Photoinduced intramolecular carbosulfonylation of alkynes: access to sulfone-containing dibenzazepines from sulfur dioxide†
Yanfang
Yao
ab,
Ziqing
Yin
a,
Fu-Sheng
He
*a,
Xuwei
Qin
a,
Wenlin
Xie
*b and
Jie
Wu
 *acd
*acd
aSchool of Pharmaceutical and Materials Engineering & Institute for Advanced Studies, Taizhou University, 1139 Shifu Avenue, Taizhou 318000, China. E-mail: jie_wu@fudan.edu.cn; 0618006@zju.edu.cn
bSchool of Chemistry and Chemical Engineering, Hunan University of Science and Technology, Xiangtan 411201, China. E-mail: xwl2000zsu@163.com
cState Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032, China
dSchool of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang 453007, China
First published on 9th February 2021
Abstract
A photoinduced three-component reaction of N-benzyl-N-(2-ethynylaryl)amides, sulfur dioxide and aryldiazonium tetrafluoroborates is developed, providing an efficient and straightforward approach for the synthesis of diverse vinylsulfonylated dibenzazepine derivatives in moderate to good yields under mild conditions. This transformation proceeds smoothly at room temperature in the presence of visible light, which shows a wide range of substrate scope with good functional group compatibility. The synthetic utility of this methodology is further demonstrated through Suzuki coupling. Mechanistic studies show that this reaction is triggered by the addition of an arylsulfonyl radical to an alkyne from sulfur dioxide, followed by vinyl radical cyclization to afford the desired sulfonated dibenzazepines.
Owing to their widespread application in the synthesis of natural products and bioactive compounds, seven-membered heterocycles and derivatives have attracted much attention from both the synthetic and medicinal community.1 Among them, the scaffold of dibenzazepine often serves as a privileged structural motif in biologically active molecules and drugs, such as Mianserin, Mirtazapine and Epinastine.2 However, traditional methods for the synthesis of the dibenzazepine skeleton often require harsh conditions or tedious steps.3 Therefore, the development of efficient protocols to access functionalized dibenzazepines is highly desirable.
The importance of sulfonyl compounds has been widely witnessed in organic chemistry and pharmaceutical chemistry.4 In particular, vinyl sulfones often serve as versatile synthons in organic synthesis,5 while they can also be used as neuroprotective agents for Parkinson's disease therapy.6 Accordingly, great efforts have been devoted to developing efficient approaches toward the synthesis of vinyl sulfones. Among them, vicinal difunctionalization of alkynes has been demonstrated as an efficient and convenient strategy for the generation of multi-substituted alkenes in recent years, in which two functional groups can be incorporated into alkyne moieties simultaneously.7 For example, by employing sulfonyl chlorides and arylboronic acids as reaction components, Nevado and co-workers reported an elegant nickel-catalyzed three-component intermolecular carbosulfonylation of alkynes for the construction of β,β-disubstituted vinyl sulfones (Scheme 1a).8 Additionally, sulfonyl radical-mediated synthesis of five- or six-membered ring compounds through sulfonylation of alkynes with the simultaneous formation of C–C bonds has been widely explored.9 Despite these achievements, to the best of our knowledge, there is no example of alkyne carbosulfonylation involving intramolecular C–H functionalization to construct sulfone-containing seven-membered ring compounds.
