Is biopolymer hair a multi-responsive smart material?
Xueliang
Xiao
ab,
Jinlian
Hu
*a,
Xiaoting
Gui
a,
Jing
Lu
a and
Hongsheng
Luo
c
aInstitute of Textiles and Clothing, the Hong Kong Polytechnic University, Hong Kong, China. E-mail: tchujl@polyu.edu.hk; Fax: +852-27731432; Tel: +852-27666347
bKey Laboratory of Eco-Textiles, Ministry of Education, Jiangnan University, Wuxi, 214122, P.R. China
cFaculty of Chemical Engineering and Light Industry, Guangdong University of Technology, Guangzhou 510006, PR China
First published on 9th August 2016
Abstract
For thousands of years, animal hairs have been merely considered as textile fibers with outstanding performances such as excellent elasticity and thermal insulation. Only recently, we have obtained indications that animal hair may be a smart natural material which displays shape memory (SM) effects responsive to four types of stimuli: heat, water, redox agents and UV-light. These smart functions of animal hair are found to be the result of its three structural components: crystals, and hydrogen (HB) and disulfide (DB) bonds among its intra- and inter-macromolecules. In this paper, camel hair was employed as one typical animal hair to investigate the SM abilities under four types of stimuli, in which the HBs were analyzed by Fourier Transform Infrared spectroscopy and the DBs by Raman spectroscopy, while the Tg and crystal structure of camel hair were determined using differential scanning calorimetry and X-ray diffraction. The shape fixation and recovery ratios were determined using an Instron tensile tester with an environmental chamber. It was discovered that the crystals in hair remain intact under the four stimuli, indicating their role as netpoints for hair SM. Under the stimuli of water and heat, the DBs of hair were characterized as unchangeable, whereas the HBs were found to vary under dry and wet conditions, indicating the roles of DBs as netpoints and HBs as switch units. For UV-light, HBs and crystals were found to be invariable and DBs were converted into thiol groups under a UV stimulus. With a redox agent containing reductant ions and aqueous molecules, it was found that DBs and HBs both worked as switch units and crystals acted as netpoints. Thus, the SM mechanism of animal hair was modelled through a twin-netpoint-switch structure. Beneficially, this natural insight can help to inspire applications for making synthetic materials with smarter functions for more environmental adaptability and impactful applications.
Introduction
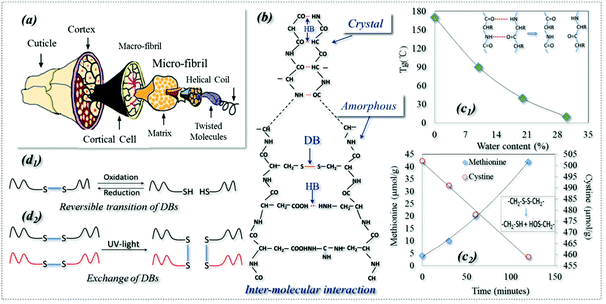
Shape memory polymers (SMPs) are a kind of smart polymer characterized by their stimuli-responsive behavior in adapting to our human demands. SMPs can be described in terms of a netpoint-switch structure as referred to in previous work,1 where their permanent shape is determined by netpoints, and reversible bonds in the amorphous region act as switch units that lead to temporary shapes. Even though there have been a few recent reports of triple stimuli-responsive SMPs,2–5 SMPs have one stimulus in most existing cases. However, Schattling et al. stressed the rising importance of developing multi-stimuli responsive polymers, called “all-in-one talents” in many industries, such as in life sciences for their comparable natural adaptability and in information technology for the parallel writing of information to give a dramatic increase in memory density.6 They believed that the addition of one more stimulus in a polymer can improve the degree of control, provide more free choice and increase the level of intricacy, which in turn enhances the adaptability of a material to different environments by more intelligent functions. However, developing such multi-responsive talented polymers requires new technologies which may be beyond our normal imagination. On the other hand, nature is full of wisdom and we may learn tricks even from a very common subject such as animal hair fiber.7 For example, an interesting shape memory (SM) phenomenon of a dry wool yarn is often encountered in the textile industry, where the yarn contracts by 20% in water after being pretreated using a reducing agent (NaHSO3) and pre-stretched to a 30% strain.8 It is believed that this is the SM of wool being responsive to redox and water.For thousands of years, animal hair has been merely considered as a textile fiber with outstanding performances for its excellent elasticity and thermal insulation. These properties are ascribed to the hair's hierarchical structure, with macro & micro-fibrils and helical coils, which are wrapped in the outside cortex and cuticles, as shown in Fig. 1a. In addition, as shown schematically in Fig. 1b, the structural components of hydrogen (HB) and disulfide (DB) bonds in crystals and amorphous regions play key roles in the properties. An HB is an electrostatic attraction between polar molecules. It is not actually a true bond but a particularly strong dipole–dipole attraction. However, DB is a strong covalent cross-linker between hair molecules (it can be characterized by Raman spectroscopy)9 that can control the hair elasticity.10,11 Keten reported that both HBs and DBs in the amorphous regions of animal hair can be reversible under certain environmental conditions, such as redox agents, water and heat,12,13 which leads to the conversion to temporary shapes and macroscope permanent shrinkage.14 In addition, as shown in Fig. 1b, ∼40% of the crystallinity in wool is proven to be too hard to cleave and has been studied by textile researchers using X-ray diffraction (XRD) which showed that the crystalline phase of wool is made of very dense HBs.15–17
HBs in polymers can be very strong, acting as dense physical cross-linkings which are difficult to cleave, such as in a hard polymer crystal phase. This is well known and they are widely used as netpoints in many SMPs, where an HB-based crystal acts as a netpoint for the purpose of shape recovery.18,19 When they are not that strong, they may be reversibly dissociated and re-formed under conditions such as heat and water,20–22 and can be used as switches in SMPs. For example, it has been found that the glass transition temperature (Tg) of wool decreases with an increase in water content (Fig. 1c1),23 due to the collapse of a large amount of HBs in the amorphous region because of aqueous molecules. It is also observed that the collapsed HBs in the amorphous region can be re-formed when the wet wool is dried. This indicates that the HBs are reversible, with and without water alternately.24 Thus, HB is the key behind a water-responsive SM and plays a main role in the water-driven shape recovery process.7,21,25 Furthermore, Jung et al. observed a water-responsive SM in a poly (ethylene glycol) (PEG)-based polyurethane where the crystalline segment was dissolved by water due to the disappearance of HBs.26 Using a high molecular weight of PEG, another shape recovery of 99% co-polymer was synthesized through HB switches.27 A simple and controllable triple-SM supramolecular composite was also developed successfully through HB switches between a polymer and mesogenic units,28 where the HBs enable broad and independent control of both Tg and cross-link density.29
It is found that DBs can be broken when a reducing agent is applied because a decreased amount of cysteine and an increased methionine were observed throughout the reducing reaction, as shown in Fig. 1c2.30 This is because DBs can be cleaved to form two thiol groups in reducing solution. In turn, the broken DBs can be reversed by an oxidation reaction.31 DBs are a type of dynamic covalent bond responsive to redox and different light triggers which form the basis of a switch in SMPs. As shown in Fig. 1d1, reversible change happens between DBs and thiols where DBs are cleaved to thiol groups by a reduction reaction and re-formed by an oxidation reaction.32 This reversible change can be found in responsive polymer capsules and micelles and gels for applications such as drug delivery.33–35 DBs have another type of reversibility: exchange reactions which can lead to the healing ability of a polymer driven by UV and visible light, as shown in Fig. 1d2.36–39 As far as SMPs are concerned, there are only two reports in the literature: a thermally-responsive SMP was reported with a semicrystalline and covalently cross-linked network where DBs were used for a self-healing function only under UV-light.40 Thus, only one SMP reported in the literature has utilized DBs as a switch responsive to redox treatment where cellulose derivatives with cross-linkable mercapto groups were used.41 Strictly speaking, the use of DBs in SMPs as netpoints has not been reported yet, but they are strong chemical bonds which are stable under some conditions and can be used as netpoints in SMPs, as evidenced in wool where they control its elasticity under normal environmental conditions.10,11 Moreover, it is also clear that DBs could act as reversible switches, having self-healing capability42 when exchange reactions take place among macro-molecules under light, particularly in UV-light conditions.
So far, crystals, HBs and DBs in polymers may form or tailor different combinations for variable SM abilities and behaviors.43 However, to date, even though occasionally there has been conversational conjecture mentioning that animal hair might have an SM effect,7 there have not been any published reports about a multi-responsive SM effect of hair fibers in terms of four stimuli as well as shape recovery, shape fixity and the above-mentioned reversible bonds or physical/chemical cross-linkers.
Materials and methods
Preparation of hair fibers
Raw hair fibers from an adult camel's back were purchased from a trade company (Sunite Right HTC villi LLC, Mongolia Autonomous Region, China). The hair samples were firstly combed to remove the mud and impurities between the fibers. Then they were screened to a diameter between 20 μm and 50 μm (fine hairs) for the following study. The samples were soaked in an ethanol/chloroform mixed solution for one minute to remove the surface fatty materials,44 which were assumed to have a negligible effect on the hair cortex and medulla. The removal of the fatty film was conducive to the efficient interaction of the hair cortex with the stimulus used in the later performance test. Then the samples had two rinses with distilled water, and they were dried at a constant 40 °C atmosphere in an oven. The moisture regain of the hair fibers was measured to be in the range of 10% to 15%.Qualitative study of SM abilities
As shown in Fig. 3a for the SM ability investigation program of the hair fibers, the conditioned straight hairs were first manually wrapped on a circular metal bar. Then the deformed hair fibers were immersed in a stimulus environment for a certain period to ensure the full interaction of the hair fibers with the stimulus factors. The deformed hair fibers were then taken out of the stimulus, and immersed in an opposite stimulus factor for a certain time. The hair shapes in each process, including the temporary shape for investigating shape fixation ability, were observed using an optical camera. When the entangled spiral dry fibers in their temporary shape encountered the stimulus, the shape recovery was recorded. This method is referred to in previous work.45Quantitative characterization of SM abilities
The SM ability of hair fiber was studied quantitatively, according to Fig. 2, with five usual steps. Instead of using temporary entangled spring shape fixation, the variation in degree of recovery from stretched hair to the original length under a stimulus is used to investigate the hair's SM ability. In Fig. 2a, the original length (L) of hair after stimulus in a particular environment was stretched up to a certain value of strain (ε0), where the ε0 is in the plastic tensile region. Then the stimulus factor was removed at time t1, and the gauge length returned to the original at time t2. Apart from the instant recovery from strain due to the elasticity, most of the strain is observed to be unrecovered as ε1. Macroscopically, the stretched hair is curved, as schematically illustrated at time t2. At t3, the stretched hair was put under the stimulus again to unlock the switch; normally, the unrecovered strain (ε1) decreased to ε2. The onset of the next tensile was from ε2 with stretching to ε0 at the second cyclic tensile. Accordingly, Fig. 2b demonstrates the cyclic tensile program based on Fig. 2a that can interpret the specific SM ability under a specific stimulus. The blue tensile circle shows the first tensile of the hair fiber where part ‘a’ means the elastic stretching and part ‘b’ means the plastic stretching up to the set strain (ε0); a sharp decrease in load ‘c’ follows and a decrease in strain ‘d’ (equivalent to ‘a’) is observed, leaving the unrecovered strains of ‘e’ and ‘f’. Once the stretched hair encounters a specific stimulus, this gives rise to the contracted strain ‘e’ and unrecovered strain ‘f’. Therefore, the onset of the second tensile of the recovered hair is from the strain of ‘f’, with a similar tensile track to the first tensile curve. The close tensile tracks from the third tensile are usually observed for most SM cases.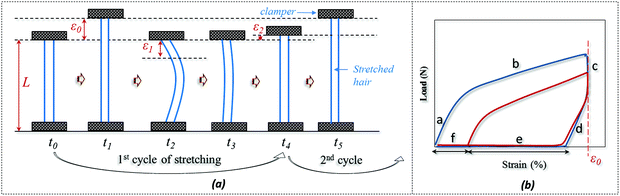 | ||
| Fig. 2 Quantitative study of SM abilities of an animal hair: (a) cyclic stretching program, (b) load-strain relationship from hair's cyclic stretching program. | ||
For Fig. 2, there are two variables that thus come across as justifying the hair's SM ability. These are Rf (eqn (1)) and Rr (eqn (2)), representing the shape fixation and recovery abilities, respectively:
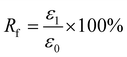 | (1) |
 | (2) |
The ideal shape recovery shows a process of ‘ε2→0’ under penetration and removal of the external stimulus into and out of the hair fibers. Physically, a greater Rf value means a more sensitive switch to be on and off, whereas a higher value of Rr implies a better SM ability of the hair fiber. During the SM investigation of each fresh sample, the ε1 and ε2 are required to be measured five times to obtain average values. The related Rf and Rr values are calculated with standard derivations. In detail, for the four stimuli which involve water, redox agent, UV-light and heat, the SM ability of camel hair was examined independently using an Instron machine (5566):46
(a) For water-induced SM: ① camel hair fibers are immersed in water at 20 °C, then one hair sample is stretched to a length in water at a proper speed; ② the stretched length of hair is kept in a dry condition, the stretched shape is then fixed without constraints; ③ the stretched hair is triggered with water, and the recovered length is measured. ④ The SM of camel is represented by Rf and Rr (eqn (1) and (2)), respectively.
