Isomaltulose alleviates acute colitis via modulating gut microbiota and the Treg/Th17 balance in mice†
Zihan
Zhou‡
a,
Shengnan
Yu‡
a,
Luwen
Cui‡
a,
Kaidi
Shao
a,
Hao
Pang
a,
Zhipeng
Wang
*b,
Ningning
He
 *a and
Shangyong
Li
*a
*a and
Shangyong
Li
*a
aSchool of Basic Medicine, Qingdao Medical College, Qingdao University, Qingdao, 266071, China. E-mail: heningning@qdu.edu.cn; lsy@qdu.edu.cn
bSchool of Marine Science and Engineering, Qingdao Agricultural University, Qingdao, 266109, China. E-mail: wangzp@ysfri.ac.cn
First published on 14th July 2022
Abstract
Food-grade isomaltulose exhibits significant modulation of gut microbiota and its metabolites in healthy populations. This study further explored the preventive therapeutic effect and anti-colitis potential of isomaltulose on dextran sulfate sodium-induced colitis in mice. Our results suggested that isomaltulose played a significant role in preventing colon shortening, reducing intestinal epithelial destruction and inhibiting inflammatory cell infiltration. Meanwhile, the isomaltulose supplement greatly reduced the production of pro-inflammatory cytokines and restored the balance between T helper type 17 (Th17) cells and regulatory T (Treg) cells. Pathway enrichment analysis for differentially expressed genes (DEGs) also indicated that the anti-inflammatory effect of isomaltulose was closely related to intestinal immunity. Moreover, the disturbed gut microbiota in ulcerative colitis (UC) was partially restored after treatment with isomaltulose. These results suggest that isomaltulose is a promising therapeutic agent for the prevention and adjunctive treatment of UC by maintaining intestinal immune homeostasis and remodeling the gut microbiota.
1. Introduction
Ulcerative colitis (UC) is a chronic recurrent inflammatory disease of the intestine involving various complications, including recurrent diarrhea, bloody stools, and tenesmus.1,2 Although its etiology is unclear, UC is closely related to the interaction between environmental factors, genetic susceptibility, and the immune system.3 Depending on the severity and course of UC, the therapeutics typically involve 5-aminosalicylic acid, corticosteroids and thiopurines, anti-TNF, anti-IL-12/23 p40 and anti-integrin or JAK inhibitors.4 These pharmacological treatments only alleviate symptomatic relief, not cure, and increase the risk of serious infection, malignant tumors and thrombosis.5 In recent years, gut microecological therapeutic strategies (e.g., probiotics and prebiotics) have played a greater role in the control and prevention of UC.Gut microbiota contains the largest reservoir of the human microbiome, providing metabolic, immunologic, and protective functions for human health.6,7 The integrity of the intestinal mucosal barrier is related to its interactions with the gut microbial ecosystem. In UC patients, the impaired intestinal epithelial barrier allows the passage of harmful gut microbiota, which activates immune cells and exacerbates the symptoms of UC. Prebiotics, as powerful modulators of gut microbiota, can directly or indirectly promote the upregulation and relocation of interepithelial tight junction proteins that form the microscopic scaffolding of the gut barrier, thereby repairing the damaged intestinal barrier in UC.7
Isomaltulose, composed of α-1,6-linked glucose and fructose, is naturally present in sugarcane or honey, and has been widely used as a sugar substitute in the food industry.8 Recent studies have reported its low glycemic index and non-carcinogenicity; thus isomaltulose was simultaneously used to improve insulin response, control hyperglycemia9 and reduce fat.10 Isomaltulose modulates the activity of specific probiotic bacteria and influences short-chain fatty acid (SCFA) production in in vitro co-culture systems.11 In addition, isomaltulose exerted beneficial effects on the regulation of gut microbiota by promoting the growth of beneficial bacteria, reducing harmful bacteria, and increasing the secretion of secondary bile acids and SCFAs in rats.12
In this study, we investigated the preventive effect of isomaltulose on dextran sulfate sodium (DSS)-induced acute colitis in mice. Our results suggested that isomaltulose significantly improved the preventative efficacy, promoted the colonic epithelial barrier integrity, modulated gut microbiota and ameliorated the regulatory T (Treg)/T helper type 17 (Th17) balance.
2. Materials and methods
2.1. Materials and supplies
The preparation and determination protocol of isomaltulose is according to our previous method.13 Antibodies against occludin (A2601) and β-actin (AC026) were purchased from ABclonal (Wuhan, China). Antibodies against zonula occludens-1 (ZO-1, A11417) were purchased from Servicebio Technology Co., Ltd (Wuhan, China). Antibodies against CD3 (100203), CD4 (100407), CD25 (102010), IL-17A (506915), Foxp3 (126407) and a mixture containing phorbol 12-myristate 13-acetate (PMA), ionomycin, Brefeldin A, etc. (423303, BioLegend, San Diego, CA, USA) were obtained from BioLegend (San Diego, CA, USA). DSS for the colitis model was purchased from MP Biomedicals (molecular weight: 36–50 kDa, Santa Ana, CA, USA). The experimental mice and standard rodent chow food were purchased from Jinan Pengyue Laboratory Animal Breeding Company (Jinan, China).2.2. Animal experiments
All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Qingdao University and experiments were approved by the Animal Ethics Committee of Medical College of Qingdao University (no. QDU-AEC-2022314). After 1 week of acclimation, C57BL/6J mice (18–20 g) were randomly grouped (n = 6) as shown in Fig. 1B: (1) NC group: no treatment for all 3 weeks; (2) DSS group: DSS-induced colitis in the last week; (3) IsoMTL group: isomaltulose (400 mg per kg per day) for all 3 weeks, and DSS-induced colitis in the last week.Acute colitis was induced by free drinking water containing 2.5% DSS. The body weight of the mice was recorded daily. Fecal samples were collected before the end of the experiment and stored at −80 °C under liquid nitrogen for gut microbiota analysis. After the mice were anesthetized with chloral hydrate, the eyeballs were removed for blood sampling. Then, the serum was obtained by centrifugation (3500 rpm, 4 °C, 30 min). Under sterile conditions, the spleen tissues of mice were collected for flow cytometry analysis. The entire colon was resected, and the length of the colon was measured. The collected serum samples and colon tissues were stored at −80 °C for further analysis.
