Phthalocyanine-induced iron active species in metal–organic framework-derived porous carbon for efficient alkaline zinc–air batteries†
Cheng
Han
a,
Xiaodeng
Zhang
a,
Qiuhong
Sun
a,
Dandan
Chen
a,
Tingting
Miao
 a,
Kongzhao
Su
a,
Kongzhao
Su
 b,
Qipeng
Li
b,
Qipeng
Li
 bc,
Shaoming
Huang
bc,
Shaoming
Huang
 d and
Jinjie
Qian
d and
Jinjie
Qian
 *ab
*ab
aKey Laboratory of Carbon Materials of Zhejiang Province, College of Chemistry and Materials Engineering, Wenzhou University, Wenzhou, Zhejiang, P. R. China. E-mail: jinjieqian@wzu.edu.cn
bState Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, Fujian, P. R. China
cCollege of Chemistry and Chemical Engineering, Zhaotong University, Zhaotong, Yunnan, P. R. China
dSchool of Materials and Energy, Guangzhou Key Laboratory of Low-Dimensional Materials and Energy Storage Devices, Guangdong University of Technology, Guangzhou, P. R. China
First published on 4th April 2022
Abstract
The oxygen reduction reaction (ORR) on the cathode is of great significance in fuel cells and metal–O2 batteries, which is well demonstrated and highly efficient in nature. Herein, iron phthalocyanine (FePc) is encapsulated into the nanosized pores of a new porous metal–organic framework (MOF) of BMM-14 to give FePc@BMM-14. It shows a series of atomically dispersed Fe-based active species embedded into N-doped Fe@NC(x+y) samples after calcination, where x and y represent the FePc concentration and temperature, respectively. During pyrolysis, the rapid volatilization of Zn contents can further expand the framework to form more loose carbon nanomaterials with abundant active components, including FeNx and ultrafine Fe-based nanoparticles. Compared to the state-of-the-art Pt/C, the obtained Fe@NC(10+900/1000) exhibits satisfactory ORR performance to give a large limiting current of −5.22/−5.16 mA cm−2 (0.3 V vs. RHE), small Tafel slope of −51.1/−38.9 mV dec−1, and excellent stability. Meanwhile, the assembled batteries based on the two above catalysts show high activity, indicative of being potential substitutes to those noble metals consolidated by the theoretical calculations. This work demonstrates phthalocyanine-induced Fe-based active species in MOF-derived graphitic carbon as efficient ORR electrocatalysts for zinc–air batteries, which can be extended into the reasonable design and preparation of cost-effective but highly efficient catalysts for various energy-related applications.
1. Introduction
Green renewable energy stands in contrast to fossil fuels and is currently attracting more attention. Globally speaking, the rapid and extensive deployment of renewable energy will result in significant energy security and economic benefits, which in turn can alleviate environmental pollution, improve public health, and reduce associated costs to a large extent.1–5 Under these circumstances, the key to the large-scale applications of various new clean energy technologies, including renewable fuel cells and secondary batteries, mainly lies in the exploitation of high-efficiency catalysts for oxygen reduction reactions (ORR) on the cathode.6–11 Although platinum-related nanomaterials have long been recognized as the best catalysts, their low durability and high price severely hinder their further development and wide commercialization.12,13 Therefore, the development of cheap, robust and high-performance non-noble metal-based substitutes has become one of the research hotspots in the scientific community.Microporous metal–organic frameworks (MOFs) have been fully demonstrated in gas storage and separation, drug delivery and release, heterogeneous catalysis, and so on.14–19 As one subclass of crystalline MOFs, zeolite imidazole frameworks (ZIFs) have also been widely investigated on account of their high porosity, adjustable structure, and rich carbon and nitrogen sources.20,21 MOF materials are structurally rich in inorganic metal units and have a high content of organic ligands that make them potential precursors for multifunctional metal carbon composites. Due to the fast volatilization of Zn species, the formation of porous N-containing carbons calcined from Zn-based ZIFs can be achieved with a great possibility.22,23 Therefore, a large number of ZIF-derived carbon nanomaterials such as carbon nanotubes and carbon nanosheets have been widely utilized in different energy-related applications, because of their large specific surface area, excellent electrical conductivity, high stability and other significant advantages.24–27 In this context, the rational design and facile preparation of porous MOF-derived carbon-based catalysts in energy conversion and storage is highly important and desired.
On the other hand, the phthalocyanine compounds show a large conjugated system of 18 electrons structurally similar to the porphyrins that exist widely in nature.28,29 Among them, the structure of iron phthalocyanine (FePc) resembles the core of hemoglobin, which is highly selective for adsorption and transport of oxygen in human blood.30–32 In addition, the atomically dispersive iron active centers have been considered to be an efficient ORR catalyst in those recent reports.33–35 For example, Dai et al. have synthesized FeNx species well confined within micropores of the hierarchically porous carbon by the direct pyrolysis of the FePc complex, which outperforms the commercial Pt/C in terms of ORR activity.36 Meanwhile, these carbon-coated iron nanoparticles (NPs) as active catalytic species could be another type of non-precious metal oxygen reduction catalysts previously demonstrated by the Gewirth group.37 Thus, the rational combination between MOF and FePc will lead to the formation of Fe-based species after calcination, namely FeNx sites as well as Fe NPs, which deserves an in-depth investigation for efficient ORR performance.
Herein, a new porous Zn-based MOF (BMM-14) is solvothermally synthesized by three different imidazole ligands; for a more detailed experimental procedure please see the ESI.† Through a simple two-solvent diffusion method, discrete FePc can be successfully dispersed into this MOF to yield FePc@BMM-14(x), where x (0.1, 1, 10 g L−1) represents the concentration of FePc within 50 mg of the MOF precursor. After being pyrolyzed at different temperatures, the morphology of FePc@BMM-14(10) exhibits a flake-like shape to prove the influence of the introduced phthalocyanine on the obtained carbon. It also shows that the volatilization of Zn species can further expand the carbon layer, which is beneficial for the formation of a more loose N-containing framework, especially for Fe@NC(10+900/1000). These as-calcined Fe,N-doped carbon nanomaterials are endowed with the advantages of high porosity and a large specific surface area, while the low content of FePc results in generating atomically dispersive FeNx centers and ultrafine Fe nanoclusters. In this case, the optimal products of Fe@NC(10+900/1000) present a satisfactory ORR activity, showing the limit current of −5.22/−5.16 mA cm−2 at 0.3 V vs. RHE, low Tafel slope of −51.1/−38.9 mV dec−1, and excellent stability compared to the state-of-the-art Pt/C. Meanwhile, the assembled zinc–air batteries (ZABs) based on the Fe@NC catalysts give a superior performance with robust long-term stability, indicative of the potential substitutes to those noble metal based catalysts that are further supported by the following theoretical models. The present work confirms the reasonable design and synthesis of transition metal based ORR electrocatalysts by low-concentration filling of phthalocyanine compounds, which would pave a new pathway to prepare novel catalysts for efficient energy storage and conversion.
