High-entropy-alloy nanoparticles synthesized by laser metallurgy using a multivariate MOF†
Wei
Yan‡
 ab,
Haoqing
Jiang‡
cd,
Wendi
Yi‡
d,
Chengbin
Zhao
b,
Yucong
Xia
b,
Hengjiang
Cong
ab,
Haoqing
Jiang‡
cd,
Wendi
Yi‡
d,
Chengbin
Zhao
b,
Yucong
Xia
b,
Hengjiang
Cong
 b,
Lin
Tang
*ac,
Gary J.
Cheng
b,
Lin
Tang
*ac,
Gary J.
Cheng
 *de,
Jianhua
He
*a and
Hexiang
Deng
*de,
Jianhua
He
*a and
Hexiang
Deng
 *abc
*abc
aThe Institute for Advanced Studies, Wuhan University, Wuhan 430072, China. E-mail: lintang@whu.edu.cn; hejianhua@whu.edu.cn; hdeng@whu.edu.cn
bCollege of Chemistry and Molecular Sciences, Wuhan University, Wuhan 430072, China
cHubei Yangtze Memory Laboratories, Wuhan 430205, China
dThe Institute of Technological Sciences, Wuhan University, Wuhan 430072, China
eSchool of Industrial Engineering, Purdue University, West Lafayette 47906, Indiana, USA. E-mail: gjcheng@purdue.edu
First published on 16th August 2022
Abstract
We report the synthesis of multivariate MOFs with combinations of metal ions, including Mn2+, Fe3+, Co2+, Ni2+, Cu2+ and Zn2+. The permanent porosity and the evenly distributed metal components make these porous crystals ideally suited for the synthesis of alloy nanoparticles. Using a laser, these MOFs are converted in air to alloy nanoparticles of binary (FeNi, FeCo), ternary (FeCoNi) and high-entropy-alloys with quinary (MnFeCoNiCu, MnFeCoNiZn) metals. One member, FeNi alloy nanoparticles with a narrow particle size distribution around 7 nm, shows a low overpotential of 282 mV at a current density of 10 mA cm−2 in the oxygen evolution reaction (OER), better than those of the single metal counterparts. This demonstrates the possibility of using MOFs to produce alloy nanoparticles with multiple metal components by a laser.
Combining various metals at atomic scale to form metal alloys is an effective way to explore their optical, magnetic and catalytic properties.1–3 The heterometals form a unique interface for the precise tuning of the Fermi energy, electron structure and work function beyond those of monometallic materials. This offers an opportunity to use alloys of natural abundant metals for the replacement of noble metals in industrial applications.4,5 It remains challenging, however, to obtain alloys with an even distribution of metals at the atomic scale, when the number of metal types becomes large. This is due to the difference of metals in their electronegativity, size and preferred packing in a lattice. Making alloys into nanoscale might possibly address the compatibility issue at the atomic scale. Typically, materials are regarded as high-entropy-alloy nanoparticles (HEANPs) when the number of metal components goes beyond 5.6–13 The formation of a high entropy compound usually involves a kinetically controlled process, with stringent requirement on the precursor and temperature.14,15
