Quaternary CsPbX3 (X = Cl1−xBrx, Br1−xIx) alloy microplates synthesized by single-step chemical vapor deposition and their two-photon absorption (TPA) properties†
Mohammad Kamal
Hossain
 ab,
Wayesh
Qarony
de,
Ying
Wang
a,
Cheuk Kai Gary
Kwok
ab,
Wayesh
Qarony
de,
Ying
Wang
a,
Cheuk Kai Gary
Kwok
 a,
Kingsley O.
Egbo
a,
Yuen Hong
Tsang
a,
Kingsley O.
Egbo
a,
Yuen Hong
Tsang
 e,
Johnny C.
Ho
e,
Johnny C.
Ho
 c and
Kin Man
Yu
c and
Kin Man
Yu
 *ac
*ac
aDepartment of Physics, City University of Hong Kong, Kowloon, Hong Kong. E-mail: kinmanyu@cityu.edu.hk
bDepartment of Physics, Comilla University, Kotbari, Cumilla 3506, Bangladesh
cDepartment of Materials Science and Engineering, City University of Hong Kong, Kowloon, Hong Kong
dDepartment of Electrical Engineering and Computer Sciences, University of California, Berkeley, CA 94720, USA
eDepartment of Applied Physics, The Hong Kong Polytechnic University, Kowloon, Hong Kong
First published on 4th January 2024
Abstract
Because of their well-defined light–matter interaction volume, high-quality single-crystalline nature, and precise bandgap tunability, all-inorganic cesium lead halide (CsPbX3 (X = Cl, Br, I)) perovskite (IHP) microplates are of fundamental and technological interest today. Here, we report the synthesis and properties of ternary CsPbX3 (X = Cl, Br, I) and quaternary CsPbX3 (X = Cl1−xBrx, Br1−xIx) alloy microplates grown by the single-step chemical vapor deposition (CVD) process. Smooth-faceted, single crystalline IHP alloy microplates with good vertical and lateral composition uniformity are achieved. These microplates exhibit strong photoluminescence and are thermally stable with no observable photodegradation. More importantly, two-photon absorption (TPA) is demonstrated in quaternary CsPb(Cl1−xBrx)3 and CsPb(Br1−xIx)3 IHP alloy microplates, which adds a new dimension to the functionalities of IHP microplates for nonlinear optical (NLO) applications. We believe that this work will open up multiple avenues for the development of microstructure compatible integrated optoelectronic and photonic applications and will certainly enhance the fundamental NLO research.
1 Introduction
Organometallic halide perovskites (OHPs) have been in the research spotlight due to their unique optoelectronic properties, including high charge carrier mobility (∼50 cm2 V−1 s−1),1 long electron–hole diffusion length (>1 μm),2 strong optical absorption,3 and direct bandgap.4 Most notably, great progress has been made in OHP solar cells in just over a decade, and a very high-power conversion efficiency (PCE) of η ≈ 26.1% has been achieved.5 However, the advancement of OHP devices is strongly hampered by several factors, such as the poor thermal stability and hygroscopic nature of the organic cation group6–9 and the formation of photoinduced trap states and ion migration in OHPs.9,10 Unlike OHPs, all-inorganic cesium lead halide perovskites (IHPs) have shown excellent open air and thermal stabilities.11–15 Moreover, similar to the OHPs, IHPs exhibit narrow-intense optical emissions (∼tens of milli-electron volts),16 high photoluminescence quantum yields (PLQY ∼90%),17 and low exciton binding energies (∼tens of milli-electron volts)18 and thus have attracted much recent attention as alternatives to OHP materials. Although solar cells fabricated using IHPs have not attained as high an efficiency as OHP cells, a PCE (η) of ∼18% has been reported.19 However, most reported studies on IHPs were based on materials synthesized by solution processes and hence the quality of materials was relatively poor. Furthermore, the ternary IHPs, CsPbCl3, CsPbBr3, and CsPbI3, have distinctive band gap emissions at ∼413 nm, ∼529 nm, and ∼715 nm, respectively. These discrete bandgaps and the lack of continuously tunable bandgap values limit their device applications.20,21It was also reported that the pure ternary CsPbX3 (X = Cl, Br, I) IHPs were vulnerable to crystalline phase stability issues.20,22,23 At high temperature (e.g., ≥47 °C for CsPbCl3; ≥130 °C for CsPbBr3; and ≥305 °C for CsPbI3), the stable phase of all IHPs is found to be cubic.20 This cubic phase usually transforms into the room temperature phase. For instance, bulk CsPbI3 has a cubic stable phase also called the black phase at ≥305 °C. As the material is cooled down gradually to room temperature, the black cubic phase of CsPbI3 transforms into an orthorhombic yellow phase.9,20,22,24 The yellow phase may also be termed as the insulating, non-perovskite or δ-phase.25 This transformation not only changes the crystal structure, but also degrades the optical properties of this ternary CsPbI3 perovskite. The black phase of CsPbI3 exhibits a smaller bandgap (Eg) compared to the yellow phase, and therefore they vary in their application practicality.9 Controlled substitution of halide anions in ternary CsPbX3 (X = Cl, Br, I), forming quaternary IHP alloys (mixed halide perovskites, e.g., CsPb(Cl1−xBrx)3), is an effective way to continuously tune the bandgaps of IHPs from ∼1.7 (CsPbI3) to 2.9 eV (CsPbCl3).11,21 Halide anion alloying of the ternary halides not only allows the tuning of the bandgap and thus the emission wavelength, but also enhances the stability of the mixed halides, namely quaternary CsPb(Cl1−xBrx)3 and CsPb(Br1−xIx)3 alloys.11–13,26 However, composition tuning of quaternary IHPs requires precise control of multiple growth parameters and hence can be a challenging task.27,28 For instance, in solution processing, the growth and composition tuning of IHPs were impeded by the poor solubility of metal halides.12 Also, in most cases, solution processing produces polycrystalline halide perovskite materials with a high density of defects.29 On the contrary, chemical vapor deposition (CVD) is a user-friendly materials synthesis technique, which produces high quality nano/micro-structured semiconductor materials.23,28 Also, good control over the growth direction, periodic twinning, crystal structure, etc. in CVD synthesis facilitates the growth of high-quality materials with well-defined morphologies.28,30 Moreover, high temperature and vacuum processed single crystalline materials obtained using the CVD growth technique exhibit improved charge transport properties with reduced trap density.31
The intriguing optoelectronic properties of IHPs are strongly influenced by the morphology and dimensionality of the materials.32,33 Investigation of a variety of structures, including microplatelets,23 microwires,34 nanoplatelets,35 nanorods,36 nanosheets,37 nanowires38 and colloidal nanocrystals,39 of different IHPs has been reported. Owing to their geometry specified properties and application practicality, these IHP structures have found potential applications in a wide range of devices.32,40 Among them, IHP microplates, because of their strong influence on local carrier lifetime and electron–hole interactions, are promising for efficient optoelectronic applications,41,42 and therefore, have attracted considerable research interest recently.
In addition to their distinctive optoelectronic features, the larger light–matter interaction volume along with tunable emission wavelengths made quaternary IHP alloy microplates ideal candidates for nonlinear optical (NLO) applications.29,43–45 Two photon or multiphoton absorption (TPA/MPA) is a non-linear optical phenomenon that involves the simultaneous absorption of two or a greater number of NIR photons with sub-bandgap energy followed by the subsequent non-radiative and radiative recombination and therefore the emission of a photon in the visible range. Since the process involves below bandgap excitation, photons penetrate deeper into the sample and the excitation occurs more in the bulk, which increases the gain length.44 Moreover, the deeper photon penetration results in reduced surface photo-absorption and scattering losses46 and thus less photodegradation.44 Therefore, materials having TPA/MPA properties have great potential for applications in light modulation,46 THz generation and detection,47 optical data storage,43 multiphoton microscopy,45 biomedical imaging and photodynamic therapy.48 Nonlinear optical (NLO) properties, like TPA and MPA, have been widely reported for organic and polymeric materials, while they have been less explored for IHPs.43 However, compared to well-explored organic NLO materials, a 5–7-fold higher MPA magnitude was reported for IHPs.43,49 So far, there have been several reports on IHP based TPA/MPA materials,29,43,49–51 including TPA in CsPbBr3 single crystals29 and MPA in (Cs, MA)PbBr3 microcrystals,50 CsPbX3 quantum dots43 and Cs4PbBr6 film.51 Very recently, enhancement of NLO properties has also been reported for halide anion engineered IHPs.43 However, from fundamental and technological viewpoints, microstructures, especially microplates, because of their well-defined morphology, high surface area to volume ratio, and larger light–matter interaction volume, are promising for TPA/MPA NLO applications.29,44,45,50 High quality mixed halide quaternary IHP alloy microplates grown by CVD could, therefore, be ideal TPA/MPA materials, which might further enhance their NLO applications. Until now, reports on the TPA and MPA properties of halide perovskite microplates were mostly restricted to ternary (Cs, MA)PbBr3 microplates,50,52,53 and no study on the TPA and MPA properties of composition tuned quaternary CsPb(Cl1−xBrx)3 and CsPb(Br1−xIx)3 alloy microplates was reported.
Here we report the structural and optical properties of high quality single crystalline all-inorganic cesium lead halide perovskite (IHP) ternary (CsPbX3 (X = Cl, Br, I)) and quaternary (CsPbX3 (X = Cl1−xBrx, Br1−xIx)) alloy microplates synthesized by a single-step CVD process. Composition tuned smooth-faceted halide perovskite alloy microplates show intense and narrow optical emissions covering the visible spectral range of 413–715 nm. In addition, non-linear optical (NLO) properties, for example, two-photon absorption (TPA), are also observed in both quaternary CsPb(Cl1−xBrx)3 and CsPb(Br1−xIx)3 alloy microplates. Our results demonstrate the viability of the quaternary IHP alloy microplates for use as frequency upconversion photonic materials, thus extending their application prospects to light manipulation and detection.
