cai, F. Jaouen and R. Li, J. Am. Chem. Soc., 2020, 142, 19602–19610 CrossRef CAS PubMed.
- S. Shaikh, M. Younis, F. Rehman, H. Jiang and X. Wang, Langmuir, 2020, 36, 9472–9480 CrossRef CAS PubMed.
- W. Chen, C. Liu, X. Ji, J. Joseph, Z. Tang, J. Ouyang, Y. Xiao, N. Kong, N. Joshi, O. Farokhzad, W. Tao and T. Xie, Angew. Chem., Int. Ed., 2022, 440, 135812 Search PubMed.
- C. Lops, A. Ancona, K. D. Cesare, B. Dumontel, N. Garino, G. Canavese, S. Hérnandez and V. Cauda, Appl. Catal., B, 2019, 243, 629–640 CrossRef CAS.
- R. Mahdavi and S. Talesh, Ultrason. Sonochem., 2019, 51, 230–240 CrossRef CAS.
- X. Wang, X. Zhong, L. Bai, J. Xu, F. Gong, Z. Dong, Z. Yang, Z. Zeng, Z. Liu and L. Cheng, J. Am. Chem. Soc., 2020, 142, 6527–6537 CrossRef CAS PubMed.
- Y. Xu, S. Zhao, Z. Weng, W. Zhang, X. Wan, T. Cui, J. Ye, L. Liao and X. Wang, ACS Appl. Mater. Interfaces, 2020, 12, 54497–54506 CrossRef CAS.
- J. Sei and T. J. Webster, Nanotechnology, 2012, 23, 495101 CrossRef PubMed.
- K. Barick, S. Nigam and D. Bahadur, J. Mater. Chem., 2010, 20, 6446–6452 RSC.
- B. Geng, S. Xu, P. Li, X. Li, F. Fang, D. Pan and L. Shen, Small, 2022, 18, 2103528 CrossRef CAS PubMed.
- X. Pan, N. Wu, S. Tian, J. Guo, C. Wang, Y. Sun, Z. Huang, F. Chen, Q. Wu, Y. Jing, Z. Yin, B. Zhao, X. Xiong, H. Liu and D. Zhou, Adv. Funct. Mater., 2022, 32, 2112145 CrossRef CAS.
- P. Gholami, L. Dinpazhoh, A. Khataee and Y. Orooji, Ultrason. Sonochem., 2019, 55, 44–56 CrossRef CAS PubMed.
- D. Xiang, Z. Liu, M. Wu, H. Liu, X. Zhang, Z. Wang, Z. Wang and L. Li, Small, 2020, 16, 1907603 CrossRef CAS PubMed.
- Y. Liu, Y. Wang, W. Zhen, Y. Wang, S. Zhang, Y. Zhao, S. Song, Z. Wu and H. Zhang, Biomaterials, 2020, 251, 120075 CrossRef CAS PubMed.
- Q. Hoang, V. Ravichandran, T. Cao, J. Kang, Y. Ko, T. Lee and M. Shim, Chem. Eng. J., 2022, 435, 135039 CrossRef.
- H. Fukui, R. Chow, J. Xie, Y. Foo, C. Yap, N. Minc, N. Mochizuki and J. Vermot, Science, 2021, 374, 351–354 CrossRef CAS PubMed.
- J. Frith, G. Kusuma, J. Carthew, F. Li, N. Cloonan, G. Gomez and J. Cooper-White, Nat. Commun., 2018, 9, 257 CrossRef PubMed.
- M. Wu, Z. Zhang, Z. Liu, J. Zhang, Y. Zhang, Y. Ding, T. Huang, D. Xiang, Z. Wang, Y. Dai, X. Wan, S. Wang, H. Qian, Q. Sun and L. Li, Nano Today, 2021, 37, 101104 CrossRef CAS.
- N. Hao, Z. Xu, Y. Nie, C. Jin, A. Closson, M. Zhang and J. Zhang, Chem. Eng. J., 2019, 378, 122222 CrossRef CAS PubMed.