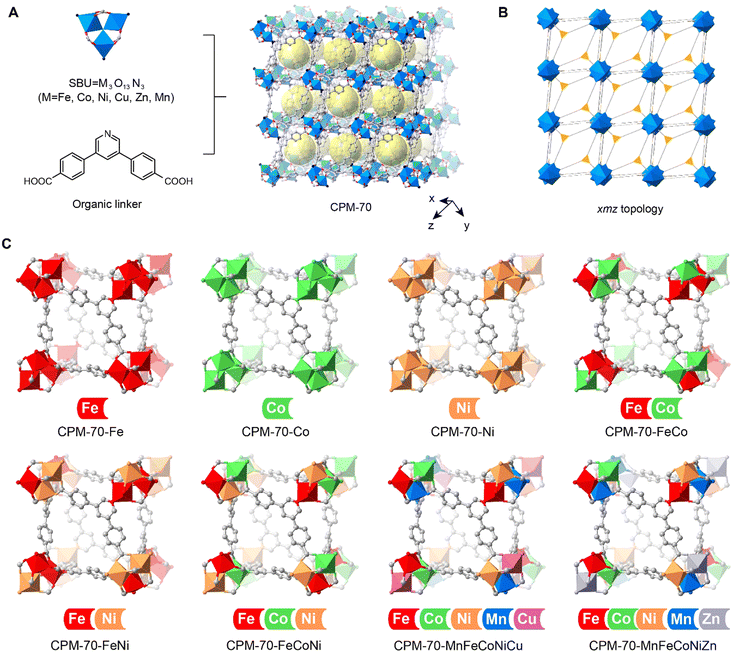
Metal–organic frameworks (MOFs) are porous crystals constructed by alternating arrangement of metal clusters and organic linkers,16,17 standing as ideal precursors with atomic precision of the metal distribution. The rich choices both in the metal clusters and the organic linkers leads to the emergence of multivariate metal–organic frameworks (MTV-MOFs), where different organic linkers and metals in the secondary building units (SBUs) can be introduced into the structure without altering the backbone.18,19 The customized pore environment in MTV-MOFs leads to excellent performances in various applications.20–22 As the precursor for the conversion to metal nanoparticles, the carbonaceous linkers adjacent to metal ions naturally produce a reductive environment for metallurgy.23 Theoretically, it's straight forward to obtain alloy nanoparticles from MTV-MOFs with multiple metal components. In fact, there are very few works reported using the MTV-MOF strategy for the fabrication of alloy nanoparticles,23–25 and the metal components have yet go beyond two types, due to the poor compatibility of various metal ions in the SBU of MTV-MOFs and/or the loss of permanent porosity. In this work, we show that a series of MTV-MOFs containing various metal ions can be applied as precursors for the production of alloy nanoparticles with up to 5 components. The corresponding alloy nanoparticles are generated by a recently developed strategy, laser metallurgy, where these MTV-MOFs are subjected to instant heating and cooling induced by a pulsed laser.23–27 The M3O13N3 SBUs with high connectivity of 9, and organic linker (4,4′-(pyridine-3,5-diyl)dibenzoic acid, PDA) with 3 connectivity sites are assembled into a series of isoreticular MOFs, CPM-70, with high stability and permanent porosity (Fig. 1A and B). We found that the trinucleate metal oxide clusters in CPM-70 exhibited excellent compatibility for various transition metals, such as Mn2+, Fe3+, Co2+, Ni2+, Cu2+ and Zn2+, leading to the successful fabrication of a series of MTV-MOFs containing unary, binary, ternary and quinary metals (Fig. 1C). When irradiated by a nanosecond pulsed laser, these MTV-MOF precursors are instantly converted to the corresponding metal nanoparticles, including high-entropy-alloy nanoparticles. The combination of the MTV-MOF strategy and the laser metallurgy method provides a new route for the synthesis of high-entropy-alloy nanoparticles in a fast and designable manner.
