A dual-mode wearable sensor with electrophysiological and pressure sensing for cuffless blood pressure monitoring†
Nan
Jiang‡
,
Gangsheng
Chen‡
,
Fan
Zhou
,
Biao
Ma
*,
Chao
Zhao
 * and
Hong
Liu
* and
Hong
Liu
 *
*
State Key Laboratory of Digital Medical Engineering, School of Biological Science and Medical Engineering, Southeast University, Nanjing 210096, China. E-mail: biaom@seu.edu.cn; czhao@seu.edu.cn; liuh@seu.edu.cn
First published on 2nd September 2024
Abstract
Wearable cuffless blood pressure sensing is essential for the monitoring of hypertension and its related cardiovascular diseases in an on-demand, timely, and comfortable manner. Measurement of the pulse transit time (PTT) has been the most employed technique to monitor blood pressure due to its noninvasiveness, low cost, and ease of device miniaturization. However, the existing PTT measurements rely on analyzing pulse and electrophysiological signals using discrete sensors that complicate the circuit design and signal processing. Herein, we report a wearable dual-mode sensor that can simultaneously monitor both the electrocardiogram and arterial pulse to simplify the measurement of the PTT. This was achieved by encapsulating a liquid metal pressure sensing circuit within the adhesive and conductive ionogel. The ionogel can not only be used for encapsulation but also as ionotronic electrodes for electrocardiogram recording, thus eliminating the use of multiple sensors in PTT sensing. We also integrated the dual-mode sensor into a printed circuit board to achieve wireless signal transmission based on Bluetooth. Upon wearing it on the wrist, the electrocardiogram and arterial pulse can be simultaneously collected. Based on these two signals, the PTT was measured to predict blood pressure, and the predicted results agree well with those of the commercial sphygmomanometer.
1. Introduction
Cardiovascular diseases impact the functions of the heart and circulatory system, resulting in the death of millions of individuals each year.1,2 Arterial blood pressure (BP) including systolic and diastolic pressures can provide information related to hemodynamics such as heart rate and cardiac output.3–5 Thus, BP monitoring is of great significance in preventing and diagnosing cardiovascular diseases and also in general health management.6,7 However, the gold standard for BP measurement is invasive intra-arterial blood pressure measurement, increasing the patient's pain and infection risk.8,9 In addition, cuff-based sphygmomanometers are widely used in the clinic but can only measure static BP.10–12 Using both these methods, it is hard to meet the needs of daily, noninvasive, and continuous BP monitoring for effective prevention and diagnosis of cardiovascular diseases.5,10,13–15Recently, the development of wearable electronics has provided a unique opportunity for noninvasive and continuous BP monitoring.16,17 Highly sensitive wearable pressure and optoelectronic sensors have been developed to detect single high-quality pulse waves for BP analysis.13,18–21 However, these wearable sensors suffer from interference of motion artifacts. Pulse wave transit time (PTT) refers to the time it takes a pulse wave to travel between two arterial sites within the same cardiac cycle,22 and PTT detection has become a promising cuffless form of BP monitoring. Compared with the analysis of single pulse waveforms,23 the PTT method based on the two cardiac signals of both pulse and electrocardiograph (ECG) waves exhibited advantages of high measuring stability and accuracy.24–26 However, the acquisition of the pulse and ECG waves typically required two discrete sensors, leading to a poor wearing experience and complex circuit design and signal processing.11,27,28 Thus, it is highly desired to develop a wearable sensor capable of simultaneous monitoring of both the pulse and ECG waves for BP measurement.
Here, we report a highly integrated wearable sensor by encapsulating the liquid metal (LM) circuit in the ionogel matrix, achieving the simultaneous monitoring of pulse and ECG signals for ambulatory BP measurement (Fig. 1). The LM circuit serves as a pressure sensor to detect pulse waves. Meanwhile, the stretchable, adhesive, and ion-conductive ionogel can conformally adhere to the skin for the ECG acquisition. The PTT was obtained by analyzing the time difference of ECG and pulse signals. Based on the PTT, we estimated the systolic and diastolic blood pressures, which show high correlation coefficients with those measured using a commercial sphygmomanometer. We believe this wearable dual-mode sensor provides a simple sensor design for daily, noninvasive, and continuous BP monitoring.
2. Results and discussion
2.1 Overview of the dual-mode sensor
A wearable sensor capable of both ECG and pulse sensing should possess a soft and conductive interface for electrophysiological signal acquisition and good pressure sensibility. The ionogels composed of solid supporting networks and inside ionic liquids exhibited good flexibility, electric conductivity, and biocompatibility,29,30 which is an ideal material for epidermal electrodes and widely used in electrophysiological signal acquisition.31–33 However, the pure ionogel exhibited poor pressure/strain sensibility and it's difficult to directly detect the pulse wave.34–37 It's required for complex microstructure design and device assemblies to improve the sensibility of the ionogel.38–41 Liquid metals (LMs) with both liquid flowability and metal conductivity are another kind of attractive soft conductors,42–44 which can sensitively respond to small pressures and strains, and have been widely used for the fabrication of pressure/strain sensors.44–48 However, the LM typically needs encapsulation to keep its function and avoid contaminating the human skin.49,50 We combine the ionogel and LM and allow them to benefit from each other to fabricate a highly integrated wearable dual-mode sensor for both electrophysiological and pressure sensing.To be specific, the dual-mode sensor consists of the ionogel film and an embedded LM circuit for electrophysiological and pressure sensing, respectively. The ionogel was synthesized via facile in situ photopolymerization of ternary mixtures of OEGA, MA, and ionic liquid composed of cation EMIM+ and anion DCA−, as shown in Fig. 2a. OEGA and MA constituted the entangled polymer network P(OEGA-co-MA). Since EMIM+ exhibited both high molar mass and strong ion–dipole interactions with the polymer P(OEGA-co-MA) to construct physically crosslinked networks, the conductivity of the as-prepared ionogel was mainly attributed to the mobile DCA−.