2.3. Flow cytometry analysis
Primary spleen cells were extracted, diluted and counted for flow cytometry analysis (BD FACSVerse™, NJ, USA).14 To determine the number of Th17 cells, collected cells were pre-treated with the PMA mixture. For Th17 and Treg cell counts, CD3, CD4 and CD25 were used as surface markers, and IL-17A and Foxp3 were used as intracellular/intranuclear cytokines, respectively.2.4. Histopathological analysis
The collected tissues were dissected, then washed with saline three times and fixed with 10% buffered formalin (pH 7.2) solution overnight. Subsequently, the tissues were rung cut and placed on a glass slide and stained with hematoxylin and eosin (H&E) and alcian blue by Servicebio Technology Co., Ltd (Wuhan, China). The images were captured using a microscope with 200× magnification (E100, Nikon, Tokyo, Japan) and an imaging system (DS-U3, Nikon, Tokyo, Japan). Mucus-producing goblet cells were observed and counted with a light microscope (OLYMPUS, Tokyo, Japan).2.5. RNA extraction and quantitative real time PCR (RT-qPCR) analysis
The extraction of total RNA and reverse transcription were performed according to the instructions of the SparkJade kit (Jinan, China). The RT-qPCR primers are shown in Table S1.† The gene expression levels were normalized using GAPDH, and the relative quantification of gene expression was calculated by the 2−ΔΔCt method.2.6. Western blot and ELISA assay
Total protein of colon tissue samples was extracted using a cold RIPA lysis buffer (Solarbio, Beijing, China). Western blot analysis was performed as previously described.15 The ELISA kits were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The levels of proinflammatory cytokines in the serum were measured according to the instructions of the ELISA kit.2.7. Transcriptome analysis
The transcriptome analysis was performed by Berry Genomics Co., Ltd (Beijing, China). In brief, the total RNA was extracted from colon tissues and the RNA library was constructed. The quantitative library was analyzed by single-end sequencing on the Illumina Genome analyzer. Data analysis was processed using R software. The raw data of counts and FPKM was available (https://bioinfogo.org/ResKsumo/data). The Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses were performed using the “clusterProfiler”, “GOplot” package from R software for the differential expression genes (DEGs), which selected the standard fold change less than or equal to 2 and the P value less than 0.05.2.8. Gut microbiota analysis
Fecal DNA extraction and dilution were performed as previously described.15 The PCR was used to amplify the V3–V4 region of the bacterial 16S rRNA gene. The sequencing library was generated and sequenced using the NovaSeq 6000 platform (Shenzhen, Guangzhou, China). The following analysis of the gut microbiota structure and distribution was performed according to our previous studiy.162.9. Statistical analysis
Data were presented as mean ± SD. Statistical significance between two groups was calculated using Student's t-test and that between multiple groups was determined using one-way analysis of variance (ANOVA). All statistical analyses were calculated using GraphPad Prism 9.3 (La Jolla, CA, USA).3. Results
3.1. Isomaltulose effectively improves clinical symptoms of acute colitis in mice
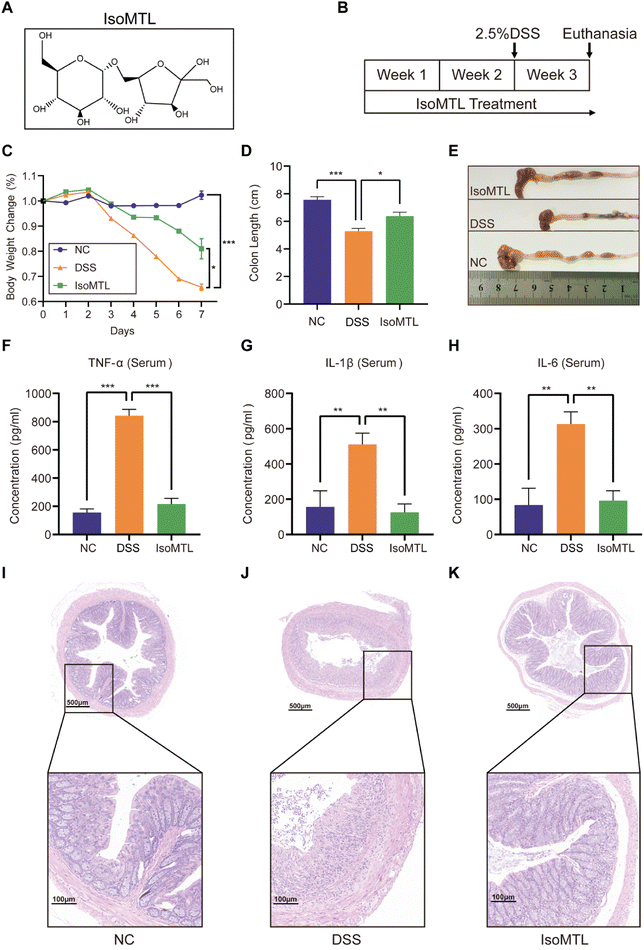
Isomaltulose is an isomer of sucrose (Fig. 1A), which was prepared by isomerisation. Briefly, the sucrose isomerase gene from Pantoea dispersa UQ68J was overexpressed in non-pathogenic yeast Yarrowia lipolytica and the sucrose isomerase was used to isomerize sucrose to produce high yields of isomaltulose. Further removal of monosaccharide by-products by converting into intracellular lipids resulted in isomaltulose with a purity of up to 97.8%.13 A DSS-induced acute colitis mouse model was used for investigating the preventive and protective effects of isomaltulose (Fig. 1B) according to our previous method.15 As shown in Fig. 1C, the body weights of mice in the DSS group decreased significantly within 7 days of DSS treatment (P < 0.001) and administration of isomaltulose (400 mg per kg per day) can significantly inhibit the weight loss caused by DSS (P < 0.05). Colon length is often evaluated as a key macroscopic indicator of colitis and is regarded as inversely associated with the severity of colitis.17 Consistent with this the colon length in the DSS group was obviously shorter (P < 0.001), which could be significantly reversed by isomaltulose (P < 0.05, Fig. 1D and E). These results indicated that isomaltulose supplementation significantly reduces the DSS-induced colitis symptoms. The pro-inflammatory cytokines (e.g. TNF-α, IL-1β and IL-6) commonly decide the onset and progression of inflammation.18 To elucidate the effect of isomaltulose on the inflammatory response, the levels of pro-inflammatory cytokines in serum were measured (Fig. 1F–H). Compared with NC group, TNF-α, IL-1β and IL-6 in the DSS group were significantly increased. Interestingly, isomaltulose supplementation significantly reduced the levels of these proinflammatory cytokines. These results suggested that the administration of isomaltulose significantly inhibited the expression of pro-inflammatory cytokines and reduced the inflammatory response in a DSS-induced colitis mouse model.To further explore the protective effect of isomaltulose on the colon, histological staining and examination of colon sections from mice were performed (Fig. 1I–K). Compared with the NC group, the histopathological examination of the DSS group showed infiltration of inflammatory cells, the absence of glands, mucosal epithelial necrosis (Fig. 1I and J). In contrast, isomaltulose supplementation significantly alleviated the severe histological damage induced by DSS, as manifested by the increased glandular numbers, mucosal epithelial integrity, and reduced inflammatory cell infiltration (Fig. 1K). Based on the above results, isomaltulose has a good protective effect on ameliorating the pathological damage of the colon caused by DSS.