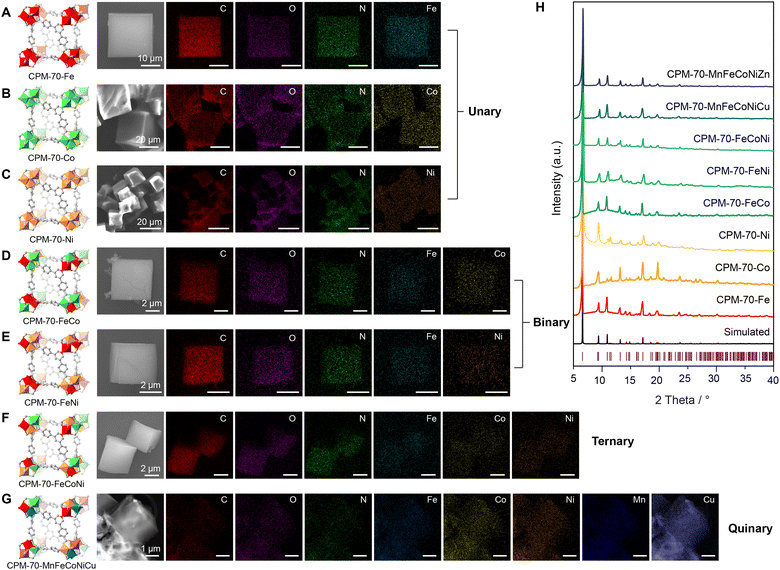
The synthesis of the isoreticular CPM-70 serial MOFs with various metal ions in the SBU was based on a reported solvothermal reaction with slight modifications.28–31 The PDA ligand was synthesized in a two-step reaction with a yield of 85% (Fig. S1–S7, ESI†). The isoreticular CPM-70 with designated metal ions in the SBU was obtained by mixing of the metal ions in the mother solvent. CPM-70 with unary metals, CPM-70-Fe, CPM-70-Co and CPM-70-Ni, were cubic crystals with size around 200 μm after solvothermal reaction of the metal ions and organic linker in DMF with an incubation time of 24 h at 150 °C (Fig. S8, ESI†). Single crystal X-ray diffraction (SCXRD) analysis of these MOF crystals showed that they had isoreticular xmz topology, and slightly varied cell parameters (Tables S1–S3, ESI†). The MTV-MOFs, such as CPM-70-MnFeCoNiCu crystals also appeared in cubic shape; however, they preserved a smaller size of around 5 μm (Fig. 2G).
The homogeneous dispersion of metal ions within MTV-MOFs was confirmed by energy-dispersive spectroscopy (EDS) in scanning electron microscopy (SEM) and transmission electron microscopy (TEM) (Fig. 2A–G and Fig. S9, ESI†). When binary and ternary metal ions were introduced in the CPM-70, such as Fe/Co, Fe/Ni, and Fe/Co/Ni, they showed identical elemental signals with C and N signals from the linker, implying that these metals were in a well-mixed manner and evenly dispersed across the MOF crystals. When the species of the metal ions increased to 5, Mn/Fe/Co/Ni/Zn or Mn/Fe/Co/Ni/Cu, for instance, they still maintained identical signal distribution with the C and N (Fig. 2G and Fig. S9, ESI†), suggesting that the trinucleate metal clusters had high compatibility with different metal ions. All the synthesized isoreticular MOFs exhibited identical powder X-ray diffraction (PXRD) patterns and matched well with the simulated one from the single crystal data (Fig. 2H), demonstrating their phase purities.
The permanent porosity of these MTV-MOFs was evaluated by nitrogen adsorption isotherms measured at 77 K. The guest molecules in the pores of these MOFs could be successfully removed by solvent exchange and supercritical CO2 activation without altering the crystallinity. This was proved by the identical PXRD patterns before and after activation (Fig. S10–S17, ESI†). High Brunauer–Emmett–Teller (BET) surface areas of 1930, 920, 1050, 1380, 1540, 1600, 1520, and 1380 m2 g−1 were observed in CPM-70-Fe, CPM-70-Co, CPM-70-Ni, CPM-70-FeCo, CPM-70-FeNi, CPM-70-FeCoNi, CPM-70-MnFeCoNiCu, and CPM-70-MnFeCoNiZn, respectively (Fig. S18–S25, ESI†). The high porosity in the MTV-MOFs benefits their conversion to alloy nanoparticles by laser metallurgy. These MTV-MOFs with high connectivity also exhibited high chemical stability, which could be revealed by the well-maintained PXRD patterns before and after immersing these MOF crystals in H2O, HCl (PH = 4) or NaOH (PH = 9) for 6 h (Fig. S26–S28, ESI†). Thermo-gravimetric analysis (TGA) uncovered that these MTV-MOFs also had high thermal stability and decomposed at around 400 °C in air (Fig. S29–S36, ESI†). The successful incorporation of various metal ions in the activated MOF precursor was analyzed by inductively coupled plasma atomic emission spectroscopy (ICP-AES) (Table S4, ESI†). When a feeding ratio of Mn/Fe/Co/Ni/Zn = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was applied in the mother solvent of the MOF, the obtained MOF exhibited a corresponding ratio of 1
1 was applied in the mother solvent of the MOF, the obtained MOF exhibited a corresponding ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.32
1.32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.54
1.54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.55
1.55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.49.