The LM circuit was fabricated using a magnetic printing method reported in our previous work,47 which is simple but effective, as shown in Fig. 2b. To be specific, we designed and fabricated the mask with a serpentine line pattern using a laser engraving machine. Then, the mask was placed on the ionogel film, and magnetic LM with 10 wt% Ni microparticles was dropped on the film. We then moved a magnet below the ionogel film to attract and spread the LM over the mask. After removing the mask, the serpentine LM circuit was obtained. Note that compared with the blade coating, this magnetic printing method can allow the intact LM circuit to adhere to the ionogel film (Fig. S1, ESI†). This is because the coating knife deformed the soft ionogel film during blade coating, which resulted in incomplete coating. To encapsulate the LM circuit, we polymerized another layer of ionogel over the LM. Consequently, the dual-mode sensor is obtained, as shown in Fig. 2c. This sensor combines with both the ionic conductivity of the ionogel and the pressure-sensing performance of the LM, enabling the simultaneous detection of both electrophysiological and pulse wave signals.
2.2 Characterization and optimization of the ionogel film
The properties of the ionogel film such as flexibility, conductivity, and adhesiveness determine the wearing comfort and ECG signal quality. To meet the needs of flexible devices, the electrode–skin interface not only requires sufficient conductivity but also needs to adapt to the modulus of human skin and have an ideal adhesive ability. Here, we optimized the ionogel properties by adjusting the content of each component. Since OEGA and MA constitute the polymer network P(OEGA-co-MA) of the main body, we changed the ratio of OEGA and MA monomers to investigate its effect on the mechanical properties of the ionogel. To be specific, we set the molar ratio of OEGA to MA at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 2
1, and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
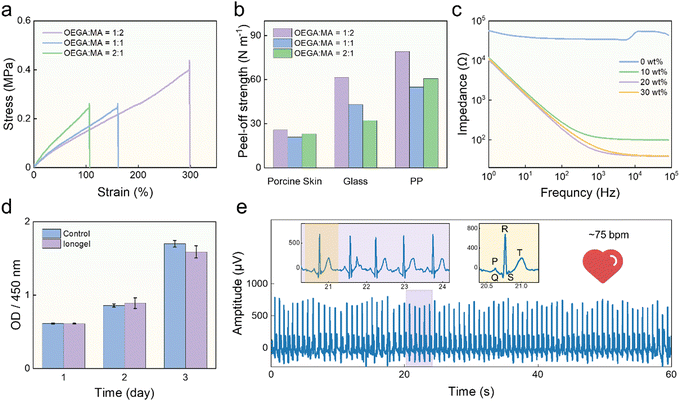
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and fixed the mass fraction of IL to 20% of the total monomer mass. With the increase of MA content, the elongation at break of the ionogel increased from 106% to 298%, as shown in Fig. 3a. The ionogel exhibited better mechanical properties at OEGA
1, and fixed the mass fraction of IL to 20% of the total monomer mass. With the increase of MA content, the elongation at break of the ionogel increased from 106% to 298%, as shown in Fig. 3a. The ionogel exhibited better mechanical properties at OEGA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MA = 1
MA = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, with a maximum tensile strength of ∼399 kPa, a toughness of ∼61 MJ m−3, and Young's modulus of ∼134 kPa. In addition, the three ionogels with different monomer ratios were also subjected to five cycles of adhesion experiment of porcine skin, glass, and polypropylene film using the standard 90° peeling method, and the averages of the results of the five cycles were used for comparison, as shown in Fig. 3b. The ionogels with the OEGA to MA ratio of 1
2, with a maximum tensile strength of ∼399 kPa, a toughness of ∼61 MJ m−3, and Young's modulus of ∼134 kPa. In addition, the three ionogels with different monomer ratios were also subjected to five cycles of adhesion experiment of porcine skin, glass, and polypropylene film using the standard 90° peeling method, and the averages of the results of the five cycles were used for comparison, as shown in Fig. 3b. The ionogels with the OEGA to MA ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 exhibited optimal adhesiveness, with the strongest peel-off strength with PP of 80 N m−1. The above experimental results suggest that the ratio of OEGA to MA is 1
2 exhibited optimal adhesiveness, with the strongest peel-off strength with PP of 80 N m−1. The above experimental results suggest that the ratio of OEGA to MA is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 is optimal, achieving better mechanical and adhesion properties. Considering the determination of the IL to the electrical conductivity of the ionogel, we changed the ionic liquid content to test its effect on the electrical properties of the ionogel with the fixed molar ratio of OEGA to MA at 1
2 is optimal, achieving better mechanical and adhesion properties. Considering the determination of the IL to the electrical conductivity of the ionogel, we changed the ionic liquid content to test its effect on the electrical properties of the ionogel with the fixed molar ratio of OEGA to MA at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. We measured the impedance of the ionogels in the wide frequency range from 1 to 105 Hz, and the impedance decreased due to the intrinsic capacitance characteristic of the ionogel.51,52 When the ionic liquid content increased from 0 wt% to 30 wt%, the electrical conductivity of the ionogel improved and reached the highest conductivity of ∼0.25 S m−1 at both 20 wt% and 30 wt% content of ionic liquid at the frequency of 105 Hz, as shown in Fig. 3c. Therefore, the ionogels with a monomer ratio of OEGA
2. We measured the impedance of the ionogels in the wide frequency range from 1 to 105 Hz, and the impedance decreased due to the intrinsic capacitance characteristic of the ionogel.51,52 When the ionic liquid content increased from 0 wt% to 30 wt%, the electrical conductivity of the ionogel improved and reached the highest conductivity of ∼0.25 S m−1 at both 20 wt% and 30 wt% content of ionic liquid at the frequency of 105 Hz, as shown in Fig. 3c. Therefore, the ionogels with a monomer ratio of OEGA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MA = 1
MA = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 20% ionic liquid content were both effective and economical, which was used in the subsequent experiments.