2. Experiment
2.1 Reagents and materials
All chemicals and solvents were used in this work without further purification; imidazole (Im, 97%, JNHH), 2-nitroimidazole (nIm, 97%, JNHH), benzimidazole (bIm, 97%, JNHH), zinc nitrate (Zn(NO3)2·3H2O, AR, 99%, Aladdin), iron phthalocyanine (FePc, AR, ≥98%, Aladdin), deionized water (DI H2O, 18 MΩ), N,N-dimethylformamide (DMF, ≥99.8%, Aladdin), n-hexane (≥99.8%, Aladdin), dimethyl sulfoxide (DMSO, ≥99.8%, Aladdin), ethanol (EtOH, >99%, GC, Aladdin), and Nafion ionomer (5 wt%, DuPont).2.2 Synthesis of Fe@BMM-14(x)
30 mg of BMM-14 was dispersed in 4 ml of n-hexane, ultrasonicated for 0.5 h and stirred for 1 h. Different concentrations of FePc were dissolved in 1 ml of DMSO solution, then added into n-hexane solution of BMM-14, stirred for 1 h, centrifuged and washed with EtOH three times, and dried overnight at 85 °C. The yield of BMM-14 is calculated to be ∼60% based on the added organic ligands.2.3 Synthesis of Fe@NC(x+y)
The Fe@NC(x+y) is synthesized by direct pyrolysis of the as-prepared Fe@BMM-14(x) in an Ar atmosphere. The furnace temperature is increased from room temperature at a heating rate of 10 °C min−1 to 700/800/900/1000 °C. After maintaining at the target temperature for 1 h, the obtained Fe@NC(x+y) is naturally cooled to room temperature. It can maintain ∼30% of its total mass at 700/800 °C and only ∼20% at 900/1000 °C.2.4 Electrochemical characterizations
The electrochemical performance of the samples was conducted in a three-electrode system using the Autolab workstation (Metrohm, Swiss) and/or the CHI760E electrochemical workstation (CH Instruments, Shanghai) for ORR, which comprise a counter electrode (graphite rod), a reference electrode (Ag/AgCl (3 M KCl)) and a working electrode (rotating ring-disk glass-carbon with catalyst, disk diameter is 5.6 mm). The working electrode is prepared by depositing the ink of catalysts. In a typical process to make up the electrode ink, 2.5 mg of Fe@NC(x+y) is dissolved in a mixed solvent containing 150 μL of EtOH, 100 μL of deionized water, and 2.5 μL of Nafion (5%) to form a homogeneous catalyst ink by ultrasonicating for 3 h. Then 15 μL of the as-prepared droplet is deposited onto the surface of the GCE electrode and dried at room temperature conditions. Before every test, the electrolyte (0.1 M KOH) is pre-purged with pure O2 for 30 min to make sure it is O2-saturated. Firstly, scanning is performed for 40 cycles of cyclic voltammetry (CV) curves until the signals are stabilized. The linear sweep voltammetry (LSV) is scanning at a potential scan rate of 5 mV s−1 at different rotational speeds from 100 to 2500 rpm for ORR. For data analysis, all the potentials are transformed with reference to a standard reversible hydrogen electrode (RHE) according to the following equation: ERHE = EAg/AgCl + 0.975 V in 0.1 M KOH. To evaluate the active surface area of catalysts, the double-layer capacitance (Cdl) is determined by measuring the CV plots from 1.00 to 1.10 V vs. RHE at various scan rate from 5 to 25 mV s−1 in N2-saturated 0.1 M KOH in the nonfaradaic potential region. By fitting the current density at 1.15 V vs. RHE at various scan rates, the linear trend is observed and found equal to the slope of the linear Cdl. All electrochemical impedance spectroscopy (EIS) measurements were recorded at the same overpotential of 0.8 V vs. RHE in the frequency range of 10−2–106 Hz with an amplitude of 5 mV. Electrocatalytic stability was obtained using the current–time (i–t) chronoamperometric curve at 0.6 V in O2-saturated 0.1 M KOH for 10 h.The electron transfer number (n) and yield of the H2O2 intermediate were calculated according to the Koutecky–Levich (K–L) eqn (1) and (2) and RRDE technologies eqn (3) and (4), as shown below:
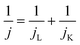 | (1) |
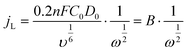 | (2) |
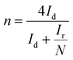 | (3) |
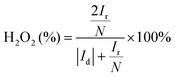 | (4) |
In these formulae, J is the experimental current density, JL is the diffusion-limited current density, and JK is the kinetic current density, respectively; ω is the rotation speed in rpm (round per minute), F is the Faraday constant (96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 485 C mol−1), C0 is the bulk concentration of oxygen (1.2 × 10−6 mol cm−3), D0 is the diffusion coefficient of oxygen in 0.1 M KOH (1.9 × 10−5 cm2 s−1), and υ is the kinetic viscosity (0.01 cm2 s−1). When the speed of rotation is also represented as rpm, 0.2 is a constant. The n can be extracted from the slope of the K–L plot. Ir is the ring current, Id is the disk current and N expresses the collection efficiency of the ring electrode (0.37).
485 C mol−1), C0 is the bulk concentration of oxygen (1.2 × 10−6 mol cm−3), D0 is the diffusion coefficient of oxygen in 0.1 M KOH (1.9 × 10−5 cm2 s−1), and υ is the kinetic viscosity (0.01 cm2 s−1). When the speed of rotation is also represented as rpm, 0.2 is a constant. The n can be extracted from the slope of the K–L plot. Ir is the ring current, Id is the disk current and N expresses the collection efficiency of the ring electrode (0.37).