1.49.
The even dispersion of different metal ions in the MTV-MOF provides an ideal precursor for the production of uniform nanoalloys by ultrafast laser nanometallurgy.24,32 The operation of laser nanometallurgy for the conversion of the MTV-MOF to the corresponding nanoalloys was achieved by irradiation of the powdery MOF precursor sandwiched between two glass slides. The nanosecond pulsed laser was operated at a wavelength of 1064 nm, frequency of 20 kHz, pulse energy of 30 mJ, beam size of 200 μm, and scribing speed of 75 mm s−1. Once laser scribed, the pristine MOF layer instantly turned black, indicating the successful conversion of the MOF precursor to a carbonaceous support. A previous study revealed that the laser nanometallurgy method could convert the transition metal ions in the MOF to the corresponding MNPs because of the localized reductive atmosphere formed around the laser irradiated spots.24 In the case of MTV-MOF with various transition metal ions in the SBU, the instant heating and cooling should lead to the formation of nanoalloys without segregation into metallic phases.7 The laser processed product, denoted as CPM-70-M-laser (M represents the metal ions in the SBU), was collected and characterized. The SEM images showed that all the CPM-70-M-laser samples exhibited an amorphous and porous structure with nanoparticles embedded (Fig. S37–S44, ESI†).
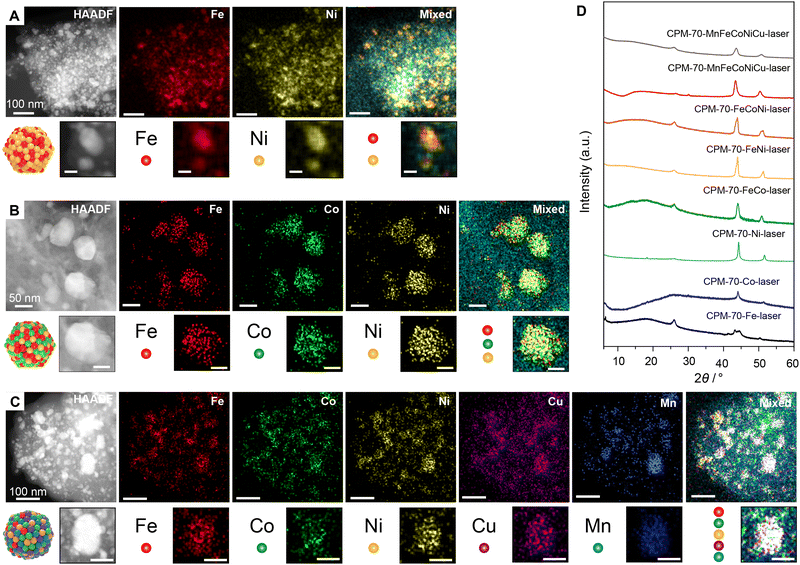
To demonstrate the well-mixed nature of different metals in the nanoalloys derived from these MTV-MOFs, elemental mappings were collected from energy dispersive spectroscopy (EDS) (Fig. 3A–C). The elemental mappings of the binary FeNi nanoalloy also confirmed the uniform distribution of Fe and Ni across the nanoparticles (Fig. 3A). The ternary FeCoNi nanoalloys also exhibited well mixed nature of the three elements across the nanoparticles (Fig. 3B). When the metal elements increased to five, in CPM-70-MnFeCoNiZn-laser for example, all the five metals showed similar distribution in each nanoparticle (Fig. 3C), suggesting the successful formation of HEANPs.7 The successful conversion of the MTV-MOF precursor to the nanoalloys was also proved by the PXRD patterns (Fig. 3D). No obvious peaks assigned to the pristine MOF were observed, suggesting their complete conversions. The broad peak at 2θ = 26° was ascribed the 001 facet of graphite and the two peaks located around 2θ = 45° and 52° were assigning to the facets of 110 and 200 of nanoalloys in fcc. No obvious peaks from the unary metals were observed in the product from MTV-MOF (Fig. S45, ESI†), demonstrating the well-mixed nature of the metals in the nanoparticles. Even encapsulated with 5 metals, the PXRD pattern of the CPM-70-MnFeCoNiCu-laser only preserved peaks assigned to fcc alloys, revealing the capability to produce HEANPs from MTV-MOF by laser metallurgy.