- T. Lee, D. Kim, M. E. Suk, G. Bang, J. Choi, J. Bae, J. Yoon, W. Moon and D. Choi, Adv. Funct. Mater., 2021, 31, 2104372 CrossRef CAS.
- J. Marmolejo-Tejada, J. Roche-Yepes, C. Pérez-López, J. Taborda, A. Ávila and A. Jaramillo-Botero, J. Chem. Inf. Model., 2021, 61, 4537–4543 CrossRef CAS PubMed.
- R. Augustine, P. Dan, A. Sosnik, N. Kalarikkal, N. Tran, B. Vincent, S. Thomas, P. Menu and D. Rouxel, Nano Res., 2017, 10, 3358–3376 CrossRef CAS.
- Z. Wang, J. Wang, J. Ayarza, T. Steeves, Z. Hu, S. Manna and A. Esser-Kahn, Nat. Mater., 2021, 20, 869–874 CrossRef CAS PubMed.
- O. Careta, J. Fornell, E. Pellicer, E. Ibañez, A. Blanquer, J. Esteve, J. Sort, G. Murillo and C. Nogués, Biomedicines, 2021, 9, 352 CrossRef CAS PubMed.
- S. Bhang, W. Jang, J. Han, J. Yoon, W. La, E. Lee, Y. Kim, J. Shin, T. Lee, H. Baik and B. Kim, Adv. Funct. Mater., 2017, 27, 1603497 CrossRef.
- J. Yoon, M. Misra, S. Yu, H. Kim, S. Bhang, S. Song, J. Lee, S. Ryu, Y. Choo, G. Jeong, S. Kwon, S. Im, T. Lee and B. Kim, Adv. Funct. Mater., 2017, 27, 1703853 CrossRef.
- Y. Qian, Y. Cheng, J. Song, Y. Xu, W. Yuan, C. Fan and X. Zheng, Small, 2020, 16, 2000796 CrossRef CAS PubMed.
- G. Murillo, A. Blanquer, C. Vargas-Estevez, L. Barrios, E. Ibáñez, C. Nogués and J. Esteve, Adv. Mater., 2017, 29, 1605048 CrossRef PubMed.
- Y. Li, L. Sun and T. Webster, J. Biomed. Nanotechnol., 2018, 14, 536–545 CrossRef CAS PubMed.
- J. Liang, H. Zeng, L. Qiao, H. Jiang, Q. Ye, Z. Wang, B. Liu and Z. Fan, ACS Appl. Mater. Interfaces, 2022, 14, 30507–30522 CrossRef CAS PubMed.
- E. Bratanis, T. Andersson, R. Lood and E. Bukowska-Faniband, Front. Microbiol., 2020, 11, 662 CrossRef PubMed.
- Y. Tang, Q. Huang, D. Zheng, Y. Chen, L. Ma, C. Huang and X. Zhang, Mater. Today, 2022, 53, 71–83 CrossRef CAS.
- N. Gogurla, B. Roy and S. Kim, Nano Energy, 2020, 77, 105242 CrossRef CAS.
- Y. Tan, K. Yang, B. Wang, H. Li, L. Wang and C. Wang, Nano Res., 2021, 14, 3969–3976 CrossRef CAS.
- G. Lee, J. H. Son, S. Lee, S. Kim, D. Kim, N. Nguyen, S. Lee and K. Cho, Adv. Sci., 2021, 8, 2002606 CrossRef CAS PubMed.
- Y. Wang, H. Zhao, J. Cheng, B. Liu, Q. Fu and Y. Wang, Nanomicro Lett., 2022, 14, 76 CrossRef CAS.
- Y. Song, F. Yin, C. Zhang, W. Guo, L. Han and Y. Yuan, Nanomicro Lett., 2021, 13, 76 Search PubMed.