The aforesaid obtained electrocatalyst ink as the cathode is dropped onto carbon paper until the loading mass is 1.0 mg cm−2 and dried in air. Besides, one Zn foil becomes the anode and the paper is used to absorb 6.0 M KOH electrolytes and separate the electrodes. Finally, these materials are assembled in perforated button-cell shells for testing. All Zn–air button battery tests are performed under ambient atmosphere at room temperature. Discharge power density is calculated from the discharge polarized profiles using the following equation:
| P = Ud × Jd |
2.5 Theoretical calculation
The constructed models of FeNx composites are optimized by the CASTEP module of the Materials Studio 8.0 software to obtain the geometry optimized structure with minimized energy, in which the functional is chosen as the Perdew–Burke–Ernzerhof (PBE) functional in generalized gradient approximation (GGA). The basic model contains four benzene rings; one of the benzene rings is removed, and Fe atoms are introduced to construct these target FeCxNy sites. In this computation, the graphene surface is modeled in a 2 × 2 supercell; the cell size is a = b = 9.839996, c = 6.8; α = β = 90°, γ = 120°, with one Fe atom doped layer and a vacuum layer of 8 Å. The Brillouin zone is sampled with 3 × 4 × 1 k-points and cut-off energy of 300 eV is used. And PDOS analysis was added in the calculation process. The adsorption energy (Ea) of O2 with FeNx is calculated by the following equation, where EO2+sub, EO2, and Esub are the energy of the FeNx substance combined with the oxygen, oxygen molecule, and FeNx substance, respectively.| Ea = EO2+sub − EO2 − Esub |
3. Results and discussion
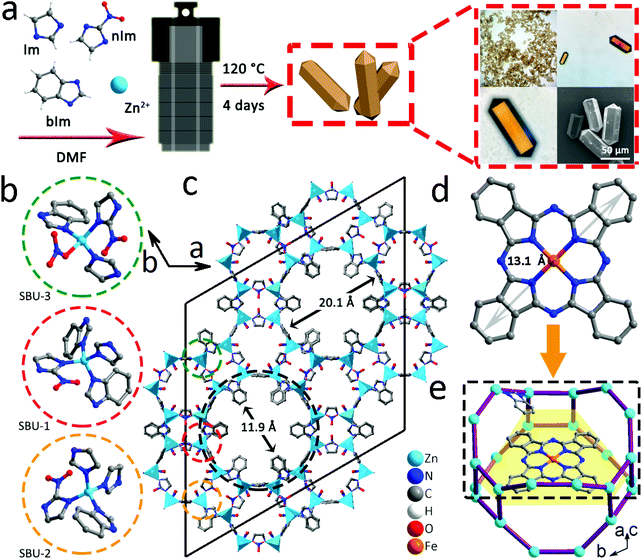
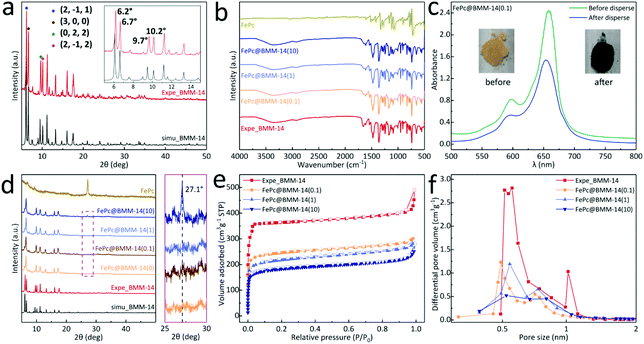
The self-assembly of imidazole (Im), nitroimidazole (nIm) and benzimidazole (bIm) together with Zn(II) cations leads to the formation of orange crystals (Fig. 1a). Meanwhile, the optical microscope and scanning electron microscope (SEM) images show that the obtained BMM-14 exhibits a prism connected to two hexagonal pyramids. Its cell unit and asymmetric unit (AU) of the single-crystal structure are depicted in Fig. 1c and S1,† respectively. As a result of various imidazole linkers, there are three different secondary building units (SBUs), namely SBU-1 (Im + 2bIm + nIm, red circle), SBU-2 (2Im + bIm + nIm, yellow circle) and SBU-3 (Im + bIm + nIm + NO3−, green circle) in Fig. 1b. In this case, the metal sites in each SBU adopt the typical ZnN4 consistent with the previously reported examples of Zn-imidazole MOFs.38 Finally, 1-dimensional tubular nanochannels are formed along the c-axis; for more information on the structure please refer to Fig. S2 and Table S1.† The bIm along the ab plane obstructs the largest channel of 20.1 Å to a small gap of around 11.9 Å, which can completely encapsulate FePc (13.1 Å) in Fig. 1c and d. The black dotted area in Fig. 1c is the largest cavity in BMM-14 that is simply selected to demonstrate the schematic diagram of FePc molecular packing (Fig. 1e). It shows that there is enough space between the two layers of Zn atoms to fill FePc, and will not be disturbed by bIm (Fig. S3†). Furthermore, the thermogravimetric analysis (TGA) under N2 flow in Fig. S4† reveals the structural change of BMM-14 in the heating process where the first weight loss is ascribed to the dehydration of physically adsorbed solvents before 320 °C, while the second loss belongs to the removal of the coordinating small molecules in 320–500 °C. The final sharp weight loss corresponds to a gradual decomposition of organic ligands into porous carbon materials.Due to the large porosity and abundant channels, a simple double-solvent dispersion method is used to encapsulate different amounts of FePc molecules.39 In Fig. 2a, the powder X-ray diffraction (PXRD) verifies that the as-made BMM-14 crystals are in good agreement with the simulated peaks, in which four strong diffraction signals at 2-theta values of 6.2°, 6.7°, 9.7°, and 10.2° are well matched with the (2, −1, 1), (3, 0, 0) (0, 2, 2) and (2, −1, 2) planes, respectively. After that, a total of 50 mg of pristine MOF is dispersed in 4 mL of n-hexane, while x (0.1, 1, 10) g L−1 of FePc in dimethyl sulfoxide is also prepared, then the above two solvents are mixed to obtain the FePc@BMM-14(x) sample. From FT-IR spectra, there are no new characteristic peaks detected after loading with different concentrations of FePc into the crystalline MOF (Fig. 2b). However, the optical color of the exchanged samples exhibits a significant change confirmed by the UV-vis spectra before and after the dispersion treatment in Fig. 2c and S5.† The PXRD patterns of FePc-encapsulated MOFs show that the obtained FePc@BMM-14(10) with the highest concentration owns a typical characteristic peak at 27.1° validating the successful loading of FePc (Fig. 2d). Meanwhile, the composite presents no obvious difference in the crystal form, of which the overall framework is almost retained. In Fig. 2e and f, these collected N2 isotherms exhibit the type-I curves, and a trend of reduced gas capacity with the filling of FePc is also observed. Furthermore, the fitted pore size distribution (PSD) curves can further confirm that all the pores and channels are centered in the microporous regions, while the number of micropores of FePc@BMM-14(x) is significantly reduced after the loading of FePc. For more specific surface area and PSD data please see Table S2.† It is indicative that the small FePc molecule has been evenly distributed in the MOF cavity that will be conducive to the formation of dispersive Fe atoms after the subsequent thermal treatment.