2 and 20% ionic liquid content were both effective and economical, which was used in the subsequent experiments.
The biocompatibility of the ionogel was also investigated by conducting an in vitro cytotoxicity assay on NIH 3T3 cells, utilizing a standard Cell Counting Kit-8 (CCK8) assay. Fig. 3d shows that the viability of the cells cocultured with the ionogel was comparable to that of the control group, indicating the good cytocompatibility of the ionogel. In addition, after wearing the ionogel for 2 hours, there was no irritation reaction, such as erythema and edema (Fig. S2, ESI†), indicating its good biocompatibility. Moreover, the dual-lead ECG signals were acquired by attaching two ionogel films to both arms of the volunteer and using a self-developed dual-channel electrophysiological acquisition device.53 The ECG signals were decomposed using wavelet transform to remove baseline drift, as shown in Fig. 3e. The peak of PQRST can be observed in ECG signals, and the amplitude of the peaks is higher than that of commercial gel electrodes, as shown in Fig. S3 (ESI†). Moreover, a low-pass filter was used to separate the noise and the effective signal to calculate the signal-to-noise ratio (SNR). The SNR of the ECG signal obtained by ionogels is 52.07 dB, which is comparable to that (50.07 dB) of the commercial gel electrodes. These results indicated that the adhesive, flexible, and conductive ionogels can serve as effective epidermal electrodes for high-quality ECG signal acquisition. Moreover, according to the design of our wearable system, we wore an ionogel–PET–ionogel sandwich film on the left wrist by touching the top ionogel by the finger of the right hand. In this condition, high-quality ECG signals were also acquired (Fig. S4, ESI†), confirming the feasibility of the ECG signal acquisition on one wrist. In addition, we conducted six hours of ECG signal acquisition using the ionogel films, all through which the signals maintained high SNRs (>50 dB), as shown in Fig. S5 (ESI†). This means that the ionogel film can serve as a long-term and effective epidermal electrode, allowing good durability and stability of the ionogel-based wearable system.
2.3 Characterization of the LM circuit
The serpentine LM circuit was encapsulated in the ionogel by the secondary polymerization of the ionogel. Due to the excellent flexibility of the LM and ionogel, the dual-mode sensor can recover to its original state after bending, stretching, and twisting, as shown in Fig. 4a. It's noted that the inside LM circuit remained intact during deformation, demonstrating that ionogels achieved good encapsulation. The LM circuit can respond to deformation by resistance change and serve as a pressure sensor. When the LM circuit is pressured and deformed, the resistance will be increased due to the decrease in the cross-section of the LM circuit. After the removal of pressure, the LM circuit and its cross-section will recover, and the resistance will recover back to the initial value, due to the good resilience of the ionogel. It's noted that the LM of the electronic conductor exhibited a high electric conductivity of 3 × 106 S m−1,54 while that of the ionogel is only ∼1 × 10−3 S m−1 at a low frequency of 1 Hz. Thus, the sensor resistance change is determined by the deformation of the LM circuit.Here, we evaluated the performance of pressure sensing of the LM circuit. To investigate the response and recovery time of the LM circuit, we applied and removed external pressure on the circuit and analyzed the resistance–time curves. We found the LM circuit exhibited different response and recovery characteristics under different pressures. Under the low pressure of 200 Pa, the resistance reached a steady-state value in a short time: 362 ms and 277 ms for response and recovery time, respectively (Fig. 4b). However, under the high pressure of 10 kPa, the response and recovery time were delayed due to the viscoelastic behavior of ionogel, which were 2.24 s and 0.72 s for 90% resistance change, respectively (Fig. S6, ESI†).55,56 Considering that the pulse is weak, the LM sensor works under low pressure during the pulse measurement. Thus, we regard 362 ms and 277 ms as the practical response and recovery time. The sensitivity of the LM circuit was evaluated in the range of 0–10 kPa at a step of 1 kPa. The resistance increased with the pressure increasing. We performed piecewise linear fitting on the response of the sensors, and the gauge factors were 0.074 kPa−1 and 0.044 kPa−1 in the range of 0–6 kPa and 6–10 kPa, respectively, as shown in Fig. 4c. In addition, to verify the stability of the pressure sensor, we tested the relative resistance response of the circuits during 500 cycles by dynamically applying a pressure of 2 kPa, as shown in Fig. 4d. The peak value is only slightly attenuated by 7% after the pressure 500 cycles, indicating the durability of the LM sensors. Moreover, we demonstrated the practical application of LM pressure sensors in pulse monitoring. The volunteer wore the flexible sensor on the left wrist and applied a load (∼15 kPa) on it with the right index finger. Meanwhile, a digital multimeter was used to collect the resistance change, as shown in Fig. 4e. The arterial pulse signals showed about 75 cycles within one minute, indicating 75 beats per minute of the heart rate (HR). Moreover, arterial pulse signals are high-quality and can show distinct three feature points: the advancing peak (P1), the reflected peak (P2), and the dicrotic wave peaks (P3). These results suggest that the LM pressure sensor has the potential for long-term and effective pulse monitoring.