- H. Peng, B. Cui, G. Li, Y. Wang, N. Li, Z. Chang and Y. Wang, Mater. Sci. Eng., C, 2015, 46, 253–263 CrossRef CAS PubMed.
- J. Wang, Z. Jia, X. Liu, J. Dou, B. Xu, B. Wang and G. Wu, Nano-Micro Lett., 2021, 13, 175 CrossRef CAS PubMed.
- H. Peng, C. Hu, J. Hu, X. Tian and T. Wu, Microporous Mesoporous Mater., 2016, 226, 140–145 CrossRef CAS.
- Z. Sun, C. Song, C. Wang, Y. Hu and J. Wu, Mol. Pharm., 2020, 17, 373–391 CAS.
- M. Carofiglio, M. Laurenti, V. Vighetto, L. Racca, S. Barui, N. Garino, R. Gerbaldo, F. Laviano and V. Cauda, Nanomaterials, 2021, 11, 2628 CrossRef CAS.
- A. B. Cook and P. Decuzzi, ACS Nano, 2021, 15, 2068–2098 CrossRef CAS PubMed.
- H. Dai, T. Sun, T. Han, Z. Guo, X. Wang and Y. Chen, Environ. Res., 2020, 191, 110086 CrossRef CAS PubMed.
- J. Wang, C. Qu, X. Shao, G. Song, J. Sun, D. Shi, R. Jia, H. An and H. Wang, Bioact. Mater., 2023, 20, 404–417 CrossRef CAS PubMed.
- H. Yang, Y. Ding, Z. Tong, X. Qian, H. Xu, F. Lin, G. Sheng, L. Hong, W. Wang and Z. Mao, Theranostics, 2022, 12, 4250–4268 CrossRef CAS PubMed.
- S. Zhang, J. Lv, P. Gao, Q. Feng, H. Wang and Y. Cheng, Nano Lett., 2021, 21, 7855–7861 CrossRef CAS PubMed.
- J. Liu, Y. Kang, S. Yin, A. Chen, J. Wu, H. Liang and L. Shao, Small, 2019, 15, 1901073 CrossRef PubMed.
- Q. Yuan, S. Hein and R. D. K. Misra, Acta Biomater., 2010, 6, 2732–2739 CrossRef CAS PubMed.
- N. Tripathy, R. Ahmad, H. Ko, G. Khang and Y. Hahn, Nanoscale, 2015, 7, 4088–4096 RSC.
- F. Muhammad, M. Guo, W. Qi, F. Sun, A. Wang, Y. Guo and G. Zhu, J. Am. Chem. Soc., 2011, 133, 8778–8781 CrossRef CAS PubMed.
- J. Zhang, D. Wu, M. Li and J. Feng, ACS Appl. Mater. Interfaces, 2015, 7, 26666–26673 CrossRef CAS PubMed.
- X. Huang, S. Wu and X. Du, Carbon, 2016, 101, 135–142 CrossRef CAS.
- X. Xiao, S. Liang, Y. Zhao, D. Huang, B. Xing, Z. Cheng and J. Lin, Nanoscale, 2020, 12, 3846–3854 RSC.
- R. Dhivya, M. Rajasekaran, J. Annaraj, J. Ranjani and J. Rajendhran, Adv. Mater. Lett., 2015, 6, 505–512 CrossRef CAS.
- M. Kundu, P. Sadhukhan, N. Ghosh, S. Chatterjee, P. Manna, J. Das and P. Sil, J. Adv. Res., 2019, 18, 161–172 CrossRef CAS PubMed.
- X. Cai, Y. Luo, W. Zhang, D. Du and Y. Lin, ACS Appl. Mater. Interfaces, 2016, 8, 22442–22450 CrossRef CAS PubMed.
- D. Depan and R. Misra, J. Biomed. Mater. Res., 2014, 102, 2934–2941 CrossRef CAS.
- F. Muhammad, M. Guo, Y. Guo, W. Qi, F. Qu, F. Sun, H. Zhao and G. Zhu, J. Mater. Chem., 2011, 21, 13406–13412 RSC.