(b) For redox-induced SM: ① 10 wt% reductant-NaHSO3 is first employed for immersing the camel hairs, and then the sample is rinsed with DI water; ② the hair is then stretched at 20 °C; ③ the deformed specimen is then fixed by an oxidation process (H2O2 dilute solution); ④ the recovery of the fixed hair is triggered by the reductant solution at 20 °C.
(c) For UV-light induced SM: ① UV-light (315 nm–400 nm, 300 mW cm−2) is used to illuminate the hair until its stress declines dramatically; ② the sample is stretched in darkness for 0.5–1 hour to fix the stretched shape; ③ the temporary shape of the hair is exposed to UV-light again to trigger its shape recovery.
(d) For heat-induced SM: ① at a moisture regain of 14%, one hair sample is elongated under a tensile load at around 80 °C, which is around 20 °C higher than its Tg, but significantly lower than the melting temperatures of the DBs and crystals of camel hair; ② it is then cooled to 20 °C and the elongation kept to fix the length; ③ it is unloaded at 20 °C and ④ the sample is heated to around 80 °C again to recover the fixed length to its original shape.
Identification of netpoint and switch unit
Over the SM investigation, the chemical functional groups and intermolecular bonds of the hair fibers were examined using Fourier Transform Infrared Spectroscopy (PerkinElmer Spectrum 100 FT-IR Spectrometer, USA) in the scan range of wavenumbers of 4000–650 cm−1 using the ATR (Attenuated-Total-Reflectance) method. The absorption spectra were recorded with eight scans at a resolution of 16 cm−1. The angle (ϕ) of incidence of the light was adjusted to 39°, the ATR crystal was diamond (refractive index n1 is 2.4), and the refractive index of the hair fiber (n2) is around 1.5. The characterized depth of penetration (DP) is in the range of 1–15 μm based on eqn (3):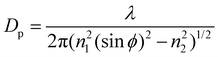 | (3) |
Here, λ is the wavelength of light. eqn (3) indicates that the IR spectra take place at the hair cortex. Raman spectra yield similar but complementary information to the Infrared spectroscopy. They rely on Raman scattering from a laser in the near infrared range. The light interacts with molecular vibrations, resulting in the energy of the laser photons being shifted up and down. The chemical cross-links, such as the disulfide bond (DB) of hair fibers under the SM key step, were characterized by a Horiba Jobin Yvon HR800 Raman spectrometer, which was equipped with an Ar laser (λ = 448 nm, 180 mW) as the excitation light source, and an Olympus BX41 microscope. The water-impenetrable crystalline phase of camel hair can be characterized by X-ray diffraction due to the Bragg regular arrangement of the crystals. Thus, the crystallinity of the hair fibers under different stimuli conditions was determined by a Rigaku Smart Lab XRD system (9 kW) that is equipped with Cu Kα radiation with a wavelength of 1.54 Å. The hair fibers were minced into short chips (powder format) to cover the stage. The test 2θ range was from 5° to 40° and recorded at a scan speed of 10°·min−1 at 40 kV and 40 mA. The structural analyses of the hair fibers in variable conditions using the above characterizations were conducted for individual hair samples that were taken from two parts of one hair fiber.
