Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Identifying subpopulations in multicellular systems by quantitative chemical imaging using label-free hyperspectral CARS microscopy†
Iestyn
Pope
 a,
Francesco
Masia
a,
Francesco
Masia
 a,
Kenneth
Ewan
a,
Kenneth
Ewan
 a,
Ana
Jimenez-Pascual
b,
Trevor C.
Dale
a,
Ana
Jimenez-Pascual
b,
Trevor C.
Dale
 a,
Florian A.
Siebzehnrubl
a,
Florian A.
Siebzehnrubl
 b,
Paola
Borri
b,
Paola
Borri
 *a and
Wolfgang
Langbein
*a and
Wolfgang
Langbein
 *c
*c
aCardiff University, School of Biosciences, Sir Martin Evans Building, Museum Avenue, Cardiff CF10 3AX, UK. E-mail: borrip@cf.ac.uk
bCardiff University, School of Biosciences, European Cancer Stem Cell Research Institute, Hadyn Ellis Building, Maindy Rd, Cardiff CF24 4HQ, UK
cCardiff University, School of Physics & Astronomy, The Parade, Cardiff CF24 3AA, UK. E-mail: langbeinww@cf.ac.uk
First published on 12th February 2021
Abstract
Quantitative hyperspectral coherent Raman scattering microscopy merges imaging with spectroscopy and utilises quantitative data analysis algorithms to extract physically meaningful chemical components, spectrally and spatially-resolved, with sub-cellular resolution. This label-free non-invasive method has the potential to significantly advance our understanding of the complexity of living multicellular systems. Here, we have applied an in-house developed hyperspectral coherent anti-Stokes Raman scattering (CARS) microscope, combined with a quantitative data analysis pipeline, to imaging living mouse liver organoids as well as fixed mouse brain tissue sections xenografted with glioblastoma cells. We show that the method is capable of discriminating different cellular sub-populations, on the basis of their chemical content which is obtained from an unsupervised analysis, i.e. without prior knowledge. Specifically, in the organoids, we identify sub-populations of cells at different phases in the cell cycle, while in the brain tissue, we distinguish normal tissue from cancer cells, and, notably, tumours derived from transplanted cancer stem cells versus non-stem glioblastoma cells. The ability of the method to identify different sub-populations was validated by correlative fluorescence microscopy using fluorescent protein markers. These examples expand the application portfolio of quantitative chemical imaging by hyperspectral CARS microscopy to multicellular systems of significant biomedical relevance, pointing the way to new opportunities in non-invasive disease diagnostics.
1. Introduction
It is becoming increasingly important in modern biomedical research to develop analytical tools capable of dissecting the complexity of multicellular systems, such as tissues and organs, without perturbing their natural state. Optical microscopy is still the only practical means to observe living specimens at sub-cellular spatial resolution in three dimensions and in real time. A major challenge of optical microscopy applied to cell biology is chemical specificity, since many biomolecules do not selectively absorb or emit light in the visible range.Albeit some endogenous biomolecules do exhibit distinguishable autofluorescence emission, for example flavins and lipo-pigments,1 a widely utilized method to introduce chemical contrast is by means of exogenous fluorescent labels with high absorption cross-sections and quantum yields attached to biomolecules of interest. However, all organic fluorescent probes are prone to photobleaching, and associated phototoxicity, severely limiting long time-course observation in living specimens. Moreover, the process of labelling is often time-consuming, invasive, and can introduce staining artefacts. Over the past two decades, vibrational microscopy exploiting the phenomenon of Raman scattering has emerged as a powerful label-free, chemically-specific modality complementing fluorescence.2 Spontaneous Raman scattering cross-sections of biomolecules are notoriously small, with corresponding limitations in imaging speed and sensitivity. The development of coherent Raman scattering (CRS) microscopy3 is partially circumventing these limitations, owing to the constructive interference of Raman light scattered by identical bonds in the focal volume, coherently driven to vibrate in phase.
Many of the earlier demonstrations of CRS microscopy4,5 showed images acquired at a single vibrational resonance, often chosen at the CH2 symmetric stretch vibration around 2850 cm−1, which is abundant in lipids. However, it is well known from Raman micro-spectroscopy that the best chemical specificity is obtained when acquiring vibrational spectra spanning a relevant range of wavenumbers, at each spatial position. Such combination of imaging with spectroscopy in CRS, often called hyperspectral CRS microscopy, has seen various experimental implementations whereby spectra at each spatial point are acquired at once6 using a spectrometer with a camera, or sequentially, by tuning the excitation wavelength7 or via spectral focusing.8 Hyperspectral acquisition has significantly widened the application space of CRS microscopy, with recent reports showing the spatial distributions of not only lipids, but also proteins, water, and nucleic acid components inside cells9–11 and tissues,6,7,12 as well as the ability to distinguish different lipid types, such as saturated versus unsaturated fatty acids,13,14 cholesterol,15 and phospholipids.16 Building from these capabilities, the application of CRS microscopy to tissue analysis for disease diagnostics in histopathology has been gaining increasing attention.17–19 Notably, the photo-stability and reproducibility of CRS micro-spectroscopy makes it a quantitative technique, with the potential to overcome qualitative and subjective decision making processes in current medical practice, suffering from high degrees of variability.
The combination of hyperspectral CRS data acquisition with quantitative image analysis algorithms is an area which has received major interest in recent years. Broadly speaking there are two main ways in which hyperspectral data sets, consisting of CRS signals as a function of spatial and spectral coordinates, are visualised. Some groups report spatially-resolved CRS intensities, or retrieved Raman-like susceptibilities, at selected wavenumbers, the latter attributed to characteristic vibrational resonances thought to be specific of certain biomolecular species.17 Pseudocolor images are then formed by assigning colours to single-frequency spatial distributions and overlay them to highlight differences in spatial patterns, which in turn can serve as markers of e.g. sub-cellular structures (such as nuclei), or diseased versus normal cells in tissues.6,12 A more advanced quantitative approach consists in factorising hyperspectral data sets into the superposition of separate components having characteristic spectra, and visualising the spatially resolved map of the components. A few methods have been proposed in the latter approach, including principal component analysis20 and independent component analysis.7 However, these methods usually are not able to make an unsupervised decomposition into individual chemical species with absolute concentration determination, which is ultimately the most meaningful quantitative representation.
We have developed over the past years a hyperspectral coherent anti-Stokes Raman scattering (CARS) microscope and an unsupervised quantitative data-analysis algorithm which allows us to retrieve Raman-like spectra and spatially-resolved concentration maps of chemical components, in physically meaningful units, with no prior knowledge.9–11,16,21 The method has so far been applied to 2D cell cultures, and its capability to dissect more complex features in multicellular systems such as 3D cell cultures and tissues has not yet been showcased. In this work, we report the application of this method on two exemplar multicellular systems, namely mouse liver organoids and mouse brain tissues xenografted with glioblastoma (GBM) cells.
Organoids are 3D micro-physiological structures of 20–400 μm diameter formed from clusters of parenchymal cells that grow and self-organise within gels formed from matrix components. The careful definition of growth factor combinations has allowed organoids to be established from a range of normal tissues and from pathological samples, using both mouse models and cells extracted from humans/patients.22 Organoids are already the system of choice for the identification of therapies in conditions such as Cystic Fibrosis and are starting to emerge as systems for cancer drug discovery in personalised medicine, since they better recapitulate patient responses than 2D cancer cell lines.
Advances in cancer research over the last decade have highlighted molecular differences at the single cell level within the same cancer and have led to a greater recognition of the degree of heterogeneity within tumors. The brain cancer glioblastoma is one of the most heterogeneous types of cancer and its rapid progression has been linked to a subpopulation of cancer cells known as GBM cancer stem cells (GSCs).23 GSCs are capable of initiating and sustaining tumor growth and are more resistant to conventional therapies.24,25 GSCs also contribute to metabolic heterogeneity in brain cancer26 and it was previously shown that GSCs preferentially utilize lipid metabolism, while non-stem GBM cells (NGC) depend on glucose.27 Hence, profound biochemical differences exist between GBM cellular subsets. Most studies investigating tumor heterogeneity in GBM and other cancers rely on transcriptional profiling or other biochemical characterization of cancer cells which necessitate sample lysis and therefore cannot be performed on living cells. Live cell imaging studies shown so far critically depend on labels (fluorescent or otherwise) to enable detection of chemical changes within cells, which is limited by the chemical sensitivity and read-out of the reporter.
Here, we have investigated whether our quantitative label-free CARS microscopy method can be used to discriminate different cellular subpopulations within organoids and brain tissues, based on their endogenous chemical content. Specifically, we address if cells can be distinguished while at different phases during the cell cycle in liver organoids, and if GSCs versus NGCs can be separated in brain tissues.
