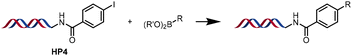Open Access Article
Open Access ArticleImprovements in micelle promoted DNA-encoded library synthesis by surfactant optimisation†
Jake A.
Odger
a,
Matthew J.
Anderson
a,
Thomas P.
Carton
a,
Bao
Nguyen
 b,
Kevin
Foote
b,
Kevin
Foote
 c and
Michael J.
Waring
c and
Michael J.
Waring
 *a
*a
aCancer Research Horizons Newcastle Drug Discovery Group, Chemistry, Newcastle University, Newcastle upon Tyne, NE1 7RU, UK. E-mail: mike.waring@ncl.ac.uk
bSchool of Chemistry, University of Leeds, Woodhouse Lane, Leeds LS2 9JT, UK
cPharmaron, West Hill Innovation Park, Hertford Road, Hoddesdon, Hertfordshire EN11 9FH, UK
First published on 13th June 2025
Abstract
DNA-encoded libraries are increasingly important in hit identification at the early stage of the drug discovery process. The approach relies on efficient methods for synthesis of drug-like compounds attached to coding DNA sequences. Many reactions employed for library synthesis are inefficient and result in significant DNA-damage, incomplete conversion and the formation of side products, which compromise the fidelity of the resulting library. We have developed a wide array of reactions that are promoted by the micelle-forming surfactant TPGS-750-M that address these issues and lead to improved efficiency. Here we demonstrate further improvements to key reactions Suzuki–Miyaura coupling, reductive amination and amide coupling by surfactant screening using principal component-based surfactant maps which lead to improved conversion for problematic substrates. This work demonstrates the utility of surfactant maps in reaction optimisation for DNA-encoded library synthesis and leads to further improvements in these important transformations.
Introduction
DNA-encoded libraries (DELs) are an emerging technology in the field of medicinal chemistry for effective identification of hit compounds.1–4 DELs consist of a vast number of organic molecules, with each member covalently attached to a specific DNA sequence, which functions as a unique, amplifiable barcode. Libraries are commonly synthesised through the use of combinatorial split-and-pool methodology (Fig. 1). This technique couples the conjugation of chemical building blocks with the enzymatic ligation of an encoding DNA oligomer, whereby each DNA sequence is specific to the chemical monomer it encodes.5 This approach allows for the generation of large libraries covering a broad range of chemical space. In fact, DELs can be synthesised with in excess of 109 library members; libraries of this size would be practically impossible to produce using traditional chemical synthesis. DELs can be screened as a mixture against an immobilised protein of interest, and polymerase chain reaction (PCR) used to amplify the DNA barcodes of any eluted binders. Next-generation sequencing (NGS) is then used to read the DNA, identifying sequences of target binders and enabling subsequent elucidation of corresponding small molecules. Hit compounds are then typically validated via off-DNA synthesis and testing in orthogonal assays.Effective hit identification through DEL technology requires high-quality libraries; i.e. containing lead-like compounds with the majority of the library correctly encoded. This must be achieved through the employment of efficient on-DNA chemical transformations in library synthesis.6 However, diversity of the libraries is often limited, owing to the challenges associated with some on-DNA synthetic methods. Conventionally, DEL reactions should be carried out in aqueous media at high dilutions, avoid damaging the integrity of the DNA barcode, proceed with high yields and afford little to no side products.7
Surfactants have been applied in organic chemistry since the 1970s to facilitate reactions in aqueous media.8 Micelle-forming surfactants can aid solubilisation and increase the effective concentration of organic reagents in an aqueous solution.9 Interest in this field has surged in recent years due to the desire to reduce the use of organic solvents.10
The use of surfactants in DEL syntheses has been shown to be advantageous. It is theorised that micellar structures localise organic reagents within the hydrophobic core of the micelles, whilst the DNA barcode remains in the aqueous compartment (Fig. 2).11 We have applied TPGS-750-M,12 a second-generation designer surfactant developed by Lipshutz et al., to various reactions on DNA, including Suzuki–Miyaura, Heck, Sonogashira and Buchwald–Hartwig cross-coupling; amide coupling; transfer hydrogenation and hydrogenolysis; and reductive amination reactions.11,13–18 Brunschweiger et al. developed sulfonic acid-containing, micelle-forming block copolymers to perform Povarov, Groebke–Blackburn–Bienaymé and Biginelli reactions.19 More recently, decarboxylative photoredox arylations were performed on DNA using cationic surfactant molecules to catalyse the reaction.20
Despite the significant application of micellar catalysis to DEL synthesis, little work has been done to evaluate the use of different surfactant molecules for these reactions. Almost all micellar chemistry on DNA has been limited to the commercially available TPGS-750-M or expensive specialist surfactants. This study aims to compare alternative readily-accessible surfactants to perform these reactions with the aim of improving the efficiencies achieved with TPGS-750-M. Nguyen et al. have recently developed a 3D surfactant map which employs the NIPALS principal component analysis algorithm, based on 22 experimental and computational descriptors of the surfactant molecules (e.g. hydrophobic and hydrophilic fragments, number of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds, and Hirshfeld charges), their interactions with water (e.g. SASA, ΔGsolv) and their micellar and emulsion properties (e.g. CMC, aggregation number, zeta potential, contact angle and HLB).21 Importantly, the designer surfactants occupy a relatively small, but central portion of surfactant space. The map has been successfully used to optimise surfactants for specific surfactant enabled reactions, therefore it was theorised that this could be applied to micellar reactions on DNA.