The morphology change of the FePc@BMM-14 series is first studied by SEM images. After continuous stirring, the bulk single crystals are cracked into micro-sized particles with an increased FePc concentration to achieve an adequate dispersion. For the control sample of FePc@BMM-14(0), there is no difference compared with the broken particles in Fig. 3a1, a2 and S6.† However, the surface of FePc@BMM-14(x) becomes significantly rough (Fig. 3b–d and S7–S9†), especially for FePc@BMM-14(10), which is reasonably selected to carry out the following thermal treatments. After calcination, these original particles will be almost destroyed which can be reflected in the products of Fe@NC(10+900/1000) with the most obvious sheet-like structure. This can be well explained by the bubbling process induced by the volatilization of Zn species in the carbon layer, thereby achieving the pore-forming effect.40 The subsequent gas sorption and PSD data verify that the obtained Fe@NC(10+900/1000) possess a higher adsorption capacity with distinct hysteresis loops in Fig. 3g, h, S13 and S14.† However, the total N2 uptake of Fe@NC(10+700/800) does not change significantly, which stemmed from the lower carbonization process in Fig. 3e, f, S11 and S12.† Meanwhile, the increased BET surface areas are in the following order Fe@NC(10+700) (60.32 m2 g−1), Fe@NC(10+800) (104.00 m2 g−1), Fe@NC(10+900) (334.75 m2 g−1), and Fe@NC(10+1000) (420.30 m2 g−1), respectively. Furthermore, the difference in PSD further proves that the carbon at higher temperature presents the larger total pore volume induced by the Zn evaporation. The enhanced micropore content of Fe@NC(10+900/1000) is indicative of the superiority of both carbon nanomaterials which are endowed with abundant active sites that would accelerate the electron transfer and mass diffusion during the electrochemical performance.
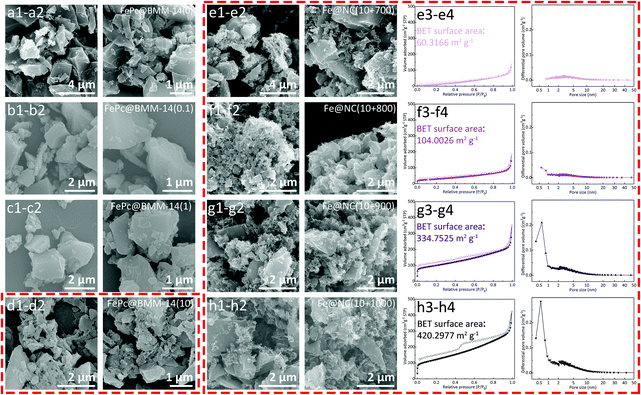 | ||
| Fig. 3 SEM images of (a–d) FePc@BMM-14(x); (e–h) SEM images, sorption isotherms and PSD curves for the calcined Fe@NC(10+y) series. | ||
Transmission electron microscopy (TEM) and high resolution TEM (HR-TEM) further reveal the nanostructure and morphological features of these carbon materials. In Fig. 4a1–d1, the Fe@NC(10+y) series present the microstructure of bulky body and sheet-shaped edge, but the nanosheet structures of Fe@NC(10+700/800) are not as obvious as Fe@NC(10+900/1000), which verifies the incompleteness of low-temperature carbonization and the characteristics of amorphous carbon. Each sample shows the tendency of the carbon layer to wrap the metal sources where the distribution of metals is relatively compact and poorly dispersive, especially for Fe@NC(10+700/800) (Fig. 4a2–d2). In this case, the metal aggregation in the graphitic carbon comes from a large amount of Zn from the MOF precursor as well as Fe from the FePc molecules. On the other hand, the ultrafine NPs are evenly distributed and atomically dispersive metals wrapped in the N-doped carbons are detected in Fe@NC(10+900/1000). Meanwhile, low-temperature carbonization leads to the formation of some inconspicuous lattice stripes of carbon (0.33 nm) in Fig. 4a3 and b3. An ordered nanostructure on the edge corresponds to the (0 0 2) plane of graphite for Fe@NC(10+800) confirming the existence of graphitized carbon, but the inner carbon maintains a disordered structure (Fig. 4b3). However, the inner/outer carbon reaches an ordered structure for Fe@NC(10+900) in Fig. 4c2 and c3, and a small number of cavities are in situ formed during pyrolysis. The same phenomenon can also be detected in Fe@NC(10+1000) where cavities in the middle of the carbon layer become more obvious. Meanwhile, the hollow carbon structure coated with Fe atoms shows orderly structure characteristics in Fig. 4d2 and d3, and for more TEM images please refer to Fig. S15–S18.† Finally, the uniform distribution of Fe@NC(x+y) can be clearly examined in the EDX spectra and elemental mappings (Fig. 4a4–d4). This is indicative of the successful formation of the thermodynamically stable FeNx species because of the low content of FePc in the microporous channels. The obtained hierarchically porous and graphitic carbon nanomaterials ensure the fast transfer of the electrolytes and electrons, and the active sites are thoroughly exposed to achieve the enhanced electrochemical performance under an extremely high atomic utilization.
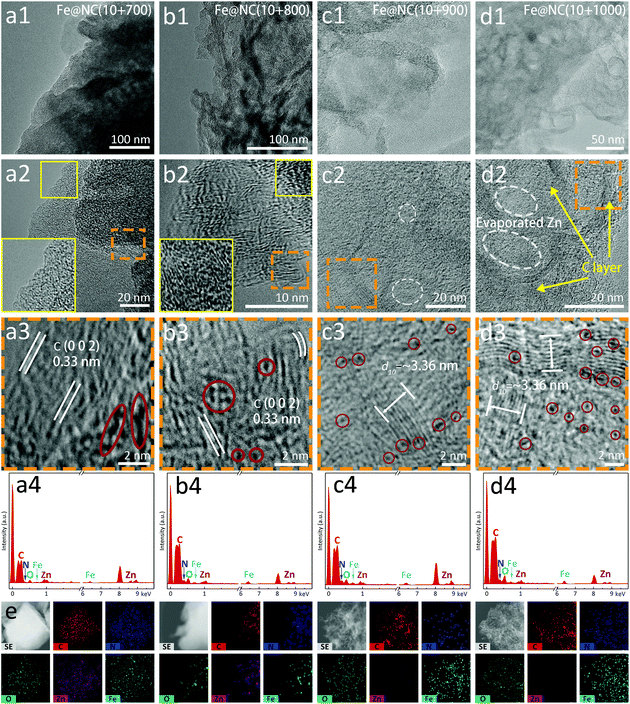 | ||
| Fig. 4 TEM/HR-TEM images, EDX spectra of (a1–a4) Fe@NC(10+700), (b1–b4) Fe@NC(10+800), (c1–c4) Fe@NC(10+900) and (d1–d4) Fe@NC(10+1000). (e) Elemental mappings of Fe@NC(10+1000). | ||
To characterize the content and coordination environment, X-ray photoelectron spectroscopy (XPS) is performed to show the coexistence of Zn, Fe, C, N, and O (Fig. 5a and S19†). Almost invisible Fe 2p peaks in the full spectra of Fe@NC(10+900/1000) confirm the presence of trace FePc introduced in the MOF pores. Meanwhile, the decrease or even disappearance of the Zn 2p signal also proves the evaporation of Zn after high temperature carbonization.41 In Fig. 5b, S20 and Table S3,† the deconvoluted O 1s peak shapes in Fe@NC(0/10+900) are almost the same where three peaks at 530.1, 531.2 and 532.2 eV are considered as the M–O, C–O and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds, respectively. The deconvoluted C 1s spectra in Fig. 5c reveal the existence of four different carbon coordination environments (C–C sp3, C
O bonds, respectively. The deconvoluted C 1s spectra in Fig. 5c reveal the existence of four different carbon coordination environments (C–C sp3, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C sp2, C–O/C–N and C
C sp2, C–O/C–N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/C
