Imparting Brønsted acidity into a zeolitic imidazole framework†
Kia
Williams
,
Le
Meng
,
Sinhye
Lee
,
Lacey
Lux
,
Wenyang
Gao
and
Shengqian
Ma
*
Department of Chemistry, University of South Florida, 4202 East Fowler Avenue, Tampa, Florida 33620, USA. E-mail: sqma@usf.edu; Fax: +1-813-974-3203; Tel: +1-813-974-5217
First published on 6th January 2016
Abstract
In this work, we demonstrate the impartment of Brønsted acidity into a zeolitic imidazole framework, ZIF-90, via post-synthetically oxidizing the aldehyde group into carboxylate group to afford ZIF-90-COOH. The preservation crystallinity and porosity after post-synthetic oxidation was confirmed by powder X-ray diffraction studies and N2 gas adsorption analysis respectively. The successful conversion of the aldehyde group into carboxylate group was verified by FT-IR studies, which resulted in remarkable enhancement of methylene blue uptake in the resultant ZIF-90-COOH due to the strong electrostatic interactions between cationic methylene blue and the anionic carboxylate group.
Metal–organic frameworks (MOFs) comprise a structurally diverse family of three-dimensional porous crystalline materials facilitated by the combination of metal nodes coordinated by organic linkers.1 Their permanent porosity, high surface area, well-defined pore sizes, and innate tunability allow for the design of these structures for various applications including gas storage,2 catalysis,3 imaging and sensing,4 and energy storage.5 The broad and sundry applications of MOFs have extended to the exploitation of Brønsted acid moieties on or within the frameworks. Heterogenous catalysis has been heavily studied amongst MOF applications, with MOFs decorated with acidic protons showing excellent catalytic activity in Friedel–Craft alkylations,6 cellulose conversion,7 and more.8 In addition to their usage in catalysis, Brønsted acid moieties in MOFs have also shown potential in proton conduction applications. In comparison to many other solid state and porous materials, MOFs hold the advantage that they are robust coordination polymers with high flexibility and tunable reactivity, depending on the linkers.1,9 This not only gives way for more controlled conductivities, but also allows for the observation of proton-conducting pathways.10 One of the less explored methods of Brønsted acid inclusion in a MOF is the addition of acidic protons directly on the coordinating linkers of the construct.11 The inclusion of polar and acidic groups within a MOF normally gives rise to Brønsted acidity and other measures related to acid strength. The mobility of the charge carriers present (i.e. carboxylates and phosphates) facilitate the Brønsted acidity and proton conductivity of the material.12
However, the embodiment of Brønsted acidity poses many challenges in the synthesis and design of MOFs. The incorporation of acidic protons can diminish structural stability or interfere with the formation of the framework, as Brønsted acid sites are typically the sites of metal coordination. Moreover, the characterization of solid acids proves arduous: the Brønsted acid sites are often sporadic and the acid–base equilibrium cannot be substantiated.13 Acid sites are generally introduced into the framework through encapsulation of the acid into the pores,14 ligating the acid onto metal sites,15 or covalently affixing the acid site onto the ligands of the framework.16 In this contribution, we report a different approach of post-synthetic oxidation to impart the Brønsted acidity into MOF as exemplified in the context of post-synthetically oxidizing the aldehyde group in a zeolitic imidazole framework (ZIF) into carboxylate group.
ZIFs, a subclass of MOFs, have shown both robust chemical and thermal stability, thereby making them good candidates for the post synthetic introduction of acid moieties under the predominantly harsh synthetic conditions required for grafting acidic protons on the framework.17 ZIF-90, a crystalline material with sodalite (sod) topology, is comprised of imidazole-2-carboxaldehyde and zinc(II) linkers and nodes, respectively. This construct was chosen for post-synthetic modification to augment Brønsted acidity present within the framework. Moreover, imidazole-2-carboxaldehyde is mono-substituted with an aldehyde functional group prior to ZIF-90 synthesis, making ZIF-90 an elementary ZIF ideal for a simple oxidation. Herein, we report a new framework, ZIF-90-COOH resulting from the post synthetic oxidation of ZIF-90 by hydrogen peroxide (Scheme 1).
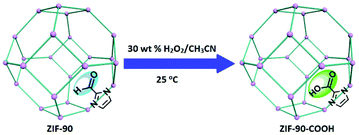 | ||
| Scheme 1 ZIF-90 was treated with 30 wt% H2O2 in acetonitrile for 72 hours at 25 °C to yield ZIF-90-COOH. | ||
ZIF-90 was synthesized via solvothermal methods18 and treated with hydrogen peroxide19 to generate a new framework, ZIF-90-COOH, decorated with –COOH groups. Due to the fragility of many MOFs and ZIFs, crystallinity must be ensured after post-synthetic modification, particularly because of the strong oxidizing nature of hydrogen peroxide. The phase purity of ZIF-90 was confirmed by powder X-ray diffraction (PXRD), and the resulting patterns are comparable to the calculated ones (Fig. 1a). Powder X-ray diffraction patterns were collected for the oxidized variant. The new construct, ZIF-90-COOH, show strong similarities to ZIF-90 while maintaining crystallinity and structural integrity. To confirm the permanent porosity of ZIF-90-COOH, gas adsorption studies were carried out. N2 isotherms at 77 K revealed that ZIF-90-COOH retains the microporous type-I adsorption behavior with a Brunauer–Emmett–Teller (BET) surface area of 376 m2 g−1 (Langmuir surface area of 421 m2 g−1), which is lower than that of the parent ZIF-90 with a BET surface area of 1166 m2 g−1 (Langmuir surface area of 1302 m2 g−1) (Fig. 1b). The observed reduction in surface area after post-synthetic oxidation should be presumably due to the conversion of the aldehyde groups to the more bulky carboxylate groups, which block the pores and reduce the pore volume. This is supported by the density functional theory (DFT) pore size distribution analysis, which shows that pore sizes of ZIF-90-COOH are predominantly distributed around 8.6 Å as compared to 11.0 Å for the parent ZIF-90 (Fig. S1†).
FT-IR was utilized to verify the carboxylic acid moiety in the new framework (Fig. 2a). A strong ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) band was observed at 1677 cm−1 in ZIF-90, indicating the carbonyl of the aldehyde. A similar v(C
O) band was observed at 1677 cm−1 in ZIF-90, indicating the carbonyl of the aldehyde. A similar v(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) of 1621 cm−1 in ZIF-90-COOH represents the retention of the carbonyl functionality in the new structure. A decrease in frequency from a standard ketone reference is attributed to the electron-donating properties of the imidazole constituents to which the carbonyls are attached. Furthermore, the hydroxyl group of the carboxylic acid was confirmed via the broad v(O–H) peak at 3293 cm−1.
O) of 1621 cm−1 in ZIF-90-COOH represents the retention of the carbonyl functionality in the new structure. A decrease in frequency from a standard ketone reference is attributed to the electron-donating properties of the imidazole constituents to which the carbonyls are attached. Furthermore, the hydroxyl group of the carboxylic acid was confirmed via the broad v(O–H) peak at 3293 cm−1.
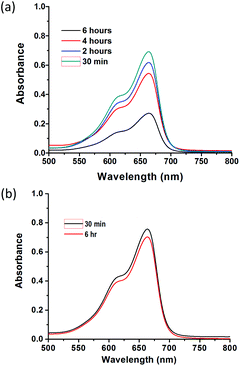
It has recently reported that MOFs containing carboxylic acid groups show remarkable methylene blue uptake from aqueous solutions due to the strong interactions between the dye and the framework.20 As such, methylene blue adsorption was employed as a technique to further verify the presence of the carboxylic acid functionality. Methylene blue removal from aqueous solutions and the related surface interactions were studied for ZIF-90-COOH and control experiments were also conducted on the parent ZIF-90. ZIF-90-COOH was soaked in 20.0 mL of a 5 ppm aqueous methylene blue solution. The adsorption was observed by UV-VIS spectroscopy (λ = 665 nm) (Fig. 2a and b). Absorbancies were taken over time, resulting in a 64% decrease in concentration after 6 hours. For comparison, the same study was carried out with the parent ZIF-90, with larger pores but lacking the carboxylic acid functionality. After 6 hours, the concentration decreased only 8%. Adsorption capacity tests reveal methylene blue uptake of 43 mg g−1 for ZIF-90-COOH, which is about ten times higher than that for ZIF-90 (4 mg g−1). Such dramatic boost of methylene blue uptake observed should be attributed to the strong interactions between methylene blue molecules and the carboxylate groups20 within ZIF-90-COOH as evidenced by the FT-IR studies (Fig. S2 and S3†). The symmetric and antisymmetric stretches of C–O on ZIF-90-COOH are characterized by the in phase and out of phase vibrational modes of νs(COO) at 1393 cm−1 and νas(COO) 1602 cm−1. The electrostatic interactions between the cationic methylene blue and anionic carboxylate group give rise to a drift of the antisymmetric vibrational mode to a stronger band at 1623 cm−1.
In summary, we have demonstrated the successful impartment of Brønsted acidity into a MOF via the post-synthetic oxidation of the aldehyde group in the zeolitic imidazole framework, ZIF-90, into carboxylate group to afford ZIF-90-COOH. The transformation from the aldehyde functionality to carboxylic acid leads to remarkable enhancement of methylene blue uptake in the resultant ZIF-90-COOH due to the strong electrostatic interactions between cationic methylene blue and the anionic carboxylate group. Moreover, the retained crystallinity and porosity of the resultant framework creates opportunity for further proton conduction studies, which will be carried out in our laboratory in the near future.
Acknowledgements
The authors acknowledge National Science Foundation (DMR-1352065) and the University of South Florida as well as the NSF Florida-Georgia financial LSAMP for support (HRD #1139850).References
- (a) H.-C. Zhou, J. R. Long and O. M. Yaghi, Chem. Rev., 2012, 112, 673 CrossRef CAS PubMed; (b) H.-C. Zhou and S. Kitagawa, Chem. Soc. Rev., 2014, 43, 5415 RSC; (c) B. Li, M. Chrzanowski, Y. Zhang and S. Ma, Coord. Chem. Rev., 2016, 307, 106 CrossRef CAS.
- (a) K. Sumida, D. L. Rogow, J. A. Mason, T. M. McDonald, E. D. Bloch, Z. R. Herm, T. H. Bae and J. R. Long, Chem. Rev., 2012, 112, 724 CrossRef CAS PubMed; (b) Y. He, W. Zhou, G. Qian and B. Chen, Chem. Soc. Rev., 2014, 43, 5657 RSC.
- (a) J. Liu, L. Chen, H. Cui, J. Zhang, L. Zhang and C. Y. Su, Chem. Soc. Rev., 2014, 43, 6011 RSC; (b) W.-Y. Gao, M. Chrzanowski and S. Ma, Chem. Soc. Rev., 2014, 43, 5841 RSC; (c) N. A. Adeel, H. Chughtai, H. A. Younus, A. Laypkovc and F. Verpoort, Chem. Soc. Rev., 2015, 44, 6804 RSC; (d) A. Dhakshinamoorthy and H. Garcia, Chem. Soc. Rev., 2014, 43, 5750 RSC.
- (a) L. E. Kreno, K. Leong, O. K. Farha, M. Allendorf, R. P. Van Duyne and J. T. Hupp, Chem. Rev., 2012, 112, 1105 CrossRef CAS PubMed; (b) Z. Hu, B. J. Deibert and J. Li, Chem. Soc. Rev., 2014, 43, 5815 RSC.
- (a) L. Lux, K. Williams and S. Ma, CrystEngComm, 2015, 17, 10 RSC; (b) S.-L. Li and Q. Xu, Energy Environ. Sci., 2013, 6, 1656 RSC.
- (a) M. Zheng, Y. Liu, C. Wang, S. Liu and W. Lin, Chem. Sci., 2012, 3, 2623 RSC; (b) B. Li, K. Leng, Y. Zhang, J. J. Dynes, J. Wang, Y. Hu, D. Ma, Z. Shi, L. Zhu, D. Zhang, Y. Sun, M. Chrzanowski and S. Ma, J. Am. Chem. Soc., 2015, 137, 4243 CrossRef CAS PubMed.
- G. Akiyama, R. Matsuda, H. Sato, M. Takata and S. Kitagawa, Adv. Mater., 2011, 23, 3294 CrossRef CAS PubMed.
- (a) L.-F. Ma, Z.-Z. Shi, F.-F. Li, J. Zhang and L.-Y. Wang, New J. Chem., 2015, 39, 810 RSC; (b) J. Jiang and O. M. Yaghi, Chem. Rev., 2015, 115, 6966 CrossRef CAS PubMed.
- (a) M. Yoon, K. Suh, S. Natarajan and K. Kim, Angew. Chem., Int. Ed., 2013, 52, 2688 CrossRef CAS PubMed; (b) T. R. Cook, Y. R. Zheng and P. J. Stang, Chem. Rev., 2013, 113, 734 CrossRef CAS PubMed; (c) P. Nugent, Y. Belmabkhout, S. D. Burd, A. J. Cairns, R. Luebke, K. Forrest, T. Pham, S. Ma, B. Space, L. Wojtas, M. Eddaoudi and M. J. Zaworotko, Nature, 2013, 495, 80 CrossRef CAS PubMed; (d) J. J. T. Perry IV, J. A. Perman and M. J. Zaworotko, Chem. Soc. Rev., 2009, 38, 1400 RSC.
- (a) M. Sadakiyo, T. Yamada, K. Honda, H. Matsui and H. Kitagawa, J. Am. Chem. Soc., 2014, 136, 7701 CrossRef CAS PubMed; (b) A. Shigematsu, T. Yamada and H. Kitagawa, J. Am. Chem. Soc., 2011, 133, 2034 CrossRef CAS PubMed.
- P. Ramaswamy, N. E. Wong and G. K. Shimizu, Chem. Soc. Rev., 2014, 43, 5913 RSC.
- (a) T. Y. Masaaki Sadakiyo and H. Kitagawa, J. Am. Chem. Soc., 2009, 131, 9906 CrossRef PubMed; (b) S. Sen, N. N. Nair, T. Yamada, H. Kitagawa and P. K. Bharadwaj, J. Am. Chem. Soc., 2012, 134, 19432 CrossRef CAS PubMed.
- G. A. Olah, G. K. S. Prakash, Á. Molnáar and J. Sommer, Super Acid Chemistry, John Wiley and Sons, Inc., Hoboken, 2nd edn, 2009 Search PubMed.
- (a) V. G. Ponomareva, K. A. Kovalenko, A. P. Chupakhin, D. N. Dybtsev, E. S. Shutova and V. P. Fedin, J. Am. Chem. Soc., 2012, 134, 15640 CrossRef CAS PubMed; (b) W. J. Phang, W. R. Lee, K. Yoo, D. W. Ryu, B. Kim and C. S. Hong, Angew. Chem., Int. Ed., 2014, 53, 8383 CrossRef CAS PubMed.
- (a) A. Vimont, J.-M. Goupil, J.-C. Lavalley, M. Daturi, S. Surble, C. Serre, F. Millange, G. Férey and N. Audebrand, J. Am. Chem. Soc., 2006, 128, 3218 CrossRef CAS PubMed; (b) J. B. DeCoste, T. J. Demasky, M. J. Katz, O. K. Farha and J. T. Hupp, New J. Chem., 2015, 39, 2396 RSC.
- (a) J. F. Van Humbeck, T. M. McDonald, X. Jing, B. M. Wiers, G. Zhu and J. R. Long, J. Am. Chem. Soc., 2014, 136, 2432 CrossRef CAS PubMed; (b) K. K. Tanabe and S. M. Cohen, Angew. Chem., Int. Ed., 2009, 48, 7424 CrossRef CAS PubMed; (c) W. J. Phang, H. Jo, W. R. Lee, J. H. Song, K. Yoo, B. Kim and C. S. Hong, Angew. Chem., Int. Ed., 2015, 54, 5142 CrossRef CAS PubMed.
- (a) K. S. Park, Z. Ni, A. P. Cote, J. Y. Choi, R. Huang, F. J. Uribe-Romo, H. K. Chae, M. O'Keeffe and O. M. Yaghi, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 10186 CrossRef CAS PubMed; (b) R. Banerjee, A. Phan, B. Wang, C. Knobler, H. Furukawa, O. M. Yaghi and M. O'Keeffe, Science, 2008, 319, 939 CrossRef CAS PubMed.
- W. Morris, C. J. Doonan, H. Furukawa, R. Banerjee and O. M. Yaghi, J. Am. Chem. Soc., 2008, 130, 12626 CrossRef CAS PubMed.
- Y. Wang, G. Ye, H. Chen, X. Hu, Z. Niu and S. Ma, J. Mater. Chem. A, 2015, 3, 15292 CAS.
- Qi Zhang, J. Yu, J. Cai, R. Song, Y. Cui, Y. Yang, B. Chen and G. Qian, Chem. Commun., 2012, 50, 14455 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5qi00229j |
| This journal is © the Partner Organisations 2016 |