2.4 Application of the dual-mode sensor for BP monitoring
As mentioned above, the dual-mode sensor we have constructed performs excellently for both ECG and pressure signal (arterial pulse wave) monitoring. Thus, non-invasive blood pressure monitoring can be achieved by simultaneously collecting these two signals and calculating the time difference. Therefore, a circuit board that matches the sensor has been designed for signal acquisition. The PCB board (4 cm in length, 3 cm in width, and a mere 0.4 mm in thickness) can be easily attached to the human wrist for signal acquisition and data transmission (Fig. 5a-i). The front and back of the PCB board are designed with round metal patches to receive ECG signals transmitted by the ionogel (Fig. S7, ESI†). Additionally, the PCB board has two metal sockets, through which copper columns can pass through the ionogel matrix to make contact with the LM circuit and then be inserted into the sockets. This allows for the application of voltage to the LM circuit and the detection of current, thereby obtaining its resistance changes. Thus, the ECG potential and resistance signals can be monitored simultaneously without interference. The main module of the wearable system is shown in Fig. 5a-ii, which consists of a dual-mode sensor composed of ionogel and LM, an analog front-end chip AD5941, a biopotential acquisition chip ADS1291, a low energy Bluetooth SoC nRF52840, and other peripherals.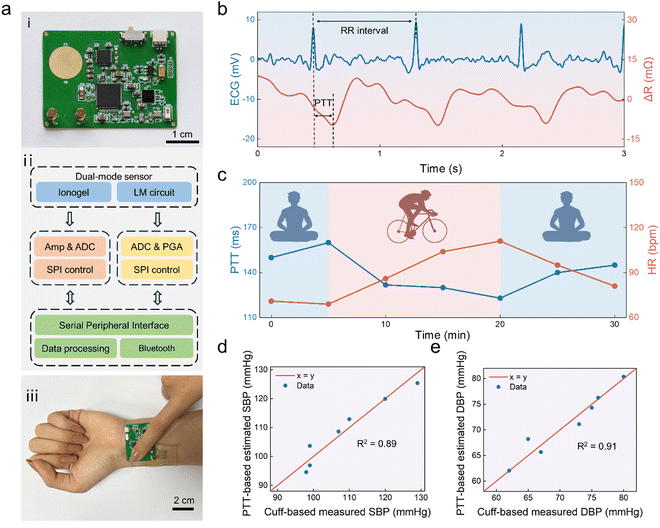 | ||
| Fig. 5 Practical application of the dual-mode sensor. (a)(i) Optical image of the PCB, (ii) simplified schematic illustration of hybrid sensors readout, (iii) Optical image of the sensor wearing on the wrist. (b) Simultaneous detection of both the ECG and pulse. (c) PTT and HR in the exercise and rest states. Results of regression analysis between measured and estimated values of the (d) SBP and (e) DBP (using eqn (1)) with extracted r-squared values. | ||
To demonstrate the practical application of this sensor for BP measurement, the wearable system was worn on the left wrist of a healthy female volunteer aged 23. The volunteer touched the top ionogel electrode with a right finger for dual-lead ECG signal acquisition, as shown in Fig. 5a-iii. Meanwhile, the volunteer can apply a load of ∼15 kPa to the top ionogel and thus the bottom dual-mode sensor for effective pulse signal acquisition. Since the load varies from person to person, the volunteers can adjust it based on their conditions. When it is necessary, the volunteer can also press the wearable system with multiple fingers. The wearable system can transmit the original pulse and ECG signals to a smartphone via Bluetooth, and real-time display was also achieved, as shown in Fig. S8 and Movie S1 (ESI†). We used the wavelet transform to remove the baseline drift of the original ECG signals and Gaussian smoothing and high-pass filtering to process pulse wave signals. The time interval between two adjacent R-peaks of the ECG signal was taken to estimate the HR, and the delayed time between the foot of the pulse wave and ECG R-peak in the same period was taken as PTT. Fig. 5b shows a typical set of simultaneous measurements of ECG and pulse wave signals from a volunteer at rest. The dual-mode sensor measured the PTT is 158 ms and HR is 71 bpm, and the latter is close to the value of 70 bpm obtained by using a commercial electronic BP monitor.
Moreover, we allowed the volunteers to exercise and rest, and data for half an hour was recorded by the wearable dual-mode sensor. Meanwhile, the commercial cuff-based BP monitor was adopted for the standard BP measurement. When using the commercial BP monitor during exercise, the volunteer was asked to stop exercising and sit. This process takes only ∼ 1 minute during which the BP and pulse are believed to be close to those of the exercising state. The volunteer started exercising as soon as the BP measurement was completed. During the exercise for 15 minutes, PTT calculated from the original signal showed a significant decrease from 150 to 123 ms and HR increased from 69 to 111 bpm, as shown in Fig. 5c. Meanwhile, the measured systolic blood pressure (SBP) and diastolic blood pressure (DBP) increased from 99 to 120 mmHg and 67 to 76 mmHg, respectively (Fig. S9, ESI†). After resting for 10 minutes, the PPT and HR decreased to 145 ms and 81 bpm, respectively. In addition, the SBP and DBP recovered to 99 and 65 mmHg, respectively. A predefined mapping model between BP and PTT values is required for the BP calculation based on PTT.23 To improve the accuracy of SBP and DBP estimation, we combined PTT and HR in the formula (eqn (1)).57,58
 | (1) |
Fig. 5d and e display the results of the linear regression analysis between measured and estimated values of SBP and DBP, along with their extracted R-squared values of 0.89 and 0.91, respectively. This illustrates the high similarity between estimated SBP/DBP values and measured values in our wearable system cases and the application of this dual-mode sensor for BP monitoring has practical feasibility. Moreover, we compared the heart rate and BP obtained by our BP wearable system to those of the commercial cuff-based electronic BP monitor, and the mean and standard deviation errors were calculated. The mean and standard deviation errors of heart rate are −2.9 bpm and 4.34 bpm, respectively (Fig. S10, ESI†). The mean and standard deviation errors of SBP are 0.0 mmHg and 2.98 mmHg, respectively, while those of DBP are 0.0 mmHg and 1.52 mmHg, respectively. These results indicated our wearable BP monitors exhibit good measurement robustness and accuracy, which are comparable to those of existing wearable BP monitoring devices.59 Moreover, our wearable BP monitors also have comprehensive advantages in simple and low-cost fabrication of sensors, good wearing experience, and integrated wireless signal transmission,21,59–61 as shown in Table S1 (ESI†).
3. Conclusions
In summary, we reported a dual-mode sensor composed of an ionogel film with an embedded LM circuit for BP monitoring. As a flexible skin interface, the ionic gel has a low Young's modulus of ∼134 kPa and good stretchability of ∼300%, as well as a good electrical conductivity of about 0.25 S m−1, resulting in a high-quality ECG signal comparable to that captured by the commercial electrodes. In addition, the LM pressure sensor showed good sensitivity and durability. Consequently, a single sensor is proposed for simultaneous acquisition of ECG and pulse waves, enabling the subsequent analysis of continuous BP measurements. The practical application of this wearable sensor in the context of daily physiological activities has been demonstrated. This integrated dual-mode sensor provides a simple device design for continuous BP monitoring.4. Experimental section
4.1 Materials
Poly(ethylene glycol) methyl ether methacrylate (OEGA) (Mn ∼ 475), methyl acrylate (MA, > 99%), and 2-hydroxy-2-methylpropiophenone (HMPP, 97%) were obtained from Aladdin (Shanghai, China). 1-Ethyl-3-methylimidazolium dicyanamide (EMIM DCA, 98%) was obtained from Macklin (Shanghai, China). Gallium (99.99%) was acquired from Dingguan Metal Technology Co., Ltd., and indium (99.99%) was sourced from Guifa Alloy Wear-Resistant Material Co., Ltd. To prepare the eutectic gallium indium (EGaIn), 75.5 g of liquid gallium and 24.5 g of indium are added to a glass beaker and then heated in an oven at 200 °C for 2 hours. Magnetic LM was obtained by adding magnetic nickel microparticles (10 wt%, mean diameter of ∼10 μm) into EGaIn via mechanical stirring in a glass mortar. NdFeB permanent magnets, measuring 12 mm in diameter and 20 mm in length with a magnetic field strength of ∼400 mT, were utilized for magnetic printing.4.2 Fabrication of the ionogel film
The monomers OEGA and MA were mixed in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio. Then 20 wt% of IL and 0.5 wt% of HMPP were added to the mixture. The mixture was vortexed for 5 min and then injected into the glass cell with a spacer thickness of 0.5 mm, followed by UV polymerization for 4 hours.
2 molar ratio. Then 20 wt% of IL and 0.5 wt% of HMPP were added to the mixture. The mixture was vortexed for 5 min and then injected into the glass cell with a spacer thickness of 0.5 mm, followed by UV polymerization for 4 hours.
4.3 Fabrication of the LM circuit
A tape mask was carved by using a laser engraving machine (CMA-4030, Han's Yueming), and then the mask was attached to the surface of the ionogel film. Next, the magnetic LM was dropped onto the release film, followed by placing the permanent NdFeB magnet under the ionogel. As the magnet moved, the magnetic LM was dispersed and adhered to the surface of the ionogel film. By removing the mask, a patterned LM circuit could be obtained.4.4 Fabrication of the dual-mode sensor
A spacer with a thickness of 0.5 mm was added to the ionogel single layer patterned with the LM circuit, and a glass slide was placed on the top. Then, the precursor was injected into the cell, followed by UV polymerization for 4 hours.4.5 Fabrication and design of PCB
The communication and processing in the final 4-layer Bluetooth-enabled PCB were facilitated by using a Nordic Semiconductor nRF52840 BLE System-on-chip. Bio-potential measurements, specifically the recording of ECG signals from the ionogels, were carried out using an ADS1291 AFE chip. This chip was then programmed through an SPI interface that was controlled by the nRF52840. Additionally, an AD5941 AFE chip was employed to determine the resistance changes of LM circuits, and similarly, it was programmed through an SPI interface driven by the nRF52840. Data from each sensor were gathered using the nRF52840 and subsequently transmitted to a Bluetooth 5.4-enabled receiver. Subsequently, a graphical interface was developed using Java to showcase the measurement results on an Android phone application. Noteworthy components utilized in this setup included a Johanson Technology 2.45 GHz chip antenna (2450AT18B100E) for wireless transmission, as well as a lithium battery (3.7 V) as the power source, regulated for the electronics via a MIC5219 boost converter.4.6 Biocompatibility test
The proliferation of MC3T3-E1 cells was assessed over 3 days under different culture conditions, both with and without the ionogel. Cytocompatibility was determined by conducting the CCK8 assay with NIH 3T3 cells. The cells were grown in high glucose Dulbecco's modified Eagle's medium (high glucose DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% streptomycin/penicillin. The ionogels were trimmed to dimensions of 0.1 cm × 0.1 cm × 0.2 cm (length, width, height), washed three times with sterilized phosphate-buffered saline (PBS), sterilized under UV light overnight, and then placed in 96-well plates. Subsequently, a suspension of NIH 3T3 cells (2 × 104 cells per mL) was co-cultured with the hydrogels and maintained at 37 °C in a 5% CO2 environment. A control group without hydrogels was also included. Cell proliferation was assessed after 1, 2, and 3 days of culture using the Cell Counting Kit-8 (CCK-8, Beyotime Biotechnology, Shanghai). At each time point, the culture medium was replaced with 100 μL of medium containing a 10% CCK-8 working concentration, followed by a 2-hour incubation at 37 °C. The absorbance at 450 nm (n = 6) was measured using a microplate reader (SYNERGY HTX) to determine the optical density (OD) value.4.7 Characterization
The adhesion performance of the ionogel was tested by using a standard 90° peeling test using an ergometer (MARK-10). The tensile properties were measured by using a tensile machine (UTM-2502, SUNS). The gauge length of the specimens was 15 mm and the loading rate was 20 mm min−1. The electrical property of the ionogel was measured by AC impedance function at an electrochemical workstation (CHI 660E) with a frequency range of 1–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Hz. The individual electrophysiological signal acquisition was using the self-developed dual-channel electrophysiological acquisition device based on the ADS1299EEGFE-PDK of the ADS1299 (Texas Instruments). Pressure blood and pulse were measured using a commercial and medical-grade blood pressure monitoring device (Yuwell, YE660D). The resistance measurements were conducted using a digital multimeter (DMM6500, Keithley).
000 Hz. The individual electrophysiological signal acquisition was using the self-developed dual-channel electrophysiological acquisition device based on the ADS1299EEGFE-PDK of the ADS1299 (Texas Instruments). Pressure blood and pulse were measured using a commercial and medical-grade blood pressure monitoring device (Yuwell, YE660D). The resistance measurements were conducted using a digital multimeter (DMM6500, Keithley).
4.8 On-body test
Before wearing the dual-mode sensor, the wrist was cleaned with 75% alcohol and deionized water, followed by removing the water using absorbent cotton. All experiments were conducted in strict compliance with the relevant laws and with the approval of the Scientific Ethical Committee of Southeast University. Informed consent was obtained from the volunteers for all the experiments involving human participants.Author contributions
Nan Jiang: conceptualization, methodology, visualization, data curation, and original draft writing. Gangsheng Chen: conceptualization, methodology, visualization, original draft writing. Fan Zhou: investigation. Chao Zhao: supervision. Biao Ma: supervision, writing review, and editing. Hong Liu: supervision, writing review, and editing. All authors have given approval to the final version of the manuscript.Data availability
Data available on request from the authors.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
This work was supported by the Natural Science Foundation of Jiangsu Province (BK20220859), the Key Research and Development Program of Jiangsu Province (BE2021700), the Science and Technology Development Program of Suzhou (SYG202117), the Jiangsu Funding Program for Excellent Postdoctoral Talent, the China Postdoctoral Science Foundation (2022M710667), the SEU Innovation Capability Enhancement Plan for Doctoral Students (CXJH_SEU 24144), and the Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX24_0473).References
- E. G. Nabel, N. Engl. J. Med., 2003, 349, 60–72 CrossRef CAS PubMed
.
- The Global Cardiovascular Risk Consortium, N. Engl. J. Med., 2023, 389, 1273–1285 CrossRef PubMed
.
- S. Mahajan, J. Gu, C. Caraballo, Y. Lu, E. S. Spatz, H. Zhao, M. Zhang, N. Sun, X. Zheng, H. Lu, H. Yuan, Z. J. Ma and H. M. Krumholz, J. Am. Geriatr. Soc., 2020, 68, 1520–1528 CrossRef PubMed
.
- S. Akselrod, D. Gordon, J. B. Madwed, N. C. Snidman, D. C. Shannon and R. J. Cohen, Am. J. Physiol. Heart Circ. Physiol., 1985, 249, H867–H875 CrossRef CAS PubMed
.
- S. N. Ahmed, F. M. Syed and D. T. Porembka, Crit. Care Med., 2007, 35, 323–329 CrossRef
.
- C. Peng, M. Chen, H. K. Sim, Y. Zhu and X. Jiang, IEEE Sens. J., 2021, 21, 2642–2650 CAS
.
- B. Ibrahim and R. Jafari, Sci. Rep., 2022, 12, 319 CrossRef CAS PubMed
.
- L. H. Peterson, R. D. Dripps and G. C. Risman, Am. Heart J., 1949, 37, 771–782 CrossRef CAS PubMed
.
- E. Chung, G. Chen, B. Alexander and M. Cannesson, Front. Med., 2013, 7, 91–101 CrossRef PubMed
.
- J. A. Pandit, E. Lores and D. Batlle, Clin. J Am. Soc. Nephrol., 2020, 15, 1531–1538 CrossRef PubMed
.
- N. Luo, W. Dai, C. Li, Z. Zhou, L. Lu, C. C. Y. Poon, S.-C. Chen, Y. Zhang and N. Zhao, Adv. Funct. Mater., 2016, 26, 1178–1187 CrossRef CAS
.
- R. Mukkamala, M. Yavarimanesh, K. Natarajan, J.-O. Hahn, K. G. Kyriakoulis, A. P. Avolio and G. S. Stergiou, Hypertension, 2021, 78, 1161–1167 CrossRef CAS
.
- Z. Yi, Z. Liu, W. Li, T. Ruan, X. Chen, J. Liu, B. Yang and W. Zhang, Adv. Mater., 2022, 34, 2110291 CrossRef CAS PubMed
.
- M. Hosanee, G. Chan, K. Welykholowa, R. Cooper, P. A. Kyriacou, D. Zheng, J. Allen, D. Abbott, C. Menon, N. H. Lovell, N. Howard, W.-S. Chan, K. Lim, R. Fletcher, R. Ward and M. Elgendi, J. Clin. Med., 2020, 9, 923 CrossRef
.
- A. Stojanova, S. Koceski and N. Koceska, J. Med. Syst., 2019, 43, 24 CrossRef PubMed
.