O/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) and there is no change in the positions of the four peaks, which confirms that the coordination environment of carbon atoms in the five material has not changed during the change of pyrolysis temperature. In this case, element N mainly comes from imidazoles in the microporous MOF in the high-resolution spectra of N 1s in Fig. 5d and Table S5.† The fitting peak positions of N in Fe@NC(10+900/1000) exhibit almost no deviations, indicating that the high-temperature carbonization poses no influence on the coordination environment of Fe and N. On the other hand, the changes in the coordination environment of Zn and Fe have been further studied in Fig. 5e and f. There is a slight negative shift trend of the 2p1/2 and 2p3/2 peak positions of Zn(II) in Fe@NC(10+700/800) compared to those in Fe@NC(10+900/1000), while the binding energies of 2p1/2 and 2p3/2 of Fe(0, II, III) tend to shift in the positive direction compared to those of Fe@NC(10+900/1000). The deconvoluted Fe 2p spectra show three pairs of weak peaks at 706/720, 709/721, and 712/724 eV for Fe(0), Fe(II), and Fe(III), respectively, implying the Fe–Nx sites in Fe@NC(10+y) samples. At higher temperatures, the binding energies of 2p1/2 and 2p3/2 of Zn(II) for Fe@NC(10+900/1000) suddenly rise as a result of the reduction and evaporation of Zn(II) species, while the binding energies of 2p1/2 and 2p3/2 of Fe(0, II, III) decrease, especially in Fe(II) and Fe(0). For more XPS data and peak changes, please refer to Fig. S19–S22 and Tables S3–S7.†
N) and there is no change in the positions of the four peaks, which confirms that the coordination environment of carbon atoms in the five material has not changed during the change of pyrolysis temperature. In this case, element N mainly comes from imidazoles in the microporous MOF in the high-resolution spectra of N 1s in Fig. 5d and Table S5.† The fitting peak positions of N in Fe@NC(10+900/1000) exhibit almost no deviations, indicating that the high-temperature carbonization poses no influence on the coordination environment of Fe and N. On the other hand, the changes in the coordination environment of Zn and Fe have been further studied in Fig. 5e and f. There is a slight negative shift trend of the 2p1/2 and 2p3/2 peak positions of Zn(II) in Fe@NC(10+700/800) compared to those in Fe@NC(10+900/1000), while the binding energies of 2p1/2 and 2p3/2 of Fe(0, II, III) tend to shift in the positive direction compared to those of Fe@NC(10+900/1000). The deconvoluted Fe 2p spectra show three pairs of weak peaks at 706/720, 709/721, and 712/724 eV for Fe(0), Fe(II), and Fe(III), respectively, implying the Fe–Nx sites in Fe@NC(10+y) samples. At higher temperatures, the binding energies of 2p1/2 and 2p3/2 of Zn(II) for Fe@NC(10+900/1000) suddenly rise as a result of the reduction and evaporation of Zn(II) species, while the binding energies of 2p1/2 and 2p3/2 of Fe(0, II, III) decrease, especially in Fe(II) and Fe(0). For more XPS data and peak changes, please refer to Fig. S19–S22 and Tables S3–S7.†
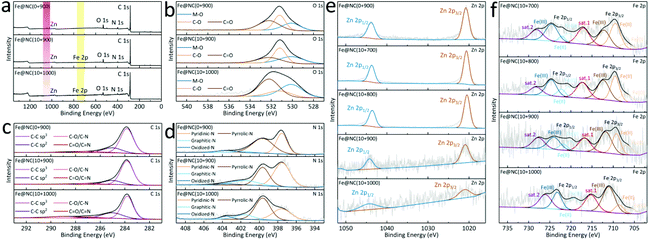 | ||
| Fig. 5 (a) Full XPS survey spectra; the deconvoluted (b) O 1s, (c) C 1s and (d) N 1s of Fe@NC(0+900) and Fe@NC(10+900/1000); the deconvoluted (e) Zn 2p and (f) Fe 2p of Fe@NC(0+900) and Fe@NC(10+y). | ||
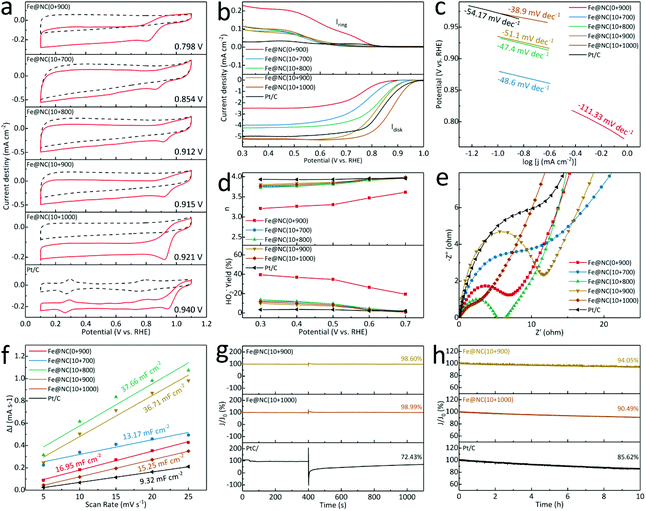
Inspired by the nanostructure as well as abundant FeNx species in Fe@NC(x+y), the electrochemical ORR performance is further evaluated in 0.1 M KOH, and all applied potentials have been calibrated to the reversible hydrogen electrode. In Fig. 6a, the cyclic voltammetry (CV) curves in N2-/O2-saturated solution at 5 mV s−1 show that the obtained Fe@NC(0+900) owns the inferior oxygen reduction peak of 0.798 V. It reveals that the position of the reduction peak has moved significantly to 0.854/0.912/0.915/0.921 V for Fe@NC(10+700/800/900/1000), respectively, but it is still lower than Pt/C (0.940 V). Meanwhile, the linear sweep voltammograms (LSV) at 1600 rpm in Fig. 6b for Fe@NC(10+900/1000) exhibit a higher onset potential and a larger diffusion limited current density (JL) of −5.22/−5.16 mV cm−2 at 0.3 V, higher than Fe@NC(0+900) (−2.49 mV cm−2), Fe@NC(10+700/800) (−3.99/−4.24 mV cm−2), and Pt/C (−4.95 mV cm−2). When compared to previous reports, the Fe@NC(10+900/1000) catalyst demonstrates a competitive ORR performance in Table S8.† The LSV curves from 100 to 2500 rpm are summarized, and the Koutecky–Levich curves of Fe@NC(10+900/1000) present almost linear and parallel fitting lines from 0.3–0.7 V (Fig. S23 and S24†). In Fig. 6c, all samples possess relatively small Tafel slopes for Fe@NC(10+700/800/900/1000) (−48.6/−47.4/−51.1/−38.9 mV dec−1) lower than Pt/C (−54.2 mV dec−1), indicating the excellent reaction kinetics with enhanced mass transfer ability. Furthermore, the calculated number of transferred electrons (n) and the HO2− yields validate the high O2 selectivity and effective 4-electron process (Fig. 6d). The electrochemical impedance spectrum (EIS) in Fig. 6e shows that the obtained Fe@NC(10+1000) is endowed with the low charge transfer resistance of 7.2 Ω in the high-frequency region, lower than for Fe@NC(0+900) (9.4 Ω), Fe@NC(10+700/800/900) (14.5/7.6/12.0 Ω) and Pt/C (18.8 Ω). On the other hand, the calculated double-layer capacitance (Cdl) shows that Fe@NC(10+800/900) (37.66/36.71 mF cm−2) owns a larger electrochemical surface area than Fe@NC(10+700/1000) (13.17/15.25 mF cm−2), Fe@NC(0+900) (16.95 mF cm−2) and Pt/C (9.32 mF cm−2), proving that both high temperature and multi-element doping can improve the ability of electron/ion transport (Fig. 6f and S25†). By inserting methanol, the excellent tolerance of Fe@NC(10+900/1000) with current retention of 98.60%/98.99% is observed compared to Pt/C of 72.43% with an obvious current attenuation in Fig. 6g. Finally, a constant current at 0.6 V is also performed at a rotation rate of 1600 rpm to evaluate the long-term ability in Fig. 6h. The loss is calculated to be 94.05% for Fe@NC(10+900) and 90.49% for Fe@NC(10+1000) better than that of 20 wt% Pt/C (85.62%), verifying the robust stability. Above all, these porous carbon nanomaterials not only behave as a rigid supporting substrate to mitigate the structural change of catalysts, but also improve the electrochemical property with well dispersed Fe-based active centers.