3.2. Effect of isomaltulose on the integrity of the intestinal barrier
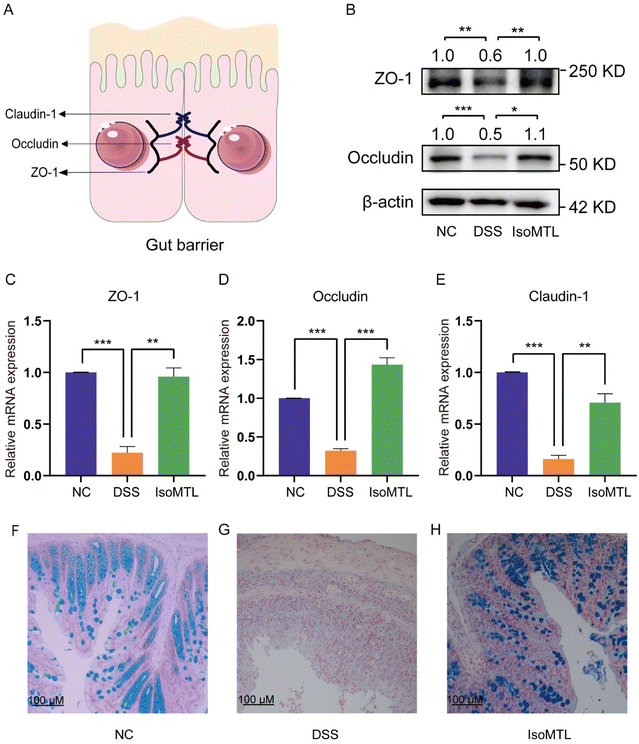
The major tight junction proteins ZO-1, occludin, and claudin-1 of the intestinal epithelium involved in mucosal healing form an important barrier in the gut, preventing the spread of potentially harmful pathogens and toxins19 (Fig. 2A). Therefore, to determine the regulatory role of isomaltulose in intestinal tight junction proteins, we measured the expression of major tight junction proteins using western blot (Fig. 2B) and RT-qPCR (Fig. 2C–E). As shown in Fig. 2B, isomaltulose significantly increased the protein expression of ZO-1 (P < 0.01) and occludin (P < 0.05). Meanwhile, isomaltulose restored the gene transcript levels of ZO-1 (P < 0.01), occludin (P < 0.001) and claudin-1 (P < 0.01) that were decreased by DSS (Fig. 2C–E).The mucus layer protects the intestine from the external environment, including pathogens.20 Alcian blue staining was further used to determine the mucosal barrier function (Fig. 2F–H). The number of mucus-producing goblet cells in the colons of mice administered with isomaltulose was significantly increased compared to mice in the DSS group. All these results suggested that isomaltulose treatment had a protective effect on the integrity of the intestinal barrier in mice with DSS-induced colitis.
3.3. Effect of isomaltulose on the intestinal immune response
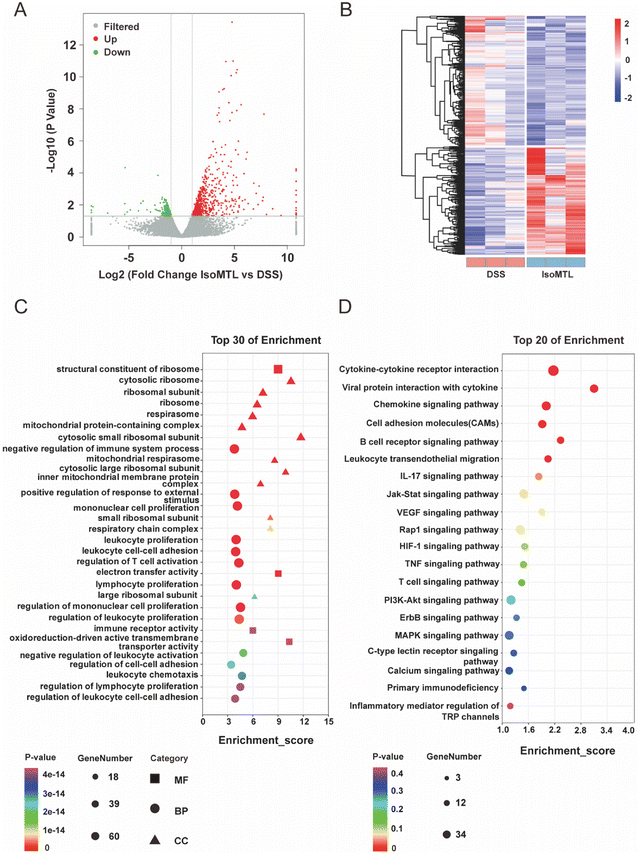
To investigate the mechanism by which isomaltulose alleviates colitis, we performed transcriptome analysis. As shown in Fig. 3A, the X-axis log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (fold change) represents the differential expression fold and up-regulation or not of DEGs is judged according to whether it is a positive value or not. Genes with larger differences were distributed closer to the two ends of the X-axis, which were the red and green dot parts. The Y-axis is represented by the −log
2 (fold change) represents the differential expression fold and up-regulation or not of DEGs is judged according to whether it is a positive value or not. Genes with larger differences were distributed closer to the two ends of the X-axis, which were the red and green dot parts. The Y-axis is represented by the −log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 p-value, which is inversely proportional to the p-value and proportional to the significance of the degree of difference. A total of 984 DEGs were obtained, including 739 upregulated and 245 downregulated genes (Fig. 3A). Furthermore, isomaltulose treatment significantly reversed the gene expression pattern of mice in DSS-induced colitis mice (Fig. 3B). GO and KEGG enrichment analyses of DEGs were performed to better understand the underlying molecular mechanism and function of isomaltulose. Most of the GO terms of the biological process (BP) were focused on the mononuclear cell proliferation, immune system processes, leukocyte cell–cell adhesion, and T cell activation (Fig. 3C). The KEGG pathway analysis for the top 20 immune related pathways showed that DEGs were involved in pathways such as the B cell receptor signaling pathway, cytokine–cytokine receptor interaction, IL-17 signaling pathway, TNF signaling pathway, T cell receptor signaling pathway and PI3K-Akt signaling pathway (Fig. 3D). In particular, enrichment in the IL-17 signaling pathway and T cell receptor signaling pathway suggests that the role of isomaltulose has an important relationship with Th17-mediated immunity.
10 p-value, which is inversely proportional to the p-value and proportional to the significance of the degree of difference. A total of 984 DEGs were obtained, including 739 upregulated and 245 downregulated genes (Fig. 3A). Furthermore, isomaltulose treatment significantly reversed the gene expression pattern of mice in DSS-induced colitis mice (Fig. 3B). GO and KEGG enrichment analyses of DEGs were performed to better understand the underlying molecular mechanism and function of isomaltulose. Most of the GO terms of the biological process (BP) were focused on the mononuclear cell proliferation, immune system processes, leukocyte cell–cell adhesion, and T cell activation (Fig. 3C). The KEGG pathway analysis for the top 20 immune related pathways showed that DEGs were involved in pathways such as the B cell receptor signaling pathway, cytokine–cytokine receptor interaction, IL-17 signaling pathway, TNF signaling pathway, T cell receptor signaling pathway and PI3K-Akt signaling pathway (Fig. 3D). In particular, enrichment in the IL-17 signaling pathway and T cell receptor signaling pathway suggests that the role of isomaltulose has an important relationship with Th17-mediated immunity.
3.4. Isomaltulose improves the balance of Treg/Th17 cells
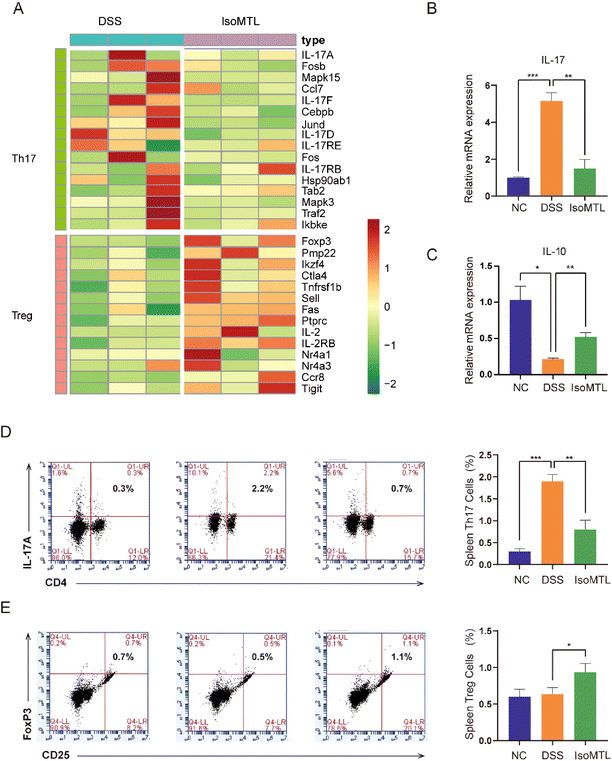
IL-10 is the main product of Treg cells and plays a key role in Treg cell-mediated remission of colitis.21 IL-17 is the main effector of Th17 cells,22 whose family includes six-member ligands (IL-17A–IL-17F) and 5 receptors (IL-17RA–IL-17RD and SEF),23 and the pathogenic role of IL-17A has been well documented in UC.24 Transcriptomic analysis of the Th17 and Treg related signaling pathways showed that DSS induction could upregulate the gene expression of the Th17 related signaling pathway and reduce the gene expression of the Treg related signaling pathway. Administration of isomaltulose can reverse the expression pattern of genes in Th17 and Treg signaling pathways (Fig. 4A). Specifically, the gene expression of IL-17A, IL-17F and IL-17RB was significantly decreased and the main effector of Treg cells Foxp3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 was highly expressed in the IsoMTL group. In addition, IL-2 inhibited Th17 differentiation25 and promoted the differentiation and function of Treg cells,26 which was significantly expressed in the IsoMTL group. Therefore, this result indicated that isomaltulose can regulate the balance of Treg/Th17 cells at the gene level. To further explore the function of isomaltulose on the balance of Treg/Th17 cells, we used RT-qPCR (Fig. 4B and C) and flow cytometry (Fig. 4D and E) to detect changes in cytokine production and Treg/Th17 cell differentiation. Compared to the NC group, the DSS treatment group significantly increased the expression of IL-17 (P < 0.001) and decreased the expression of IL-10 (P < 0.05). Compared with the DSS group, isomaltulose supplementation significantly increased the expression of IL-10 (P < 0.01), while greatly decreasing the expression of IL-17 (P < 0.01) in colitis mice (Fig. 4B and C). Consistent with this isomaltulose significantly decreased the number of Th17 cells (P < 0.01, Fig. 4D) and increased the number of Treg cells in the spleen of the DSS-induced colitis model (P < 0.05, Fig. 4E). These results indicated that isomaltulose could restore intestinal immune homeostasis in the mouse model of UC by rebalancing the Treg/Th17 cell populations and the production of pro-inflammatory and anti-inflammatory cytokines.
22 was highly expressed in the IsoMTL group. In addition, IL-2 inhibited Th17 differentiation25 and promoted the differentiation and function of Treg cells,26 which was significantly expressed in the IsoMTL group. Therefore, this result indicated that isomaltulose can regulate the balance of Treg/Th17 cells at the gene level. To further explore the function of isomaltulose on the balance of Treg/Th17 cells, we used RT-qPCR (Fig. 4B and C) and flow cytometry (Fig. 4D and E) to detect changes in cytokine production and Treg/Th17 cell differentiation. Compared to the NC group, the DSS treatment group significantly increased the expression of IL-17 (P < 0.001) and decreased the expression of IL-10 (P < 0.05). Compared with the DSS group, isomaltulose supplementation significantly increased the expression of IL-10 (P < 0.01), while greatly decreasing the expression of IL-17 (P < 0.01) in colitis mice (Fig. 4B and C). Consistent with this isomaltulose significantly decreased the number of Th17 cells (P < 0.01, Fig. 4D) and increased the number of Treg cells in the spleen of the DSS-induced colitis model (P < 0.05, Fig. 4E). These results indicated that isomaltulose could restore intestinal immune homeostasis in the mouse model of UC by rebalancing the Treg/Th17 cell populations and the production of pro-inflammatory and anti-inflammatory cytokines.
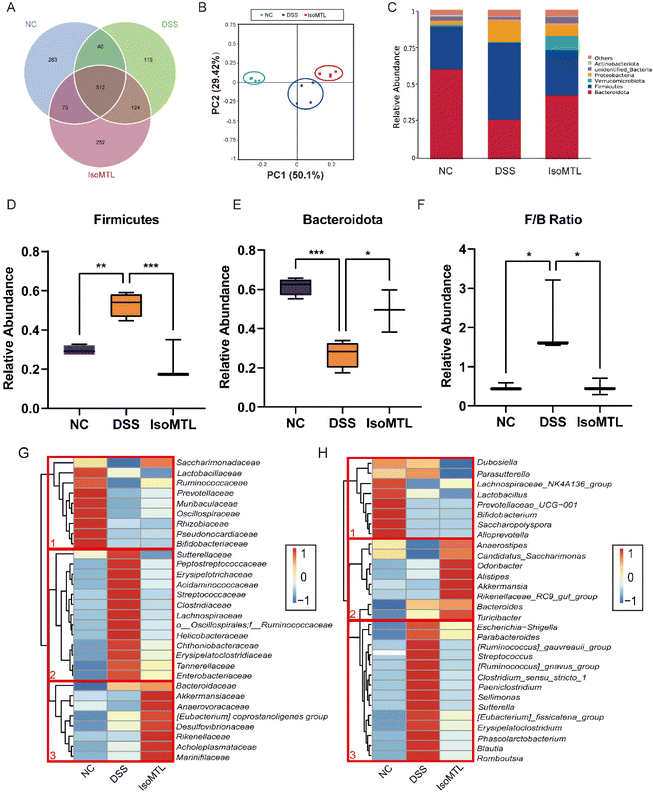
3.5. Isomaltulose alters the relative abundance of gut microbiota
The regulatory effect of isomaltulose on gut microbiota was further studied by 16S rRNA sequencing. The common and unique OTUs were displayed to prove that DSS induction and isomaltulose treatment changed the composition of gut microbiota (Fig. 5A). The microbiota composition in each group was clearly clustered using weighted UniFrac principal coordinates analysis (PCoA). In PCoA analysis, the percentages presented along the axes represent the overall fraction of dissimilarity captured by PC1 (50.1%) and PC2 (29.42%) (Fig. 5B). Compared to the NC group, the DSS administration significantly shifted the structure of gut microbiota and isomaltulose reversed this shift to some extent. Simultaneously, the relative abundance of gut bacteria was compared at the phylum level (Fig. 5C). We observed that isomaltulose significantly reversed the DSS-induced changes in Firmicutes, Bacteroidota, and Proteobacteria. As shown in Fig. 5D–F, mice fed with DSS presented a drastic reduction in the F/B ratio, and isomaltulose treatment reduced the relative abundance of Firmicutes and increased the relative abundance of Bacteroidota caused by DSS. Compared to the DSS group, the relative abundance of Proteobacteria and Actinobacteriota was partly reversed after the administration of isomaltulose (Fig. S1A–C†). It is worth noting that the relative abundance of Verrucomicrobiota was significantly increased, which could increase the content of SCFAs.In order to further determine the composition differences of gut microbiota in mice, the changes of gut microbiota at the level of family and genus were observed (Fig. 5G and H). The DSS group showed clear differences from the NC group at both the family and genus levels, indicating that the DSS-induced colitis mice caused obvious gut dysbiosis. At the family level, the gut microbiota of cluster 2 increased after DSS treatment, while the gut microbiota of cluster 1 decreased (Fig. 5G). For cluster 1, the abundance of Bifidobacteriaceae, Pseudonocardiaceae, and Lactobacillaceae was higher in the NC group than in the DSS and IsoMTL groups. After the treatment with isomaltulose, the changes of gut microbiota in cluster 2 were obviously reversed, and the bacteria were partially restored to the level of the NC group. Meanwhile, the gut microbiota of cluster 3 was obviously elevated under the effect of isomaltulose, which mainly consisted of beneficial bacteria (Akkermansiaceae, Marinifilaceae, Anaerovoracaceae, etc.). At the genus level, the gut microbiota in cluster 1 was decreased and that in cluster 3 was increased after DSS treatment (Fig. 5H). After isomaltulose treatment, the gut microbiota of cluster 3 was restored to the control level, and the gut microbiota of cluster 2 was significantly increased (e.g., Akkermansia and Alistipes). These results further suggested that isomaltulose might reduce the effect of DSS on gut microbiota by increasing partially the content of beneficial bacteria, as well as restoring the dysbiosis of gut microbiota.
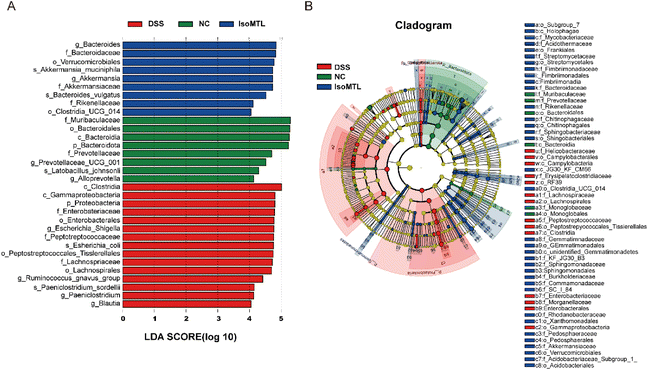
The LEfSe method was used to analyze the difference between the samples, which could further show the microbial diversity (Fig. 6A and B). The larger the LDA score, the greater the influence of species abundance on the difference effect. Setting LDA score >2 and P < 0.05 as a meaningful cut-off, LEfSe identified 32 bacterial taxa which were significantly different between the three groups and could represent typical characteristics of different groups. As Fig. 6A shows, the DSS group had 15 dominant microorganisms, while the IsoMTL group had the other 9 dominant microorganisms, including families Bacteroidaceae, genus Bacteroides and phylum Gemmatimonadetes, etc., which was also verified by the differential-abundance microbial cladogram (Fig. 6B). Thus, LEfSe analysis illustrated that multiple taxonomic differences were found among three groups of DSS, NC and IsoMTL.
3.6 Association between gut microbiota and UC-related symptoms and Treg/Th17-related immunity
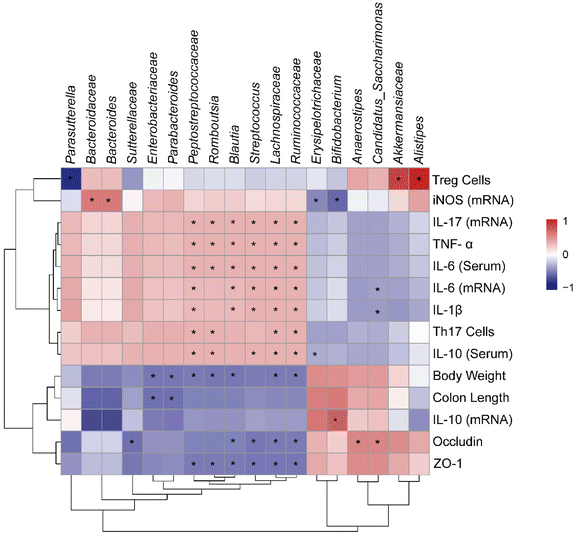
Correlation analysis can be used to verify the correlation between different data, so we used Spearman correlation analysis to examine the relationship between inflammatory factors and gut microbiota. Based on our results, inflammatory factors (TNF-α, IL-6, IL-17 and IL-1β) and tight junction proteins (ZO-1 and occludin) were highly correlated with changes in gut microbiota. As shown in Fig. 7, genus of Peptostreptococcaceae, Romboutsia, Blautia, Streptococcus, Lachnospiraceae and Ruminococcaceae showed a significantly positive correlation with inflammation factors and displayed a negative correlation with tight junction proteins. Meanwhile, the presence of Enterobacteriaceae and Parabacteroides significantly reduced the body weight and colon length. Notably, Akkermansiaceae and Alistipes were significantly positively correlated with Treg cells, while Parasutterella was significantly negatively correlated. Besides, there was a significantly positive correlation between the abundance of Bacteroidaceae and Bacteroides with the level of iNOS. These results clearly reveal the relationship between changes in different bacteria and host biological effects, indicating that changes in the gut microbiota are closely associated with inflammatory responses, especially tight junction proteins.4. Discussion
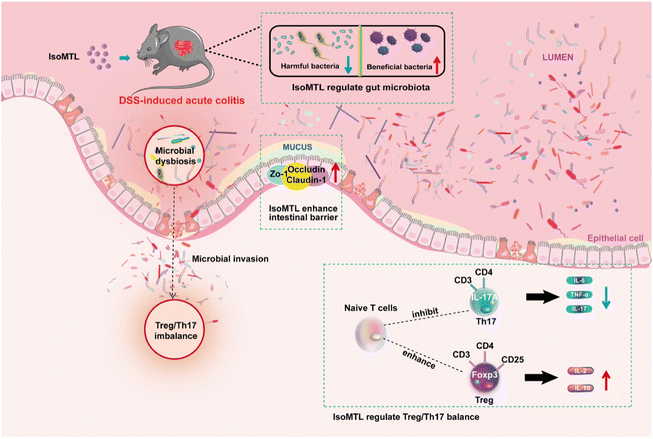
Isomaltulose is an isomer of sucrose27 derived from natural sources such as honey (<1%) and sugarcane,28 whose sweetness is approximately half that of sucrose (0.4–0.45).29 We previously developed engineered yeast to obtain high-purity isomaltulose.13 In healthy and obese patients, isomaltulose supplementation had been reported to reduce insulin resistance and elevated blood pressure, while benefiting endothelial function and cardiovascular health.30,31 Herein, we further indicated that isomaltulose supplementation was significantly effective in improving the prevention of acute colitis in mice, promoting colonic epithelial barrier integrity, reducing inflammatory cytokines, modulating gut microbiota and ameliorating Treg/Th17 balance (Fig. 8). These results suggest that isomaltulose is a promising therapeutic agent for the prevention and adjuvant treatment of UC by maintaining intestinal homeostasis.The pathogenesis of UC is closely related to a compromised intestinal barrier, where hyperpermeability of the gut increases pathogen invasion and leads to translocation of the gut microbiota.32 Tight junction proteins (e.g., ZO-1, claudin-1 and occludin) play an important role in maintaining cellular integrity and regulating intestinal barrier permeability by regulating the paracellular permeability of water, ions and macromolecules in adjacent cells.33 Reduced expression of intestinal tight junction proteins can cause local or systemic inflammatory and immune responses in the gut, as well as endotoxin invasion, intestinal bacterial translocation and disruption of the intestinal mucosal barrier, which can further exacerbate local or systemic inflammatory and immune responses in the gut.34–37 Previous studies have identified the role of isomaltulose in reduction of intestinal pathogens.12 However, only a few studies have focused on the effects of isomaltulose on tight junctions and related intestinal barrier. In this study, morphology (Fig. 1I–J), transcript and protein analysis (Fig. 2) all demonstrated the excellent therapeutic role of isomaltulose in improving the intestinal barrier and tight junctions disrupted by DSS, further explaining the action model of its role in UC adjuvant therapy.
Recently, isomaltulose has been shown to alter the structure and composition of the gut microbiota, thereby increasing the abundance of beneficial microorganisms, suggesting its potential prebiotic activity in the normal polulation.12 Herein, we demonstrated that isomaltulose treatment greatly modulated the overall structural and genus/species-specific changes in the dysfunctional gut microbiota of DSS-induced colitis mice (Fig. 5 and 6). An increase in Lachnospiraceae can cause immune overresponse triggering intestinal inflammation.38 Meanwhile, a study had shown that Lachnospiraceae and Streptococcaceae were increased in the gut in mice with colitis,39 which was consistent with our results (Fig. 5G). After treatment with isomaltulose, the number of Lachnospiraceae and Streptococcaceae were significantly reduced. He et al. proposed that prebiotic unsaturated alginate oligosaccharides could improve colitis via modulating gut microbiota, such as reducing the abundance of Acidaminococcaceae and Lachnospiraceae, while increasing the abundance of Anaerobiospirillum, which showed a similar pattern to isomaltulose.40 Furthermore, isomaltulose treatment significantly increased the number of some anti-inflammatory bacteria (e.g., Bacteroides, etc.) and the amount of Akkermansia, a typically beneficial bacteria known to be positively associated with gut integrity.41 These findings could contribute to the development of isomaltulose as a specialty health food for UC patients.
Treg/Th17 cell imbalance is a key factor in the development of UC,42 hence targeting Treg/Th17 cell regulation has emerged as a potential strategy for the prevention and treatment of UC.43–45 Bursts of inflammation are the main factor in the pathogenesis of UC, and the production of cytokine storms contributes directly to this process.46,47 In this study, the changes of Treg and Th17 indicated that isomaltulose treatment improved the balance of Treg/Th17 cells, which has a similar immune mechanism to an active ingredient of Chinese herbal medicine used to treat UC called baicalin.48 Spearman analysis further showed that changes in the gut microbiota were strongly correlated with associated changes in the gut barrier, inflammatory factors and Treg/Th17 cells (Fig. 7).
5. Conclusion
In this study, a food-grade supplement of isomaltulose significantly ameliorates DSS-induced UC symptoms and gut microbiota dysbiosis in mice. Meanwhile, the changes of specific families/genus in gut microbiota were significantly associated with UC-related symptoms, tight junction proteins, inflammatory factors, and the Treg/Th17 cell balance. Our results suggest that isomaltulose is a promising therapeutic agent for UC prevention and adjuvant treatment via maintaining intestinal immune homeostasis and remodeling intestinal microbiota.Abbreviations
| DSS | Dextran sodium sulfate |
| ELISA | Enzyme linked immunosorbent assay |
| H&E | Hematoxylin and eosin |
| IL | Interleukins |
| SCFAs | Short chain fatty acids |
| Th17 | T helper 17 cell |
| TNF | Tumor necrosis factor |
| Treg | T regulatory cell |
| ZO-1 | Zonula occludens-1 |
| DEGs | Differentially expressed genes |
| PMA | Phorbol 12-myristate 13-acetate |
| GO | Gene ontology |
| KEGG | Kyoto encyclopedia of genes and genomes |
Author contributions
S. L. and N. H. designed the study; Z. Z., S. Y., L. C., H. P. and K. S. performed the experiments; Z. Z. and N. H. analyzed the data and made figures; Z. Z., L. C. and S. Y. wrote the manuscript. S. L., N. H., Z. Z., L. C. and S. Y. reviewed/edited the manuscript. All authors approved the final manuscript.Conflicts of interest
The authors state no conflicts of interest.Acknowledgements
This research was supported by the National Natural Science Foundation of China (NSFC) (Grant No. 31900031), the Natural Science Foundation of Shandong Province (Grant No. ZR2019BD027), and the Shandong Youth Science and Technology Innovation Team.References
- S. M. Adams and P. H. Bornemann, Ulcerative colitis, Am. Fam. Physician, 2013, 87, 699–705 Search PubMed.
- R. J. Xavier and D. K. Podolsky, Unravelling the pathogenesis of inflammatory bowel disease, Nature, 2007, 448, 427–434 CrossRef CAS PubMed.
- M. Eisenstein, Ulcerative colitis: towards remission, Nature, 2018, 563, S33 CrossRef CAS PubMed.
- T. Kobayashi, B. Siegmund, C. Le Berre, S. C. Wei, M. Ferrante, B. Shen, C. N. Bernstein, S. Danese, L. Peyrin-Biroulet and T. Hibi, Ulcerative colitis, Nat. Rev. Dis. Primers, 2020, 6, 73 CrossRef PubMed.
- R. Al-Horani, E. Spanudakis and B. Hamad, The market for ulcerative colitis, Nat. Rev. Drug Discovery, 2022, 21, 15–16 CrossRef CAS PubMed.
- X. Y. Guo, X. J. Liu and J. Y. Hao, Gut microbiota in ulcerative colitis: insights on pathogenesis and treatment, J. Dig. Dis., 2020, 21, 147–159 CrossRef PubMed.
- S. M. Jandhyala, R. Talukdar, C. Subramanyam, H. Vuyyuru, M. Sasikala and D. Nageshwar Reddy, Role of the normal gut microbiota, World J. Gastroenterol., 2015, 21, 8787–8803 CrossRef CAS PubMed.
- T. Amano, S. Katayama, Y. Okamoto, J. Otsuka, N. Fujii, G. P. Kenny, T. Nishiyasu, Y. Enoki and D. Maejima, Comparisons of isomaltulose, sucrose, and mixture of glucose and fructose ingestions on postexercise hydration state in young men, Eur. J. Nutr., 2021, 60, 4519–4529 CrossRef CAS PubMed.
- E. Sokołowska, A. Sadowska, D. Sawicka, I. Kotulska-Bąblińska and H. Car, A head-to-head comparison review of biological and toxicological studies of isomaltulose, d-tagatose, and trehalose on glycemic control, Crit. Rev. Food Sci. Nutr., 2021, 1–26 Search PubMed.
- H. Lightowler, L. Schweitzer, S. Theis and C. J. Henry, Changes in Weight and Substrate Oxidation in Overweight Adults Following Isomaltulose Intake During a 12-Week Weight Loss Intervention: A Randomized, Double-Blind, Controlled Trial, Nutrients, 2019, 11, 2367 CrossRef CAS PubMed.
- H.-H. Su, R. Xu, Z.-D. Yang, Y. Guo, J. Gao, L. Mo, Y.-F. Gao, H. Cheng, P.-J. Zhang and J.-S. Huang, Green synthesis of isomaltulose from cane molasses by an immobilized recombinant Escherichia coli strain and its prebiotic activity, LWT–Food Sci. Technol., 2021, 143, 111054 CrossRef CAS.
- Z. D. Yang, Y. S. Guo, J. S. Huang, Y. F. Gao, F. Peng, R. Y. Xu, H. H. Su and P. J. Zhang, Isomaltulose Exhibits Prebiotic Activity, and Modulates Gut Microbiota, the Production of Short Chain Fatty Acids, and Secondary Bile Acids in Rats, Molecules, 2021, 26, 2464 CrossRef CAS PubMed.
- P. Zhang, Z. P. Wang, J. Sheng, Y. Zheng, X. F. Ji, H. X. Zhou, X. Y. Liu and Z. M. Chi, High and efficient isomaltulose production using an engineered Yarrowia lipolytica strain, Bioresour. Technol., 2018, 265, 577–580 CrossRef CAS PubMed.
- M. Sendler, C. van den Brandt, J. Glaubitz, A. Wilden, J. Golchert, F. U. Weiss, G. Homuth, L. L. De Freitas Chama, N. Mishra, U. M. Mahajan, L. Bossaller, U. Völker, B. M. Bröker, J. Mayerle and M. M. Lerch, NLRP3 Inflammasome Regulates Development of Systemic Inflammatory Response and Compensatory Anti-Inflammatory Response Syndromes in Mice With Acute Pancreatitis, Gastroenterology, 2020, 158, 253–269 CrossRef CAS PubMed .e214.
- N. He, Y. Wang, Z. Zhou, N. Liu, S. Jung, M. S. Lee and S. Li, Preventive and Prebiotic Effect of α-Galacto-Oligosaccharide against Dextran Sodium Sulfate-Induced Colitis and Gut Microbiota Dysbiosis in Mice, J. Agric. Food Chem., 2021, 69, 9597–9607 CrossRef CAS PubMed.
- S. Li, L. Wang, B. Liu and N. He, Unsaturated alginate oligosaccharides attenuated obesity-related metabolic abnormalities by modulating gut microbiota in high-fat-diet mice, Food Funct., 2020, 11, 4773–4784 RSC.
- X. Zhang, Q. Zou, B. Zhao, J. Zhang, W. Zhao, Y. Li, R. Liu, X. Liu and Z. Liu, Effects of alternate-day fasting, time-restricted fasting and intermittent energy restriction DSS-induced on colitis and behavioral disorders, Redox Biol., 2020, 32, 101535 CrossRef CAS PubMed.
- M. Coskun, S. Vermeire and O. H. Nielsen, Novel Targeted Therapies for Inflammatory Bowel Disease, Trends Pharmacol. Sci., 2017, 38, 127–142 CrossRef CAS PubMed.
- B. Allam-Ndoul, S. Castonguay-Paradis and A. Veilleux, Gut Microbiota and Intestinal Trans-Epithelial Permeability, Int. J. Mol. Sci., 2020, 21, 6402 CrossRef CAS PubMed.
- M. E. Johansson and G. C. Hansson, Immunological aspects of intestinal mucus and mucins, Nat. Rev. Immunol., 2016, 16, 639–649 CrossRef CAS PubMed.
- M. C. Fantini, C. Becker, I. Tubbe, A. Nikolaev, H. A. Lehr, P. Galle and M. F. Neurath, Transforming growth factor beta induced FoxP3+ regulatory T cells suppress Th1 mediated experimental colitis, Gut, 2006, 55, 671–680 CrossRef CAS PubMed.
- T. Van Nguyen, C. H. Piao, Y. J. Fan, D. U. Shin, S. Y. Kim, H. J. Song, C. H. Song, H. S. Shin and O. H. Chai, Anti-allergic rhinitis activity of α-lipoic acid via balancing Th17/Treg expression and enhancing Nrf2/HO-1 pathway signaling, Sci. Rep., 2020, 10, 12528 CrossRef CAS PubMed.
- N. C. Brembilla, L. Senra and W. H. Boehncke, The IL-17 Family of Cytokines in Psoriasis: IL-17A and Beyond, Front. Immunol., 2018, 9, 1682 CrossRef PubMed.
- S. L. Gaffen, R. Jain, A. V. Garg and D. J. Cua, The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing, Nat. Rev. Immunol., 2014, 14, 585–600 CrossRef CAS PubMed.
- A. Laurence, C. M. Tato, T. S. Davidson, Y. Kanno, Z. Chen, Z. Yao, R. B. Blank, F. Meylan, R. Siegel, L. Hennighausen, E. M. Shevach and J. O'Shea, Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation, Immunity, 2007, 26, 371–381 CrossRef CAS PubMed.
- R. Ghosh, R. Dey, R. Sawoo and B. Bishayi, Neutralization of IL-17 and treatment with IL-2 protects septic arthritis by regulating free radical production and antioxidant enzymes in Th17 and Tregs: An immunomodulatory TLR2 versus TNFR response, Cell. Immunol., 2021, 370, 104441 CrossRef CAS PubMed.
- W. Mu, W. Li, X. Wang, T. Zhang and B. Jiang, Current studies on sucrose isomerase and biological isomaltulose production using sucrose isomerase, Appl. Microbiol. Biotechnol., 2014, 98, 6569–6582 CrossRef CAS PubMed.
- Z. P. Wang, Q. Q. Wang, S. Liu, X. F. Liu, X. J. Yu and Y. L. Jiang, Efficient Conversion of Cane Molasses Towards High-Purity Isomaltulose and Cellular Lipid Using an Engineered Yarrowia lipolytica Strain in Fed-Batch Fermentation, Molecules, 2019, 24, 1228 CrossRef PubMed.
- B. A. Lina, D. Jonker and G. Kozianowski, Isomaltulose (Palatinose): a review of biological and toxicological studies, Food Chem. Toxicol., 2002, 40, 1375–1381 CrossRef CAS PubMed.
- H. Arai, A. Mizuno, M. Sakuma, M. Fukaya, K. Matsuo, K. Muto, H. Sasaki, M. Matsuura, H. Okumura, H. Yamamoto, Y. Taketani, T. Doxxi and E. Takeda, Effects of a palatinose-based liquid diet (Inslow) on glycemic control and the second-meal effect in healthy men, Metabolism, 2007, 56, 115–121 CrossRef CAS PubMed.
- E. de Groot, L. Schweitzer and S. Theis, Efficacy of Isomaltulose Compared to Sucrose in Modulating Endothelial Function in Overweight Adults, Nutrients, 2020, 12, 141 CrossRef CAS PubMed.
- M. Coskun, Intestinal epithelium in inflammatory bowel disease, Front. Med., 2014, 1, 24 Search PubMed.
- Y. Jian, D. Zhang, M. Liu, Y. Wang and Z. X. Xu, The Impact of Gut Microbiota on Radiation-Induced Enteritis, Front. Cell. Infect. Microbiol., 2021, 11, 586392 CrossRef CAS PubMed.
- L. W. Kaminsky, R. Al-Sadi and T. Y. Ma, IL-1β and the Intestinal Epithelial Tight Junction Barrier, Front. Immunol., 2021, 12, 767456 CrossRef CAS PubMed.
- E. Russo, F. Giudici, C. Fiorindi, F. Ficari, S. Scaringi and A. Amedei, Immunomodulating Activity and Therapeutic Effects of Short Chain Fatty Acids and Tryptophan Post-biotics in Inflammatory Bowel Disease, Front. Immunol., 2019, 10, 2754 CrossRef CAS PubMed.
- G. P. Ramos and K. A. Papadakis, Mechanisms of Disease: Inflammatory Bowel Diseases, Mayo Clin. Proc., 2019, 94, 155–165 CrossRef CAS PubMed.
- S. Li, M. Jin, Y. Wu, S. Jung, D. Li, N. He and M. S. Lee, An efficient enzyme-triggered controlled release system for colon-targeted oral delivery to combat dextran sodium sulfate (DSS)-induced colitis in mice, Drug Delivery, 2021, 28, 1120–1131 CrossRef CAS PubMed.
- M. N. Quraishi, M. Sergeant, G. Kay, T. Iqbal, J. Chan, C. Constantinidou, P. Trivedi, J. Ferguson, D. H. Adams, M. Pallen and G. M. Hirschfield, The gut-adherent microbiota of PSC-IBD is distinct to that of IBD, Gut, 2017, 66, 386–388 CrossRef PubMed.
- H. Zeng, S. L. Ishaq, F. Q. Zhao and A. G. Wright, Colonic inflammation accompanies an increase of β-catenin signaling and Lachnospiraceae/Streptococcaceae bacteria in the hind gut of high-fat diet-fed mice, J. Nutr. Biochem., 2016, 35, 30–36 CrossRef CAS PubMed.
- N. He, Y. Yang, H. Wang, N. Liu, Z. Yang and S. Li, Unsaturated alginate oligosaccharides (UAOS) protects against dextran sulfate sodium-induced colitis associated with regulation of gut microbiota, J. Funct. Foods, 2021, 83, 104536 CrossRef CAS.
- A. Everard, C. Belzer, L. Geurts, J. P. Ouwerkerk, C. Druart, L. B. Bindels, Y. Guiot, M. Derrien, G. G. Muccioli, N. M. Delzenne, W. M. de Vos and P. D. Cani, Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 9066–9071 CrossRef CAS PubMed.
- A. Ueno, L. Jeffery, T. Kobayashi, T. Hibi, S. Ghosh and H. Jijon, Th17 plasticity and its relevance to inflammatory bowel disease, J. Autoimmun., 2018, 87, 38–49 CrossRef CAS PubMed.
- R. Yu, F. Zuo, H. Ma and S. Chen, Exopolysaccharide-Producing Bifidobacterium adolescentis Strains with Similar Adhesion Property Induce Differential Regulation of Inflammatory Immune Response in Treg/Th17 Axis of DSS-Colitis Mice, Nutrients, 2019, 11, 782 CrossRef CAS PubMed.
- S. Wen, L. He, Z. Zhong, R. Zhao, S. Weng, H. Mi and F. Liu, Stigmasterol Restores the Balance of Treg/Th17 Cells by Activating the Butyrate-PPARγ Axis in Colitis, Front. Immunol., 2021, 12, 741934 CrossRef CAS PubMed.
- C. Wei, J. Y. Wang, F. Xiong, B. H. Wu, M. H. Luo, Z. C. Yu, T. T. Liu, D. F. Li, Q. Tang, Y. X. Li, D. G. Zhang, Z. L. Xu, H. T. Jin, L. S. Wang and J. Yao, Curcumin ameliorates DSS-induced colitis in mice by regulating the Treg/Th17 signaling pathway, Mol. Med. Rep., 2021, 23, 34 CAS.
- T. Takagawa, A. Kitani, I. Fuss, B. Levine, S. R. Brant, I. Peter, M. Tajima, S. Nakamura and W. Strober, An increase in LRRK2 suppresses autophagy and enhances Dectin-1-induced immunity in a mouse model of colitis, Sci. Transl. Med., 2018, 10, eaan8162 CrossRef PubMed.
- S. Schreiber, K. Aden, J. P. Bernardes, C. Conrad, F. Tran, H. Höper, V. Volk, N. Mishra, J. I. Blase, S. Nikolaus, J. Bethge, T. Kühbacher, C. Röcken, M. Chen, I. Cottingham, N. Petri, B. B. Rasmussen, J. Lokau, L. Lenk, C. Garbers, F. Feuerhake, S. Rose-John, G. H. Waetzig and P. Rosenstiel, Therapeutic Interleukin-6 Trans-signaling Inhibition by Olamkicept (sgp130Fc) in Patients with Active Inflammatory Bowel Disease, Gastroenterology, 2021, 160, 2354–2366 CrossRef CAS PubMed .e2311.
- L. Zhu, L. Z. Xu, S. Zhao, Z. F. Shen, H. Shen and L. B. Zhan, Protective effect of baicalin on the regulation of Treg/Th17 balance, gut microbiota and short-chain fatty acids in rats with ulcerative colitis, Appl. Microbiol. Biotechnol., 2020, 104, 5449–5460 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2fo01157c |
| ‡ These authors have contributed equally to this work and share first authorship. |
| This journal is © The Royal Society of Chemistry 2022 |