 | ||
| Scheme 1 Intermolecular and intramolecular carbosulfonylation of alkynes through sulfonyl radical addition. | ||
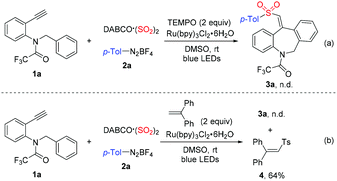
It is known that DABCO (SO2)2 and potassium/sodium metabisulfite are efficient sulfur dioxide surrogates, which have been successfully applied to the synthesis of sulfonyl compounds.10 Consequently, the chemistry of sulfur dioxide insertion has become an attractive strategy for the generation of highly functionalized sulfonyl-containing molecules in organic synthesis.11–13 For instance, our group recently described a four-component fluoroalkylsulfonylation of terminal alkynes with fluoroalkyl radicals, DABCO (SO2)2 and hydrazines, giving rise to (E)-ethyl 2,2-difluoro-4-aryl-4-sulfamoylbut-3-enoates.14 Prompted by the advances in the construction of seven-membered ring compounds via radical processes15 and our continuous interest in sulfonylation from sulfur dioxide, herein, we report a photoinduced three-component reaction of N-benzyl-N-(2-ethynylaryl)amides, DABCO (SO2)2 and aryldiazonium tetrafluoroborates. This protocol provides a range of sulfonated dibenzazepines in moderate to good yields under mild conditions (Scheme 1b). Additionally, this sulfonyl radical-mediated multicomponent reaction represents a rare example of carbosulfonylation of alkynes involving the insertion of sulfur dioxide and intramolecular C–H bond functionalization to furnish sulfone-containing dibenzazepines. Driven by the importance of dibenzazepines and sulfones, we envisioned that such a transformation might be beneficial for organic synthesis and drug discovery.
We began our studies by choosing N-benzyl-N-(2-ethynylphenyl)-2,2,2-trifluoroacetamide 1a, DABCO (SO2)2 and 4-methylphenyldiazonium tetrafluoroborate 2a as the model substrates for the optimization of the reaction conditions. Initially, the reaction was performed in DMSO at room temperature without the addition of any catalysts. To our delight, the desired sulfonated dibenzazepine 3a was obtained in 21% yield (Table 1, entry 1). The structure of compound (Z)-3a was confirmed by X-ray diffraction analysis.16 However, the yield could not be improved when the solvent or temperature was changed (data not shown in Table 1). We envisioned that an oxidative single electron transfer might be involved during the reaction process. Thus, we considered to introduce a photocatalyst, and this reaction was performed under visible light irradiation of 30 W blue LEDs. Gratifyingly, the corresponding product 3a was afforded in 60% yield by employing Eosin-Y as the photocatalyst (Table 1, entry 2). Encouraged by this result, other photocatalysts were evaluated, and the results showed that Ru(bpy)3Cl2·6H2O was the best choice, providing the corresponding product 3a in 78% yield (Table 1, entries 3 and 4). Subsequently, we investigated the solvent effect in this transformation. As a result, no better yields were obtained when the reaction was performed in DMF or DMA (Table 1, entries 5 and 6). Additionally, the yield of product 3a was decreased when the amount of 4-methylphenyldiazonium tetrafluoroborate 1a, DABCO (SO2)2 or the concentration of the reaction was changed (Table 1, entries 7–10). We also explored the reaction by using a white LED (35 W) instead of a blue LED (30 W) as the light source, and the corresponding product 3a was generated in 57% yield (Table 1, entry 11).
| Entry | Photocatalyst | Solvent | Yieldb (%) |
|---|---|---|---|
| a Reactions conditions: N-benzyl-N-(2-ethynylphenyl)-2,2,2-trifluoroacetamide 1a (0.2 mmol), 4-methylphenyldiazonium tetrafluoroborate 2a (0.3 mmol), DABCO (SO2)2 (0.2 mmol), photocatalyst (2 mol%), solvent (2.0 mL), stirred at room temperature for 24 h under blue LED (30 W) irradiation. b Isolated yield based on N-benzyl-N-(2-ethynylphenyl)-2,2,2-trifluoroacetamide 1a. c Compound 2a (0.4 mmol). d Compound 2a (0.2 mmol). e DABCO (SO2)2 (0.3 mmol). f DMSO (4.0 mL). g White LED (35 W) instead of blue LED. | |||