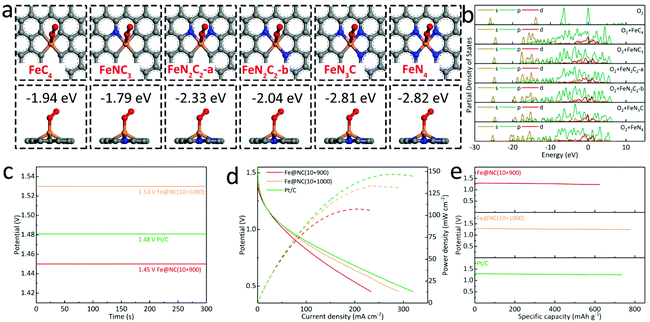
In order to better understand the improvement of ORR performance in the Fe@NC(x+y) series, the theoretical calculations are used to clarify the adsorption behavior of the oxygen molecule on different types of Fe/N-doped graphitic carbon (FeNxCy). There are a total of six differentiated situations for Fe and N coordination environments, namely FeC4, FeNC3, FeN2C2-a, FeN2C2-b, FeN3C and FeN4. First of all, the standard model of the O2 unit cell is established, and its calculated energy is about −867.91 eV (Fig. S26†). In Fig. 7a, S27 and S28,† it shows that FeNxCy sites doped with N have lowered the adsorption energy of O2 loaded on the active sites as FeC4 (−1.94 eV), FeNC3 (−1.79 eV), FeN2C2-a (−2.33 eV), FeN2C2-b (−2.04 eV), FeN3C (−2.81 eV) and FeN4 (−2.82 eV). In this case, FeNC3 exhibits a higher adsorption energy than the pristine FeC4 caused by an inevitable breakdown of structural symmetry.42 With greater N-doping, the overall adsorption energy can be further reduced with more π electrons, which are experimentally supported by the high-efficiency ORR activity of FeNx sites in our work. In Fig. 7b, the partial density of states (PDOS) for FeNxCy at approximately 0, −6.42, and −13.87 eV are significantly broadened by valence hybridization compared to the isolated O2 molecule. Furthermore, FeNC3, FeN2C2-a/b, FeN3C and FeN4 are also endowed with obviously lower energies than that of FeC4, indicating the transfer of the partial charge from oxygen to the metal atom in the N-coordinated centers.43 In addition, we have further applied the optimal samples to assemble Zn–air batteries (ZABs). In Fig. 7c, the Fe@NC(10+1000) provides the largest open circuit voltage (OCV) of 1.53 V, higher than Pt/C (1.47 V) and Fe@NC(10+900) (1.45 V). Meanwhile, the maximum current density and peak power density of Fe@NC(10+900) in Fig. 7d are calculated to be 233.6 mA cm−2 and 107.1 mW cm−2, which is inferior to that of Fe@NC(10+1000) (291.0 mA cm−2, 133.0 mW cm−2), while the Pt/C based ZAB exhibits the best performance of 319.7 mA cm−2 and 146.8 mW cm−2. Furthermore, the specific capacity of the Pt/C-based battery is 734.0 mA h g−1, lower than that of Fe@NC(10+1000) at 777.6 mA h g−1 (Fig. 7e). These above results confirm the high catalytic ORR performance and high stability in alkaline media for MOF-derived porous carbons with abundant FeNx sites as well as Fe-based NPs. And the long-term charge and discharge curves of the ZABs with Fe@NC(10+1000) and Pt/C show a high round-trip efficiency of 52% and 54% within 100 cycles to confirm their robust stability for possible practical devices (Fig. S29†).
4. Conclusion
All in all, we have successfully obtained one new Zn-based BMM-14 with three different imidazole linkers and 1-dimensional large pores, which are so large that they can be efficiently filled with discrete FePc complexes without agglomeration and accumulation. By adjusting various carbonization temperatures, the FePc-encapsulating FePc@BMM-14(x) can be thermally converted into a series of Fe-based active centers embedded into 2-dimensional N-doped carbon nanomaterials, denoted as Fe@NC(x+y). In addition, the high-temperature evaporation of Zn species leads to the formation of large voids and abundant active materials (including atomically dispersive FeNx and ultrafine Fe-based NPs) in the graphitic carbon layer. They are more conducive to the exposure of active centers and fast mass transfer during electrochemical reactions. Therefore, the most optimal catalysts of Fe@NC(10+900/1000) are better than the commercial Pt/C in terms of mass activity, long-term durability and methanol tolerance, and are used as cathode materials for efficient alkaline ZABs and further confirmed by the theoretical calculations. This work proposes an effective method to introduce 0-dimensional transition metal active materials into large size-compatible pores of a N-containing MOF precursor that is further pyrolyzed into highly active and stable metal carbon oxygen reduction electrocatalysis. It will shed light on the rational design and synthesis of cost-effective, high-performance and robust electrocatalysts for energy-related applications in the near future.Author contributions
The manuscript was written through the contributions of all authors. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was financially supported by the Special Basic Cooperative Research Programs of Yunnan Provincial Undergraduate Universities Association (202101BA070001-042 and 202101BA070001-031), the BUCT-WZU Joint Fund (KH2012031), and the Basic Science and Technology Research Project of Wenzhou, Zhejiang Province (G20190007).References
- S. Chaemchuen, N. A. Kabir, K. Zhou and F. Verpoort, Metal-organic frameworks for upgrading biogas via CO2 adsorption to biogas green energy, Chem. Soc. Rev., 2013, 42, 9304–9332 RSC.