C bonds, and Hirshfeld charges), their interactions with water (e.g. SASA, ΔGsolv) and their micellar and emulsion properties (e.g. CMC, aggregation number, zeta potential, contact angle and HLB).21 Importantly, the designer surfactants occupy a relatively small, but central portion of surfactant space. The map has been successfully used to optimise surfactants for specific surfactant enabled reactions, therefore it was theorised that this could be applied to micellar reactions on DNA.
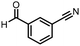
Three reactions that we have previously optimised using TPGS-750-M as the surfactant were chosen for this study: Suzuki–Miyaura cross-coupling (Fig. 3a), reductive amination (Fig. 3b) and “reverse” amide coupling (i.e. DNA-tagged carboxylic acid, Fig. 3c). These reactions are well-established and offer good to excellent conversions for a wide range of substrates. The preliminary reactions used substrates that were selected based on their being relatively low-yielding when TPGS-750-M was employed.
Results and discussion
Suzuki–Miyaura cross-coupling
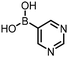
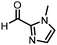
Coupling of a DNA-tagged bromopyrazole (HP1) with boronic acid 1 was chosen for the initial Suzuki–Miyaura cross-coupling reaction (previous conversion 55%).11 Seven commercially available surfactants were applied to this reaction alongside TPGS-750-M (Table 1). This set of 8 surfactants was chosen to represent all areas of the surfactant map using the previously reported Python code to maximise the area covered by the selected surfactants within the 3D map (Fig. 4, with projection onto 3 × 2D planes to show the chemical space covered by the selection).21 The designer surfactant TPGS-750-M was used as the origin point for the algorithm, due to its known performance in our reactions. The principal component PC3 is mainly influenced by the nominal charge of the surfactant molecule. The most significant contributors to PC1 are the volume, surface area and flexibility of the hydrophobic and hydrophilic fragments of each surfactant. PC2 was mainly derived from a combination of contact angles, zeta potential and volume/area/flexibility properties of the hydrophobic fragment.Firstly, replacing the surfactant from the reaction with just water resulted in the expected slight decrease in conversion to desired product compared with the use of TPGS-750-M (Table 1, entries 1 and 2). Conversions are determined by taking the integral of the peak areas in the total ion chromatogram, desired product conversion expressed as a percentage of all species present.
Several surfactants (PEG5C12, Tween 65, Brij 700 and Brij S20) led to a significant decrease in conversion (26–41%, Table 1, entries 3–6). The use of Triton-X-405 and TTAC yielded comparable conversions to TPGS-750-M (59% and 62% conversion observed for Triton-X-405 and TTAC respectively, Table 1, entries 7 and 8). Finally, utilising sulfobetaine-16 resulted in an improvement in conversion to 70% (Table 1, entry 9).
From these results, based on the surfactant map, a further subset of surfactants was assessed (Table 2). From this subset of four more surfactants, polysorbate 60 afforded a significantly reduced conversion (30%) compared with TPGS-750-M (Table 2, entry 1). Both (lauryldimethylammonio)acetate and dodecylamino-3-propane-1-sulfonate (DAPS) resulted in similar conversions to desired product (65% and 60%, respectively) compared with TPGS-750-M (Table 2, entries 2 and 3). Employing 4-dodecylbenzene sulfonic acid as the surfactant in this reaction led to the highest conversion across all surfactants tested (75%, Table 2, entry 4).