2 Results and discussion
Previously we reported whispering gallery mode (WGM) lasing and demonstrated the on-chip white light emission of CVD grown ternary CsPbX3 (X = Cl, Br and I) perovskite alloy microplates.23 Here, in addition to the ternary CsPbX3 microplates, their mixed-anion halide quaternary extensions, CsPb(Cl1−xBrx)3 and CsPb(Br1−xIx)3, are also synthesized by the single step CVD route. A detailed discussion on the CVD growth can be found in the ESI† or elsewhere in the literature.4,20,23,28,34,35,54 The properties and prospects of the quaternary halide perovskite alloy microplates are presented in the following subsections.2.1 Structural and elemental analyses
The morphologies of the ternary CsPbX3 (X = Cl, Br, I) and quaternary CsPbX3 (X = Cl1−xBrx, Br1−xIx) alloy microplates were initially investigated by optical and scanning electron microscopy (SEM). Well-defined square like microplates were grown on both c-Al2O3 (sapphire) and SiO2 coated Si substrates (SiO2/Si). Sharp-edged square microplates of IHP alloys with smooth surfaces observed by both low- and high-resolution SEM imaging suggest that they have good crystalline quality.20,27 SEM images of typical CsPb(Cl1−xBrx)3, CsPb(Br1−xIx)3 and ternary CsPbX3 (X = Cl, Br, I) microplates are shown in Fig. 1(b), (c), and in Fig. S2 (ESI†), respectively. The lateral size of CsPb(Cl1−xBrx)3 alloy microplates varies between ∼5 and 30 μm, with a majority of these structures being <10 μm in lateral dimensions. In comparison, although CsPb(Br1−xIx)3 alloy microplates shown in Fig. 1(c) appear to be very similar to CsPb(Cl1−xBrx)3, they have a more uniform lateral dimension of ∼10–20 μm. Despite lateral size variations, the thickness of both types of alloy microplates lies in the range of ∼300–500 nm. We note that the lateral dimensions and the vertical height profiles of the alloy microplates can be synergistically controlled by tuning the deposition time, precursor quantity in the source materials, and the carrier gas flow rate. Atomic force microscopy (AFM) images showing the smooth surfaces and well-defined morphologies of typical CsPb(Br1−xIx)3 and CsPb(Cl1−xBrx)3 alloy microplates are shown in Fig. 1(d) and Fig. S3 (in the ESI†), respectively. The corresponding thickness profiles are presented respectively in Fig. 1(e) and in Fig. S3(b) (ESI†).X-ray diffraction (XRD) studies confirm that the CsPbCl3, CsPbBr3 and CsPbI3 microplates have, respectively, tetragonal, monoclinic, and orthorhombic crystalline structures. The same crystal structures of pure ternary halides have been reported in the literature.20 It is known that at high temperature (e.g., ≥47 °C for CsPbCl3; ≥130 °C for CsPbBr3; and ≥305 °C for CsPbI3) the stable phase of all IHPs is cubic. Since all CVD growth processes were carried out at a high temperature of >450 °C, the IHP microplates were first grown in the cubic phase and when they were cooled down to room temperature, they underwent phase transitions to their respective stable room temperature phases.20,27 XRD patterns of the ternary and quaternary alloy microplate structures are shown in Fig. 1(f). Notice that the (100) diffraction peaks from the quaternary alloys are shifted to diffraction angles between their respective ternary IHPs, indicating the formation of random alloys.
From Fig. 1(f), the lattice parameter of quaternary alloy microplates can be obtained from the shift of the (100) diffraction peak. Hence, the alloy composition ‘x’ in CsPb(Cl1−xBrx)3 and CsPb(Br1−xIx)3 microplates can be calculated based on the XRD patterns, assuming the virtual crystal approximation (Vegard's law) for their lattice parameters. Elemental composition of the quaternary IHP alloy microplates was also studied by energy dispersive X-ray spectroscopy (EDS) in combination with SEM for both CsPb(Cl1−xBrx)3 and CsPb(Br1−xIx)3 alloys. EDS data obtained for various square microplates of a particular sample and for different positions on each square microplate show consistent results, confirming the compositional uniformity of the quaternary halide alloy microplates. EDS analysis and the elemental maps of the representative quaternary CsPb(Cl1−xBrx)3 and CsPb(Br1−xIx)3 IHP alloy microplates are shown in Fig. 2. The atomic ratios of constituting elements in the respective alloys are given in the corresponding EDS spectra shown in Fig. 2(a) and (b). EDS results show that the atomic ratios of the two alloys are Cs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pb:(Cl
Pb:(Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Br) = 18.7
Br) = 18.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16.1: (31.4
16.1: (31.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33.8) and Cs
33.8) and Cs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pb:(Br
Pb:(Br![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) I) = 19.8
I) = 19.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17.3: (52.2
17.3: (52.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10.6), which are in general agreement with the halide perovskite formulation, ABX3, and x values of CsPb(Cl1−xBrx)3 and CsPb(Br1−xIx)3 are estimated to be 0.60 and 0.17, respectively. The corresponding x values obtained from the XRD calculations are respectively 0.70 and 0.11, which are slightly different from values obtained by EDS analysis. This discrepancy may be attributed to the surface sensitivity of EDS. It should also be noted that x obtained by XRD corresponds to substitutional atoms responsible for changing the lattice parameter, while that by EDS represents atoms present but not necessarily in the lattice. The elemental maps of typical quaternary CsPb(Cl1−xBrx)3 and CsPb(Br1−xIx)3 alloy microplates shown in Fig. 2(a) and (b) reveal the even distribution of the constituting elements with no obvious clustering throughout the microplates. In the following, alloy composition x obtained by XRD is used.
10.6), which are in general agreement with the halide perovskite formulation, ABX3, and x values of CsPb(Cl1−xBrx)3 and CsPb(Br1−xIx)3 are estimated to be 0.60 and 0.17, respectively. The corresponding x values obtained from the XRD calculations are respectively 0.70 and 0.11, which are slightly different from values obtained by EDS analysis. This discrepancy may be attributed to the surface sensitivity of EDS. It should also be noted that x obtained by XRD corresponds to substitutional atoms responsible for changing the lattice parameter, while that by EDS represents atoms present but not necessarily in the lattice. The elemental maps of typical quaternary CsPb(Cl1−xBrx)3 and CsPb(Br1−xIx)3 alloy microplates shown in Fig. 2(a) and (b) reveal the even distribution of the constituting elements with no obvious clustering throughout the microplates. In the following, alloy composition x obtained by XRD is used.