- K. Hartlieb, J. Holcroft, P. Moghadam, N. Vermeulen, M. Algaradah, M. Nassar, Y. Botros, R. Snurr and J. Stoddart, CD-MOF: A Versatile Separation Medium, J. Am. Chem. Soc., 2016, 138, 2292–2301 CrossRef CAS.
- D. Li, M. Kassymova, X. Cai, S.-Q. Zang and H.-L. Jiang, Photocatalytic CO2 reduction over metal-organic framework-based materials, Coord. Chem. Rev., 2020, 412, 213262 CrossRef CAS.
- X. Cui, S. Shyshkanov, T. Nguyen, A. Chidambaram, Z. Fei, K. Stylianou and P. Dyson, CO2 Methanation via Amino Alcohol Relay Molecules Employing a Ruthenium Nanoparticle/Metal Organic Framework Catalyst, Angew. Chem., Int. Ed., 2020, 59, 16371–16375 CrossRef CAS PubMed.
- X. Zhang, Y. Zhang, Q. Li, X. Zhou, Q. Li, J. Yi, Y. Liu and J. Zhang, Highly efficient and durable aqueous electrocatalytic reduction of CO2 to HCOOH with a novel bismuth-MOF: experimental and DFT studies, J. Mater. Chem. A, 2020, 8, 9776–9787 RSC.
- X. F. Lu, B. Y. Xia, S. Q. Zang and X. W. D. Lou, Metal-Organic Frameworks Based Electrocatalysts for the Oxygen Reduction Reaction, Angew. Chem., Int. Ed., 2020, 59, 4634–4650 CrossRef CAS PubMed.
- Z. Liang, H. Guo, G. Zhou, K. Guo, B. Wang, H. Lei, W. Zhang, H. Zheng, U.-P. Apfel and R. Cao, Metal-Organic-Framework-Supported Molecular Electrocatalysis for the Oxygen Reduction Reaction, Angew. Chem., Int. Ed., 2021, 60, 8472–8476 CrossRef CAS.
- L. Yang, X. Zeng, W. Wang and D. Cao, Recent Progress in MOF-Derived, Heteroatom-Doped Porous Carbons as Highly Efficient Electrocatalysts for Oxygen Reduction Reaction in Fuel Cells, Adv. Funct. Mater., 2018, 28, 1704537 CrossRef.
- I. T. Kim, S. Shin and M. W. Shin, Development of 3D interconnected carbon materials derived from Zn-MOF-74@carbon nanofiber web as an efficient metal-free electrocatalyst for oxygen reduction, Carbon, 2018, 135, 35–43 CrossRef CAS.
- H. F. Wang, L. Chen, H. Pang, S. Kaskel and Q. Xu, MOF-derived electrocatalysts for oxygen reduction, oxygen evolution and hydrogen evolution reactions, Chem. Soc. Rev., 2020, 49, 1414–1448 RSC.
- Y. Wang, J. Wang, D. Wei and M. Li, A “MOF-Protective-Pyrolysis” Strategy for the Preparation of Fe-N-C Catalysts and the Role of Fe, N, and C in the Oxygen Reduction Reaction in Acidic Medium, ACS Appl. Mater. Interfaces, 2019, 11, 35755–35763 CrossRef CAS.
- L. Zhang, H. Li and J. Zhang, Kinetics of oxygen reduction reaction on three different Pt surfaces of Pt/C catalyst analyzed by rotating ring-disk electrode in acidic solution, J. Power Sources, 2014, 255, 242–250 CrossRef CAS.
- X. Yan, Y. Jia, L. Zhang and X. Yao, Platinum stabilized by defective activated carbon with excellent oxygen reduction performance in alkaline media, Chin. J. Catal., 2017, 38, 1011–1020 CrossRef CAS.
- H. Li, K. Wang, Y. Sun, C. T. Lollar, J. Li and H.-C. Zhou, Recent advances in gas storage and separation using metal-organic frameworks, Mater. Today, 2018, 21, 108–121 CrossRef CAS.
- B. Li, H. M. Wen, W. Zhou and B. Chen, Porous Metal-Organic Frameworks for Gas Storage and Separation: What, How, and Why?, J. Phys. Chem. Lett., 2014, 5, 3468–3479 CrossRef CAS PubMed.
- M. Kotzabasaki and G. E. Froudakis, Review of computer simulations on anti-cancer drug delivery in MOFs, Inorg. Chem. Front., 2018, 5, 1255–1272 RSC.
- Y. Xie, X. Liu, X. Ma, Y. Duan, Y. Yao and Q. Cai, Small Titanium-Based MOFs Prepared with the Introduction of Tetraethyl Orthosilicate and Their Potential for Use in Drug Delivery, ACS Appl. Mater. Interfaces, 2018, 10, 13325–13332 CrossRef CAS PubMed.
- L. Ma, C. Abney and W. Lin, Enantioselective catalysis with homochiral metal-organic frameworks, Chem. Soc. Rev., 2009, 38, 1248–1256 RSC.
- A. H. Chughtai, N. Ahmad, H. A. Younus, A. Laypkov and F. Verpoort, Metal-organic frameworks: versatile heterogeneous catalysts for efficient catalytic organic transformations, Chem. Soc. Rev., 2015, 44, 6804–6849 RSC.
- J. Yang, Y.-B. Zhang, Q. Liu, C. Trickett, E. Gutierrez-Puebla, M. Monge, H. Cong, A. Aldossary, H. Deng and O. Yaghi, Principles of Designing Extra-Large Pore Openings and Cages in Zeolitic Imidazolate Frameworks, J. Am. Chem. Soc., 2017, 139, 6448–6455 CrossRef CAS PubMed.
- W. Morris, J. Doonan, H. Furukawa, R. Banerjee and O. Yaghi, Crystals as Molecules: Postsynthesis Covalent Functionalization of Zeolitic Imidazolate Frameworks, J. Am. Chem. Soc., 2008, 130, 12626–12627 CrossRef CAS PubMed.
- L. Chai, Q. Huang, H. Cheng, X. Wang, L. Zhang, T.-T. Li, Y. Hu, J. Qian and S. Huang, Bottom-up preparation of hierarchically porous MOF-modified carbon sphere derivatives for efficient oxygen reduction, Nanoscale, 2020, 12, 8785–8792 RSC.