2. Materials and methods
2.1. Sample preparation – Fucci2aR liver bile duct organoids
2.2. Sample preparation – brain tissues with GBM
Human glioblastoma cell line L0 was a kind gift from Prof. Brent Reynolds (University of Florida) and was cultured as described previously.31 Briefly, cells were lentivirally transduced to express the green fluorescent protein eGFP and maintained as sphere cultures in N2 medium containing 20 ng ml−1 EGF. For orthotopic implantation of GSC and NGC populations, glioblastoma cells were dissociated, and single cell suspensions immunostained using a mouse monoclonal antibody against FGFR1 (Thermo Fischer, 1:50 dilution) and appropriate secondary antibody (Thermo Fischer, 1:500), and purified by FACS.31 Orthotopic xenografts of FGFR1 + (GSC) and FGFR1-(NGC) populations (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per animal each) were performed in 4–6 weeks old female SCID mice.31 Animal care and handling, and all procedures were performed in accordance with FELASA and institutional guidelines of Cardiff University and approved by the Animal Welfare Ethical Review Body of Cardiff University and the UK home office (PPL30/3331). Mice were maintained under Isoflurane anesthesia during procedures. Mice were monitored daily for the development of neurological signs and body weight loss. Animals reaching endpoint criteria were transcardially perfused using 2% paraformaldehyde and the brains removed for histology. Tissue preparation and cryosectioning was performed as described by Jimenez-Pascual et al.31 Briefly, 30 μm thick sections were cut on a cryomicrotome and stored in cryoprotectant until use. For CARS microscopy, tissue sections were mounted onto glass slides and air dried before being reconstituted with double-distilled water (ddH2O). Prior to imaging, sections were covered with a #1.5, 24 × 24 mm coverslip (Menzel Gläser, Agar Scientific AGL46S24-15) and sealed with nail varnish. Endogenous eGFP expression in GBM cells was used to discriminate between tumour and host cells.
000 cells per animal each) were performed in 4–6 weeks old female SCID mice.31 Animal care and handling, and all procedures were performed in accordance with FELASA and institutional guidelines of Cardiff University and approved by the Animal Welfare Ethical Review Body of Cardiff University and the UK home office (PPL30/3331). Mice were maintained under Isoflurane anesthesia during procedures. Mice were monitored daily for the development of neurological signs and body weight loss. Animals reaching endpoint criteria were transcardially perfused using 2% paraformaldehyde and the brains removed for histology. Tissue preparation and cryosectioning was performed as described by Jimenez-Pascual et al.31 Briefly, 30 μm thick sections were cut on a cryomicrotome and stored in cryoprotectant until use. For CARS microscopy, tissue sections were mounted onto glass slides and air dried before being reconstituted with double-distilled water (ddH2O). Prior to imaging, sections were covered with a #1.5, 24 × 24 mm coverslip (Menzel Gläser, Agar Scientific AGL46S24-15) and sealed with nail varnish. Endogenous eGFP expression in GBM cells was used to discriminate between tumour and host cells.
2.3. Optical micro-spectroscopy
Imaging of both the living organoids and fixed glioblastoma tissues was performed on the same inverted microscope (Nikon, Eclipse Ti-U). Alongside conventional wide-field epi-fluorescence and DIC imaging, the microscope has been modified to enable two-photon fluorescence (TPF) and hyperspectral CARS imaging modalities, described in our previous work.8 A home-built environmental chamber surrounds the microscope allowing a constant temperature of 37 °C with a 5% CO2/air-blend environment to be maintained for live cell imaging. Both sets of measurements used a 40 × 1.15 NA water-immersion objective (Nikon, MRD77410 CFI Plan Apochromat λS series) with a 1.0 × tube lens. A 1.34 NA oil-immersion condenser lens (Nikon, MEL41410) was used with the fixed glioblastomas, while a 0.72 NA dry condenser (Nikon, MEL56100) was used with the organoids to accommodate the low profile dish and lid of the CO2 chamber. xy sample motion and z objective motion are automated by stepper motors (Prior, ProScan III controller (V31XYZ), xy stage (H117NN2N) and z drive (PS3H122)).DIC illumination was provided by a halogen tungsten lamp (Nikon, V2-A LL 100 W) followed by a blue-green filter (Schott, BG40) to block near-infrared light, for which the DIC polarisers do not have sufficient extinction. A red bandpass filter (ThorLabs, FB650-40 (650 ± 20) nm) is used to define the wavelength range; the longer (red) wavelengths penetrate further into the organoids and do not excite fluorescence, avoiding photobleaching. A de Sénarmont compensator (Nikon, MEN51941 T-P2 DIC Polarizer HT) is used for DIC offset phase adjustment, consisting of a polarizer and a quarter-wave plate in the illumination beam path. The angle θ of the polarizer with respect to the fast axis of the quarter-wave plate is adjustable. The compensator is followed by a Nomarski prism in the condenser unit (Nikon, MEH52500 HNA N2 Oil, with the 1.34 NA oil-immersion condenser, or Nikon, MEH52400 CLWD N2, with the 0.72 NA dry condenser). A second Nomarski prism is placed after the objective (MBH76245 D-C DIC Slider 40X-II, Nikon), followed by a linear polariser (Nikon, MEN51980 Ti-A-E DIC Analyzer Block) in the filter turret. DIC phase angle setting for the organoids was θ = 45°, and for the brain tissues was θ = 0° (no background transmission) and θ = 40°. An exposure time of 0.1 s was used for all DIC experiments. The lamp illumination intensity was set to result in a maximum image pixel value around 15 ke, corresponding to about 2 mW cm−2 at the sample for the 40° and 45° rotation angles and about 70 mW cm−2 for the 0° rotation angle.
For the brain tissue samples, wide-field epi-fluorescence excitation of eGFP was provided by a metal–halide lamp (Prior Scientific, Lumen L200/D) set at 10% of the maximum power. A suitable exciter/emitter/dichroic filter cube (Semrock, GFP-A-Basic) was used, resulting in an illumination intensity of about 3 W cm−2 at the sample. Fluorescence images were acquired with an exposure time of 0.03 s.
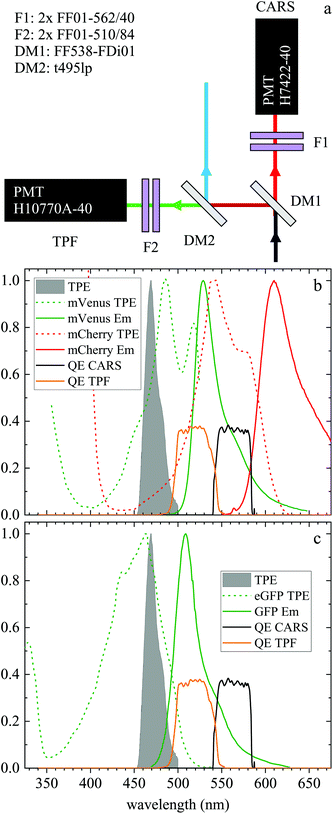 | ||
| Fig. 1 (a) Forward detection layout. A dichroic beamsplitter (DM1) transmits the CARS emission at wavelengths >540 nm and reflects the TPF emission at shorter wavelengths. In the CARS detection path, a pair of bandpass filters (F1) rejects unwanted signals from reaching the CARS PMT. The signal reflected by DM1 travels through a second dichroic mirror (DM2) which reflects wavelengths below 495 nm. A pair of bandpass filters (F2) rejects unwanted signals from reaching the TPF PMT. (b) Normalized two-photon excitation (dashed lines) and emission (solid lines) spectra for the mVenus32 (green) and mCherry33 (red) fluorescent proteins in the Fucci2aR system. (c) Normalized two-photon excitation33 (dashed line) and emission (solid line) spectrum of eGFP used in the glioblastoma tumour cells. Also plotted are the normalized TPF excitation pulse spectrum (grey infill curve, at half its wavelength) and the quantum efficiency of the TPF (orange) and CARS (black) PMT detection from the entrance into DM1. | ||
2.3.2.1. Organoid acquisition settings. Organoids were first located using DIC, as described above. TPF and hyperspectral CARS images were acquired sequentially to avoid bleaching of TPF by the CARS excitation, as well as bleed through of TPF into the CARS detection, see results section for discussion. TPF images and hyperspectral CARS z-stacks were acquired at 1 μm and 5 μm intervals respectively, over a z-range of 30 μm. The pixel size in the x, y plane was (86 nm)2. A pixel dwell time of 100 μs and 1 μs and PMT gains of 106 and 104.6 were used for TPF and CARS, respectively. Typical excitation powers at the sample were 41 mW (pump), 31 mW (Stokes) and 11 mW (TPF). Hyperspectral (x, y and wavenumber) CARS scans were acquired over the range 2600–3800 cm−1, with 5 cm−1 step size. Following each scan, an image was acquired with the pump and Stokes pulses out of time overlap (all other settings identical) to measure non-CARS background.
2.3.2.2. Brain tissue acquisition settings. Wide-field fluorescence and DIC were first used to locate GSCs and NGCs within the brain section. TPF and hyperspectral CARS images were acquired sequentially to avoid bleed through of the strong fluorescence signal into the CARS detection, see results section for discussion. PMT gains of 104.8 and 105 were used for TPF and CARS, respectively with a pixel dwell time of 10 μs for both. The pixel size in the x, y plane was (80 nm)2. Typical excitation powers at the sample were 31 mW (pump), 23 mW (Stokes) and 8 mW (TPF). Hyperspectral CARS scans were acquired over the range 2600–3700 cm−1, with 5 cm−1 step size. Following each hyperspectral CARS scan, an image was acquired with the pump and Stokes pulses out of time overlap (all other settings identical) to determine background offset levels.
2.4. CARS hyperspectral analysis
Hyperspectral CARS datasets can be difficult to visualise and interpret, and for this purpose we have developed the Hyperspectral Image Analysis (HIA) software.9 HIA enables the retrieval of the imaginary part of the CARS susceptibility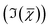 and the factorisation of the spectra into a specified number of chemical components with corresponding concentration maps. Briefly, the HIA pipeline operates as follows.9,35,36 Firstly, a background image acquired with the pump and Stokes beams out of time overlap is subtracted from each image of the hyperspectral CARS scan. As well as subtracting backgrounds e.g. from the electronics, this step also removes potential fluorescence contributions generated from the individual pulses. Singular-value decomposition (SVD) is then employed to denoise the data. In order to correct for the varying temporal overlap of the pump and Stokes beams, data are divided by the spectrum of a non-resonant material (glass), acquired with the same laser power and acquisition settings. If there is a region filled with water present in the image, this may be used as reference material, combined with a previously calibrated glass/water ratio. If there is no such region present (as with the brain tissue samples), a glass spectrum is measured in the glass coverslip immediately after the acquisition of the CARS data on the sample. To enable quantitative analysis, the imaginary part of the CARS susceptibility
and the factorisation of the spectra into a specified number of chemical components with corresponding concentration maps. Briefly, the HIA pipeline operates as follows.9,35,36 Firstly, a background image acquired with the pump and Stokes beams out of time overlap is subtracted from each image of the hyperspectral CARS scan. As well as subtracting backgrounds e.g. from the electronics, this step also removes potential fluorescence contributions generated from the individual pulses. Singular-value decomposition (SVD) is then employed to denoise the data. In order to correct for the varying temporal overlap of the pump and Stokes beams, data are divided by the spectrum of a non-resonant material (glass), acquired with the same laser power and acquisition settings. If there is a region filled with water present in the image, this may be used as reference material, combined with a previously calibrated glass/water ratio. If there is no such region present (as with the brain tissue samples), a glass spectrum is measured in the glass coverslip immediately after the acquisition of the CARS data on the sample. To enable quantitative analysis, the imaginary part of the CARS susceptibility 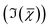 is retrieved using a phase-corrected Kramers–Kronig (PCKK) procedure, which recovers a Raman-like spectrum. Finally, the retrieved spatially-resolved susceptibility spectra are factorised into spectra and spatially-resolved concentration maps of chemical components (a method which we called FSC3), based on non-negative matrix factorisation. We use an iterative algorithm37 where concentration maps and spectra are sequentially varied to minimise the factorisation error. Importantly, the factorisation is unsupervised, i.e. it does not require prior knowledge of the sample's chemical composition. The number of components used in the factorisation can be varied, with the optimal number being the minimum required to provide unique spatial and spectral features. Typically, in the range of 6 to 7 components are needed, with 5 to 6 components being physically meaningful, and 1 or 2 components accounting for spurious noise and imaging artefacts. To reduce the importance of water, which is dominating the
is retrieved using a phase-corrected Kramers–Kronig (PCKK) procedure, which recovers a Raman-like spectrum. Finally, the retrieved spatially-resolved susceptibility spectra are factorised into spectra and spatially-resolved concentration maps of chemical components (a method which we called FSC3), based on non-negative matrix factorisation. We use an iterative algorithm37 where concentration maps and spectra are sequentially varied to minimise the factorisation error. Importantly, the factorisation is unsupervised, i.e. it does not require prior knowledge of the sample's chemical composition. The number of components used in the factorisation can be varied, with the optimal number being the minimum required to provide unique spatial and spectral features. Typically, in the range of 6 to 7 components are needed, with 5 to 6 components being physically meaningful, and 1 or 2 components accounting for spurious noise and imaging artefacts. To reduce the importance of water, which is dominating the 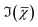 spectra above 3100 cm−1, the spectral range analysed by FSC3 is typically limited11 to a maximum of 3150 cm−1, which retains the relevant vibrational resonances of the organic material. FSC3 may be applied to hyperspectral data sets individually, or multiple sets can be analysed together. It is also possible to take components from a selected factorisation analysis, and use these as a base to factorise other data sets. This is referred to as projection, and uses a single step of the factorisation algorithm which calculates the concentration maps, while keeping the spectra fixed to the given base.
spectra above 3100 cm−1, the spectral range analysed by FSC3 is typically limited11 to a maximum of 3150 cm−1, which retains the relevant vibrational resonances of the organic material. FSC3 may be applied to hyperspectral data sets individually, or multiple sets can be analysed together. It is also possible to take components from a selected factorisation analysis, and use these as a base to factorise other data sets. This is referred to as projection, and uses a single step of the factorisation algorithm which calculates the concentration maps, while keeping the spectra fixed to the given base.
3. Results and discussion
3.1. Fucci2aR liver bile duct organoids
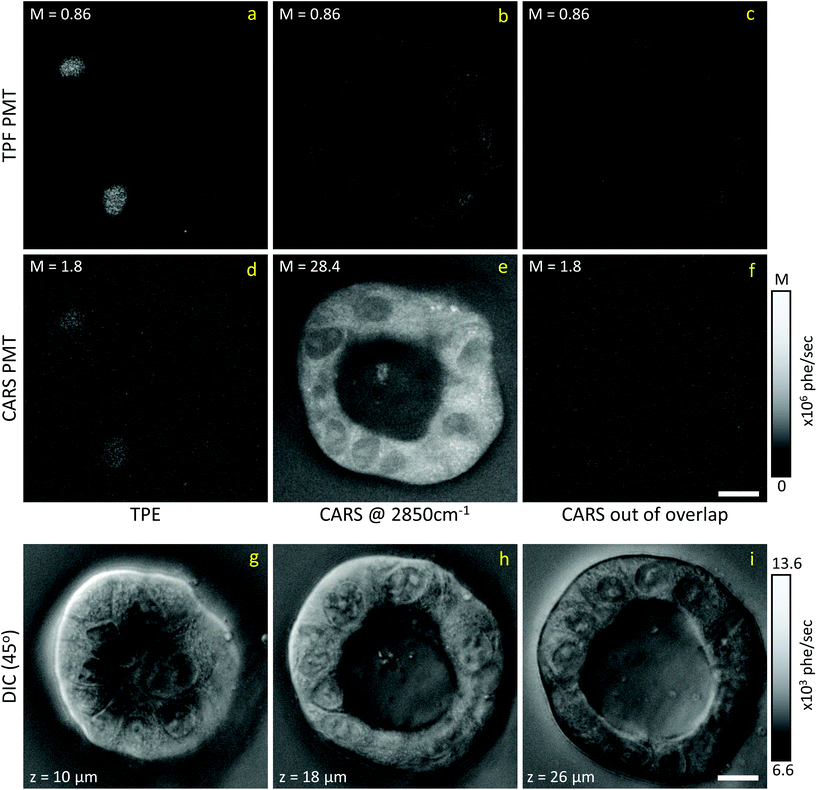
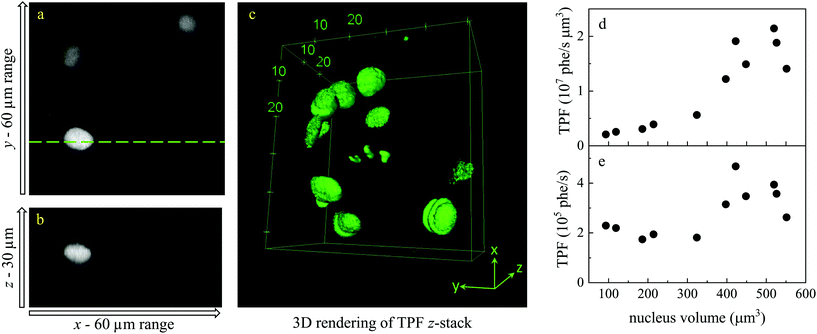
Liver organoids investigated here incorporated the fluorescence ubiquitination-based cell cycle indicator (Fucci2aR) system (see section 2.1.1). This is a fluorescent protein-based construct that employs a red (mCherry) and a green (mVenus) fluorescent protein fused to two cell cycle dependent proteins, Cdt1 and geminin, respectively. In eukaryotic cells, Cdt1 level peaks during G1 phase and plummets upon S entry. Conversely, the geminin level is high during S, G2 and M phases, and is degraded in the subsequent G1 phase. Therefore, when cells are in the G1 phase, the high level of mCherry gives rise to red-fluorescent cell nuclei, whilst in the S/G2/M phase the high level of mVenus results in green-fluorescent nuclei. We used this construct as a fluorescence marker, enabling direct correlation of TPF and hyperspectral CARS imaging on the same living organoid, to investigate whether hyperspectral CARS is able to distinguish subpopulation of cells at different phases in the cell cycle. The experiment was set-up to avoid bleed-through between fluorescence emission and CARS detection, as detailed in the following. The two-photon excitation and emission spectra for mCherry33 and mVenus32 are displayed in Fig. 1(b) overlaid with the spectrum of the TPE beam, and with the TPF and CARS PMT detection quantum efficiency (QE) spectra for the optical setup. mCherry is excited only weakly by the TPE beam, and its emission lies above the detection ranges of both the TPF and CARS PMTs. Therefore, no fluorescence signal is observed from cells in the G1 phase under these excitation/detection conditions. Conversely, the mVenus TPE spectrum overlaps well with the TPE pulse spectrum, and the peak of its emission falls within the detection range of the TPF PMT. There is also an overlap of the mVenus emission spectrum with the CARS PMT detection range. Under TPE beam excitation, we therefore observe a strong signal detected by the TPF PMT, and a weaker signal from the CARS PMT, from those cells that are in the S/G2/M phase of their cell cycle. This is demonstrated by the images in Fig. 2(a and d) which show the signal in an xy plane sectioning the organoid, detected by the TPF and CARS PMTs when the sample is excited only by the TPE beam. For comparison, Fig. 2(b and e) illustrates the detected signal when the sample is excited only by the CARS (pump and Stokes) beams at 2850 cm−1 IFD. Here, we observe a strong signal in the CARS PMT from lipids and proteins in the cell cytoplasm, with the cell nucleus appearing dark. The organoid forms a hollow structure, as can also be seen in the DIC images (Fig. 2(g–i)) taken at different z-planes through the organoid. A signal from the cell cytoplasm is observed in the TPF PMT under CARS excitation. This is originating from two-photon excited auto-fluorescence, as can be seen in Fig. 2(c and f) which illustrates the observed signal when the sample is excited by the pump and Stokes beams out of time overlap (i.e. there is no CARS signal generated). Such autofluorescence is predominantly generated by the pump beam, considering the pump pulse TPE peak at around 340 nm.38Fig. 2(c and f) also demonstrates that there is no fluorescence detected by the CARS PMT under pump and Stokes excitation when the pulses are not in temporal overlap. To maintain such de-coupling, TPF and CARS images were acquired sequentially, rather than simultaneously, throughout the experiments.TPF images were acquired in 3D, as z-stacks with 1 μm intervals, while hyperspectral CARS z-stacks were acquired at 5 μm steps through the organoid (see section 2.3.2.1). This choice was made in order to compromise between 3D imaging and the time required for hyperspectral CARS acquisition, while minimising motion artefacts and photodamage, since organoids were alive. The TPF z-stack is used to positively identify the cells in the S/G2/M phase of their cell cycle and generate a 3D reconstruction of their position for correlation with the hyperspectral CARS data. A xy slice with z-projection, along with a 3D rendering of the TPF z-stack is presented in Fig. 3. An overview of all xy planes is shown in Fig. S1.† The TPF volumetric dataset was then quantitatively analysed, as follows. Cell nuclei volumes were calculated from the 3D reconstruction using the 3D Objects Counter plugin39 for the image analysis software ImageJ to segment the nuclei (see section S1 ii and Fig. S2†). The fluorescence intensity integrated over the nucleus volume is given in Fig. 3(d)versus nucleus volume for each cell which showed localization of TPF in the nucleus. A clear trend of increasing fluorescence intensity with increasing nucleus volume is observed, consistent with the progressive increase of the size of a cell and its nucleus during the eukaryotic cell cycle,40–42 and that mVenus-geminin is highly expressed in the later phases of the cycle, once the cell has started its DNA replication. Notably, we observe a step in the intensity increase for intermediate cell volumes between 300 μm3 and 400 μm3, which is clearly visible in the average intensity over the nucleus volume, as shown in Fig. 3(e). The correlation between nucleus size and mVenus fluorescence intensity suggests that low-intensity small-nucleus cells are at the G1/S phase, intermediate sized nuclei with high intensity are in the S/G2 phase, and large nuclei are in the G2/M phase.
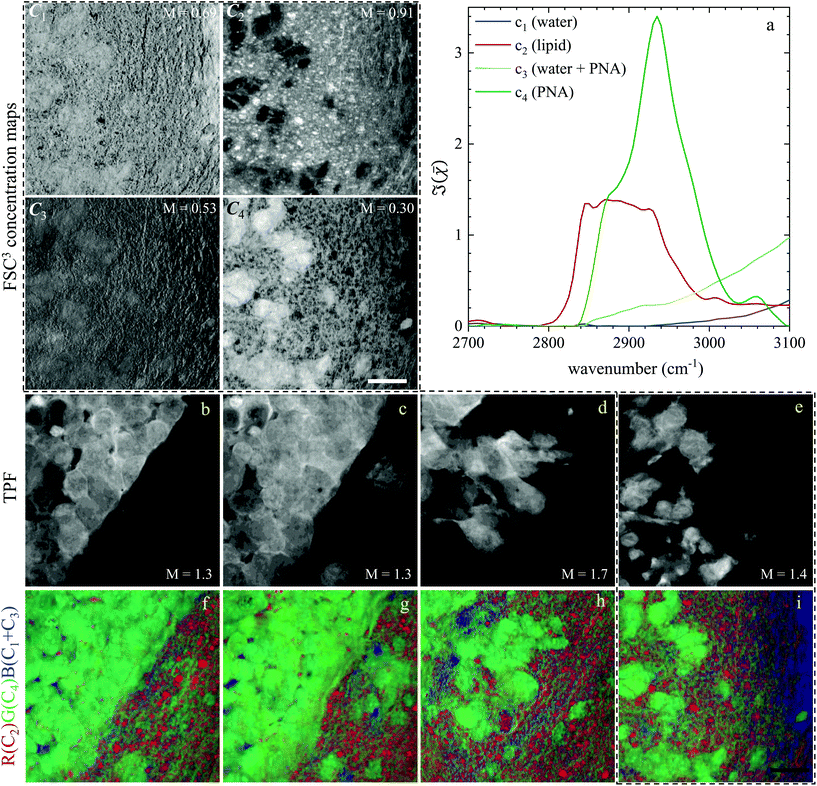
Hyperspectral CARS was quantitatively analysed using the HIA/FSC3 pipeline as described in section 2.4. The resultant susceptibility spectra and concentration maps measured at the same xy plane as shown in Fig. 3 are presented in Fig. 4. Seven spectral components were used. Based on the retrieved susceptibility spectra and the spatial profile of the concentration maps, the first six components were attributed to c1: water, c2: protein, c3: nucleic acids + protein (PNA), c4: DNA, c5: lipid, c6: lipid + protein. This is consistent with published reports9,11,43 showing that: (i) water has a Raman spectrum peaked at around 3300 cm−1, hence appears as a tail in the 2800–3200 cm−1 range; (ii) the ratio between the CH2 symmetric stretch vibration (2850 cm−1) and the CH3 resonance (2930 cm−1) discriminates between lipids (rich in CH2 bonds) and proteins (peaked at 2930 cm−1); (iii) nucleic acids exhibit Raman spectra peaked at 2950 cm−1 and DNA is confined to the cell nucleus. We note that all components contain a significant amount of water additional to the attributed organic material. c4 is dominated by a water contribution. The water-subtracted spectrum of this component (see Fig. S9†) shows a spectral distribution between 2900 and 3100 cm−1 consistent with the main features of DNA (see ref. 43Fig. 1B). The spectral shape and concentration map of c7 indicates that this is not a physically meaningful chemical component, and is attributed to imaging or sample motion artefacts (see Fig. S3, and S6† for an overview of concentration and spectral errors). Inspection of the concentration maps reveals that there is no obvious chemical component that can be directly correlated with the mVenus marker. Indeed, while during the cell cycle it is known that the amount of proteins and nucleic acids is growing in preparation for mitosis, at the same time, the cell increases its volume at a steady rate, hence it is unclear whether the concentration of these components should be significantly altered during the cycle. On the other hand, it has been shown, using yeast as model systems of the eukaryotic cell cycle,41,42 that despite the sharp increase in DNA copy number during DNA replication in the S-phase, the size of the cell nucleus does not abruptly increase in parallel, suggesting that the DNA concentration could be significantly higher at the end of the S phase.
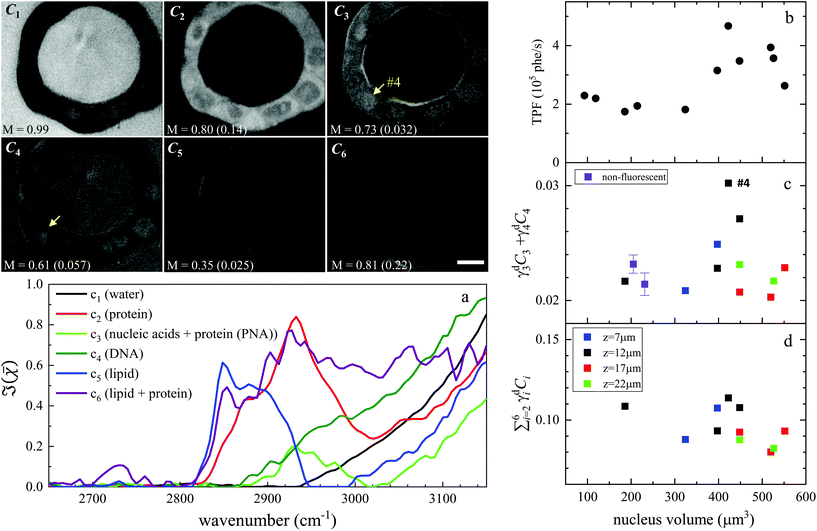 | ||
| Fig. 4 Overview of the FSC3 analysis on Fucci2aR liver bile duct organoids. Images C1 to C6 are the concentration maps of the corresponding components, on a grey scale from 0 to M as indicated in vol/vol units. The corresponding dry vol/vol concentrations are given in parenthesis. Images are shown for the plane corresponding to the TPF xy section in Fig. 3, and the strongly fluorescent cell visible in the TPF section is indicated as #4, for reference. (a) Susceptibility spectra of the FSC3 components. (b–d) Dry concentrations of components averaged over the nuclei areas, for the cells that exhibited mVenus TPF in the nucleus, as a function of the nucleus volume (each data point represents an individual nucleus). Different z-planes were evaluated (indicated by colours), and data are shown for the planes showing the largest nucleus area (see text). Purple symbols show the mean (bar: standard error of the mean) of the dry components for two sets of cells having no detectable mVenus fluorescence in the nucleus (see text). The TPF intensity per nucleus volume is plotted again (same data as in Fig. 3), for direct comparison. Scale bar 10 μm. | ||
To investigate this point, we analysed all available hyperspectral CARS datasets for the different z-planes, using the seven components shown in Fig. 4 as common basis (projection analysis, see Materials and methods). On these data sets, we identified those cells that exhibited TPF of mVenus in the nucleus, and calculated the average concentration of each component over the nucleus area. Since hyperspectral CARS z-stacks were performed with coarse 5 μm steps, well above Nyquist sampling, we could not examine the retrieved concentration components volumetrically in 3D. For each cell, we therefore selected the z-planes where the nucleus area appeared largest, as to avoid z-planes too close to the nucleus top/bottom edges (see also Fig. S10†). This is to ensure that the measured concentrations are only sampling the nucleus region, considering the 1.5 μm wide axial point-spread function of 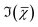 for the data shown. Concentrations of c2 to c6 were scaled to represent dry values, by removing the water contribution, as described in our recent work.11 Briefly, for each component, we calculated the dry volume fraction γdi, to obtain the corresponding dry vol/vol concentration γdiCi. Details on the determination of γdi are given in section S1 iv.† Fig. S11† shows γdiCi averaged over the nucleus area, for each cell that exhibited TPF of mVenus in the nucleus, taken at the selected z-plane (as explained above), as a function of the nucleus volume. Since c3 and c4 both are localized in the nucleus and have a spectrum comprising DNA, we considered the sum of these two components, which is shown in Fig. 4(c). Notably, we see that this sum γd3C3 + γd4C4 sharply increases with increasing nucleus volume around 350 μm3, following a similar trend as the TPF intensity, which we attributed as signature of the S/G2 phase. The subsequent decrease of this concentration for large nuclei volumes in the G2/M phase, when the DNA replication has ended, is in line with the expansion of the nucleus size in preparation for mitosis while no longer increasing its DNA content. The spread of concentration values for different z-planes of one cell around 450 μm3 nucleus volume could also be indicative of an asymmetric distribution of DNA in preparation for mitosis. The total dry concentration
for the data shown. Concentrations of c2 to c6 were scaled to represent dry values, by removing the water contribution, as described in our recent work.11 Briefly, for each component, we calculated the dry volume fraction γdi, to obtain the corresponding dry vol/vol concentration γdiCi. Details on the determination of γdi are given in section S1 iv.† Fig. S11† shows γdiCi averaged over the nucleus area, for each cell that exhibited TPF of mVenus in the nucleus, taken at the selected z-plane (as explained above), as a function of the nucleus volume. Since c3 and c4 both are localized in the nucleus and have a spectrum comprising DNA, we considered the sum of these two components, which is shown in Fig. 4(c). Notably, we see that this sum γd3C3 + γd4C4 sharply increases with increasing nucleus volume around 350 μm3, following a similar trend as the TPF intensity, which we attributed as signature of the S/G2 phase. The subsequent decrease of this concentration for large nuclei volumes in the G2/M phase, when the DNA replication has ended, is in line with the expansion of the nucleus size in preparation for mitosis while no longer increasing its DNA content. The spread of concentration values for different z-planes of one cell around 450 μm3 nucleus volume could also be indicative of an asymmetric distribution of DNA in preparation for mitosis. The total dry concentration  is shown in Fig. 4(d), and appears approximately constant versus nucleus volume. The overview of each component given in Fig. S11† shows that individual components do not exhibit a well defined trend. Indeed, only γd3C3 + γd4C4 appears to correlate with the TPF intensity dependence, suggesting that this combination could be used as a marker of the S/G2 phase in the cell cycle. For consistency, we also examined the cells imaged by hyperspectral CARS that did not exhibit a significant mVenus TPF in the nucleus. Fig. 4(c) shows the mean of γd3C3 + γd4C4 over these cells (purple data points). We considered two cases: all the non-fluorescent nuclei areas, and a sub-set whereby we excluded nuclei with very small areas (<
is shown in Fig. 4(d), and appears approximately constant versus nucleus volume. The overview of each component given in Fig. S11† shows that individual components do not exhibit a well defined trend. Indeed, only γd3C3 + γd4C4 appears to correlate with the TPF intensity dependence, suggesting that this combination could be used as a marker of the S/G2 phase in the cell cycle. For consistency, we also examined the cells imaged by hyperspectral CARS that did not exhibit a significant mVenus TPF in the nucleus. Fig. 4(c) shows the mean of γd3C3 + γd4C4 over these cells (purple data points). We considered two cases: all the non-fluorescent nuclei areas, and a sub-set whereby we excluded nuclei with very small areas (<![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 μm2), which could be too close to the top/bottom nucleus edges, as well as nuclei with large areas (>
22 μm2), which could be too close to the top/bottom nucleus edges, as well as nuclei with large areas (>![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 53 μm2), to exclude cells beyond the G1 phase. The two purple data points represent the mean for these two sets. An overview of γd3C3 + γd4C4 for each cells in the two sets, and the resulting mean, standard deviation, and standard error of the mean is shown in Fig. S12.† For the non-fluorescent cells we used the nucleus area A to calculate the volume as V = A3/2, to position these data points in Fig. 4. Considering all non-fluorescent cells, we obtained a mean of γd3C3 + γd4C4 which is slightly higher than when selecting cells as described above (see also Fig. S12†), but in both cases the values are below those observed for the fluorescent cells attributed to the S/G2 phase, and in line with the lower values seen in the other phases, consistent with our interpretation. It should be noted that the limited number of fluorescent cells available in this study implies that the analysis has a modest statistical significance, hence the results are to be taken as indicative. The emphasis here is on the quantitative data analysis pipeline presented, offering a valuable methodology for investigating quantitative chemically signatures of the cell cycle with hyperspectral CARS microscopy.
53 μm2), to exclude cells beyond the G1 phase. The two purple data points represent the mean for these two sets. An overview of γd3C3 + γd4C4 for each cells in the two sets, and the resulting mean, standard deviation, and standard error of the mean is shown in Fig. S12.† For the non-fluorescent cells we used the nucleus area A to calculate the volume as V = A3/2, to position these data points in Fig. 4. Considering all non-fluorescent cells, we obtained a mean of γd3C3 + γd4C4 which is slightly higher than when selecting cells as described above (see also Fig. S12†), but in both cases the values are below those observed for the fluorescent cells attributed to the S/G2 phase, and in line with the lower values seen in the other phases, consistent with our interpretation. It should be noted that the limited number of fluorescent cells available in this study implies that the analysis has a modest statistical significance, hence the results are to be taken as indicative. The emphasis here is on the quantitative data analysis pipeline presented, offering a valuable methodology for investigating quantitative chemically signatures of the cell cycle with hyperspectral CARS microscopy.
3.2. Brain tissue with GBM
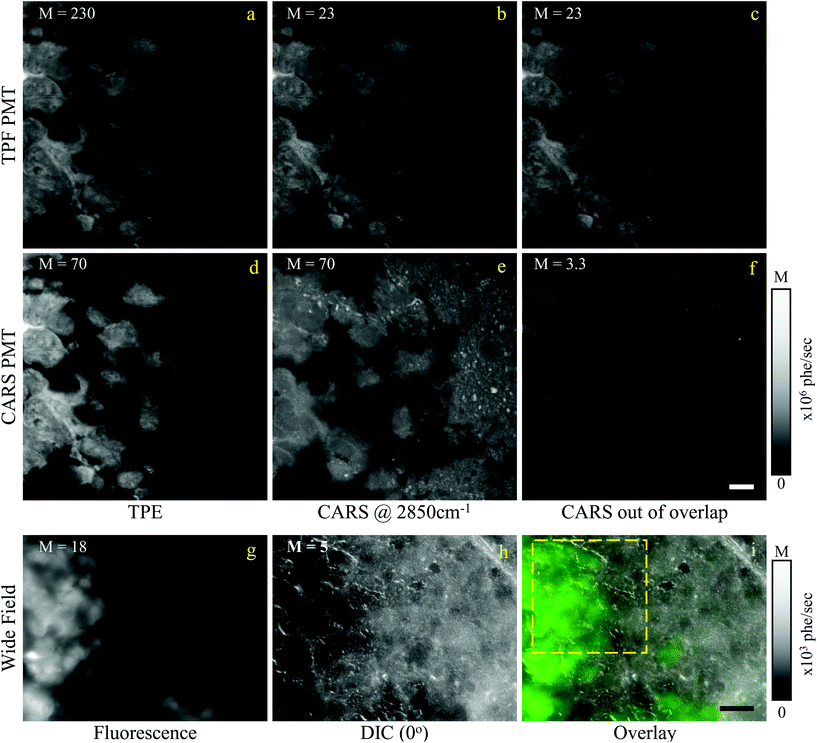
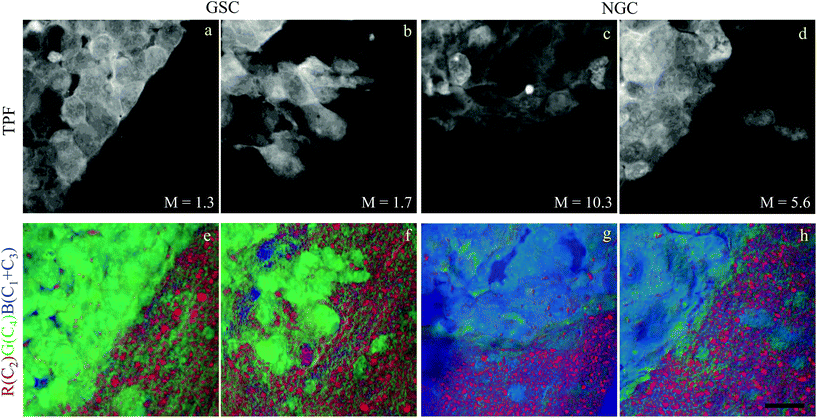
In this section, we report our work on brain tissues obtained from mice xenografted with either eGFP-labelled GBM stem cells (GSC) or eGFP-labelled non-stem GBM cells (NGC). The aim was to investigate if hyperspectral CARS microscopy can separate normal brain tissue from cancerous cells, and if tumours derived from GSC versus NGC can be distinguished. eGFP labelling enables positive correlation with CARS imaging. As discussed in section 3.1, signal bleed through between CARS and TPF can occur and needs to be corrected for. The two-photon excitation and emission spectra for eGFP are displayed in Fig. 1(c) together with the spectra of the TPE beam and the TPF and CARS PMT QE. The TPE beam spectrum overlaps well with the eGFP excitation spectrum, and the eGFP emission overlaps well with the TPF PMT QE. There is also a significant overlap of the eGFP emission with the CARS PMT QE. We can therefore expect to see eGFP emission in both the TPF and CARS PMTs. This is confirmed experimentally, as reported in Fig. 5(a and d), showing the TPF and CARS PMT signals when the sample is excited only by the TPE beam. Fig. 5(b and e) illustrates the detected signal when the sample is excited only by the CARS (pump and Stokes) beam, at 2850 cm−1 IFD. We observe a strong signal in the CARS PMT from lipids and proteins in the cell cytoplasm. In the TPF PMT, in addition to the two-photon excited auto-fluorescence, predominantly generated by the pump pulse (similar to what described in section 3.1), we also see a substantial contribution from the eGFP emission. This is a result of the significant spectral overlap of the TPE by the pump beam around 340 nm and the two-photon excitation of eGFP (see Fig. 1). Fig. 5(c and f) shows the observed signal when the sample is excited by the pump and Stokes beams out of time overlap. We can see a weak fluorescence detected in the CARS PMT, due to two-photon excitation from the individual pump and Stokes pulses. Although about 20 times weaker than the CARS signal, this contribution is taken into account in the HIA/FSC3 pipeline analysis as described in section 2.4. In the experiments, TPF excited by the TPE beam and CARS excited by the CARS beams were acquired sequentially.Regions of interest (ROIs) containing both cancer cells and normal tissue were initially found using wide-field epi-fluorescence and DIC imaging, see Fig. 5(g–i) for tissues from mice implanted with GSC cells. A z-position approximately in the centre of the 30 μm thick section was located using TPF imaging. TPF and hyperspectral CARS scans were acquired on multiple ROIs (6 for GCS-derived tumours and 2 for NGC-derived tumours) as described in section 2.3.2.2. Hyperspectral CARS scans were then processed through our HIA/FSC3 pipeline, as described in section 2.4. For these samples, using five components in the FSC3 was found to be optimal. All 6 ROIs in tissues from mice implanted with GSC cells were analysed together, hence spectra are a common basis in the factorisation. Exemplar concentration maps for one of the ROIs are shown in Fig. 6(C1–C4) in grey scale (all ROIs are given in Fig. S13 and S15–S20†).
Based on the spectral shape and the spatially-resolved concentration maps, the components were assigned to c1: water, c2: lipid, c3: water + PNA, c4: PNA. Component c5 exhibited a spectrum that is not physically meaningful, and thus is attributed to artefacts (see Fig. S14†). Fig. 6 shows the susceptibility spectra and concentration maps of components c1 to c4 for the GSC-derived tumour cells. RGB overlays of the concentration maps of components c2 (red), c4 (green) and c1 + c3 (blue) are presented in Fig. 6(f–i) for four ROIs. Comparing them with the corresponding TPF images shown in the figure on the row above, there is a clear separation between the GSC-derived cancer and normal brain tissue. Tumour cells in the colour concentration maps appear green-blue, hence richer in water and proteins compared to normal tissue. Notably, this finding is consistent with the fact that tumour cells proliferate faster than normal cells, hence they need to make more proteins, a point well recognised in the cell biology literature (for a review see ref. 44). It is also known that malignant tissue contains more water than normal tissue. Some of this water is between cells (interstitial), but there are reports45 showing that tumour cells contain more water than normal tissue. This could be explained since, in most instances, tumour cells are larger than normal cells, albeit this point is not yet fully understood.
Brain sections containing NGC-derived tumours were also imaged, at two ROIs, in the same way as described for the sections containing GSC-derived cancerous tissue. In the HIA/FSC3 analysis pipeline, the spectral components determined from the ROIs with GSC-derived cancer cells were used as a basis (projected FSC3, see section 2.4), allowing for a direct comparison between the two data sets. Concentration errors and spectral errors for this factorisation analysis are shown in Fig. S15–S20† and Fig. S21, S22.† Notably, the spectral errors are similar across the GSC and NGC data sets, showing that both datasets are well represented by the chosen common spectral basis. Colour overlays were generated using the same components mapped in the same RGB order, and are presented in Fig. 7 alongside the colour maps for two ROIs in the GSC-derived tumour sections, on the same colour scale for direct comparison. Firstly, we again note the clear distinction between cancer cells and the normal tissue. Secondly, when comparing the GSC-derived and NGC-derived tumours in the false colour maps we note the GSC-derived cells appear significantly greener. This shows that tumour cells derived from GSCs contain more PNA than those derived from NGCs. A quantitative analysis of the dry concentrations and the water concentration is shown in Fig. 8 confirming this conclusion. Indeed, the spatially-resolved dry PNA concentration γd4C4, across all available ROIs, revealed a mean value of 14.6% vol/vol (with a standard deviation (SD) of 0.9%) for the GSC-derived tumour regions compared to a mean of 9% vol/vol (SD 0.5%) in the NGC-derived tumour regions (corresponding to a 1.6 ratio), and a mean of 7.8% vol/vol (SD 0.6%) in the normal tissue regions (see section S2 iii†). Therefore, our quantitative hyperspectral CARS technique not only distinguishes between cancerous versus normal brain tissue, but is also able to dissect the heterogeneity of cancer cells, and to distinguish tumours derived from stem cells versus non-stem cells.
 | ||
| Fig. 7 Comparison of the hyperspectral CARS FSC3 analysis on brain sections with GSC-derived and NGC-derived tumours. Samples with NGC-derived tumours were analysed using as spectral basis the 5 components of the FSC3 analysis from samples with GSC-derived tumours (see Fig. 6). (a–d) TPF images identifying GSC-derived and NGC-derived cancer cells. Intensity grey scale from 0 to M × 108 photoelectrons per second. (e–h) False colour maps generated from the overlay of the concentrations of components c2 (red), c4 (green), c1 + c3 (blue). RGB channel range 0 to M (vol/vol) adjusted as in Fig. 6. Scale bar 20 μm. | ||
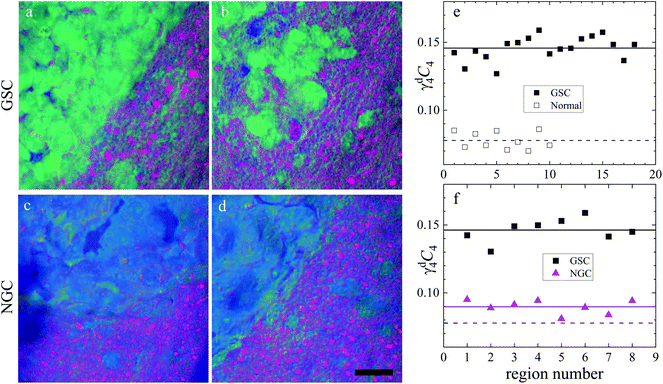 | ||
Fig. 8 Dry component analysis on brain sections with GSC-derived and NGC-derived tumours. False colour dry concentration maps of the FSC3 components lipid γd2C2 (red) and PNA γd4C4 (green), and the total water concentration 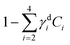 (blue). (a and b) GSC and (c and d) NGC samples. Images are on the same RGB scale, from 0 to a colour maximum scale M (vol/vol) with Mred = 0.18, Mgreen = 0.22, Mblue = 1.48. Scale bar: 20 μm. (e and f) Dry PNA concentration of component γd4C4 spatially averaged over selected areas identified from TPF and false colour maps (see Fig. S24 and S25† for region maps). (e) GSC-derived tumour regions (solid black squares) compared with regions of normal tissue (hollow squares). The lines show averages for GSC-derived tumour regions (solid); and normal tissue regions (dashed). (f) GSC-derived (black squares) and NGC-derived (magenta triangles) tumour regions for the two GSC and two NGC ROIs shown in (a–d). The solid magenta line shows the average of the NGC-derived tumour regions; the solid and dashed black lines are the same as in (e). (blue). (a and b) GSC and (c and d) NGC samples. Images are on the same RGB scale, from 0 to a colour maximum scale M (vol/vol) with Mred = 0.18, Mgreen = 0.22, Mblue = 1.48. Scale bar: 20 μm. (e and f) Dry PNA concentration of component γd4C4 spatially averaged over selected areas identified from TPF and false colour maps (see Fig. S24 and S25† for region maps). (e) GSC-derived tumour regions (solid black squares) compared with regions of normal tissue (hollow squares). The lines show averages for GSC-derived tumour regions (solid); and normal tissue regions (dashed). (f) GSC-derived (black squares) and NGC-derived (magenta triangles) tumour regions for the two GSC and two NGC ROIs shown in (a–d). The solid magenta line shows the average of the NGC-derived tumour regions; the solid and dashed black lines are the same as in (e). | ||
4. Conclusions
In conclusion, we have investigated two types of complex multicellular systems, namely liver organoids and brain tissue sections, using correlative TPF and hyperspectral CARS microscopy combined with our recently developed quantitative and unsupervised data analysis pipeline.Organoids were maintained alive under hyperspectral CARS measurements, owing to carefully designed culture and imaging conditions. They contained a fluorescence construct (Fucci2aR) which enabled us to selectively tag cells in the S/G2/M phase via the fluorescent protein mVenus in the nucleus. Quantitative analysis of the volumetric TPF datasets showed a correlation between increasing fluorescence intensity and nucleus volume, which we used to separate the early S stage from the late S/G2 and G2/M stages. Hyperspectral CARS microscopy measurements acquired on a sub-set of the z-planes imaged by TPF provided chemical components, representing water, proteins, DNA, and lipids, with susceptibility spectra and concentration maps in physically meaningful units. An analysis of the average nucleus concentration of the dry components rich in DNA showed a correlation with the trend observed in TPF versus nucleus size. The results suggest that label-free chemically specific hyperspectral CARS microscopy can highlight the cell subgroup in the S/G2 phase, when DNA replication has occurred while the cell nucleus size has not yet significantly expanded, such that the DNA concentration is highest.
Brain tissues were sectioned from mice that had been implanted with eGFP-labelled glioblastoma stem cells and non-stem cells. They were imaged with correlative TPF and hyperspectral CARS, the latter analysed to extract meaningful chemical components, namely water, proteins and nucleic acids, lipids, and their concentration maps. Here, we found a correlation between the TPF image contrast from regions containing eGFP-tagged cancer cells and the CARS chemical contrast from regions rich in proteins, clearly distinct from areas containing normal tissue. Remarkably, we were also able to distinguish cancer cells derived from implanted GSCs versus NGCs, on the basis of the relative protein content. Specifically we found that tumour cells derived from GSCs are significantly richer in proteins and nucleic acids (by a factor of 1.6 in mean PNA concentration value) compared to those derived from NGCs.
Overall, these results highlight the potential of quantitative hyperspectral CARS microscopy to become an enabling technology in biomedical research, capable of dissecting the complexity of living organs and tissues via the label-free identification of chemically distinct subcellular populations non-invasively, offering significant opportunities in e.g. 3D cancer biology.
Data availability
Information about the data created during this research, including how to access it, is available from Cardiff University data archive at http://doi.org/10.17035/d.2020.0123251197.Author contributions
T.D., F.S., P.B., W.L. conceptualized the work and methodology. I.P., P.B. and W.L. developed the microscope setup. I.P. acquired the data. F.M. and W.L. developed the analysis software. I.P. and F.M. analysed the data. K.E. prepared and handled the organoids. A.J. and F.S. prepared, stained, and handled the brain slices. I.P. P.B. and W.L. wrote the manuscript. F.M., F.S., and T.D. reviewed the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was funded by Cancer Research UK (grant n. C368/A22099), the MRC (MR/S007709/1) and the Wellcome Trust (Cardiff University Institutional Fund ISSF3 grant n. 204824/Z/16/Z). The hyperspectral CARS microscope was funded by the UK Research Council BBSRC grant n. BB/H006575/1, and the EPSRC grant n. EP/M028313/1. F. M. acknowledges the Ser Cymru II programme (Case ID 80762-CU-148) which is funded by Cardiff University and the European Regional Development Fund through the Welsh Government.References
- M. Monici, Biotechnology Annual Review, Elsevier, 2005, pp. 227–256 Search PubMed.
- J.-X. Cheng and X. S. Xie, Science, 2015, 350, aaa8870 CrossRef.
- C. Zhang and J.-X. Cheng, APL Photonics, 2018, 3, 090901 CrossRef.
- C. L. Evans and X. S. Xie, Annu. Rev. Anal. Chem., 2008, 1, 883–909 CrossRef CAS.
- A. Zumbusch, W. Langbein and P. Borri, Prog. Lipid Res., 2013, 52, 615–632 CrossRef CAS.
- C. H. Camp, Y. J. Lee, J. M. Heddleston, C. M. Hartshorn, A. R. H. Walker, J. N. Rich, J. D. Lathia and M. T. Cicerone, Nat. Photonics, 2014, 8, 627–634 CrossRef.
- Y. Ozeki, W. Umemura, Y. Otsuka, S. Satoh, H. Hashimoto, K. Sumimura, N. Nishizawa, K. Fukui and K. Itoh, Nat. Photonics, 2012, 6, 845–851 CrossRef CAS.
- I. Pope, W. Langbein, P. Watson and P. Borri, Opt. Express, 2013, 21, 7096–7106 CrossRef CAS.
- F. Masia, A. Glen, P. Stephens, P. Borri and W. Langbein, Anal. Chem., 2013, 85, 10820–10828 CrossRef CAS.
- F. Masia, I. Pope, P. Watson, W. Langbein and P. Borri, Anal. Chem., 2018, 90, 3775–3785 CrossRef CAS.
- A. Karuna, F. Masia, M. Wiltshire, R. Errington, P. Borri and W. Langbein, Anal. Chem., 2019, 91, 2813–2821 CrossRef CAS.
- C. H. Camp and M. T. Cicerone, Nat. Photonics, 2015, 9, 295–305 CrossRef CAS.
- H. A. Rinia, K. N. J. Burger, M. Bonn and M. Müller, Biophys. J., 2008, 95, 4908–4914 CrossRef CAS.
- C. Di Napoli, I. Pope, F. Masia, W. Langbein, P. Watson and P. Borri, Anal. Chem., 2016, 88, 3677–3685 CrossRef CAS.
- P. Wang, J. Li, P. Wang, C.-R. Hu, D. Zhang, M. Sturek and J.-X. Cheng, Angew. Chem., Int. Ed., 2013, 52, 13042–13046 CrossRef CAS.
- A. Nahmad-Rohen, D. Regan, F. Masia, C. McPhee, I. Pope, W. Langbein and P. Borri, Anal. Chem., 2020, 92, 14657–14666 CrossRef CAS.
- M. T. Cicerone, J. Charles and H. Camp, Analyst, 2018, 143, 33–59 RSC.
- S. A. Boppart, S. You, L. Li, J. Chen and H. Tu, APL Photonics, 2019, 4, 100901 CrossRef.
- B. Sarri, F. Poizat, S. Heuke, J. Wojak, F. Franchi, F. Caillol, M. Giovannini and H. Rigneault, Biomed. Opt. Express, 2019, 10, 5378–5384 CrossRef CAS.
- S. Satoh, Y. Otsuka, Y. Ozeki, K. Itoh, A. Hashiguchi, K. Yamazaki, H. Hashimoto and M. Sakamoto, Pathol. Int., 2014, 64, 518–526 CrossRef CAS.
- C. D. Napoli, I. Pope, F. Masia, P. Watson, W. Langbein and P. Borri, Biomed. Opt. Express, 2014, 5, 1378–1390 CrossRef.
- J. Kim, B.-K. Koo and J. A. Knoblich, Nat. Rev. Mol. Cell Biol., 2020, 21, 571–584 CrossRef CAS.
- J. D. Lathia, S. C. Mack, E. E. Mulkearns-Hubert, C. L. L. Valentim and J. N. Rich, Genes Dev., 2015, 29, 1203–1217 CrossRef CAS.
- S. Bao, Q. Wu, R. E. McLendon, Y. Hao, Q. Shi, A. B. Hjelmeland, M. W. Dewhirst, D. D. Bigner and J. N. Rich, Nature, 2006, 444, 756–760 CrossRef CAS.
- F. A. Siebzehnrubl, D. J. Silver, B. Tugertimur, L. P. Deleyrolle, D. Siebzehnrubl, M. R. Sarkisian, K. G. Devers, A. T. Yachnis, M. D. Kupper, D. Neal, N. H. Nabilsi, M. P. Kladde, O. Suslov, S. Brabletz, T. Brabletz, B. A. Reynolds and D. A. Steindler, EMBO Mol. Med., 2013, 5, 1196–1212 CrossRef CAS.
- C. E. Badr, D. J. Silver, F. A. Siebzehnrubl and L. P. Deleyrolle, Cell. Mol. Life Sci., 2020, 77, 5101–5119 CrossRef CAS.
- L. B. Hoang-Minh, F. A. Siebzehnrubl, C. Yang, S. Suzuki-Hatano, K. Dajac, T. Loche, N. Andrews, M. Schmoll Massari, J. Patel, K. Amin, A. Vuong, A. Jimenez-Pascual, P. Kubilis, T. J. Garrett, C. Moneypenny, C. A. Pacak, J. Huang, E. J. Sayour, D. A. Mitchell, M. R. Sarkisian, B. A. Reynolds and L. P. Deleyrolle, EMBO J., 2018, 37, e98772 CrossRef.
- R. L. Mort, M. J. Ford, A. Sakaue-Sawano, N. O. Lindstrom, A. Casadio, A. T. Douglas, M. A. Keighren, P. Hohenstein, A. Miyawaki and I. J. Jackson, Cell Cycle, 2014, 13, 2681–2696 CrossRef CAS.
- H. Ireland, R. Kemp, C. Houghton, L. Howard, A. R. Clarke, O. J. Sansom and D. J. Winton, Gastroenterology, 2004, 126, 1236–1246 CrossRef CAS.
- M. Huch, C. Dorrell, S. F. Boj, J. H. van Es, V. S. W. Li, M. van de Wetering, T. Sato, K. Hamer, N. Sasaki, M. J. Finegold, A. Haft, R. G. Vries, M. Grompe and H. Clevers, Nature, 2013, 494, 247–250 CrossRef CAS.
- A. Jimenez-Pascual, J. S. Hale, A. Kordowski, J. Pugh, D. J. Silver, D. Bayik, G. Roversi, T. J. Alban, S. Rao, R. Chen, T. M. McIntyre, G. Colombo, G. Taraboletti, K. O. Holmberg, K. Forsberg-Nilsson, J. D. Lathia and F. A. Siebzehnrubl, Cancer Discovery, 2019, 9, 1574–1589 CrossRef CAS.
- H. Hashimoto, K. Isobe, A. Suda, F. Kannari, H. Kawano, H. Mizuno, A. Miyawaki and K. Midorikawa, Appl. Opt., 2010, 49, 3323 CrossRef CAS.
- M. Drobizhev, N. S. Makarov, S. E. Tillo, T. E. Hughes and A. Rebane, Nat. Methods, 2011, 8, 393–399 CrossRef CAS.
- I. Rocha-Mendoza, W. Langbein and P. Borri, Appl. Phys. Lett., 2008, 93, 201103 CrossRef.
- F. Masia, A. Karuna, P. Borri and W. Langbein, J. Raman Spectrosc., 2015, 46, 727–734 CrossRef CAS.
- A. Karuna, F. Masia, P. Borri and W. Langbein, J. Raman Spectrosc., 2016, 47, 1167–1173 CrossRef CAS.
- J. Kim and H. Park, SIAM J. Sci. Comput., 2011, 33, 3261–3281 CrossRef.
- B.-H. Li and S.-S. Xie, World J. Gastroenterol., 2005, 11, 3931–3934 CrossRef CAS.
- S. Bolte and F. P. Cordèlieres, J. Microsc., 2006, 224, 213–232 CrossRef CAS.
- B. Alberts, A. Johnson, J. Lewis, M. Raff, K. Roberts and P. Walter, Molecular Biology of the Cell, Garland Science, New York, 4th edn, 2002 Search PubMed.
- P. Jorgensen, N. P. Edgington, B. L. Schneider, I. Rupes, M. Tyers and B. Futcher, Mol. Biol. Cell, 2007, 18, 3523–3532 CrossRef CAS.
- F. R. Neumann and P. Nurse, J. Cell Biol., 2007, 179, 593–600 CrossRef CAS.
- F.-K. Lu, S. Basu, V. Igras, M. P. Hoang, M. Ji, D. Fu, G. R. Holtom, V. A. Neel, C. W. Freudiger, D. E. Fisher and X. S. Xie, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 11624–11629 CrossRef CAS.
- M. Buszczak, R. A. Signer and S. J. Morrison, Cell, 2014, 159, 242–251 CrossRef CAS.
- K. F. A. Ross and R. E. Gordon, J. Microsc., 1982, 128, 7–21 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/D0AN02381G |
| This journal is © The Royal Society of Chemistry 2021 |