- E. Song, Z. Xie, W. Bai, H. Luan, B. Ji, X. Ning, Y. Xia, J. M. Baek, Y. Lee, R. Avila, H.-Y. Chen, J.-H. Kim, S. Madhvapathy, K. Yao, D. Li, J. Zhou, M. Han, S. M. Won, X. Zhang, D. J. Myers, Y. Mei, X. Guo, S. Xu, J.-K. Chang, X. Yu, Y. Huang and J. A. Rogers, Nat. Biomed. Eng., 2021, 5, 759–771 CrossRef CAS PubMed
.
- X. Yu, Z. Xie, Y. Yu, J. Lee, A. Vazquez-Guardado, H. Luan, J. Ruban, X. Ning, A. Akhtar, D. Li, B. Ji, Y. Liu, R. Sun, J. Cao, Q. Huo, Y. Zhong, C. Lee, S. Kim, P. Gutruf, C. Zhang, Y. Xue, Q. Guo, A. Chempakasseril, P. Tian, W. Lu, J. Jeong, Y. Yu, J. Cornman, C. Tan, B. Kim, K. Lee, X. Feng, Y. Huang and J. A. Rogers, Nature, 2019, 575, 473–479 CrossRef CAS PubMed
.
- H. Li, Y. Ma, Z. Liang, Z. Wang, Y. Cao, Y. Xu, H. Zhou, B. Lu, Y. Chen, Z. Han, S. Cai and X. Feng, Natl. Sci. Rev., 2020, 7, 849–862 CrossRef
.
- M. Elgendi, R. Fletcher, Y. Liang, N. Howard, N. H. Lovell, D. Abbott, K. Lim and R. Ward, npj Digit. Med., 2019, 2, 60 CrossRef PubMed
.
- C. Liu, J.-T. Kim, S. S. Kwak, A. Hourlier-Fargette, R. Avila, J. Vogl, A. Tzavelis, H. U. Chung, J. Y. Lee, D. H. Kim, D. Ryu, K. B. Fields, J. L. Ciatti, S. Li, M. Irie, A. Bradley, A. Shukla, J. Chavez, E. C. Dunne, S. S. Kim, J. Kim, J. B. Park, H. H. Jo, J. Kim, M. C. Johnson, J. W. Kwak, S. R. Madhvapathy, S. Xu, C. M. Rand, L. E. Marsillio, S. J. Hong, Y. Huang, D. E. Weese-Mayer and J. A. Rogers, Adv. Healthcare Mater., 2021, 10, 2100383 CrossRef CAS PubMed
.
- S. Min, D. H. Kim, D. J. Joe, B. W. Kim, Y. H. Jung, J. H. Lee, B.-Y. Lee, I. Doh, J. An, Y.-N. Youn, B. Joung, C. D. Yoo, H.-S. Ahn and K. J. Lee, Adv. Mater., 2023, 35, 2301627 CrossRef CAS PubMed
.
- S. Hoshide, A. Yoshihisa, F. Tsuchida, H. Mizuno, H. Teragawa, T. Kasai, H. Koito, S. Ando, Y. Watanabe, Y. Takeishi and K. Kario, Hypertens. Res., 2022, 45, 1001–1007 CrossRef PubMed
.
- X.-R. Ding, N. Zhao, G.-Z. Yang, R. I. Pettigrew, B. Lo, F. Miao, Y. Li, J. Liu and Y.-T. Zhang, IEEE J. Biomed. Health Inform., 2016, 20, 1455–1465 Search PubMed
.
- I. Sharifi, S. Goudarzi and M. B. Khodabakhshi, Artif. Intell. Med., 2019, 97, 143–151 CrossRef PubMed
.
- J. Ji, M. Dong, Q. Lin and K. C. Tan, IEEE Trans. Cybern., 2023, 53, 4162–4174 Search PubMed
.
- Z.-B. Zhou, T.-R. Cui, D. Li, J.-M. Jian, Z. Li, S.-R. Ji, X. Li, J.-D. Xu, H.-F. Liu, Y. Yang and T.-L. Ren, Materials, 2023, 16, 2133 CrossRef CAS PubMed
.
- J. Choi, Y. Kang, J. Park, Y. Joung and C. Koo, Sensors, 2023, 23, 1684 CrossRef
.
- F. Miao, Z.-D. Liu, J.-K. Liu, B. Wen, Q.-Y. He and Y. Li, IEEE J. Biomed. Health Inform., 2020, 24, 79–91 Search PubMed
.
- C.-C. Yan, W. Li, Z. Liu, S. Zheng, Y. Hu, Y. Zhou, J. Guo, X. Ou, Q. Li, J. Yu, L. Li, M. Yang, Q. Liu and F. Yan, Adv. Funct. Mater., 2024, 34, 2314408 CrossRef CAS
.
- W. Zhao, Z. Lei and P. Wu, Adv. Sci., 2023, 10, 2300253 CrossRef CAS
.
- Z. Yu and P. Wu, Adv. Funct. Mater., 2021, 31, 2107226 CrossRef CAS
.
- K. Le, X. Sun, J. Chen, J. V. John, A. Servati, H. Heidari, A. Khademhosseini, F. Ko, F. Jiang and P. Servati, Chem. Eng. J., 2023, 471, 144675 CrossRef CAS
.
- T. Chen, G. Ye, H. Wu, S. Qi, G. Ma, Y. Zhang, Y. Zhao, J. Zhu, X. Gu and N. Liu, Adv. Funct. Mater., 2022, 32, 2206424 CrossRef CAS
.
- L. Wang, W. Xia, Y. Yu, S. Liu, Y. Peng, Z. Wu and H. Chen, J. Mater. Chem. C, 2023, 11, 6627–6634 RSC
.
- N. Jiang, X. Chang, D. Hu, L. Chen, Y. Wang, J. Chen and Y. Zhu, Chem. Eng. J., 2021, 424, 130418 CrossRef CAS
.
- A. K. Padhan, D. Sharma, T. S. Thomas, A. P. Sinha, A. N. Mallick and D. Mandal, J. Mater. Chem. A, 2024, 12, 9508–9517 RSC
.
- H. Ren, X. He, Y. Long, Q. Li, S. Li and X. Zhou, J. Mater. Chem. C, 2024, 12, 4737–4750 RSC
.
- M. Zhang, M. Gu, L. Shao, G. Cheng, H. Gao, B. Sun, S. Li, T. Tang, N. Li, Y. Yi, D. Wei, C. Yang and D. Wei, ACS Appl. Mater. Interfaces, 2023, 15, 15884–15892 CrossRef CAS
.
- Y. Huang, L. Zhao, M. Cai, J. Zhu, L. Wang, X. Chen, Y. Zeng, L. Zhang, J. Shi and C. F. Guo, Adv. Healthcare Mater., 2023, 12, 2301838 CrossRef CAS PubMed
.
- J. H. Kwon, Y. M. Kim and H. C. Moon, ACS Nano, 2021, 15, 15132–15141 CrossRef CAS
.
- Y. Guo, F. Yin, Y. Li, G. Shen and J.-C. Lee, Adv. Mater., 2023, 35, 2300855 CrossRef CAS
.
- T. Daeneke, K. Khoshmanesh, N. Mahmood, I. A. de Castro, D. Esrafilzadeh, S. J. Barrow, M. D. Dickey and K. Kalantar-zadeh, Chem. Soc. Rev., 2018, 47, 4073–4111 RSC
.
- N. Li, X. Yuan, Y. Li, G. Zhang, Q. Yang, Y. Zhou, M. Guo and J. Liu, Adv. Mater., 2024, 2404330 CrossRef CAS PubMed
.
- S. Chen, Z. Cui, H. Wang, X. Wang and J. Liu, Appl. Phys. Rev., 2023, 10, 021308 CAS
.
- M. D. Dickey, Adv. Mater., 2017, 29, 1606425 CrossRef
.
- Y. Luo, H. Fan, X. Lai, Z. Zeng, X. Lan, P. Lin, L. Tang, W. Wang, Y. Chen and Y. Tang, Biosens. Bioelectron., 2024, 246, 115905 CrossRef CAS PubMed
.
- B. Ma, C. Xu, J. Chi, J. Chen, C. Zhao and H. Liu, Adv. Funct. Mater., 2019, 29, 1901370 CrossRef
.
- H. Yu, L. Yu, X. Qi, J. Cui, K. Wu, Y. Liu, L. Chen and X. Li, J. Mater. Chem. C, 2023, 11, 10455–10463 RSC
.
- V. Timosina, T. Cole, H. Lu, J. Shu, X. Zhou, C. Zhang, J. Guo, O. Kavehei and S.-Y. Tang, Biosens. Bioelectron., 2023, 235, 115414 CrossRef CAS PubMed
.
- R. Tang, C. Zhang, B. Liu, C. Jiang, L. Wang, X. Zhang, Q. Huang, J. Liu and L. Li, Biosens. Bioelectron., 2022, 216, 114600 CrossRef CAS PubMed
.
- Y. M. Chi, T.-P. Jung and G. Cauwenberghs, IEEE Rev. Biomed. Eng., 2010, 3, 106–119 Search PubMed
.
- S. Yao and Y. Zhu, JOM, 2016, 68, 1145–1155 CrossRef
.
- Y. Wang, W. Tian, J. Xu, Y. Tian, C. Xu, B. Ma, Q. Hao, C. Zhao and H. Liu, IEEE Sens. J., 2023, 23, 21767–21775 Search PubMed
.
- J. Ma, F. Krisnadi, M. H. Vong, M. Kong, O. M. Awartani and M. D. Dickey, Adv. Mater., 2023, 35, 2205196 CrossRef CAS
.
- H. Souri, H. Banerjee, A. Jusufi, N. Radacsi, A. A. Stokes, I. Park, M. Sitti and M. Amjadi, Adv. Intell. Syst., 2020, 2, 2000039 CrossRef
.
- M. Amjadi, K.-U. Kyung, I. Park and M. Sitti, Adv. Funct. Mater., 2016, 26, 1678–1698 CrossRef CAS
.
- F. Heydari, M. P. Ebrahim, J.-M. Redoute, K. Joe, K. Walker and M. Rasit Yuce, Inform. Fusion, 2020, 54, 119–127 CrossRef
.
- D. Buxi, J.-M. Redouté and M. R. Yuce, Physiol. Meas., 2015, 36, R1 CrossRef PubMed
.
- J. Li, H. Jia, J. Zhou, X. Huang, L. Xu, S. Jia, Z. Gao, K. Yao, D. Li, B. Zhang, Y. Liu, Y. Huang, Y. Hu, G. Zhao, Z. Xu, J. Li, C. K. Yiu, Y. Gao, M. Wu, Y. Jiao, Q. Zhang, X. Tai, R. H. Chan, Y. Zhang, X. Ma and X. Yu, Nat. Commun., 2023, 14, 5009 CrossRef CAS PubMed
.
- S. Yang, Y. Zhang, S.-Y. Cho, R. Correia and S. P. Morgan, Opt. Quantum Electron., 2021, 53, 93 CrossRef
.
- D. Kireev, K. Sel, B. Ibrahim, N. Kumar, A. Akbari, R. Jafari and D. Akinwande, Nat. Nanotechnol., 2022, 17, 864–870 CrossRef CAS
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4tc02494j |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |