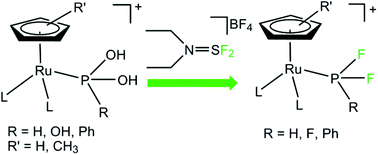
The transformation of phosphorous oxyacids into the corresponding fluorophosphines was mediated by [RuCp(PPh3)2Cl] under mild reaction conditions using a soft deoxofluorinating agent. The reaction is selective, proceeds with high yields and can be extended to a wide range of phosphorous oxyacids once coordinated to the ruthenium synthon [RuCp(PPh3)2]+ as their hydroxyphosphine tautomer. Deoxofluorination of phenylphosphinic acid was also mediated by [RuCpR(CH3CN)3]PF6, where CpR: Cp = C5H5, Cp* = C5Me5, and [Ru(η6-p-cymene)(μ-Cl)Cl]2. X-Ray single crystal structures of the two new derivatives, [RuCp(PPh3)2{PhP(OH)2}]CF3SO3 and [Ru(η6-p-cymene)Cl2{PhP(OH)2}] have been determined.