Results and discussion
Multi-responsive SM abilities of hair fibers
Fig. 3a shows the general program of camel hair under a particular stimulus for investigating its SM ability. The results for camel hairs under four types of stimuli are given with key images in Fig. 3b. It is noted that the stimuli of water and redox agent show remarkably corresponding SM abilities so that the final recovered shape of the hair is close to its original. It is believed that the aqueous molecules play an important role in the SM of hairs.7 Furthermore, the reductant ions (SO32−) break down the DBs between branches of keratin macromolecules; thus one netpoint candidate may be destroyed after the shape fixation process. This is manifested through the relatively poorer shape recovery (Rec.) from the redox stimulus. In comparison, the stimuli of heat and UV-light endow camel hairs with a good shape fixation ability. Nonetheless, both stimuli to the deformed hairs perform with poor ability in terms of shape recovery, as shown by the unrecovered residues of temporarily fixed spiral circles. Theoretically, the stimuli of heat and UV-light give rise to hairs in a much drier state; whereas the former result in hair with less structural water and HBs, and the latter breaks down the DBs,36 so that the temporarily shape fixed hairs become relatively difficult to recover within the same recovery duration compared with the stimuli of water and redox. Referring to the characterizations of structural morphology and components of other animal hairs in previous work,7 different types of hairs would manifest different degrees of SM abilities under the four stimuli, depending on the contents of the three structural components in hair.Quantitatively, according to eqn (1) and (2) for Fig. 4a–d, the calculated shape fixation ratio (Rf) values show that water and redox stimuli endow the hair with the highest fixed strains, i.e. 0.84 and 0.875 respectively, which are much higher than the values from heat (0.73), UV-light (0.65) and the original state (0.65). This is ascribed to the increased number of HBs from penetrated aqueous molecules after stretching, which act as switch units that lock the temporary shape during the shape fixation process. For the shape recovery ratios (Rr), water leads to the largest degree of shape recovery (0.76), followed by heat stimulus (0.547), redox stimulus (0.525), and UV-light stimulus (0.225). It should be noted that all the tests were under the same tensile speed, which means the recovery times are the same for each stimulus. Water, in such a case, unlocks the biggest number of switch units that cause the biggest recovery by stress release from netpoints. To different extents, heat, redox agent and UV-light can open the corresponding switches to different degrees, leading to different recovery abilities, as manifested in Fig. 4(a)–(d). The shape recovery for the original hair is ascribed to the relaxation of protein macromolecular chains under a stress-free state. However, due to the plastic stretching of hair, it is inevitable there will be a large unrecovered strain for a short recovery time. Exposure of camel hair to UV-light can break down partial DBs that promote strain recovery, as shown by the increased 3.5% of recovered strain from the original hair. Stimuli of heat and redox both work on the HBs that result in a larger recovered strain, indicating that HBs (to a much larger degree than DBs) are the key switch in the SM of hair fibers.
Mechanical properties of camel hairs under multi-stimuli
Because of the unlocking effect of the stimuli on a switch, the stretching of camel hair manifests variable performance based on the breakage degree of the switch, as shown by the tensile curves in Fig. 5(a and b). Because of the complete existence of crystalline phase, chemical crosslinkers (DBs) and HBs for taking tensile load, the dry camel hair performs with the highest breaking force. The stimuli, to some extent, destroy one or two kinds of crosslinkers to lower the tensile strength: for instance, the thermal energy reduces the amount of structural water (forming HBs) from the hair cortex, thus the stretching force decreases remarkably. Similarly, camel hair soaked in water also breaks down the amorphous HBs due to plasticization by aqueous molecules, resulting in a breaking strength lower than that of dry hair.47 A slight increase in strain may be ascribed to the swelling of macromolecule chains filled with free aqueous molecules, like a lubrication effect. However, the intact structure of netpoint and chemical crosslinkages constrains the occurrence of higher breaking forces.For hair under the stimulus of a reducing agent, the breaking strain is increased twofold compared with it in a dry state. It is known that the reducing reaction can decrease the amount of DBs and HBs since the reaction occurs in a solution environment. This can increase the mobility of neighbor macromolecules significantly for the observed large breaking strain; meantime, the tensile force is decreased remarkably from its dry state for the lower number of bonds. Fig. 5b compares the collapsed amount of DBs on the basis of hair sample exposure to UV-light for different durations. In contrast to the reducing reaction to the hair, UV-light only breaks down the DBs inside the hair fiber without breaking the HBs; thus, the hair sample after UV-light illumination still maintains a relatively high tensile strength. However, owing to the slight breakage of DBs, the breaking strain becomes higher with an increase in UV-light illumination time, as shown by the higher breaking strain of UV-5 h (5 hours of illumination) than UV-1 h.
Based on the two-phase model, Fig. 5d refers to a modified structural model48 for the original camel hair that contains a discrete crystalline phase and macromolecular chains and branches in amorphous area:
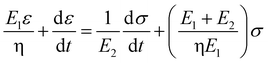 | (4) |
 | (5) |
Here, E1, E2 and E3 relate to the elastic component, such as the contributions from HBs, DBs or discrete crystalline crosslinkers, the symbol “⋯” means other elastic candidates. The multi-stimulus, to some extent, decreases the modulus of the corresponding elastic component, as shown in Fig. 5c. For example, the stimulus of UV-light reduces the number of DBs inside the hair fiber that leads to a decrease in E2 value, causing the hair modulus (Eu) to decrease from the original modulus (Eo), as shown in the inset of Fig. 5c; therefore, the same value of σ would have a larger tensile strain ε. For the stimulus of a redox agent, the hair fiber was reduced in two components (DBs and HBs); thus the modulus of the hair shows the lowest value of all. However, qualitatively, this structural model can only interpret well the macroscopic phenomenon of tensile abilities related to E1, E2 and E3 of HBs, DBs or discrete crystalline cross-linkers, respectively, because the decrease in type and amount of modulus under certain stimuli varies. Thus, the schematic illustrations for the effects of four stimuli on the key crosslinking components are given for the observed tensile curves, where the detailed effect of every stimulus on the key components is characterized in the following section.
Identification of netpoint and switch of hair fibers
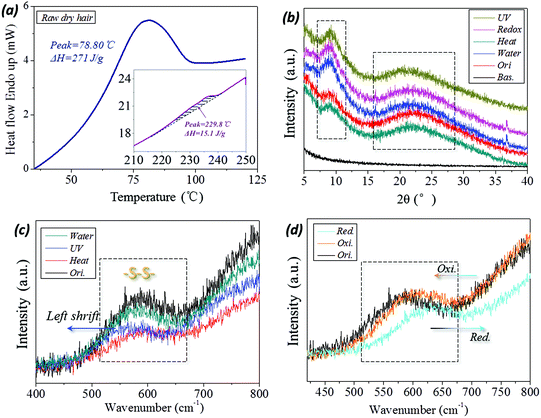
The variation in internal structure of hair samples under UV-light, reductant solution, high temperature and wet conditions have been evaluated from a few experimental aspects. In DSC scanning of raw camel hair, two thermal absorption peaks appear, as shown by the peak configuration in Fig. 6a. The first broad endothermic peak with temperature at 78.8 °C and the enthalpy of 270.1 J g−1 relate to the removal of structural/free water (forming HBs) from the fiber.49 The second DSC endothermic singlet at 230 °C, as shown by the inset, is due to the denaturation of ordered α-helical keratin,50 where the enthalpy is decreased to 15.1 J g−1. This means the existence of the crystalline phase in animal hair and the volume content account for 10%–20% of the whole fiber for the netpoints in hair's SM performance. The XRD patterns of camel hair under five states are shown in Fig. 6b. The diffraction shoulders and peaks from the original state and those that arise by lighting, reducing, heating and hydration with sharp peaks, especially at the abscissa of 2θ = 9°(0.98 nm) and 21°(0.46 nm), indicate the strong characteristic α-helix and β-keratin crystalline phases, respectively. The nearly identical intensities of both characteristic peaks at five hair states (original (ori.), heating (hea.), wetting (wet), reducing (redox) and lighting (UV)) indicate the invariable amount of crystalline phase during the hair multi-stimuli induced SM performance.The corresponding processes, such as UV-illumination and hydration, have scarcely any effect on the hair crystal characteristic peaks. Thus, the existing XRD peaks of hair under the four stimuli conditions indicate that the crystalline phase can take a netpoint role in the multi-stimuli SM behaviors of hair.