- D. Ye, Y. Ma, W. Zhao, H. Cao, J. Kong, H. Xiong and H. Möhwald, ACS Nano, 2016, 10, 4294–4300 CrossRef CAS PubMed.
- Y. Wang, S. Song, J. Liu, D. Liu and H. Zhang, Angew. Chem., Int. Ed., 2015, 54, 536–540 CAS.
- X. Cai, Y. Luo, H. Yan, D. Du and Y. Lin, ACS Appl. Mater. Interfaces, 2017, 9, 5739–5747 CrossRef CAS PubMed.
- X. Chen, T. Niu, Y. Gao, X. Liang, S. Li, L. Zhang, L. Li, T. Wang, Z. Su and C. Wang, Chem. Eng. J., 2019, 371, 443–451 CrossRef CAS.
- J. Wang, J. Lee, D. Kim and L. Zhu, ACS Appl. Mater. Interfaces, 2017, 9, 39971–39984 CrossRef CAS PubMed.
- T. Cui, Z. Yan, H. Qin, Y. Sun, J. Ren and X. Qu, Small, 2019, 15, 1903323 CrossRef CAS PubMed.
- J. Wang, S. Yu, Q. Wu, X. Gong, S. He, J. Shang, X. Liu and F. Wang, Angew. Chem., Int. Ed., 2021, 60, 10766–10774 CrossRef CAS PubMed.
- W. Liang, J. Cheng, J. Zhang, Q. Xiong, M. Jin and J. Zhao, ACS Nano, 2022, 16, 2762–2773 CrossRef CAS PubMed.
- Y. Huang, Q. Gao, C. Li, X. Chen, X. Li, Y. He, Q. Jin and J. Ji, Adv. Funct. Mater., 2022, 32, 2109011 CrossRef CAS.
- P. Qiu, M. Huang, S. Wu, M. Wen, N. Yu and Z. Chen, ACS Appl. Mater. Interfaces, 2022, 14, 29537–29549 CrossRef CAS PubMed.
- M. Yi, F. Wang, W. Tan, J. Hsieh, E. Egelman and B. Xu, J. Am. Chem. Soc., 2022, 144, 13055–13059 CrossRef CAS PubMed.
- L. Qiu, W. Zhang, S. Wang, X. Zhang, Y. Zhao, L. Cao and L. Sun, Mater. Sci. Eng., C, 2017, 81, 485–491 CrossRef CAS.
- L. Luo, D. D. Nguyen, C. Huang and J. Lai, Chem. Eng. J., 2022, 429, 132409 CrossRef CAS.
- X. Zhou, S. Wang, C. Zhang, Y. Lin, J. Lv, S. Hu, S. Zhang and M. Li, Mikrochim. Acta, 2021, 188, 56 CrossRef CAS PubMed.
- L. Feng and Y. Zhao, View, 2020, 1, e15 CrossRef.
- J. Chen, F. Xue, W. Du, H. Yu, Z. Yang, Q. Du and H. Chen, Nano Lett., 2022, 22, 6156–6165 CrossRef CAS PubMed.
- L. Zhu, K. Yuan, J. Yang, C. Hang, H. Ma, X. Ji, A. Devi, H. Lu and D. Zhang, Microsyst. Nanoeng., 2020, 6, 30 CrossRef CAS PubMed.
- T. Hang, J. Wu, S. Xiao, B. Li, H. Li, C. Yang, C. Yang, N. Hu, Y. Xu, Y. Zhang and X. Xie, Microsyst. Nanoeng., 2020, 6, 41 CrossRef CAS PubMed.
- M. Salimi and S. Hosseini, Sens. Actuators, B, 2021, 344, 130127 CrossRef CAS.
- X. Li, C. Luo, Q. Fu, C. Zhou, M. Ruelas, Y. Wang, J. He, Y. Wang, Y. Zhang and J. Zhou, Adv. Mater., 2020, 32, 2000060 CrossRef CAS PubMed.
- X. Pan, Y. Qi, Z. Du, J. He, S. Yao, W. Lu, K. Ding and M. Zhou, J. Nanobiotechnol., 2021, 19, 392 CrossRef CAS PubMed.
- H. Di, Z. Mi, Y. Sun, X. Liu, X. Liu, A. Li, Y. Jiang, H. Gao, P. Rong and D. Liu, Theranostics, 2020, 10, 9303–9314 CrossRef CAS PubMed.
- S. Zhou, T. Hu, G. Han, Y. Wu, X. Hua, J. Su, W. Jin, Y. Mou, X. Mou, Q. Li and S. Liu, Small, 2020, 10, e2004492 CrossRef PubMed.
- L. Ding, L. Liu, L. He, C. Y. Effah, R. Yang, D. Ouyang, N. Jian, X. Liu, Y. Wu and L. Qu, Anal. Chem., 2021, 93, 15200–15208 CrossRef CAS PubMed.
- C. Wu, J. Dai, X. Li, L. Gao, J. Wang, J. Liu, J. Zheng, X. Zhan, J. Chen, X. Cheng, M. Yang and J. Tang, Nat. Nanotechnol., 2021, 16, 288–295 CrossRef CAS PubMed.
- H. Zhang, Z. Li, C. Gao, X. Fan, Y. Pang, T. Li, Z. Wu, H. Xie and Q. He, Sci. Robot., 2021, 6, 9519 CrossRef PubMed.
- X. Shi, Y. Zhang, Y. Tian, S. Xu, E. Ren, S. Bai, X. Chen, C. Chu, Z. Xu and G. Liu, Small Methods, 2021, 5, 2000416 CrossRef CAS PubMed.
- X. Yi, W. Zeng, C. Wang, Y. Chen, L. Zheng, X. Zhu, Y. Ke, X. He, Y. Kuang and Q. Huang, Nano Res., 2022, 15, 1205–1212 CrossRef CAS.
- J. Ma, J. Ren, Y. Jia, Z. Wu, L. Chen, N. O. Haugen, H. Huang and Y. Liu, Nano Energy, 2019, 62, 376–383 CrossRef CAS.
- F. Shi, M. Ding, H. Tong, Y. Yang, J. Zhang, L. Wang, H. Li and Y. Huo, J. Environ. Chem. Eng., 2022, 10, 107385 CrossRef CAS.
- X. Xu, R. Zhang, X. Yang, Y. Lu, Z. Yang, M. Peng, Z. Ma, J. Jiao and L. Li, Adv. Healthcare Mater., 2021, 10, 2100518 CrossRef CAS PubMed.
- J. Shen, M. Ma, M. Shafiq, H. Yu, Z. Lan and H. Chen, Angew. Chem., Int. Ed., 2022, 61, e202113703 CAS.
- Q. Cai, D. Yang, L. Zhong and P. Yang, Chem. Mater., 2020, 32, 7492–7506 CrossRef CAS.
- C. Mao, Y. Xiang, X. Liu, Z. Cui, X. Yang, K. Yeung, H. Pan, X. Wang, P. Chu and S. Wu, ACS Nano, 2017, 11, 9010–9021 CrossRef CAS PubMed.
- Y. Liang, M. Wang, Z. Zhang, G. Ren, Y. Liu, S. Wu and J. Shen, Chem. Eng. J., 2019, 378, 122043 CrossRef CAS.
- X. Qu, M. Wang, Mi. Wang, H. Tang, S. Zhang, H. Yang, W. Yuan, Y. Wang, J. Yang and B. Yue, Adv. Mater., 2022, 34, 2200096 CrossRef CAS PubMed.
- J. Zhang, Y. Lin, Z. Lin, Q. Wei, J. Qian, R. Ruan, X. Jiang, L. Hou, J. Song, J. Ding and H. Yang, Adv. Sci., 2022, 9, 2103444 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |