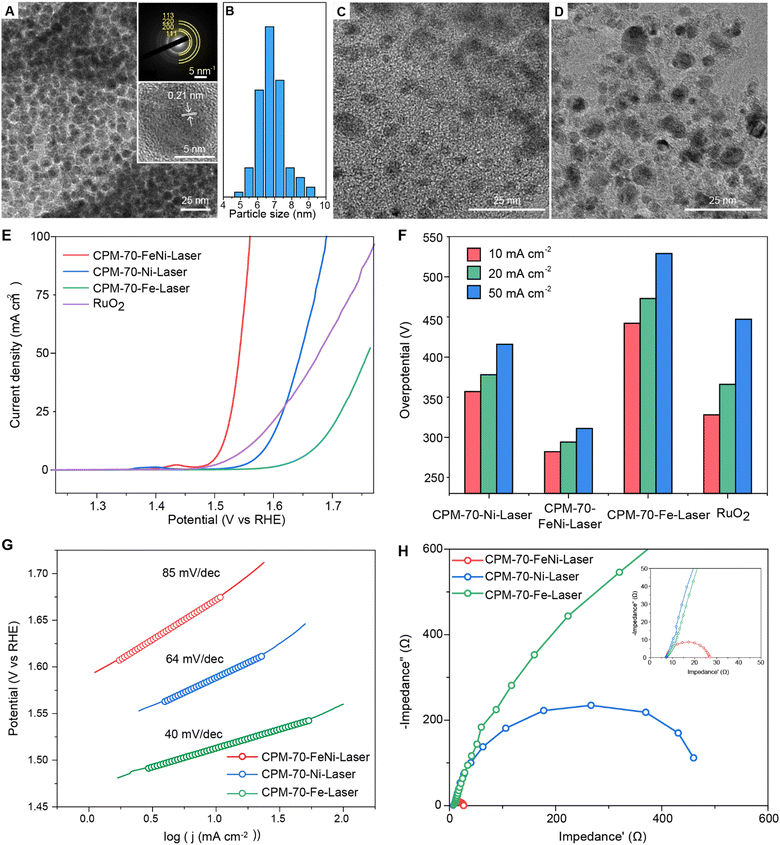
We found that uniform binary FeNi nanoalloys with an averaged size of 7 nm could be successfully synthesized by laser metallurgy from the CPM-70-FeNi precursor (Fig. 4A and B). The high-resolution TEM images revealed the lattice distance of 0.21 nm (Fig. 3A), consistent with the 111 lattice plane of the FeNi nanoalloy. The selected area electron diffraction (SAED) pattern of the FeNi nanoalloys also revealed their fcc phase. Notably, larger particles of FeC and Ni were observed from the corresponding unary MOF precursors (Fig. 4C and D), suggesting that the MTV-MOF strategy can regulate the morphology of the nanoparticles. The chemical state of the elements in the nanoalloys was analyzed by X-ray photoelectron spectroscopy (XPS) (Fig. S53, ESI†). We found that most of the transition metals, Mn, Fe, Co, Ni, Zn, and Cu, were in zero oxidation state with partial oxidation on the surface, consistent with previous reports.7 The existence of the carbonaceous support was proved by the Raman spectra of the CPM-70-M-laser samples (Fig. S54, ESI†). Two peaks located at Raman shifts of 1050 and 1350 cm−1 are ascribed to the D and G band of graphite.32 The existence of a peak at 2600 cm−1 suggested the formation of few-layered graphene in the product, which would benefit the electron transfer in electrochemical reactions.
Nanoalloys are widely used as catalysts in energy and environmental fields.4–6 The catalytic performance of the uniform FeNi nanoalloys from laser nanometallurgy was demonstrated in the oxygen evolution reaction (OER) for electrochemical water splitting. Linear sweep voltammetry (LSV) curves of laser-induced samples were recorded to evaluate their OER performance (Fig. 4E). The binary CPM-70-FeNi-laser exhibited the lowest potential at a fixed current density of 10 mA cm−2, smaller than that of CPM-70-Ni-laser, CPM-70-Fe-laser and the commercial RuO2 catalyst. The overpotentials measured at a current density of 10, 20 and 50 mA cm−2 are listed in Fig. 4F. The binary CPM-70-FeNi-laser catalyst delivered the smallest overpotential of 282 mV at a fixed current density of 10 mA cm−2, while CPM-70-Ni-laser, CPM-70-Fe-laser and RuO2 catalysts showed higher overpotentials of 357 mV, 442 mV and 328 mV, respectively, under identical conditions. A similar trend was found when the current density increased to 20 and 50 mA cm−2 (Fig. 4F). The Tafel curves of the samples were recorded to investigate the reaction kinetics of the catalysts (Fig. 4G). The binary CPM-70-FeNi-laser catalyst showed a smallest slope of 40 mV dec−1 among these samples, suggesting its fast oxygen evolution kinetics. Electrochemical impedance spectroscopy (EIS) was further performed at a fixed potential of 1.51 V vs. RHE to uncover the charge transfer impedance in the catalysts (Fig. 4H). The binary CPM-70-FeNi-laser catalyst exhibited the smallest charge transfer resistance (Rct) in the three catalysts, indicating the excellent electron transfer in the nanoalloy catalysts. The alloying strategy by laser nanometallurgy of MTV-MOF provides rich choices for the fabrication of nanoalloys with excellent catalytic performance.
In summary, the MTV-MOF strategy was successfully applied to incorporate various metal ions in a series of isoreticular MOFs for the synthesis of alloy nanoparticles. The high compatibility of the metal clusters in these MTV-MOFs provides exceptional dispersion of the metal ions in the precursor and the laser metallurgy method benefits the ultrafast reaction kinetics. We believe that this method will shed new light on the fabrication of HEANPs derived from MOFs because of the huge MOF family and their MTV-MOF variants.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the Core Research Facilities of College of Chemistry and Molecular Sciences for assistance with material characterizations. We acknowledge support from the National Natural Science Foundation of China (22025106 and 21971199), the National Key Research and Development Project (2018YFA0704000), the Innovation Team of Wuhan University (2042017kf0232) and the Postdoctoral innovation research positions in Hubei Province.References
- P. C. Chen, X. Liu, J. L. Hedrick, Z. Xie, S. Wang, Q. Y. Lin, M. C. Hersam, V. P. Dravid and C. A. Mirkin, A. Polyelemental nanoparticle libraries, Science, 2016, 352, 1565–1569 CrossRef CAS PubMed
.
- C. Xie, Z. Niu, D. Kim, M. Li and P. Yang, Surface and interface control in nanoparticle catalysis, Chem. Rev., 2019, 120, 1184–1249 CrossRef PubMed
.
- H. Duan, D. Wang and Y. Li, Green chemistry for nanoparticle synthesis, Chem. Soc. Rev., 2015, 44, 5778–5792 RSC
.
- X. Huang, Z. Zhao, L. Cao, Y. Chen, E. Zhu, Z. Lin, M. Li, A. Yan, A. Zettl, Y. M. Wang, X. Duan, T. Mueller and Y. Huang, High-performance transition metal-doped Pt3Ni octahedra for oxygen reduction reaction, Science, 2015, 348, 1230–1234 CrossRef CAS PubMed
.
- Q. L. Zhu, J. Li and Q. Xu, Immobilizing metal nanoparticles to metal-organic frameworks with size and location control for optimizing catalytic performance, J. Am. Chem. Soc., 2013, 135, 10210–10213 CrossRef CAS PubMed
.
- W. T. Koo, J. E. Millstone, P. S. Weiss and I. D. Kim, The design and science of polyelemental nanoparticles, ACS Nano, 2020, 14, 6407–6413 CrossRef CAS PubMed
.
- Y. Yao, Z. Huang, P. Xie, S. D. Lacey, R. J. Jacob, H. Xie, F. Chen, A. Nie, T. Pu, M. Rehwoldt, D. Yu, M. R. Zachariah, C. Wang, R. S. Yassar, J. Li and L. Hu, Carbothermal shock synthesis of high-entropy-alloy nanoparticles, Science, 2018, 359, 1489–1494 CrossRef CAS PubMed
.
- H. Jiang, X. Liu, M. N. Zhu, J. Xu, L. An, P. F. Sui, J. L. Luo and G. J. Cheng, Nanoalloy libraries from laser-induced thermionic emission reduction, Sci. Adv., 2022, 8, eabm6541 CrossRef CAS PubMed
.
- D. Wu, K. Kusada, Y. Nanba, M. Koyama, T. Yamamoto, T. Toriyama, S. Matsumura, O. Seo, I. Gueye, J. Kim, L. S. R. Kumara, O. Sakata, S. Kawaguchi, Y. Kubota and H. Kitagawa, Noble-Metal High-Entropy-Alloy Nanoparticles: Atomic-Level Insight into the Electronic Structure, J. Am. Chem. Soc., 2022, 144, 3365–3369 CrossRef CAS PubMed
.
- L. Tao, M. Sun, Y. Zhou, M. Luo, F. Lv, M. Li, Q. Zhang, L. Gu, B. Huang and S. Guo, A General Synthetic Method for High-Entropy Alloy Subnanometer Ribbons, J. Am. Chem. Soc., 2022, 144, 10582–10590 CrossRef CAS PubMed
.
- S. Gao, S. Hao, Z. Huang, Y. Yuan, S. Han, L. Lei, X. Zhang, R. S.-Yassar and J. Lu, Synthesis of high-entropy alloy nanoparticles on supports by the fast moving bed pyrolysis, Nat. Commun., 2020, 11, 1–10 CrossRef PubMed
.
- N. L. Broge, M. Bondesgaard, F. S. Pedersen, M. Roelsgaard and B. B. Iversen, Autocatalytic Formation of High-Entropy Alloy Nanoparticles, Angew. Chem., Int. Ed., 2020, 132, 22104–22108 CrossRef
.
- H. Qiao, M. T. Saray, X. Wang, S. Xu, G. Chen, Z. Huang, C. Chen, G. Zhong, Q. Dong, M. Hong, H. Xie, R. S. Yassar and L. Hu, Scalable synthesis of high entropy alloy nanoparticles by microwave heating, ACS Nano, 2021, 15, 14928–14937 CrossRef CAS PubMed
.
- E. P. George, D. Raabe and R. O. Ritchie, High-entropy alloys, Nat. Rev. Mater., 2019, 4, 515–534 CrossRef CAS
.
- G. Pacchioni, High-entropy materials go nano, Nat. Rev. Mater., 2022, 7, 156 CrossRef
.
- H. C. Zhou, J. R. Long and O. M. Yaghi, Introduction to metal-organic frameworks, Chem. Rev., 2012, 112, 673–674 CrossRef CAS PubMed
.
- H. C. Zhou and S. Kitagawa, Metal–organic frameworks (MOFs), Chem. Soc. Rev., 2014, 43, 5415–5418 RSC
.
- H. Deng, C. J. Doonan, H. Furukawa, R. B. Ferreira, J. Towne, C. B. Knobler, B. Wang and O. M. Yaghi, Multiple functional groups of varying ratios in metal–organic frameworks, Science, 2010, 327, 846–850 CrossRef CAS PubMed
.
- L. J. Wang, H. Deng, H. Furukawa, F. Gándara, K. E. Cordova, D. Peri and O. M. Yaghi, Synthesis and Characterization of Metal–Organic Framework-74 Containing 2, 4, 6, 8, and 10 Different Metals, Inorg. Chem., 2014, 53, 5881–5883 CrossRef CAS PubMed
.
- K. A. White, D. A. Chengelis, K. A. Gogick, J. Stehman, N. L. Rosi and S. Petoud, Near-Infrared Luminescent Lanthanide MOF Barcodes, J. Am. Chem. Soc., 2009, 131, 18069–18071 CrossRef CAS PubMed
.
- B. Tu, L. Diestel, Z.-L. Shi, W. R. L. Nisansala Bandara, Y. Chen, W. Lin, Y.-B. Zhang, S. G. Telfer and Q. Li, Harnessing Bottom-Up Self-Assembly To Position Five Distinct Components in an Ordered Porous Framework, Angew. Chem., Int. Ed., 2019, 58, 5348–5353 CrossRef CAS PubMed
.
- C. C. Blas, N. L. Salas, M. C. Gutiérrez, I. P. Orench, E. G. Puebla, M. L. Ferrer, M. Á. Monge and F. Gándara, Encoding Metal–Cation Arrangements in Metal−Organic Frameworks for Programming the Composition of Electrocatalytically Active Multimetal Oxides, J. Am. Chem. Soc., 2019, 141, 1766–1774 CrossRef PubMed
.
- H. F. Wang, L. Chen, H. Pang, S. Kaskel and Q. Xu, MOF-derived electrocatalysts for oxygen reduction, oxygen evolution and hydrogen evolution reactions, Chem. Soc. Rev., 2020, 49, 1414–1448 RSC
.
- H. Jiang, S. Jin, C. Wang, R. Ma, Y. Song, M. Gao, X. T. Liu, A. G. Shen, G. J. Cheng and H. Deng, Nanoscale laser metallurgy and patterning in air using MOFs, J. Am. Chem. Soc., 2019, 141, 5481–5489 CrossRef CAS PubMed
.
- R. Ma, H. Jiang, C. Wang, C. Zhao and H. Deng, Multivariate MOFs for laser writing of alloy nanoparticle patterns, Chem. Commun., 2020, 56, 2715–2718 RSC
.
- W. Zhang, W. Yan, H. Jiang, C. Wang, Y. Zhou, F. Ke, H. Cong and H. Deng, Uniform Bi-Sb alloy nanoparticles synthesized from MOFs by laser metallurgy for sodium-ion batteries, ACS Sustainable Chem. Eng., 2020, 8, 335–342 CrossRef CAS
.
- Q. Wang and D. Astruc, State of the art and prospects in metal-organic framework (MOF)-based and MOF-derived nanocatalysis, Chem. Rev., 2019, 120, 1438–1511 CrossRef PubMed
.
- X. Zhao, C. Mao, K. T. Luong, Q. Lin, Q. G. Zhai, P. Feng and X. Bu, Framework Cationization by Preemptive Coordination of Open Metal Sites for Anion-Exchange Encapsulation of Nucleotides and Coenzymes, Angew. Chem., Int. Ed., 2016, 55, 2768–2772 CrossRef CAS PubMed
.
- S. Yao, D. Wang, Y. Cao, G. Li, Q. Huo and Y. Liu, Two stable 3D porous metal-organic frameworks with high performance for gas adsorption and separation, J. Mater. Chem. A, 2015, 3, 16627–16632 RSC
.
- J. Jia, X. Lin, C. Wilson, A. J. Blake, N. R. Champness, P. Hubberstey, G. Walker, E. J. Cussen and M. Schröder, Twelve-connected porous metal-organic frameworks with high H2 adsorption, Chem. Commun., 2007, 840–842 RSC
.
- Y. S. Wei, K. J. Chen, P. Q. Liao, B. Y. Zhu, R. B. Lin, H. L. Zhou, B. Y. Wang, W. Xue, J. P. Zhang and X. M. Chen, Turning on the flexibility of isoreticular porous coordination frameworks for drastically tunable framework breathing and thermal expansion, Chem. Sci., 2013, 4, 1539–1546 RSC
.
- H. Jiang, L. Tong, H. Liu, J. Xu, S. Jin, C. Wang, X. Hu, L. Ye, H. Deng and G. J. Cheng, Graphene-metal-metastructure monolith via laser shock-induced thermochemical stitching of MOF crystals, Matter, 2020, 2, 1535–1549 CrossRef
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2qm00588c |
| ‡ These authors contributed equally to this work. |
| This journal is © the Partner Organisations 2022 |