With respect to the comparison of camel hair fibers for Raman spectra under four stimuli (‘water’, ‘UV’, ‘heat’ and redox (‘red. and oxi.’)) conditions, it should be noted that the original (‘ori.’), ‘water’, ‘heat’ curves can be viewed as being almost coincident in the Raman scanned regions (abscissa values of Fig. 6c and d). Specifically, the symmetrical DB mode from 500–580 cm−1 can be found as a broad characteristic peak which is associated with several molecular conformations,9,51i.e. g–g–g (510 cm−1), g–g–t (525 cm−1) and t–g–t (540 cm−1) (g and t denote gauche and trans) conformations. Fig. 6c suggests that the present heating process and aqueous molecules have a negligible effect on the DBs in hair, indicating this chemical crosslinker may act as a netpoint in hair heat- and water- induced SM behaviors. However, it should be noted that the abscissa of the broad peak moves forward in the left and right directions for the camel hair under stimuli of UV-light and reducing agent, indicating the band breaking mechanism for DBs is different for the two stimuli. However, it is proved that the DBs were both switched on after the two stimuli on the hair fiber. Curiously, the camel hair under a reducing agent and oxidation environment endow the Raman spectra of camel hair with opposite motions of the peak abscissa and intensity ratio, indicating the symmetrical vibrations of DBs in on (thiol groups) and off (DBs) states. This suggests that DBs can act as switches in some stimuli-induced SM behaviors.
In Fig. 7(a and b), a broad absorption band at around 3400 cm−1 corresponding to free water (polar group of –OH) is introduced to the ATR-FTIR curve of the wet sample.52 This includes the stimuli of water and reducing solution. In particular, both the characteristic peaks of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching (Amide band I) and N–H bending (Amide band II) vibrations are shifted to higher wavenumbers from 1630 cm−1 to 1633 cm−1 and 1531 cm−1 to 1533 cm−1, respectively, as shown by the dashed frame (for the band of O⋯H) in Fig. 7(a and b) and the measured values in Table 1. This implies that the intermolecular HBs are formed between the residues and aqueous molecules during hydration, which is consistent with the results reported previously.53 Therefore, the absorbed aqueous molecules within the biopolymer hair exist in two distinct states of free water and bound water.
O stretching (Amide band I) and N–H bending (Amide band II) vibrations are shifted to higher wavenumbers from 1630 cm−1 to 1633 cm−1 and 1531 cm−1 to 1533 cm−1, respectively, as shown by the dashed frame (for the band of O⋯H) in Fig. 7(a and b) and the measured values in Table 1. This implies that the intermolecular HBs are formed between the residues and aqueous molecules during hydration, which is consistent with the results reported previously.53 Therefore, the absorbed aqueous molecules within the biopolymer hair exist in two distinct states of free water and bound water.
| Hair states | IR peak position/wave number (cm−1) | Peak intensity ratio | |
|---|---|---|---|
| Ori. dry/Def. wet | 1630/1633 | 1531/1543 | 0.979/0.854 |
| Ori. dry/Def. heat | 1630/1634 | 1531/1515 | 0.979/0.994 |
| Ori. dry/Def. UV | 1630/1629 | 1531/1532 | 0.979/0.988 |
| Ori. dry/Def. red. | 1630/1633 | 1531/1539 | 0.979/0.834 |
| Ori. dry/Rec. oxi. | 1630/1628 | 1531/1532 | 0.979/0.831 |
In detail, from the viewpoint of the SM program, four ATR-IR curves for the case of camel hair under stimuli of heating (or UV-illumination) for shape fixation and water (or reductant solution) for shape recovery are given as labeled in Fig. 7c. Consistent with the dashed frame in Fig. 7a, it should be noted that the intensity ratio of characteristic peaks of N–H bending to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations are evidently different for the hair in dry (original and heated) and wet (water, redox agent) conditions. Moreover, the wavenumber shifting and varied intensity ratio of two characteristic peaks between key SM steps for four stimuli have been calculated, as shown by the values in Table 1. In respect of wavenumber shifting, the characteristic peak of C
O stretching vibrations are evidently different for the hair in dry (original and heated) and wet (water, redox agent) conditions. Moreover, the wavenumber shifting and varied intensity ratio of two characteristic peaks between key SM steps for four stimuli have been calculated, as shown by the values in Table 1. In respect of wavenumber shifting, the characteristic peak of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching is increased by 2–4 cm−1 from dry to wet states; in turn, the wavenumber is decreased by 2–4 cm−1 from wet to dry states. Similarly, the characteristic peak of N–H bonding is increased and decreased by shifts of 8 cm−1 and 16 cm−1, respectively. This reversible shifting related to the conversion between the original and heated, wet and dry conditions suggests that the intermolecular HBs undergo reversible destruction and formation processes, accordingly.24 However, the stimulus of UV illumination has a negligible effect on wavenumber shifting for C
O stretching is increased by 2–4 cm−1 from dry to wet states; in turn, the wavenumber is decreased by 2–4 cm−1 from wet to dry states. Similarly, the characteristic peak of N–H bonding is increased and decreased by shifts of 8 cm−1 and 16 cm−1, respectively. This reversible shifting related to the conversion between the original and heated, wet and dry conditions suggests that the intermolecular HBs undergo reversible destruction and formation processes, accordingly.24 However, the stimulus of UV illumination has a negligible effect on wavenumber shifting for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and N–H vibrations, indicating that HBs are not switch units for UV-stimulated SM of camel hair.
O and N–H vibrations, indicating that HBs are not switch units for UV-stimulated SM of camel hair.
Furthermore, the change in intensity ratio between the two characteristic peaks of camel hair, identically, shows the increase in the ratio from the hair states of dry (Ori. dry = 97.9%) to heat (Def. hea. = 99.40%) and wet (Rec. wet = 85.45% (water), 83.4% and 83.1% (redox stimulus)) to dry (Rec. dry = 97.42%) reverses, respectively. This can be interpreted from the schematic illustration of Fig. 7d on the basis of a molecular motion viewpoint, which corresponds to the six steps of the multi-stimuli sensitive SM program. The calculation shows that the intensity ratio of ‘Def. hea.’ and ‘Def. dry.’ (④) almost equals one, which means the intensities of characteristic peaks at 1620–1640 cm−1 and 1510–1535 cm−1 are observed to equal each other, indicating approximately the same amounts of carbonyl group (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯) and imino group (N–H⋯). This indicates that the excess of free aqueous molecules has been removed out of the hair shaft during the heating or drying process.54 The slight decrease in the peak intensity ratio of hair in its original and deformed dry states from its heated dry state (① and ⑥) may be ascribed to a tiny amount of free aqueous molecules attracting the imino group under normal moisture regain. When the temporarily deformed hairs soak in water/solution, the penetrated aqueous molecules can disrupt the HB formed between carbonyl and imino groups (③ and ⑤). Under this dynamic state, each aqueous molecule was attached to each imino group for the polar attraction between the atoms of hydrogen (N–
O⋯) and imino group (N–H⋯). This indicates that the excess of free aqueous molecules has been removed out of the hair shaft during the heating or drying process.54 The slight decrease in the peak intensity ratio of hair in its original and deformed dry states from its heated dry state (① and ⑥) may be ascribed to a tiny amount of free aqueous molecules attracting the imino group under normal moisture regain. When the temporarily deformed hairs soak in water/solution, the penetrated aqueous molecules can disrupt the HB formed between carbonyl and imino groups (③ and ⑤). Under this dynamic state, each aqueous molecule was attached to each imino group for the polar attraction between the atoms of hydrogen (N–![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ) and oxygen (H–
) and oxygen (H–![[O with combining low line]](https://www.rsc.org/images/entities/char_004f_0332.gif) –H). This leads to the decreased number of discrete amino groups for 1510–1535 cm−1 and the remarkably reduced intensity ratio between the two characteristic groups. This soaking process of hair in the free state switches on the HBs-locking shape, and recovers the innate shape with the aid of netpoints. Removal of and encountering water/solution in the hair enable the same interaction to take place among the atoms of hydrogen and oxygen on the groups of amino, carbonyl and hydroxyl in hair, respectively.55 It should be pointed out that the interaction of polar molecules between macromolecule chains only demonstrates the transformation process of each SM step, the interaction dynamics (speed of switch on and off at the macroscopic scale) is not involved in the illustration. On the other hand, the variation of DBs cannot be characterized using an FT-IR approach; thus, the UV-light and Redox stimulated SM of camel hair are not accessed herein for investigating the DBs as switch units in corresponding SM performance. The function of DBs as switch units can be referred to in Fig. 6d, where the variation of DBs under a redox stimulus proves the possibility of DBs as switch units.
–H). This leads to the decreased number of discrete amino groups for 1510–1535 cm−1 and the remarkably reduced intensity ratio between the two characteristic groups. This soaking process of hair in the free state switches on the HBs-locking shape, and recovers the innate shape with the aid of netpoints. Removal of and encountering water/solution in the hair enable the same interaction to take place among the atoms of hydrogen and oxygen on the groups of amino, carbonyl and hydroxyl in hair, respectively.55 It should be pointed out that the interaction of polar molecules between macromolecule chains only demonstrates the transformation process of each SM step, the interaction dynamics (speed of switch on and off at the macroscopic scale) is not involved in the illustration. On the other hand, the variation of DBs cannot be characterized using an FT-IR approach; thus, the UV-light and Redox stimulated SM of camel hair are not accessed herein for investigating the DBs as switch units in corresponding SM performance. The function of DBs as switch units can be referred to in Fig. 6d, where the variation of DBs under a redox stimulus proves the possibility of DBs as switch units.
In summary of Fig. 7, two characteristic IR peaks of camel hair appearing at 1620–1640 cm−1 and 1510–1535 cm−1 undergo higher wavenumber shifting and decreased peak intensity ratio from the process of hair samples being deformed heated/reduced, recovered wet to the final recovered dry state, as shown in the tendency summary of the inset in Fig. 7c. The wavenumber shifting and regular variation of peak intensity indicate that HBs in animal hairs can lock the hair's temporary shape by removal of its internal water using heating/drying and recover its innate shape by an encounter with an aqueous environment. This reversible cycle of HB implies its switch unit role in heat-, water- and reductant- sensitive SM of animal hairs.