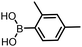
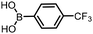
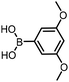
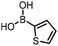
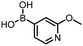
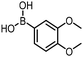
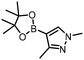
The performance of the three best-performing surfactants (sulfobetaine-16, (lauryldimethylammonio)acetate and 4-dodecylbenzenesulfonic acid) was assessed against a wider range of substrates. DNA-tagged aryl iodide headpiece (HP4) was reacted with ten boronic acid/ester substrates (Table 3). All three surfactants afforded an overall improvement in reaction outcome across the set of substrates compared to TPGS-750-M. 4-Dodecylbenzenesulfonic acid offered the highest average conversion across all ten substrates. Therefore, 4-dodecylbenzenesulfonic acid was profiled across a wider range of boronic acid/esters (Table 4). 13 boronate substrates with varying chemical moieties and reactivities were chosen. Only one substrate gave rise to a reduced conversion with 4-dodecylbenzenesulfonic acid relative to TPGS-750-M (Table 4, entry 1). For 7 of the substrates, the conversion to product using TPGS-750-M and 4-dodecylbenzenesulfonic acid was comparable (within 5%, Table 4, entries 2–8), the remaining five substrates exhibited improved conversion (Table 4, entries 8–13). Hence, across all the substrates tested for the Suzuki–Miyaura reaction, 4-dodecylbenzenesulfonic acid as the surfactant offers significant improvement. Of the 24 substrates tested, 11 were found to be improved by the use of 4-dodecylbenzenesulfonic acid, 12 yielded the same conversion (within 5%) for both, whilst TPGS-750-M was preferred for only one substrate. The overall average conversion across all boronate substrates tested was improved from 72% with TPGS-750-M to 79% with 4-dodecylbenzenesulfonic acid.
Reductive amination
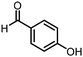
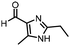
The same approach was taken for the reductive amination reaction. The primary reaction tested was that of a DNA-tagged amine (HP2) with aldehyde 3. The same initial set of surfactants used previously were screened (Table 5). Previous work has proven the effectiveness of the use of surfactant in this reaction, which provides significant improvement upon conversions to desired product.18 The TPGS-750-M promoted reaction was somewhat better than anticipated (85%, Table 5, entry 1, compared to 77% observed previously). Two surfactants, PEG5C12 and Tween 65, gave reduced conversion (74% and 45% respectively, entries 2 and 3), Brij 700 and Brij S20, were comparable (86% and 91% respectively, Table 5, entries 4 and 5). The remainder of the surfactants all showed significantly improved results, with sulfobetaine-16 yielding 97% and Triton-X-405 and TTAC affording 100% conversion to desired product (Table 5, entries 6–8). Therefore, we determined that a follow up surfactant screen was not necessary. Instead, the three best-performing surfactants (sulfobetaine-16, Triton-X-405 and TTAC) were assessed for their wider limited substrate scope. Four diverse aldehydes were selected due to their previously observed moderate conversions to desired product (Table 6). Both Triton-X-405 and sulfobetaine-16 in these reactions were detrimental to conversion for all four aldehydes compared to TPGS-750-M. However, TTAC offered significant improvement in desired product conversion for every substrate, as well as a marked increase in the average conversion. Therefore, TTAC was selected as the optimal surfactant for the reductive amination and was hence applied to an extended selection of 20 aldehydes (Table 7). 4 aldehydes afforded reduced conversion to desired product with TTAC versus TPGS-750-M (Table 7, entries 1–4). A further 4 were comparable (within 5% conversion, Table 7, entries 5–8). However, the remaining 12 substrates provided increased conversions to desired product with TTAC, 9 of which gave an improvement of >10% conversion. In total, 16 of 25 total aldehydes explored exhibited an improved conversion to desired product upon changing the surfactant in the reaction from TPGS-750-M to TTAC. 5 of the aldehydes tested performed comparably (conversions within 5%), whilst only 4 aldehydes afforded reduced conversions to desired product following the implementation of TTAC. The overall average conversion across all aldehyde substrates was improved from 67% using TPGS-750-M to 75% using TTAC as the surfactant.Reverse amide coupling
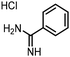
Finally, the process was repeated for a “reverse” amide coupling reaction of DNA-tagged acid (HP3) with amine 5, with an expected conversion to desired product of 58%.16 The initial surfactant scope was performed using the same surfactants as previously (Table 8). The first thing to note is that the use of TPGS-750-M in this reaction afforded a much lower conversion to desired product (15%) than we had previously observed (58%), equivalent to no surfactant (Table 8, entries 1 and 2). Many of the initial surfactants (Tween 65, Brij 700, Brij S20 and Triton-X-405) offered some improvement in conversion compared with TPGS-750-M (20–27%, Table 8, entries 4–7). However, when both TTAC and sulfobetaine-16 were implemented in the reaction, a significant improvement in conversion was observed (44% and 36% respectively, Table 8, entries 8 and 9). From these initial results, a further subset of surfactants selected using the map was explored (Table 9). None of these surfactants offered any improvement on the reactions, with conversions to desired product ranging from 11–17%. Therefore, TTAC and sulfobetaine-16 were selected as the two best-performing surfactants to assess further. 6 amines occupying a range of chemical space with varying conversions were selected (Table 10). The use of TTAC as the surfactant in this reaction across this subset of substrates led to a significant reduction in the average conversion to desired product compared with using TPGS-750-M. However, sulfobetaine-16 gave a slight improvement and was profiled against further substrates (Table 11).Only one of the further substrates performed slightly worse when sulfobetaine-16 was utilised (Table 11, entry 1). 4 of the substrates performed similarly (within 5%, entries 2–5). The remaining 7 substrates all afforded improved conversions with sulfobetaine-16 (entries 6–12). Overall, across 19 substrates, conversions were improved for 9 amines when sulfobetaine-16 was used in place of TPGS-750-M. A further 9 substrates afforded the same conversion (within 5%) when either sulfobetaine-16 or TPGS-750-M was chosen as the surfactant, whilst only one substrate afforded a reduced conversion (>5%), albeit only 7% in magnitude. Average conversion across all amine substrates was improved from 53% using TPGS-750-M to 62% with sulfobetaine-16.
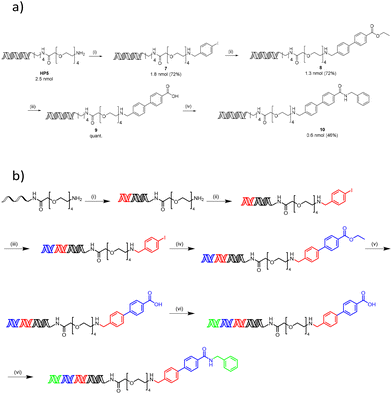
Synthesis of a DNA-conjugated multicycle compound
To demonstrate the applicability of this work to DEL synthesis, a representative encoded compound was synthesised using a 3-cycle sequence of each reaction utilising the optimal surfactant (Scheme 1a). A reductive amination reaction of PEG-amine (HP5) with 4-iodobenzaldehyde using TTAC yielded compound 7. Suzuki–Miyaura cross-coupling of 7 with (4-(ethoxycarbonyl)phenyl)boronic acid in the presence of 4-dodecylbenzenesulfonic acid led to compound 8, and subsequent ester hydrolysis using aqueous LiOH produced acid 9. Finally, reverse amide coupling with benzylamine and sulfobetaine-16 formed the final compound 10. All reactions proceeded with excellent conversions to desired products and moderate recovery of DNA (comparable to the equivalent reactions using TPGS-750-M) to give final product 10 with a quantitative conversion (Fig. S201–204†) and an overall recovery of 18%.This synthetic sequence was repeated, incorporating a DNA ligation prior to each chemical transformation to encode each chemical building block and form a representative 1 × 1 × 1 library (Scheme 1b). For each ligation, success was confirmed by the appearance of a major band at the expected number of base pairs upon gel electrophoresis (Fig. S206–208†). PCR amplification with NGS elongation primers also resulted in the expected major band at 161 base pairs (Fig. S209†), which suggests efficient amplification following the synthesis of the library. NGS of the library confirmed that the integrity of the DNA barcodes remained intact, with 78% of >80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 reads corresponding to the expected sequence.
000 reads corresponding to the expected sequence.
Effect of surfactants
Overall, this study has shown that the published surfactant map can be employed for rational and efficient optimisation of surfactant for a wide range of reactions. In most cases, a readily available surfactant was found as a superior replacement for the commonly used TPGS-750-M. This effect could be attributed to the central location of designer surfactants in the map. In the past it has been shown that surfactants occupying this region are sufficiently good at many reactions, but not often the optimal surfactant for each of them.21For the three reactions in this study, ionic surfactants were found to the best choices, i.e. 4-dodecylbenzenesulfonic acid, TTAC and sulfobetaine-16 (zwitterionic) (Fig. 5). Cationic and zwitterionic surfactants are known to interact with DNAs and can absorb them into micelles.22,23 In the case of zwitterionic surfactants, the interaction has been shown to change the shape of DNAs.24–26 These can have significant impact on the steric environment around the reaction centres. Given that our reactions were performed at much lower concentrations compared to typical surfactant-enabled organic reactions, the changes to DNAs through micellar interactions are particularly relevant.
It is also noteworthy that the improved surfactants do not contain hydrolytically labile linkers in contrast to TPGS-750-M. TPGS-750-M is known to degrade rapidly under hydrolytic conditions at high temperatures27 and this may lead to their reduced effectiveness with prolonged reactions times. This is less likely to be a consideration for the any of the improved surfactants.
Conclusions
In summary, three established on-DNA micelle-mediated reactions have been improved to afford greater conversions to desired product across a broad substrate scope through the implementation of alternative surfactants to catalyse the reaction, compared to TPGS-750-M, the typical surfactant used in these reactions.4-Dodecylbenzenesulfonic acid was employed to improve a Suzuki–Miyaura reaction, in which 11 of 24 boronate substrates were improved whilst only one substrate afforded a reduction in conversion. A reductive amination was improved through the use of TTAC as the surfactant in the reaction, in which 16 of the 25 aldehyde substrates gave rise to improved conversions when TTAC was employed, whilst only four substrates yielded reduced conversions compared with TPGS-750-M. Finally, a reverse amide coupling of a DNA-tagged acid with various amines was improved through the use of sulfobetaine-16 as the surfactant in the reaction, whereby conversion was improved for 9 amines and was only reduced for 1 substrate. No damage to the duplex DNA tags was detected in these reactions, even with the use of ionic surfactants. The surfactant map was demonstrated to be an effective tool for surfactant selection and optimisation in ‘micellar catalysis.21
Finally, a representative DNA-conjugated compound was successfully synthesised using the optimal surfactant for each of the investigated reactions, and PCR amplification followed by NGS confirmed that the conditions did not cause a detrimental effect to the DNA barcode integrity, highlighting applicability to library synthesis.
Author contributions
JAO carried out experimental work and co-wrote the manuscript, MJA and TPC carried out experimental work, BN designed the surfactant screens, KF co-supervised JAO, MJW supervised JAO, MJA and TPC and co-wrote the manuscript. All authors reviewed the manuscript prior to publication.Conflicts of interest
There are no conflicts to declare.Data availability
Full supporting data and experimental details of this study are available within the article and the ESI.†Acknowledgements
The authors gratefully acknowledge EPSRC MoSMed CDT (EP/S022791/1, studentship awarded to JAO, MJA and TPC), Pharmaron, Inc. (studentship awarded to JAO), Exscientia, a wholly owned subsidiary of Recursion (studentship awarded to TPC), and Cancer Research UK (DRCDDRPGMApr2020\100002, Newcastle Drug Discovery Unit Programme Grant) for funding.References
- S. Brenner and R. A. Lerner, Proc. Natl. Acad. Sci. U. S. A., 1992, 89, 5381–5383 CrossRef CAS PubMed.
- R. A. Lerner, R. A. Lerner and D. Neri, Biochem. Biophys. Res. Commun., 2020, 527, 757–759 CrossRef CAS PubMed.
- I. F. S. F. Castan, J. S. Graham, C. L. A. Salvini, H. A. Stanway-Gordon and M. J. Waring, Bioorg. Med. Chem., 2021, 43, 116273 CrossRef CAS PubMed.
- R. A. Goodnow, C. E. Dumelin and A. D. Keefe, Nat. Rev. Drug Discovery, 2017, 16, 131–147 CrossRef CAS PubMed.
- A. Litovchick, M. A. Clark and A. D. Keefe, Artif. DNA PNA XNA, 2014, 5, e27896-1–e27896-11 Search PubMed.
- A. Gironda-Martínez, E. J. Donckele, F. Samain and D. Neri, ACS Pharmacol. Transl. Sci., 2021, 4, 1265–1279 CrossRef PubMed.
- M. L. Malone and B. M. Paegel, ACS. Comb. Sci., 2016, 18, 182–187 CrossRef CAS PubMed.
- E. J. Fendler and J. H. Fendler, Adv. Phys. Org. Chem, 1970, 8, 271–406 CrossRef CAS.
- C. Tang and B. T. McInnes, Molecules, 2022, 27, 5611 CrossRef CAS PubMed.
- G. La Sorella, G. Strukul and A. Scarso, Green. Chem, 2015, 17, 644–683 RSC.
- J. H. Hunter, L. Prendergast, L. F. Valente, A. Madin, G. Pairaudeau and M. J. Waring, Bioconjugate Chem., 2020, 31, 149–155 CrossRef CAS PubMed.
- B. H. Lipshutz, S. Ghorai, A. R. Abela, R. Moser, T. Nishikata, C. Duplais, A. Krasovskiy, R. D. Gaston and R. C. Gadwood, J. Org. Chem, 2011, 76, 4379–4391 CrossRef CAS PubMed.
- H. A. Stanway-Gordon, J. A. Odger and M. J. Waring, Bioconjugate Chem, 2023, 34, 756–763 CAS.
- J. S. Graham, H. A. Stanway-Gordon and M. J. Waring, Chem. – Eur. J., 2023, 29, e202300603 CrossRef CAS PubMed.
- J. S. Graham, J. H. Hunter and M. J. Waring, J. Org. Chem., 2021, 86, 17257–17264 CrossRef CAS PubMed.
- J. H. Hunter, M. J. Anderson, I. F. S. F. Castan, J. S. Graham, C. L. A. Salvini, H. A. Stanway-Gordon, J. J. Crawford, A. Madin, G. Pairaudeau and M. J. Waring, Chem. Sci., 2021, 12, 9475–9484 RSC.
- H. A. Stanway-Gordon, J. S. Graham and M. J. Waring, Angew. Chem., Int. Ed., 2022, 61, e202111927 CrossRef CAS PubMed.
- M. J. Anderson, T. P. Carton, C. L. A. Salvini, J. J. Crawford, G. Pairaudeau and M. J. Waring, Chem. – Eur. J., 2024, 30, e202400239 CrossRef CAS PubMed.
- M. K. Škopić, K. Götte, C. Gramse, M. Dieter, S. Pospich, S. Raunser, R. Weberskirch and A. Brunschweiger, J. Am. Chem. Soc., 2019, 141, 10546–10555 CrossRef PubMed.
- P. R. Chheda, N. Simmons and Z. Shi, Org. Lett., 2024, 26, 59 Search PubMed.
- K. Sharma, A. McCorry, S. Boobier, J. Mottram, R. Napier, I. W. Ashworth, A. J. Blacker, N. Kapur, S. L. Warriner, M. H. Wright and B. N. Nguyen, Chem. Sci., 2024, 15, 5764–5774 RSC.
- S. Husale, W. Grange, M. Karle, S. Bürgi and M. Hegner, Nucleic Acids Res., 2008, 36, 1443–1449 CrossRef CAS PubMed.
- S. Gromelski and G. Brezesinski, Langmuir, 2006, 22, 6293–6301 CrossRef CAS PubMed.
- R. S. Dias, L. M. Magno, A. J. M. Valente, D. Das, P. K. Das, S. Maiti, M. G. Miguel and B. Lindman, J. Phys. Chem. B, 2008, 112, 14446–14452 CrossRef CAS PubMed.
- L. Feng, L. Xu, J. Hao and S. Dong, Colloids Surf., A, 2016, 501, 65–74 CrossRef CAS.
- L. Feng, L. Xu, S. Dong and J. Hao, Soft Matter, 2016, 12, 7495–7504 RSC.
- C. Krell, R. Schreiber, L. Hueber, L. Sciascera, X. Zheng, A. Clarke, R. Haenggi, M. Parmentier, H. Baguia, S. Rodde and F. Gallou, Org. Process Res. Dev., 2021, 25, 900–915 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ob00864f |
| This journal is © The Royal Society of Chemistry 2025 |