| 1 | — | DMSO | 21 |
| 2 | Eosin Y | DMSO | 60 |
| 3 | fac-Ir(ppy)3 | DMSO | 69 |
| 4 | Ru(bpy)3Cl2·6H2O | DMSO | 78 |
| 5 | Ru(bpy)3Cl2·6H2O | DMF | 63 |
| 6 | Ru(bpy)3Cl2·6H2O | DMA | 68 |
| 7c | Ru(bpy)3Cl2·6H2O | DMSO | 52 |
| 8d | Ru(bpy)3Cl2·6H2O | DMSO | 60 |
| 9e | Ru(bpy)3Cl2·6H2O | DMSO | 57 |
| 10f | Ru(bpy)3Cl2·6H2O | DMSO | 60 |
| 11g | Ru(bpy)3Cl2·6H2O | DMSO | 57 |
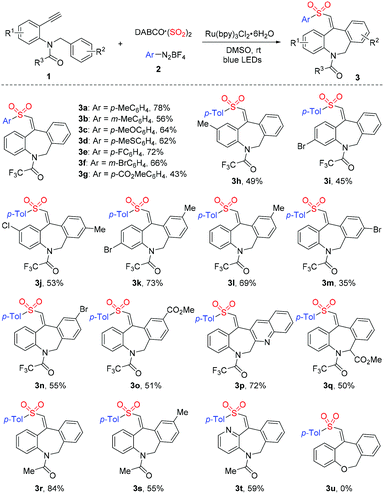
With the above optimized conditions in hand, we further evaluated the reaction scope of this three-component reaction of N-benzyl-N-(2-ethynylaryl)amides 1, DABCO (SO2)2 and aryldiazonium tetrafluoroborates 2. As shown in Table 2, the reaction could tolerate various substituents on the aromatic ring of aryldiazonium tetrafluoroborates 2, leading to the corresponding products 3a-3g in moderate to good yields. Different functional groups including methyl, methoxy, methylthio, fluoro, bromo and ester units were found to be compatible under the standard conditions. Subsequently, we investigated the substitution effect on the aromatic ring of the N-(2-ethynylaryl) moiety of substrate 1. As expected, the desired sulfone-containing dibenzazepines 3h-3k were obtained efficiently with respect to electron-donating and electron-withdrawing substituents. Additionally, diverse substrates 1 with substituents on the aryl ring of the N-benzyl moiety were evaluated, affording the desired products in moderate to good yields. For instance, sulfonated products 3l and 3n bearing a methyl or a bromo group on the para-position of the N-benzyl ring were generated in 69% and 55% yield, respectively. The bromo-substituent in 3n was further applied in the Suzuki coupling reaction for further transformation (see the ESI†). The reaction of the 2-(bromomethyl)quinolone-derived substrate also proceeded smoothly to deliver the product 3p in 72% yield. Moreover, N-benzyl-N-(2-ethynylphenyl)amide bearing an ester group at the benzylic position could be employed as the substrate, and the corresponding product 3q was produced in 50% yield. Notably, the replacement of a trifluoroacetyl group by an acetyl group on the nitrogen resulted in the formation of products 3r-3t in 55–84% yields. Unfortunately, the ether substrate 1-(benzyloxy)-2-ethynylbenzene was not suitable for this reaction to provide the dibenzoxepine 3u. However, from the 1H NMR spectra, it seemed that a small amount of amide rotamers would be present.
| a Isolated yield based on N-benzyl-N-(2-ethynylaryl)amide 1. |
|---|
|
Since the combination of DABCO (SO2)2 and aryldiazonium tetrafluoroborate would provide an arylsulfonyl radical,10 to gain further insights into the mechanism of this three-component reaction, several control experiments were carried out under the standard conditions. As expected, the model reaction of N-benzyl-N-(2-ethynylphenyl)-2,2,2-trifluoroacetamide 1a, DABCO (SO2)2 and 4-methylphenyldiazonium tetrafluoroborate 2a was completely hampered in the presence of 2 equivalents of 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO), which suggested that a radical process was involved in this reaction (Scheme 2, eqn (a)). Additionally, to confirm the radical intermediates generated in this transformation, 2 equivalents of 1,1-diphenylethylene were added to the model reaction (Scheme 2, eqn (b)). As a result, no desired product 3a was observed, and (2-tosylethene-1,1-diyl)dibenzene 4 was obtained in 64% yield, revealing that the 4-methylphenylsulfonyl radical was trapped by 1,1-diphenylethylene.
With the above experimental results in hand and inspired by previous reports,10 a plausible reaction mechanism was proposed for this photoinduced alkyne carbosulfonylation process. As shown in Scheme 3, 4-methylphenyl diazonium tetrafluoroborate 2a would react with DABCO (SO2)2 to generate 4-methylphenyl sulfonyl radical A and a tertiary amine radical cation with the release of molecular nitrogen. In the meantime, photoexcitation of Ru(II) would give rise to the excited-state Ru(II)*, which could be oxidized to Ru(III) by the tertiary amine radical cation. Alternatively, 4-methylphenyl diazonium tetrafluoroborate 2a would undergo a single-electron transfer by the photoexcited Ru(II)* to give a 4-methylphenyl radical, which would capture sulfur dioxide to form 4-methylphenyl sulfonyl radical A. Then, intermediate A would attack the triple bond of N-benzyl-N-(2-ethynylphenyl)-2,2,2-trifluoroacetamide 1a to provide vinyl radical B, which would undergo 7-ortho cyclization to form a radical intermediate C. Subsequently, radical intermediate C would proceed through a single-electron oxidation by Ru(III) to produce cation intermediate D and regenerate the photocatalyst. Finally, the resulting cation D would undergo deprotonation by DABCO leading to the desired product 3a.
In summary, we have developed an efficient and straightforward approach for the construction of sulfonylated dibenzazepines through a visible-light induced three-component reaction of N-benzyl-N-(2-ethynylaryl)amides, sulfur dioxide and aryldiazonium tetrafluoroborates. This protocol features mild reaction conditions, broad substrate scope and good functional group compatibility, giving rise to the corresponding products in moderate to good yields. Notably, by employing this method, both the vinyl sulfone moiety and the dibenzazepine scaffold can be introduced in the one-pot process, which represents a valuable strategy to access sulfonated seven-membered rings. Preliminary mechanistic studies show that this transformation proceeds through a radical addition/cyclization cascade with the insertion of sulfur dioxide.
The financial support from the National Natural Science Foundation of China (No. 21871053), Natural Science Foundation of Zhejiang Province (LQ21B020002), the Leading Innovative and Entrepreneur Team Introduction Program of Zhejiang (No. 2019R01005) and the Open Research Fund of School of Chemistry and Chemical Engineering, Henan Normal University (2020ZD04) is gratefully acknowledged.
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) A. S. Kende, J. I. M. Hernando and J. B. J. Milbank, Org. Lett., 2001, 3, 2505 CrossRef CAS; (b) A. Fürstner and O. R. Thiel, J. Org. Chem., 2000, 65, 1738 CrossRef; (c) F. E. Dutton, B. H. Lee, S. S. Johnson, E. M. Coscarell and P. H. Lee, J. Med. Chem., 2003, 46, 2057 CrossRef CAS; (d) E. Vitaku, D. T. Smith and J. T. Njardarson, J. Med. Chem., 2014, 57, 10257 CrossRef CAS.
- (a) A. Aoyama, K. Endo-Umeda, K. Kishida, K. Ohgane, T. Noguchi-Yachide, H. Aoyama, M. Ishikawa, H. Miyachi, M. Makishima and Y. Hashimoto, J. Med. Chem., 2012, 55, 7360 CrossRef CAS; (b) P. Roszkowski, J. K. Maurin and Z. Czarnocki, Beilstein J. Org. Chem., 2015, 11, 1509 CrossRef CAS; (c) D. V. N. S. Rao, R. Dandala, V. K. Handa, M. Sivakumaran, A. V. R. Reddy and A. Naidu, Org. Prep. Proced. Int., 2007, 39, 399 CrossRef CAS; (d) R. A. Al-Qawasmeh, Y. Lee, M.-Y. Cao, X. Gu, S. Viau, J. Lightfoot, J. A. Wright and A. H. Young, Bioorg. Med. Chem. Lett., 2009, 19, 104 CrossRef CAS.
- (a) T. Zou, X.-G. Zhang, J.-H. Li, C.-L. Deng and R.-Y. Tang, Adv. Synth. Catal., 2012, 354, 889 CrossRef CAS; (b) D. Solé and F. Mariani, J. Org. Chem., 2013, 78, 8136 CrossRef.
- For selected examples, see: (a) N. S. Simpkins, Sulfones in Organic Synthesis, Pergamon Press, Oxford, UK, 1993 Search PubMed; (b) The Chemistry of Sulfones and Sulfoxides, ed. S. Patai, Z. Rappoport and C. Stirling, Wiley, Chichester, UK, 1988 Search PubMed; (c) A.-N. R. Alba, X. Companyó and R. Rios, Chem. Soc. Rev., 2010, 39, 2018 RSC; (d) A. El-Awa, M. N. Noshi, X. Mollat du Jourdin and P. L. Fuchs, Chem. Rev., 2009, 109, 2315 CrossRef CAS; (e) K. A. Scott and J. T. Njardarson, Top. Curr. Chem., 2018, 376, 5 CrossRef.
- (a) J. T. Palmer, D. Rasnick, J. L. Klaus and D. Brömme, J. Med. Chem., 1995, 38, 3193 CrossRef CAS; (b) D. C. Meadows, T. B. Mathews, T. W. North, M. J. Hadd, C. L. Kuo, N. Neamati and J. Gervay-Hague, J. Med. Chem., 2005, 48, 4526 CrossRef CAS; (c) D. C. Meadows and J. Gervay-Hague, Med. Res. Rev., 2006, 26, 793 CrossRef CAS; (d) R. Ettari, E. Nizi, M. E. Di Francesco, M. A. Dude, G. Pradel, R. Vicik, T. Schirmeister, N. Micale, S. Grasso and M. Zappala, J. Med. Chem., 2008, 51, 988 CrossRef CAS; (e) R. van der Westhuyzen and E. Strauss, J. Am. Chem. Soc., 2010, 132, 12853 CrossRef CAS.
- S. Y. Woo, J. H. Kim, M. K. Moon, S. H. Han, S. K. Yeon, J. W. Choi, B. K. Jang, H. J. Song, Y. G. Kang, J. W. Kim, J. Lee, D. J. Kim, O. Hwang and K. D. Park, J. Med. Chem., 2014, 57, 1473 CrossRef CAS.
- (a) P. Gao, X.-R. Song, X.-Y. Liu and Y.-M. Liang, Chem. – Eur. J., 2015, 21, 7648 CrossRef CAS; (b) H.-L. Hua, Y.-T. He, Y.-F. Qiu, Y.-X. Li, B. Song, P. Gao, X.-R. Song, D.-H. Guo, X.-Y. Liu and Y.-M. Liang, Chem. – Eur. J., 2015, 21, 1468 CrossRef CAS; (c) P. Gao, Y.-W. Shen, R. Fang, X.-H. Hao, Z.-H. Qiu, F. Yang, X.-B. Yan, Q. Wang, X.-J. Gong, X.-Y. Liu and Y.-M. Liang, Angew. Chem., Int. Ed., 2014, 53, 7629 CrossRef CAS; (d) Y.-P. Xiong, M.-Y. Wu, X.-Y. Zhang, C.-L. Ma, L. Huang, L.-J. Zhao, B. Tan and X.-Y. Liu, Org. Lett., 2014, 16, 1000 CrossRef CAS; (e) Z. Li, A. García-Domínguez and C. Nevado, J. Am. Chem. Soc., 2015, 137, 11610 CrossRef CAS; (f) M. Israr, H. Xiong, Y. Li and H. Bao, Org. Lett., 2019, 21, 7078 CrossRef CAS.
- A. GarcÍa-DomÍnguez, S. Müller and C. Nevado, Angew. Chem., Int. Ed., 2017, 56, 9949 CrossRef.
- (a) D. Zheng, J. Yu and J. Wu, Angew. Chem., Int. Ed., 2016, 55, 11925 CrossRef CAS; (b) D. Sun, K. Yin and R. Zhang, Chem. Commun., 2018, 54, 1335 RSC; (c) X.-Y. Qin, L. He, J. Li, W.-J. Hao, S.-J. Tu and B. Jiang, Chem. Commun., 2019, 55, 3227 RSC; (d) Y. Liu, Q.-L. Wang, Z. Chen, H. Li, B.-Q. Xiong, P.-L. Zhang and K.-W. Tang, Chem. Commun., 2020, 56, 3011 RSC; (e) N. Zhou, M. Wu, K. Kuang, S. Wu and M. Zhang, Adv. Synth. Catal., 2020, 362, 5391 CrossRef CAS.
- For selected reviews, see: (a) G. Qiu, K. Zhou, L. Gao and J. Wu, Org. Chem. Front., 2018, 5, 691 RSC; (b) K. Hofman, N. Liu and G. Manolikakes, Chem. – Eur. J., 2018, 24, 11852 CrossRef CAS; (c) G. Liu, C. Fan and J. Wu, Org. Biomol. Chem., 2015, 13, 1592 RSC; (d) G. Qiu, L. Lai, J. Cheng and J. Wu, Chem. Commun., 2018, 54, 10405 RSC; (e) G. Qiu, K. Zhou and J. Wu, Chem. Commun., 2018, 54, 12561 RSC; (f) D. Zheng and J. Wu, Sulfur Dioxide Insertion Reactions for Organic Synthesis Nature, Springer, Berlin, 2017 CrossRef; (g) S. Ye, G. Qiu and J. Wu, Chem. Commun., 2019, 55, 1013 RSC; (h) S. Ye, M. Yang and J. Wu, Chem. Commun., 2020, 56, 4145 RSC; (i) D. Zeng, M. Wang, W.-P. Deng and X. Jiang, Org. Chem. Front., 2020, 7, 3956 RSC.
- For selected examples, see: (a) D. Zheng, Y. An, Z. Li and J. Wu, Angew. Chem., Int. Ed., 2014, 53, 2451 CrossRef CAS; (b) X. Gong, M. Wang, S. Ye and J. Wu, Org. Lett., 2019, 21, 1156 CrossRef CAS; (c) S. Ye, D. Zheng, J. Wu and G. Qiu, Chem. Commun., 2019, 55, 2214 RSC; (d) S. Ye, Y. Li, J. Wu and Z. Li, Chem. Commun., 2019, 55, 2489 RSC; (e) X. Gong, X. Li, W. Xie, J. Wu and S. Ye, Org. Chem. Front., 1863, 2019, 6 Search PubMed; (f) F.-S. He, X. Gong, P. Rojsitthisak and J. Wu, J. Org. Chem., 2019, 84, 13159 CrossRef CAS; (g) S. Ye, X. Li, W. Xie and J. Wu, Asian J. Org. Chem., 2019, 8, 893 CrossRef CAS; (h) S. Ye, K. Zhou, P. Rojsitthisak and J. Wu, Org. Chem. Front., 2020, 7, 14 RSC; (i) J. Zhang, M. Yang, J.-B. Liu, F.-S. He and J. Wu, Chem. Commun., 2020, 56, 3225 RSC; (j) F.-S. He, Y. Yao, W. Xie and J. Wu, Chem. Commun., 2020, 56, 9649 Search PubMed; (k) F.-S. He, Y. Yao, W. Xie and J. Wu, Adv. Synth. Catal., 2020, 362, 4744 CrossRef CAS.
- For recent selected examples, see: (a) F.-S. He, Y. Wu, X. Li, H. Xia and J. Wu, Org. Chem. Front., 1873, 2019, 6 Search PubMed; (b) X. Wang, Y. Kuang, S. Ye and J. Wu, Chem. Commun., 2019, 55, 14962 RSC; (c) X. Gong, M. Yang, J.-B. Liu, F.-S. He, X. Fan and J. Wu, Green Chem., 2020, 22, 1906 RSC; (d) X. Gong, M. Yang, J.-B. Liu, F.-S. He and J. Wu, Org. Chem. Front., 2020, 7, 938 RSC; (e) X. Wang, Y. Lin, J.-B. Liu, F.-S. He, Y. Kuang and J. Wu, Chin. J. Chem., 2020, 38, 1098 CrossRef CAS; (f) Y. Liu, Q.-L. Wang, Z. Chen, P. Chen, K.-W. Tang, Q. Zhou and J. Xie, Org. Biomol. Chem., 2019, 17, 10020 RSC; (g) Y. Liu, Q.-L. Wang, Z. Chen, Q. Zhou, P.-L. Zhang and K.-W. Tang, Chem. Commun., 2019, 55, 12212 RSC; (h) K. Zhou, J.-B. Liu, W. Xie, S. Ye and J. Wu, Chem. Commun., 2020, 56, 2554 RSC; (i) B. Ni, B. Zhang, J. Han, B. Peng, Y. Shan and T. Niu, Org. Lett., 2020, 22, 670 CrossRef CAS; (j) Y. He, J. Yang, Q. Liu, X. Zhang and X. Fan, J. Org. Chem., 2020, 85, 15600 CrossRef CAS.
- For selected examples, see: (a) F. Liu, J.-Y. Wang, P. Zhou, G. Li, W.-J. Hao, S.-J. Tu and B. Jiang, Angew. Chem., Int. Ed., 2017, 56, 15570 CrossRef CAS; (b) H. Wang, S. Sun and J. Cheng, Org. Lett., 2017, 19, 5844 CrossRef CAS; (c) G. Li, Z. Gan, K. Kong, X. Dou and D. Yang, Adv. Synth. Catal., 2019, 361, 1808 CrossRef CAS; (d) T.-H. Zhu, X.-C. Zhang, X.-L. Cui, Z.-Y. Zhang, H. Jiang, S.-S. Sun, L.-L. Zhao, K. Zhao and T.-P. Loh, Adv. Synth. Catal., 2019, 361, 3593 CrossRef CAS; (e) Y. Meng, M. Wang and X. Jiang, Angew. Chem., Int. Ed., 2020, 59, 1346 CrossRef CAS; (f) S. Chen, Y. Li, M. Wang and X. Jiang, Green Chem., 2020, 22, 322 RSC; (g) X. Marset, J. Torregrosa-Crespo, R. M. Martinez-Espinosa, G. Guillena and D. J. Ramón, Green Chem., 2019, 21, 4127 RSC; (h) K. Chen, W. Chen, B. Han, W. Chen, M. Liu and H. Wu, Org. Lett., 2020, 22, 1841 CrossRef CAS; (i) Y. Liu, D. Yu, Y. Guo, J.-C. Xiao, Q.-Y. Chen and C. Liu, Org. Lett., 2020, 22, 2281 CrossRef CAS; (j) T. Zhang, M.-K. Pang, Z.-D. Chen, B. Zhang, J. Weng and G. Lu, Org. Lett., 2020, 22, 3072 CrossRef; (k) Q. Lin, Z. Ma, C. Zheng, X.-J. Hu, Y. Guo, Q.-Y. Chen and C. Liu, Chin. J. Chem., 2020, 38, 1107 CrossRef CAS; (l) K. Zhou, J. Chen and J. Wu, Chin. Chem. Lett., 2020, 31, 2996 CrossRef CAS; (m) Y. Yao, Z. Yi, W. Chen, W. Xie, F.-S. He and J. Wu, Adv. Synth. Catal., 2021, 363, 570 CrossRef.
- (a) Y. Xiang, Y. Li, Y. Kuang and J. Wu, Chem. – Eur. J., 2017, 23, 1032 CrossRef CAS; (b) Y. Li, Y. Xiang, Y. Kuang and J. Wu, Org. Chem. Front., 2016, 3, 1493 RSC.
- (a) P. Xiong, H.-H. Xu, J. Song and H.-C. Xu, J. Am. Chem. Soc., 2018, 140, 2460 CrossRef CAS; (b) Y. Zhao, J.-R. Chen and W.-J. Xiao, Org. Lett., 2018, 20, 224 CrossRef CAS; (c) H. Wang, B. Wang, S. Sun and J. Cheng, Org. Chem. Front., 2018, 5, 2547 RSC; (d) D. Liu, M.-J. Jiao, X.-Z. Wang and P.-F. Xu, Org. Lett., 2019, 21, 4745 CrossRef CAS; (e) T.-T. Zhang, M.-J. Luo, F. Teng, Y. Li, M. Hu and J.-H. Li, Adv. Synth. Catal., 2020, 362, 4645 Search PubMed.
- CCDC 2046979 contains the supplementary crystallographic data for this paper.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and spectral data, and copies of 1H and 13C NMR spectra. CCDC 2046979. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0cc07927h |
| This journal is © The Royal Society of Chemistry 2021 |