Multi-responsive SM mechanism of animal hairs
Based on the investigation of SM abilities of camel hair under four external stimuli, it is learned that animal hair is a smart natural biopolymer material that has a shape memory function responsive to heat, water, redox agent and UV-light. These smart functions of animal hair are due to its three structural components: crystals, HBs and DBs among its intra- and inter- macromolecules. Specifically, animal hair has reversible amino-carboxyl HBs and DBs in cysteine in amorphous regions and crystals where the HBs are more difficult to cleave. From an SMP netpoint-switch structural point of view,1 a twin-net-switch model is proposed for hair's SM in which the crystalline phase and DBs act as netpoints while HBs in amorphous regions and DBs work together as switches. Thus, there are two sets of netpoints and two switches that were never proposed previously for such a smart polymer structure. For simplicity, this is called a twin-netpoint-switch model, as shown in Fig. 8a (Original). In this model, DBs can be regarded as both switches and netpoints, depending on the type of external stimulus applied, e.g., as netpoints when exposed to water which cannot break DBs, but as switches when exposed to a reducing agent and UV-light when the DBs can be dissociated into thiol groups. When a stimulus acts on amorphous regions, a temporary shape of animal hair can be programmed due to the opening of a switch while the netpoints can ensure recovery back to the original shape. Vividly, Fig. 8(a and b) shows that, for the stimuli of water and heat, HBs act as the single switch unit whereas crystals and DBs both work as twin-netpoints. For the stimulus of UV-light, DBs act as a switch unit and crystals work as single netpoint. However, for the stimulus of reductant solution, DBs and HBs are both on and off due to the aqueous molecules and ions of SO32− during the related SM program; thus they both act as switch units, whereas crystals without change can work as a single netpoint for SM ability. With the help of the proposed model, it is believed that there is a possibility of developing an all-in-one smart SMP with quadruple-stimulus responsiveness by the novel twin-netpoint-switch structure, inspired by our rediscovery of animal hair fibers.Conclusions
In this work, camel hair as one type of animal hair was investigated for its multi-stimuli responsive SM behaviors, and for corresponding mechanisms in molecular and structural networks. The innate shape of a camel hair can be recovered in varying degrees through different stimuli effects on the fixed deformation, which is typical for a multi-stimuli SM material. High temporary shape fixation (>0.8) and shape recovery (>0.5) of camel hair after exposure to water and a redox agent demonstrated that animal hairs are smart α-keratin fibers stimulated by water and redox. However, low temporary shape fixation under heat (0.55) and low shape recovery (0.23) under UV-illumination, indicate that animal hairs have less SM abilities under stimuli of heat and UV-light. The single tensile of camel hair interpreted well for the effect of each stimulus on the switch (assuming netpoints intact after stimuli) opening and closing. XRD and DSC characterizations of camel hair under four stimuli showed that the crystalline phase can be viewed as an invariable component during the SM program. However, Raman spectra showed the DBs breaking when camel hair faces UV-light and reductant ions, and invariant under the stimuli of water and heat, indicating these bonds act as switch units for stimuli of UV-light and redox agent, and as netpoinst for water and heat. FTIR characterization for HBs of camel hair through the variations of characteristic peaks in wavenumber shifting and intensity ratio showed that these bonds could act as switches for the stimuli of heat, water and redox agents in each SM behavior. Based on this, a twin-netpoint-switch structure model for animal hair was proposed for interpreting the different SM abilities of the hair when exposed to different external stimuli, where a twin-netpoint/single-switch structure is for the stimulus of water, heat and UV-light, and a single-netpoint/twin-switch structure is for the stimulus of a redox agent. Thus, this study is believed to be able to provide inspiration for making more remarkable synthetic SMPs responsive to many stimuli.Competing financial interests
The authors declare no competing financial interests.Acknowledgements
The authors gratefully acknowledge the financial support from the National Natural Science Foundation of China (Grant No. 51373147), Natural Science Foundation of Jiangsu Province (Grant No. BK20160157), and the Hong Kong General Research Fund (RGC Project No. 15209815).Notes and references
- J. L. Hu and S. J. Chen, J. Mater. Chem., 2010, 20(17), 3346–3355 RSC.
- J. R. Kumpfer and S. J. Rowan, J. Am. Chem. Soc., 2011, 133, 12866–12874 CrossRef CAS PubMed.
- L. Wang, X. Yang, H. Chen, G. Yang, T. Gong, W. Li and S. Zhou, Polym. Chem., 2013, 4, 4461–4468 RSC.
- Z. Tao, K. Peng, Y. Fan, Y. Liu and H. Yang, Polym. Chem., 2016, 7, 1405–1412 RSC.
- J. Chen, S. Zhang, F. Sun, N. Li, K. Cui, J. He, D. Niu and Y. Li, Polym. Chem., 2016, 7, 2947–2954 RSC.
- P. Schattling, F. D. Jochum and P. Theato, Polym. Chem., 2014, 5, 25–36 RSC.
- X. Xiao and J. Hu, Sci. Rep., 2016, 6, 26393 CrossRef CAS PubMed.
- J. L. Hu, Y. Zhu, H. H. Huang and J. Lu, Prog. Polym. Sci., 2012, 37(12), 1720–1763 CrossRef CAS.
- W. Akhtar and H. G. M. Edwards, Spectrochim. Acta, Part A, 1997, 53(7), 1021–1031 CrossRef.
- M. Harris, L. R. Mizell and L. Fourt, Ind. Eng. Chem., 1942, 34(7), 833–838 CrossRef CAS.
- H. Lindley, Text. Res. J., 1957, 27, 690–695 CrossRef CAS.
- S. Keten, C. C. Chou, A. C. T. Van Duin and M. J. Buehler, J. Mech. Behav. Biomed. Mater., 2012, 5, 32–40 CrossRef CAS PubMed.
- B. M. Chapman, J. Text. Inst., 1970, 61(9), 448–457 CrossRef CAS.
- W. T. Astbury and H. J. Woods, Philos. Trans. R. Soc., A, 1934, 232, 333–394 CrossRef.
- C. Samways and G. W. Hastings, Nature, 1970, 225, 634–635 CrossRef CAS PubMed.
- S. S. Lotay and P. T. Speakman, Nature, 1977, 265, 274–276 CrossRef CAS PubMed.
- B. Wu, Y. Yi, T. Xu and H. Lei, 2nd Int. Conf. Electr. & Mech. Eng. Inf. Tech. (EMEIT- 2012), Altantis Press, Paris, France, 2012, pp. 1405–1408.
- S. D'hollander, G. Van Assche, B. Van Mele and F. Du Prez, Polymer, 2009, 50, 4447–4454 CrossRef.
- T. Zhang, Z. Wen, Y. Hui, M. Yang, K. Yang, Q. Zhou and Y. Wang, Polym. Chem., 2015, 6, 4177–4184 RSC.
- D. Ratna and J. Karger-Kocsis, J. Mater. Sci., 2008, 43, 254–269 CrossRef CAS.
- S. Zhang, Z. J. Yu, T. Govender, H. Y. Luo and B. J. Li, Polymer, 2008, 49(15), 3205–3210 CrossRef CAS.
- W. M. Huang, B. Yang, L. An, C. Li and Y. S. Chan, Appl. Phys. Lett., 2005, 86, 114101–114103 CrossRef.
- F. J. Wortmann, B. J. Rigby and D. G. Phillips, Text. Res. J., 1984, 54, 6–8 CrossRef CAS.
- J. D. Eaves, J. J. Loparo, C. J. Fecko, S. T. Roberts, A. Tokmakoff and P. L. Geissler, Proc. Natl. Acad. Sci. U. S. A., 2005, 102(37), 13019–13022 CrossRef CAS PubMed.
- K. Fan, W. M. Huang, C. C. Wang, Z. Ding, Y. Zhao, H. Purnawali, K. C. Liew and L. X. Zheng, eXPRESS Polym. Lett., 2011, 5(5), 409–416 CrossRef CAS.
- Y. C. Jung and H. H. So, J. Macromol. Sci., Phys., 2006, 45(6), 1189–1189 CrossRef CAS.
- G. Liu, C. Guan, H. Xia, F. Guo, X. Ding and Y. Peng, Macromol. Rapid Commun., 2006, 27, 1100–1104 CrossRef CAS.
- H. Chen, Y. Liu, T. Gong, L. Wang, K. Zhao and S. Zhou, RSC Adv., 2013, 3, 7048–7056 RSC.
- T. Ware, K. Hearon, A. Lonnecker, K. L. Wooley, D. J. Maitland and W. Voit, Macromolecules, 2012, 45, 1062–1069 CrossRef CAS PubMed.
- M. Yao, Textile Materials, China Textile Press, Beijing, China, 2009 Search PubMed.
- W. J. Wedemeyer, E. Welker, M. Narayan and H. A. Scheraga, Biochemistry, 2000, 39(15), 4208–4216 CrossRef.
- M. Zheng, F. Aslund and G. Storz, Science, 1998, 279, 1718–1721 CrossRef CAS PubMed.
- Y. Yan, Y. Wang, J. K. Heath, E. C. Nice and F. Caruso, Adv. Mater., 2011, 23, 3916–3921 CrossRef CAS PubMed.
- Y. Kakizawa, A. Harada and K. Kataoka, J. Am. Chem. Soc., 1999, 121, 11247–11248 CrossRef CAS.
- E. A. Dailing, D. P. Nair, W. K. Setterberg, K. A. Kyburz, C. Yang, T. D'Ovidio, K. S. Anseth and J. W. Stansbury, Polym. Chem., 2016, 7, 816–825 RSC.
- H. Otsuka, S. Nagano, Y. Kobashi, T. Maeda and A. Takahara, Chem. Commun., 2010, 46, 1150–1152 RSC.
- J. Canadell, H. Goossens and B. Klumperman, Macromolecules, 2011, 44(8), 2536–2541 CrossRef CAS.
- B. D. Fairbanks, S. P. Singh, C. N. Bowman and K. S. Anseth, Macromolecules, 2011, 44(8), 2444–2450 CrossRef CAS PubMed.
- Y. Amamoto, H. Otsuka, A. Takahara and K. Matyjaszewski, Adv. Mater., 2012, 24, 3975–3980 CrossRef CAS PubMed.
- B. T. Michael, C. A. Jaye, E. J. Spencer and S. J. Rowan, ACS Macro Lett., 2013, 2, 694–699 CrossRef.
- D. Aoki, Y. Teramoto and Y. Nishio, Biomacromolecules, 2007, 8, 3749–3757 CrossRef CAS PubMed.
- R. Chang, Y. Huang, G. Shan, Y. Bao, X. Yun, T. Dong and P. Pan, Polym. Chem., 2015, 6, 5899–5910 RSC.
- S. Ponyrko, R. K. Donato and L. Matejka, Polym. Chem., 2016, 7, 560–572 RSC.
- F. A. Nagia and R. S. R. EL-Mohamedy, Dyes Pigm., 2007, 75(3), 550–555 CrossRef CAS.
- Z. Q. Liu, D. Jiao and Z. F. Zhang, Biomaterials, 2015, 65, 13–21 CrossRef CAS PubMed.
- Q. Song, H. Chen, S. Zhou, K. Zhao, B. Wang and P. Hu, Polym. Chem., 2016, 7, 1739–1746 RSC.
- X. Xiao and J. Hu, Int. J. Chem. Eng., 2016, 1, 4803254 Search PubMed.
- X. Xiao, J. Hu and D. Hui, Composites, Part B, 2016, 91, 559–568 CrossRef CAS.
- C. Tonetti, A. Varesano, C. Vineis and G. Mazzuchetti, J. Therm. Anal. Calorim., 2015, 119, 1445–1451 CrossRef CAS.
- F. J. Wortmann and H. Deutz, J. Appl. Polym. Sci., 1993, 48(1), 137–150 CrossRef CAS.
- M. Richard-Lacroix and C. Pellerin, Macromolecules, 2013, 46(14), 5561–5569 CrossRef CAS.
- J. Yao, Y. Liu, S. Yang and J. Liu, J. Eng. Fibers Fabr., 2008, 3(2), 1–10 Search PubMed.
- Y. Ishida, L. Chabanne, M. Antonietti and M. Shalom, Langmuir, 2014, 30, 447–451 CrossRef CAS PubMed.
- G. Li, H. Meng and J. Hu, J. R. Soc., Interface, 2012, 9(77), 3279–3287 CrossRef CAS PubMed.
- S. Chen, J. Hu and H. Zhuo, Compos. Sci. Technol., 2010, 70(10), 1437–1443 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |