Open Access Article
Open Access ArticleSelectivity of hosts for guests by liquid-assisted grinding: differences between solution and mechanochemistry†
Jean
Lombard
 ,
Heinrich
Laker
,
Heinrich
Laker
 ,
Francis
Prins
,
Helene
Wahl
,
Francis
Prins
,
Helene
Wahl
 ,
Tanya
le Roex
,
Tanya
le Roex
 and
Delia A.
Haynes
and
Delia A.
Haynes
 *
*
Department of Chemistry & Polymer Science, Stellenbosch University, P. Bag X1, Matieland, 7602, South Africa. E-mail: dhaynes@sun.ac.za
First published on 6th October 2021
Abstract
Selectivity for particular guests in two host–guest systems has been investigated using both solution-based and mechanochemical methods. Selectivity was shown to occur in both systems when using mechanochemistry. The selectivity profile obtained using mechanochemistry can be different from that observed when carrying out analogous selectivity experiments in solution.
The selectivity of host–guest systems for particular guests has long been a topic of interest to solid-state chemists.1 Hosts that exhibit selectivity for one guest over another have potential use in for example separation,2 or removal of impurities or toxins.3 Understanding, and ultimately controlling, such selectivity is an essential part of developing such systems for applications.
Recently, our group has become interested in host–guest systems based on the pamoate ion. We have studied the porosity4,5 of one such system, 3,4-lutidine pamoate hemihydrate (crystallised as its THF solvate), and have also investigated the selectivity of this material with respect to the sorption of particular guests from both the gas and liquid phases.6 We have also studied the selectivity behaviour of a related system, the isostructural solvates of 4-phenylpyridinium pamoate, when crystallised from mixtures of particular solvents.7
Mechanochemical synthesis is known to result in different chemical reactivity, different reaction pathways and different selectivity in terms of the product of the reaction when compared to solution methods.8 Control of polymorphic form can also be achieved using mechanochemical methods,9 and mechanochemistry is becoming an essential screening tool for different solid forms in the pharmaceutical industry.10 There have been several reports on competitive selection of co-former in co-crystals using mechanochemistry.11 However, there have been very few studies reported investigating the selectivity of hosts for particular guests using mechanochemistry. In fact we are aware of only one such study: Caira et al. reported the selectivity behaviour of an organic host molecule (1,1,6,6-tetraphenylhexa-2,4-diyne-1,6-diol) towards 2-, 3- and 4-aminobenzonitrile.12 The authors reported similar selectivity profiles from solution and solid-state methods for two solvents, and somewhat different selectivity profiles for the third solvent. The authors comment that the mechanochemical reactions ‘follow the trends obtained in the solution experiments’.12
Our combined interest in the host–guest chemistry of pamoate salts, and in mechanochemistry, led us to further investigate the selectivity behaviour of a new pamoate-based host system, 1,10-phenanthrolinium pamoate (1, Scheme 1) using mechanochemistry.
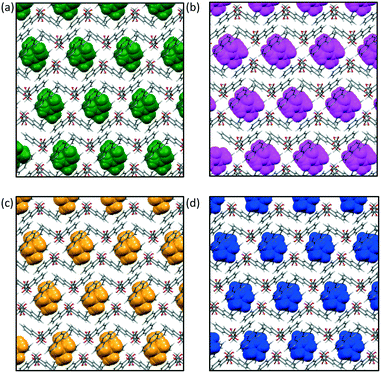
Salt 1, 1,10-phenanthrolinium pamoate, was first crystallised in our group as its THF solvate, 1·THF, from a THF-water mixture. Analysis of the crystal structure revealed that 1·THF is isostructural to its previously reported DMF solvate, 1·DMF (CSD refcode QEXJEJ13). Further investigations resulted in the characterisation of two more isostructural solvates, 1·DMA and 1·DMSO. Details of the crystallography are given in the ESI.†
The crystal structures of this isostructural series of solvates consist of hydrogen-bonded chains of pamoate anions which pack alongside one another to give layers. The cation hydrogen bonds to the solvent (not the anion), and these units pack in columns between the layers of anions. The solvent molecules are in pockets in this structure, with two solvent molecules in each pocket (Fig. 1).
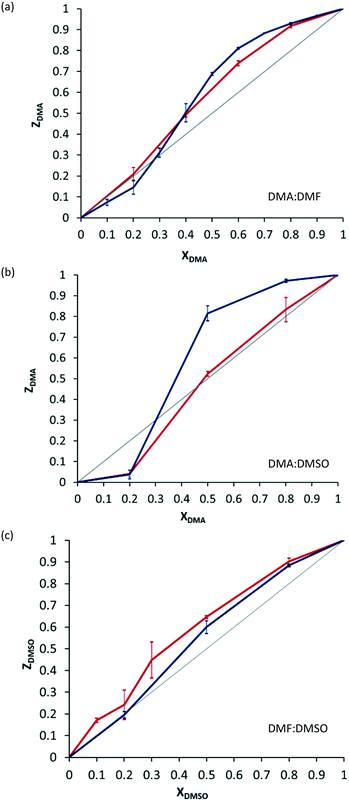
The characterisation of this isostructural series of salts prompted us to investigate whether the material would show selectivity for one solvent over another during the crystallisation process. We therefore carried out a series of crystallisations from mixtures of solvents of different mole ratios (see ESI† for details). This revealed some degree of concentration-dependent selectivity, with the preference of solvents in the order DMA>DMSO>DMF (Fig. 2, blue lines).14 The selectivity for particular solvents could not easily be rationalised: there is no apparent trend in density of the solvates, or in the strength of hydrogen bonding between the cation and the solvent (see ESI†).
All four isostructural solvates of 1 could easily be synthesised mechanochemically, via liquid-assisted grinding (LAG) in a mortar and pestle (PXRD in ESI†). The selectivity for one solvent over another using mechanochemical synthesis was thus investigated. The selectivity observed by mechanochemistry is not pronounced, and neither is the difference in selectivity between synthetic methods. There is, nonetheless, a reproducible difference between the selectivity profiles obtained from solution and those obtained using mechanochemical methods (Fig. 2, red lines).
These interesting results, showing that selectivity during mechanochemical synthesis of a host–guest system can differ from the selectivity in solution synthesis, prompted us to extend the investigation to a second system.
We selected 3,4-lutidinium pamoate hemihydrate (2), which crystallises as its THF solvate. We have investigated this system extensively, and have found that the THF can be exchanged for a variety of solvents and volatile solids, leading to a series of crystallographically-characterised isostructural solvates.5 We also found that exposure of 2·THF to mixtures of solvents in either solution or the gas phase shows preferential inclusion of some solvents over others (note that in this case, selectivity is observed during solvent exchange, rather than during crystallisation, as is the case for salt 1).6 This system therefore seemed an ideal candidate to further our investigation, specifically by investigating whether selectivity via guest exchange would also differ if experiments are carried out mechanochemically.
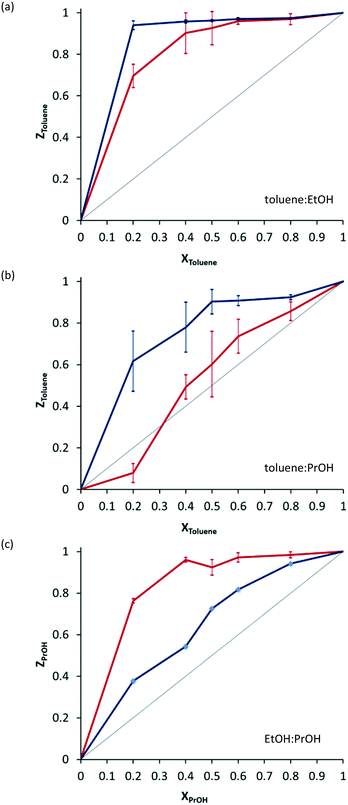
Crystals of 2·THF were immersed in mixtures of ethanol and toluene, propanol and toluene and ethanol and propanol with varying mole fractions. Analysis of the crystals after exposure to solvent mixtures showed that the system preferentially includes toluene over ethanol at all mole ratios (Fig. 3a, blue line). There is a marked preference for inclusion of toluene over propanol (Fig. 3b, blue line), and a preference for propanol over ethanol (Fig. 3c, blue line). Attempts to determine the crystal structures of crystals isolated from mixed solvent systems were inconclusive – we were unable to convincingly model both solvents in the channels.
When the experiments are carried out mechanochemically in a ball mill, by grinding crystalline 2·THF with solvent mixtures, the selectivity profiles are quite different (Fig. 3, red lines). The preferential inclusion of toluene over ethanol is still observed, but it is less pronounced. The preferential inclusion of propanol over ethanol is far more pronounced. Most surprisingly, the preference for toluene over propanol is reversed at some mole ratios: when the material is prepared mechanochemically, there is a slight preference for inclusion of propanol over toluene when the mole fraction of toluene is low.
Preferential inclusion of toluene over other solvents can be explained on the basis of shape-filling: toluene molecules fill space more effectively in the solvate, resulting in a higher-density more stable crystal. Why the selectivity profile should change when synthesis is carried out mechanochemically is more difficult to explain. The most likely situation is that one solvate is kinetically favoured over the other. This would then be the solvate that is isolated in greater quantities from mechanochemical experiments.
A link between solvent volatility and preferential inclusion in mechanochemistry was also considered on the grounds that perhaps whichever solvent evaporates more rapidly is included in lower amounts during a mechanochemical exchange experiment. The boiling points of toluene, ethanol and propanol are 110.6, 78.4 and 97 °C respectively. One might expect toluene to be preferentially included to a larger degree when using mechanochemistry, however in our experiments the opposite is observed: in both cases the preference for inclusion of toluene is less pronounced, and in some cases even reversed.
Conclusions
A series of isostructural solvates, which can all be synthesised by crystallisation from solution as well as mechanochemically by LAG, were investigated in terms of their selectivity for different guests. It was found that while the selectivity profiles obtained using solution and mechanochemical methods were similar, there were reproducible differences between them.A second system was investigated which showed more significant differences when selectivity experiments were carried out in solution and by mechanochemistry. In some cases the selectivity for a particular guest was significantly more or less pronounced, and in one case the selectivity was reversed at certain mole fractions of a particular pair of guests. The guest which was preferentially included during solution experiments, due to space-filling, is not favoured to the same extent when carrying out selectivity experiments mechanochemically. It seems most likely that these observed differences are due to the solvates which are less favoured in terms of space-filling being kinetically favoured, and therefore being formed to a greater extent during mechanochemical experiments.
We have demonstrated for the first time that it is possible to alter the selectivity profile of a particular host–guest system by carrying out experiments mechanochemically. This is of interest as it means different methods of synthesis could be used in order to obtain a desired selectivity in a particular system. Further investigation is needed to identify trends in this behaviour, but in systems where the kinetically-favoured product is different to the thermodynamically-favoured product, it may be more likely that a particular product could be selectively synthesised by changing the synthetic method.
Experimental
The solvates of 1 were prepared by combining pamoic acid and 1,10-phenanthroline hydrate in the respective solvent and crystallising by slow evaporation. Details are given in the ESI.† The crystallisation conditions for these solvates had in some cases previously been established, however, they were crystallised once more to obtain the accurate NMR data that were needed for subsequent selectivity studies.Crystals of 2·THF were prepared as previously described.4
Solvent mixtures were prepared by mixing the solvents in various mole ratios. Data obtained for individual selectivity experiments are tabulated in the ESI.†
Selectivity experiments in solution (1)
Pamoic acid (106 mg, 0.273 mmol) and 1,10-phenanthroline hydrate (54 mg, 0.272 mmol) were dissolved in the specific solvent mixtures with stirring and heating. Solutions were prepared in glass vials, keeping the caps partially on to prevent evaporation. Vials were capped and allowed to crystallise, either in the refrigerator or at room temperature. Differences between results obtained at different temperatures were not large, and all results have been included. Crystallisation times depended on the solvent system. The crushed crystalline product (13 mg) was dissolved in 0.6 ml DMSO-d6 and analysed via 1H NMR (300 MHz). The relative intensities of the solvent peaks were used to determine the ratios between the included solvents.Selectivity experiments by mechanochemistry (1)
Pamoic acid (53 mg, 0.137 mmol) and 1,10-phenanthroline hydrate (27 mg, 0.136 mmol) were combined. The reagents were ground together in an agate mortar and pestle for 10 minutes whilst adding a few drops of the relevant solvent mixture. The dry powdered product (13 mg) was dissolved in 0.6 ml DMSO-d6 and analysed via 1H NMR (300 MHz). The relative intensities of solvent peaks were used to determine the ratios between the included solvents.Selectivity experiments in solution (2)
Fresh crystals of 2·THF were taken from the mother liquor and dried on filter paper to get rid of any surface solvent. The crystals were then gently placed in a glass vial, after which the desired solvent mixture was slowly added (2–3 mL) to the crystals with a glass pipette. The glass vial was then closed with a lid and sealed with parafilm, to minimize solvent evaporation, and left to stand for 2–3 days. After 2–3 days, the crystals were removed and analysed by NMR, PXRD and single crystal diffraction.Selectivity experiments by mechanochemistry (2)
Fresh crystals of 2·THF were taken from the mother liquor and dried on filter paper to remove any surface solvent. Approximately 80 mg of the crystals were weighed out and placed in the grinding vial, after which 80 μL of the desired solvent mixture was added with a micropipette. The grinding vial was then placed in a Form-Tech Scientific Shaker Mill® and the sample was ground for 15 minutes at 1000 rpm. The resulting powder was analysed by NMR.Author contributions
J. L., H. L. and F. P. were involved in investigation, validation and visualisation. H. W. was also involved in investigation. T. l. R. and D. A. H. conceptualised the study and supervised. D. A. H. wrote the article, with editorial comments from all authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We would like to acknowledge the Wilhelm Frank Scholarship Fund, the National Research Foundation of South Africa and Stellenbosch University for funding. NMR experiments were carried out on instruments managed by the Central Analytical Facility at Stellenbosch University.References
- L. R. Nassimbeni, Acc. Chem. Res., 2003, 36, 631–637 CrossRef CAS PubMed.
- P. Li, Y. He, J. Guang, L. Weng, J. C. G. Zhao, S. Xiang and B. Chen, J. Am. Chem. Soc., 2014, 136, 547–549 CrossRef CAS PubMed.
- C. García-Simón, M. Garcia-Borràs, L. Gómez, T. Parella, S. Osuna, J. Juanhuix, I. Imaz, D. Maspoch, M. Costas and X. Ribas, Nat. Commun., 2014, 5, 5557–5566 CrossRef PubMed.
- H. Wahl, D. A. Haynes and T. le Roex, Chem. Commun., 2012, 48, 1775–1777 RSC.
- H. Wahl, D. A. Haynes and T. le Roex, Cryst. Growth Des., 2017, 17, 4377–4383 CrossRef CAS.
- H. Wahl, D. A. Haynes and T. le Roex, Cryst. Growth Des., 2018, 18, 944–953 CrossRef CAS.
- H. Wahl, D. A. Haynes and T. le Roex, CrystEngComm, 2015, 17, 1549–1555 RSC.
- (a) J. G. Hernández and C. Bolm, J. Org. Chem., 2017, 82, 4007–4019 CrossRef PubMed; (b) J. L. Howard, Q. Cao and D. L. Browne, Chem. Sci., 2018, 9, 3080–3094 RSC.
- (a) A. M. Belenguer, G. I. Lampronti, A. J. Cruz-Cabeza, C. A. Hunter and J. K. M. Sanders, Chem. Sci., 2016, 7, 6617–6627 RSC; (b) D. Hasa, E. Miniussi and W. Jones, Cryst. Growth Des., 2016, 16, 4582–4588 CrossRef CAS; (c) S. Aitipamula, P. S. Chow and R. B. H. Tan, CrystEngComm, 2010, 12, 3691–3697 RSC; (d) F. Fischer, A. Heidrich, S. Greiser, S. Benemann, K. Rademann and F. Emmerling, Cryst. Growth Des., 2016, 16, 1701–1707 CrossRef CAS; (e) Y. Yuan, L. Wang, D. Li, Z. Deng and H. Zhang, Cryst. Growth Des., 2018, 18, 7244–7247 CrossRef CAS.
- D. Hasa and W. Jones, Adv. Drug Delivery Rev., 2017, 117, 147–161 CrossRef CAS PubMed.
- (a) F. Fischer, M. Joester, K. Rademann and F. Emmerling, Chem. – Eur. J., 2015, 21, 14969–14974 CrossRef CAS PubMed; (b) S. Lukin, I. Lončarić, M. Tireli, T. Stolar, M. V. Blanco, P. Lazić, K. Užarević and I. Halasz, Cryst. Growth Des., 2018, 18, 1539–1547 CrossRef CAS.
- M. R. Caira, L. R. Nassimbeni, F. Toda and D. Vujovic, J. Am. Chem. Soc., 2000, 122, 9367–9372 CrossRef CAS.
- T.-M. Shang, Q.-F. Zhou and J.-H. Sun, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2007, 63, o506 CrossRef CAS.
- Selectivity experiments involving THF were not carried out due the rapid evaporation of this solvent, complicating both experimental design and the analysis of the resulting crystals by NMR.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, crystallographic data, PXRD, selectivity results. CCDC 2111529–2111532. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1ce01286j |
| This journal is © The Royal Society of Chemistry 2021 |