- L. Chai, L. Zhang, X. Wang, Z. Hu, Y. Xu, T.-T. Li, Y. Hu, J. Qian and S. Huang, Cube-shaped metal-nitrogen-carbon derived from metal-ammonia complex-impregnated metal-organic framework for highly efficient oxygen reduction reaction, Carbon, 2020, 158, 719–727 CrossRef CAS.
- L. Chai, Z. Hu, X. Wang, L. Zhang, T.-T. Li, Y. Hu, J. Pan, J. Qian and S. Huang, Fe7C3 nanoparticles with in situ grown CNT on nitrogen doped hollow carbon cube with greatly enhanced conductivity and ORR performance for alkaline fuel cell, Carbon, 2021, 174, 531–539 CrossRef CAS.
- F. Li, T. Qin, Y. Sun, R. Jiang, J. Yuan, X. Liu and A. P. O'Mullane, Preparation of a one-dimensional hierarchical MnO@CNT@Co-N/C ternary nanostructure as a high-performance bifunctional electrocatalyst for rechargeable Zn–air batteries, J. Mater. Chem. A, 2021, 9, 22533–22543 RSC.
- T. Liu, S. Feng, J. Huo, Q. Li, C. Xie and S. Wang, Crystalline-Water/Coordination Induced Formation of 3D Highly Porous Heteroatom-Doped Ultrathin Carbon Nanosheet Networks for Oxygen Reduction Reaction, ChemCatChem, 2018, 10, 4562–4568 CrossRef CAS.
- Y. Guan, N. Li, Y. Li, L. Sun, Y. Gao, Q. Zhang, C. He, J. Liu and X. Ren, Two dimensional ZIF-derived ultra-thin Cu-N/C nanosheets as high performance oxygen reduction electrocatalysts for high-performance Zn-air batteries, Nanoscale, 2020, 12, 14259–14266 RSC.
- R. Baker, D. P. Wilkinson and J. Zhang, Electrocatalytic activity and stability of substituted iron phthalocyanines towards oxygen reduction evaluated at different temperatures, Electrochim. Acta, 2008, 53, 6906–6919 CrossRef CAS.
- H. Jia, Y. Yao, J. Zhao, Y. Gao, Z. Luo and P. Du, A novel two-dimensional nickel phthalocyanine-based metal-organic framework for highly efficient water oxidation catalysis, J. Mater. Chem. A, 2018, 6, 1188–1195 RSC.
- X. Zhang, R. Lin, X. Meng, W. Li, F. Chen and J. Hou, Iron Phthalocyanine/Two-Dimensional Metal-Organic Framework Composite Nanosheets for Enhanced Alkaline Hydrogen Evolution, Inorg. Chem., 2021, 60, 9987–9995 CrossRef CAS PubMed.
- Z. Zeng, X. Fang, W. Miao, Y. Liu, T. Maiyalagan and S. Mao, Electrochemically Sensing of Trichloroacetic Acid with Iron(II) Phthalocyanine and Zn-Based Metal Organic Framework Nanocomposites, ACS Sens., 2019, 4, 1934–1941 CrossRef CAS.
- A. Morozan, M. T. Sougrati, V. Goellner, D. Jones, L. Stievano and F. Jaouen, Effect of Furfuryl Alcohol on Metal Organic Framework-based Fe/N/C Electrocatalysts for Polymer Electrolyte Membrane Fuel Cells, Electrochim. Acta, 2014, 119, 192–205 CrossRef CAS.
- G. Song, Z. Wang, L. Wang, G. Li, M. Huang and F. Yin, Preparation of MOF(Fe) and its catalytic activity for oxygen reduction reaction in an alkaline electrolyte, Chin. J. Catal., 2014, 35, 185–195 CrossRef CAS.
- J.-W. Huang, Y.-B. Chen, J.-M. Yang, H.-B. Zhu and H. Yang, Boosting the oxygen reduction performance of MOF-5-derived Fe-N-C electrocatalysts via a dual strategy of cation-exchange and guest-encapsulation, Electrochim. Acta, 2021, 366, 137408 CrossRef CAS.
- W. Yang, Y. Zhang, X. Liu, L. Chen and J. Jia, In situ formed Fe-N doped metal organic framework@carbon nanotubes/graphene hybrids for a rechargeable Zn-air battery, Chem. Commun., 2017, 53, 12934–12937 RSC.
- J. Zhou, P. N. Duchesne, Y. Hu, J. Wang, P. Zhang, Y. Li, T. Regier and H. Dai, Fe-N bonding in a carbon nanotube-graphene complex for oxygen reduction: an XAS study, Phys. Chem. Chem. Phys., 2014, 16, 15787–15791 RSC.
- J. A. Varnell, E. M. Tse, C. Schulz, T. Fister, R. Haasch, J. Timoshenko, A. Frenkel and A. Gewirth, Identification of carbon-encapsulated iron nanoparticles as active species in non-precious metal oxygen reduction catalysts, Nat. Commun., 2016, 7, 12582 CrossRef CAS.
- X. C. Huang, Y. Y. Lin, J. P. Zhang and X. M. Chen, Ligand-directed strategy for zeolite-type metal-organic frameworks: zinc(II) imidazolates with unusual zeolitic topologies, Angew. Chem., Int. Ed., 2006, 45, 1557–1559 CrossRef CAS PubMed.
- Q. Yang, Q. Xu and H. L. Jiang, Metal-organic frameworks meet metal nanoparticles: synergistic effect for enhanced catalysis, Chem. Soc. Rev., 2017, 46, 4774–4808 RSC.
- Y. Guo, A. Dong, Q. Huang, Q. Li, Y. Hu, J. Qian and S. Huang, Hierarchical N-doped CNTs grafted onto MOF-derived porous carbon nanomaterials for efficient oxygen reduction, J. Colloid Interface Sci., 2020, 606, 1833–1841 CrossRef.
- Q. Huang, Y. Guo, X. Wang, L. Chai, J. Ding, L. Zhong, T.-T. Li, Y. Hu, J. Qian and S. Huang, In-MOF-derived ultrathin heteroatom-doped carbon nanosheets for improving oxygen reduction, Nanoscale, 2020, 12, 10019–10025 RSC.
- H. Xu, S. Xi, J. Li, S. Liu, P. Lyu, W. Yu, T. Sun, D.-C. Qi, Q. He, H. Xiao, M. Lin, J. Wu, J. Zhang and J. Lu, Chemical design and synthesis of superior single-atom electrocatalysts via in situ polymerization, J. Mater. Chem. A, 2020, 8, 17683–17690 RSC.
- J. P. McClure, O. Borodin, M. Olguin, D. Chu and P. S. Fedkiw, Sensitivity of Density Functional Theory Methodology for Oxygen Reduction Reaction Predictions on Fe-N4-Containing Graphitic Clusters, J. Phys. Chem. C, 2016, 120, 28545–28562 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2130792. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2qi00394e |
| This journal is © the Partner Organisations 2022 |