2.2 Composition tuning of quaternary IHP alloy microplates
Since the ternary CsPbX3 (X = Cl, Br, I) IHPs have bandgap values of 3.0 eV (X = Cl), 2.3 eV (X = Br) and 1.7 eV (X = I), anion substitution of these materials is expected to produce materials with tunable bandgaps and hence tunable emission peaks covering the entire visible spectrum from 1.7 to 3.0 eV (or λ from 715 to 413 nm). Both quaternary CsPb(Cl1−xBrx)3 and CsPb(Br1−xIx)3 IHPs with halide anion content, x, ranging from 0 to 1 were grown by the one step CVD process. Steady state PL measurements were performed on the quaternary IHP alloys using a home-built PL system. Fig. 3(a) shows the continuous shifting of the PL peaks with the change in the alloy composition, x = Br and I, of CsPb(Cl1−xBrx)3 and CsPb(Br1−xIx)3 alloys, respectively. Corresponding true color photographs covering violet (∼3.0 eV; 413 nm) to green (∼2.3 eV; 529 nm) to red (∼1.7 eV; 715 nm) emissions are also shown in the inset of Fig. 3(a). The violet, green and red luminescence colors correspond to emissions from the pure ternary halide compounds CsPbCl3, CsPbBr3, and CsPbI3, respectively.20,23 The emission peak width (FWHM) values of the alloy microplates are in the range from 85–150 meV, comparable to or even better than the values of those obtained for IHP nano-microstructures grown by hot injection colloidal and CVD syntheses in previous reports.16,20 These relatively narrow peak widths suggest that the alloy microplates have good crystalline quality with low defect density and small composition fluctuation.20In addition to the steady state PL study, spectrophotometric analysis of the IHP alloy microplates was also performed to estimate the bandgaps of different alloy compositions. A comparison of the room temperature absorbance and steady state PL emission peaks of typical CsPb(Cl1−xBrx)3 alloy microplates with x = 0.60 shown in Fig. 3(b) reveals that the absorption edge closely matches with the PL peak. A very similar phenomenon was also observed for a representative CsPb(Br1−xIx)3 alloy microplate sample (Fig. S5, ESI†). This suggests that the PL emission corresponds to the band to band emission and is a good measure of the bandgap of the mixed halide quaternary alloys. More generally, for resistive materials like ternary and quaternary halide perovskite microstructures, free carrier effects (Burstein–Moss shift) are insignificant, and the optical bandgap is the same as the intrinsic bandgap. Since the PL emission energy agrees with the absorption edge (as shown in Fig. 3(b)), the PL emission energy corresponds to the fundamental bandgap of the alloy microplates. Here, it is necessary to mention that the probe beam size (∼2 mm) in the spectrophotometry setup is much larger than the microplate dimension. Since microplates are randomly dispersed on but do not fully cover the substrate surface, a large fraction of the incident beam goes directly through the substrate. Hence, accurate absorption coefficients (α) of the quaternary microplates cannot be obtained.
As mentioned earlier, the composition of the quaternary alloy microplates was determined by EDS and XRD. We relate the bandgap measured by PL spectroscopy and the composition x calculated from the XRD spectra of both the quaternary CsPb(Cl1−xBrx)3 and CsPb(Br1−xIx)3 alloys in Fig. 3(c). A monotonous decrease in the bandgap is observed (from ∼3.0 eV (CsPbCl3) to ∼1.7 eV (CsPbI3)) with the increase in the x of the respective CsPb(Cl1−xBrx)3 and CsPb(Br1−xIx)3 alloys. The composition dependency of the bandgap follows a linear virtual crystal approximation (Vegard's law) with small bowing parameters b = 0.39 eV and 0.68 eV for CsPb(Cl1−xBrx)3 and CsPb(Br1−xIx)3, respectively.27 The best fits of the bandgap data using these b values are also shown in Fig. 3(c) as pink solid lines. These bowing parameter values are in good agreement with the previously reported first principles calculated values of 0.48 eV and 0.32 eV obtained for the same materials and the experimentally obtained bowing parameter value of b = 0.33 for MAPb(I1−xBrx)3.26,55
2.3 Composition uniformity and stability of quaternary alloy microplates
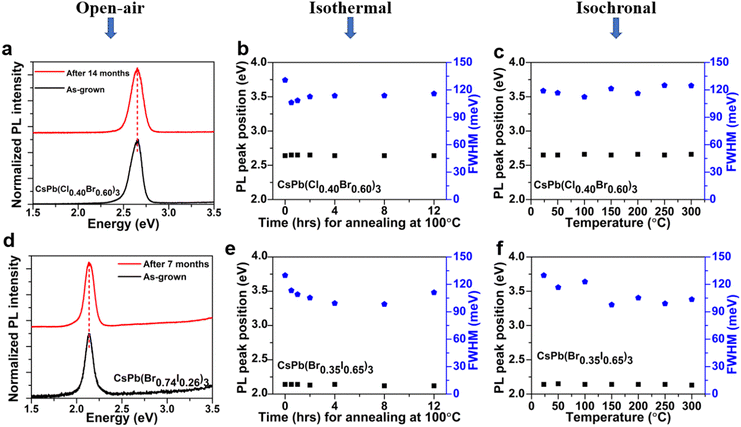
A systematic investigation of the lateral and vertical uniformity of the quaternary alloy microplates was performed using a home-built steady state PL setup and a confocal micro-PL mapping facility. Firstly, using a continuous wave (cw) He–Cd laser with λexc = 320 nm, each sample was illuminated from the front and the back at the same specific location on the sample surface for samples deposited on sapphire substrates as illustrated in the schematic drawing in Fig. 4(a). A similar approach was then adopted and repeated for the full series of CsPb(Cl1−xBrx)3 and CsPb(Br1−xIx)3 alloys to obtain the PL emission spectra from various sites on the same sample surface. It is estimated that the penetration depth (90% absorption) of the laser is ∼100 nm. The emission peaks from different locations on the same sample are the same with a variation of <10 meV and practically overlap when illuminated from the front and back at the same location, suggesting that the alloy composition is vertically uniform (Fig. 4(b) and (c)). The overlapping of the front and back side PL spectra obtained separately for typical CsPb(Cl1−xBrx)3 and CsPb(Br1−xIx)3 alloy compositions is shown in Fig. S4(a) and (b) (ESI†). Secondly, a confocal μ-PL setup, WITec alpha300 R, equipped with an excitation source, λexc = 532 nm, a charge coupled detector (CCD), and a scanning stage was used to scan the lateral uniformity of typical CsPb(Br1−xIx)3 microplates. However, our current setup with λexc = 532 nm is unable to probe CsPb(Cl1−xBrx)3 microplates with bandgaps >2.3 eV (λ < 530 nm). Note that mixed halide perovskites, especially the iodine based alloys, are known to be sensitive to photoexcitation and photoinduced phase separation.56 In order to avoid any photoinduced degradation or phase segregation, μ-PL mapping was performed using a laser beam size of ∼0.7 μm with an excitation power of 5.0 μW and a mapping time of 5 min. The relatively uniform PL intensity distribution throughout the CsPb(Br1−xIx)3 (x∼0.17) alloy microplate surface shown in Fig. 4(d) suggests that quaternary IHP alloy microplates with uniform material quality are obtained by the single-step CVD process.57In addition to the wide variety of optoelectronic applications, it has also been reported that IHPs exhibit excellent potential for non-linear optical applications, e.g., lasers,23 frequency up-conversion lasers,46etc. However, all these applications require high photostability of the materials because of the strong non-linear light–matter interactions involved.58 Although IHPs are considered to be more stable compared to their OHP counterparts,12 the stability of the quaternary IHP alloys is less well understood. Here, we performed a study of the stability of representative quaternary IHP alloy microplates by PL spectroscopy. PL measurements were performed several times a month on CsPb(Cl1−xBrx)3 (Fig. 5(a)) and CsPb(Br1−xIx)3 (Fig. 5(d)) microplates within time periods of 14 and 7 months, respectively. No significant change in both the PL peak positions and peak widths is observed for both alloys. It is important to note that after taking out of the CVD chamber, the samples are stored in a dry box maintained at a relative humidity of ∼40%.
The thermal stability of the quaternary microplates was further studied by isothermal and isochronal annealing in air. The isothermal annealing studies were carried out at 100 °C from 0 to 12 hours, while the isochronal annealing studies were performed for 5 min up to 300 °C. Fig. 5(b) and (c), respectively, show PL peak positions and widths of a typical CsPb(Cl1−xBrx)3 alloy microplate sample after isothermal and isochronal annealing. No significant shift in the PL peak position or broadening of the peak width is observed in Fig. 5(b) and (c), suggesting that the CsPb(Cl1−xBrx)3 alloy microplates are thermally stable at least up to 300 °C. Similarly, Fig. 5(e) and (f) show that the CsPb(Br1−xIx)3 alloy sample is thermally stable after both isothermal and isochronal annealing with no shift in the PL peak position. However, the PL peak width of CsPb(Br1−xIx)3 decreases slightly by ∼30 nm after both isothermal and isochronal annealing. This may be due to the removal of defects after post-growth annealing. Note that during all measurements, samples underwent several laser irradiation exposures, at ∼100–190 mW cm−2 under ≥ AM 1.5 one sun solar irradiation. The insignificant changes observed in the PL peak shift and the PL FWHM values (Fig. 5(e) and (f)) suggest that the quaternary IHP alloy microplates grown by the single-step CVD process also have a high resistance to photodegradation.
2.4 Quaternary CsPbX3 (X = Cl1−xBrx; Br1−xIx) alloy microplates as NLO materials
To understand the NLO properties of quaternary CsPb(Cl1−xBrx)3 and CsPb(Br1−xIx)3 alloy microplates, in addition to the normal one-photon absorption (OPA) PL emission, two-photon absorption (TPA) based PL emission properties were investigated. Fig. 6(a) shows a schematic energy band diagram of the two-photon absorption (TPA) and the relevant PL processes. Unlike conventional one-photon absorption, where a single photon with sufficient energy is absorbed, TPA involves the combined energy of two lower-energy photons to achieve the same effect. In the TPA process, two photons with energy lower than the bandgap are absorbed nearly simultaneously (Δt = 10−15 s), causing an electron to move from the valence band to the conduction band. This process is nonlinear and requires high photon intensity. Subsequent radiative recombination results in the emission of a higher energy photon (corresponding to the bandgap energy) in the visible range as illustrated in Fig. 6(a). The TPA mechanism involved in quaternary CsPb(Cl1−xBrx)3 and CsPb(Br1−xIx)3 alloy microplates is initiated by the absorption of an incident photon with Ephoton = 1.55 eV at (λexc = 800 nm), which excites a valence band electron to a virtual state, while momentarily (Δt ∼ 10−15 s) a second photon with the same energy triggers the virtual state electron to an upper state in the conduction band. The virtual state is a temporary, non-physical intermediate state that exists only for a very short duration, providing a quantum mechanical pathway for the TPA process. It allows the system to absorb the combined energy of two low energy photons and transition to a higher energy state. The excited electron loses its energy through thermalization and hence reaches the conduction band minimum. Finally, the subsequent radiative recombination gives rise to the emission of a visible photon with λemission = 473 nm for CsPbCl1.5Br1.5 and λemission = 584 nm for CsPbBr2.4I0.6 alloy microplates.Fig. 6(b) and (c) reveal the TPA related PL spectra of typical CsPb(Cl1−xBrx)3 and CsPb(Br1−xIx)3 alloy microplates, respectively. For the CsPbCl1.5Br1.5 microplates, normal single photon excitation with λexc = 320 nm results in a PL emission peak at around 468 nm corresponding to a bandgap of Eg ∼ 2.64 eV. However, when the microplates were excited with sub-bandgap photons with λexc = 800 nm (Ephoton = 1.55 eV), the emission peak is red shifted by 5 nm to 473 nm. Likewise, for CsPbBr2.4I0.6 microplates, the PL peak for the TPA process with λexc = 800 nm is red shifted by ∼20 nm relative to the OPA PL obtained for λexc = 532 nm (Fig. 6(c)). The bandgap of the quaternary CsPbBr2.4I0.6 alloy microplates estimated from the OPA PL measurements using λexc = 532nm is ∼2.21 eV (λemission = 561 nm). The red shift of TPA PL emission can be attributed to the increased reabsorption effects due to the larger penetration depth in the TP excitation.29,52,59,60 The comparatively weaker absorption intensity of the NIR photons (λexc = 800 nm) allows them to penetrate deeper into the material and therefore excite largely from the bulk. In contrast, above band gap UV (λexc = 320 nm) or visible photons (λexc = 532 nm) will be strongly absorbed within a shallow region, which usually causes surface confined excitation and emission.44 The surface confined excitation and the excitation from the bulk may trigger the OPA and TPA related PL peak shift.17,61 Moreover, maintaining the excitation at λexc = 800 nm a series of TPA emission spectra were collected for both CsPbCl1.5Br1.5 and CsPbBr2.4I0.6 by varying the excitation power. The power dependent fluorescence spectra of the two quaternary alloys are shown in Fig. S6(a) and (b) in the ESI.† Note that the fluorescence peak intensities at ∼470 nm and ∼580 nm respectively for CsPbCl1.5Br1.5 and CsPbBr2.4I0.6 increase with the excitation power. The relative fluorescence intensities as a function of the excitation power for the two alloys are plotted in Fig. S6(c) and (d) (ESI†), respectively, for the two alloys. The red lines in Fig. S6(c) and (d) (ESI†) show linear fits of the logarithmic plots. The quadratic dependence of the PL intensity on the excitation power clearly confirms that the emission under 800 nm excitation of the quaternary CsPbCl1.5Br1.5 and CsPbBr2.4I0.6 all-inorganic halide perovskite alloy microplates is a TPA induced phenomenon.17,58,59,62 These observations, therefore, demonstrate that both quaternary CsPb(Cl1−xBrx)3 and CsPb(Br1−xIx)3 alloy microplates can act as frequency upconversion NLO materials and have high prospects for their integration into optoelectronic applications, especially in light manipulation and detection related photonic devices. We note that for a complete understanding of the NLO properties of these alloy microplates, the TPA cross–section and structure–property relationship should also be extracted and these will be studied in our future work.
3 Conclusion
We report the properties of ternary CsPbX3 (X = Cl, Br, I) and quaternary CsPbX3 (X = Cl1−xBrx, Br1−xIx) all-inorganic halide perovskite (IHP) alloy microplates synthesized by the single-step chemical vapor deposition (CVD) process. In particular, the non-linear two photon absorption effect of the quaternary microplates is investigated. The CVD growth can be well controlled to allow us to realize high-quality, single-crystalline alloy microplates with tunable compositions and hence tunable emission wavelengths in the spectral range of 413–715 nm (1.7 to 3.0 eV). These quaternary IHP microplates are compositionally uniform both vertically and laterally, exhibiting intense and narrow PL emissions. Moreover, they are thermally stable and highly resistant to photodegradation. Furthermore, two-photon absorption (TPA) properties are demonstrated for mixed halide quaternary CsPb(Cl1−xBrx)3 and CsPb(Br1−xIx)3 alloy microplates, which add a new dimension to the functionalities of these all-inorganic halide perovskites (IHPs) as nonlinear optical (NLO) materials. We believe that this work will open up new avenues for the development of microplate compatible integrated optoelectronic and photonic applications and will certainly enhance the fundamental NLO research.Data availability
Data are available from the corresponding author only upon request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by CityU SGP (no. 9380076).References
- E. Edri, S. Kirmayer, D. Cahen and G. Hodes, J. Phys. Chem. Lett., 2013, 4, 897–902 CrossRef CAS PubMed.
- P. F. Ndione, Z. Li and K. Zhu, J. Mater. Chem. C, 2016, 4, 7775–7782 RSC.
- W. J. Yin, Y. Yan and S. H. Wei, J. Phys. Chem. Lett., 2014, 5, 3625–3631 CrossRef CAS PubMed.
- K. Park, J. W. Lee, J. D. Kim, N. S. Han, D. M. Jang, S. Jeong, J. Park and J. K. Song, J. Phys. Chem. Lett., 2016, 7, 3703–3710 CrossRef CAS PubMed.
- https://www.nrel.gov/pv/assets/pdfs/best-research-cell-efficiencies.pdf, visited on 29 August 2023.
- J. Song, J. Li, X. Li, L. Xu, Y. Dong and H. Zeng, Adv. Mater., 2015, 27, 7162–7167 CrossRef CAS PubMed.
- Y. Jiang, M. R. Leyden, L. Qiu, S. Wang, L. K. Ono, Z. Wu, E. J. Juarez-Perez and Y. Qi, Adv. Funct. Mater., 2018, 28, 1703835 CrossRef.
- Z. Li, M. Yang, J. S. Park, S. H. Wei, J. J. Berry and K. Zhu, Chem. Mater., 2016, 28, 284–292 CrossRef CAS.
- X. Li, F. Cao, D. Yu, J. Chen, Z. Sun, Y. Shen, Y. Zhu, L. Wang, Y. Wei, Y. Wu and H. Zeng, Small, 2017, 13, 1603996 CrossRef PubMed.
- E. T. Hoke, D. J. Slotcavage, E. R. Dohner, A. R. Bowring, H. I. Karunadasa and M. D. McGehee, Chem. Sci., 2015, 6, 613–617 RSC.
- T. Xu, L. Chen, Z. Guo and T. Ma, Phys. Chem. Chem. Phys., 2016, 18, 27026–27050 RSC.
- J. B. Hoffman, A. L. Schleper and P. V. Kamat, J. Am. Chem. Soc., 2016, 138, 8603–8611 CrossRef CAS PubMed.
- J. K. Nam, S. U. Chai, W. Cha, Y. J. Choi, W. Kim, M. S. Jung, J. Kwon, D. Kim and J. H. Park, Nano Lett., 2017, 17, 2028–2033 CrossRef CAS PubMed.
- Q. Ma, S. Huang, S. Chen, M. Zhang, C. F. J. Lau, M. N. Lockrey, H. K. Mulmudi, Y. Shan, J. Yao, J. Zheng, X. Deng, K. Catchpole, M. A. Green and A. W. Y. Ho-Baillie, J. Phys. Chem. C, 2017, 121, 19642–19649 CrossRef CAS.
- L. Zhang, X. Yang, Q. Jiang, P. Wang, Z. Yin, X. Zhang, H. Tan, Y. M. Yang, M. Wei, B. R. Sutherland, E. H. Sargent and J. You, Nat. Commun., 2017, 8, 15640 CrossRef CAS PubMed.
- L. Protesescu, S. Yakunin, M. I. Bodnarchuk, F. Krieg, R. Caputo, C. H. Hendon, R. X. Yang, A. Walsh and M. V. Kovalenko, Nano Lett., 2015, 15, 3692–3696 CrossRef CAS PubMed.
- Y. Wang, X. Li, X. Zhao, L. Xiao, H. Zeng and H. Sun, Nano Lett., 2016, 16, 448–453 CrossRef CAS PubMed.
- N. Yantara, S. Bhaumik, F. Yan, D. Sabba, H. A. Dewi, N. Mathews, P. P. Boix, H. V. Demir and S. Mhaisalkar, J. Phys. Chem. Lett., 2015, 6, 4360–4364 CrossRef CAS PubMed.
- Y. Wang, M. Ibrahim Dar, L. K. Ono, T. Zhang, M. Kan, Y. Li, L. Zhang, X. Wang, Y. Yang, X. Gao, Y. Qi, M. Grätzel and Y. Zhao, Science, 2019, 365, 591–595 CrossRef CAS PubMed.
- Y. Wang, X. Guan, D. Li, H. C. Cheng, X. Duan, Z. Lin and X. Duan, Nano Res., 2017, 10, 1223–1233 CrossRef CAS.
- A. Zhang, C. Dong and J. Ren, J. Phys. Chem. C, 2017, 121, 13314–13323 CrossRef CAS.
- S. Dastidar, D. A. Egger, L. Z. Tan, S. B. Cromer, A. D. Dillon, S. Liu, L. Kronik, A. M. Rappe and A. T. Fafarman, Nano Lett., 2016, 16, 3563–3570 CrossRef CAS PubMed.
- P. Guo, M. K. Hossain, X. Shen, H. Sun, W. Yang, C. Liu, C. Y. Ho, C. K. Kwok, S. W. Tsang, Y. Luo, J. C. Ho and K. M. Yu, Adv. Opt. Mater., 2018, 6, 1700993 CrossRef.
- H. Wang, H. Bian, Z. Jin, H. Zhang, L. Liang, J. Wen, Q. Wang, L. Ding and S. F. Liu, Chem. Mater., 2019, 31, 6231–6238 CrossRef CAS.
- R. E. Beal, D. J. Slotcavage, T. Leijtens, A. R. Bowring, R. A. Belisle, W. H. Nguyen, G. F. Burkhard, E. T. Hoke and M. D. McGehee, J. Phys. Chem. Lett., 2016, 7, 746–751 CrossRef CAS PubMed.
- Z. Zhou, Y. Cui, H. X. Deng, L. Huang, Z. Wei and J. Li, Appl. Phys. Lett., 2017, 110, 113901 CrossRef.
- M. K. Hossain, P. Guo, W. Qarony, Y. H. Tsang, C. Liu, S. W. Tsang, J. C. Ho and K. M. Yu, Nano Res., 2020, 13, 2939–2949 CrossRef CAS.
- H. Zhou, S. Yuan, X. Wang, T. Xu, X. Wang, H. Li, W. Zheng, P. Fan, Y. Li, L. Sun and A. Pan, ACS Nano, 2017, 11, 1189–1195 CrossRef CAS.
- C. Zhao, W. Tian, J. Liu, Q. Sun, J. Luo, H. Yuan, B. Gai, J. Tang, J. Guo and S. Jin, J. Phys. Chem. Lett., 2019, 10, 2357–2362 CrossRef CAS.
- L. Gil-Escrig, C. Momblona, M. G. La-Placa, P. P. Boix, M. Sessolo and H. J. Bolink, Adv. Energy Mater., 2018, 8, 1703506 CrossRef.
- W. Tian, H. Zhou and L. Li, Small, 2017, 13, 1702107 CrossRef PubMed.
- K. Wang, G. Xing, Q. Song and S. Xiao, Adv. Mater., 2020, 33, 2000306 CrossRef.
- T. Qiu, Y. Hu, F. Xu, Z. Yan, F. Bai, G. Jia and S. Zhang, Nanoscale, 2018, 10, 20963–20989 RSC.
- Y. Li, Z. Shi, L. Lei, Z. Ma, F. Zhang, S. Li, D. Wu, T. Xu, X. Li, C. Shan and G. Du, ACS Photonics, 2018, 5, 2524–2532 CrossRef CAS.
- Q. Zhang, R. Su, X. Liu, J. Xing, T. C. Sum and Q. Xiong, Adv. Funct. Mater., 2016, 26, 6238–6245 CrossRef CAS.
- T. Yang, Y. Zheng, Z. Du, W. Liu, Z. Yang, F. Gao, L. Wang, K. C. Chou, X. Hou and W. Yang, ACS Nano, 2018, 12, 1611–1617 CrossRef CAS PubMed.
- J. Shamsi, Z. Dang, P. Bianchini, C. Canale, F. Di Stasio, R. Brescia, M. Prato and L. Manna, J. Am. Chem. Soc., 2016, 138, 7240–7243 CrossRef CAS PubMed.
- M. K. Hossain, R. dos Reis, W. Qarony, Y. H. Tsang, J. C. Ho and K. M. Yu, J. Mater. Chem. C, 2021, 9, 3229–3238 RSC.
- S. Yakunin, L. Protesescu, F. Krieg, M. I. Bodnarchuk, G. Nedelcu, M. Humer, G. De Luca, M. Fiebig, W. Heiss and M. V. Kovalenko, Nat. Commun., 2015, 6, 8056 CrossRef CAS PubMed.
- W. Qarony, M. K. Hossain, M. I. Hossain, L. Zeng, S. Ma, K. M. Yu, A. Salleo, D. Knipp, C. T. Yip and Y. H. Tsang, Thin Solid Films, 2021, 737, 138950 CrossRef CAS.
- D. W. DeQuilettes, S. M. Vorpahl, S. D. Stranks, H. Nagaoka, G. E. Eperon, M. E. Ziffer, H. J. Snaith and D. S. Ginger, Science, 2015, 348, 683–686 CrossRef CAS PubMed.
- G. Grancini, A. R. Srimath Kandada, J. M. Frost, A. J. Barker, M. De Bastiani, M. Gandini, S. Marras, G. Lanzani, A. Walsh and A. Petrozza, Nat. Photonics, 2015, 9, 695–701 CrossRef CAS PubMed.
- A. Pramanik, K. Gates, Y. Gao, S. Begum and P. Chandra Ray, J. Phys. Chem. C, 2019, 123, 5150–5156 CrossRef CAS.
- Z. Y. Zhang, H. Y. Wang, Y. X. Zhang, K. J. Li, X. P. Zhan, B. R. Gao, Q. D. Chen and H. B. Sun, Phys. Chem. Chem. Phys., 2017, 19, 2217–2224 RSC.
- F. O. Saouma, D. Y. Park, S. H. Kim, M. S. Jeong and J. I. Jang, Chem. Mater., 2017, 29, 6876–6882 CrossRef CAS.
- C. Huang, K. Wang, Z. Yang, L. Jiang, R. Liu, R. Su, Z. K. Zhou and X. Wang, J. Phys. Chem. C, 2017, 121, 10071–10077 CrossRef CAS.
- M. Savoini, L. Huber, H. Cuppen, E. Abreu, M. Kubli, M. J. Neugebauer, Y. Duan, P. Beaud, J. Xu, T. Rasing and S. L. Johnson, ACS Photonics, 2018, 5, 671–677 CrossRef CAS.
- A. Vangara, A. Pramanik, Y. Gao, K. Gates, S. Begum and P. C. Ray, ACS Appl. Bio Mater., 2018, 1, 298–309 CrossRef CAS PubMed.
- T. He, J. Li, X. Qiu, S. Xiao, C. Yin and X. Lin, Adv. Opt. Mater., 2018, 6, 1800843 CrossRef.
- H. He, E. Ma, X. Chen, D. Yang, B. Chen and G. Qian, Small Methods, 2019, 3, 1900396 CrossRef CAS.
- K. N. Krishnakanth, S. Seth, A. Samanta and S. Venugopal Rao, Nanoscale, 2019, 11, 945–954 RSC.
- Y. Gao, S. Wang, C. Huang, N. Yi, K. Wang, S. Xiao and Q. Song, Sci. Rep., 2017, 7, 45391 CrossRef CAS.
- G. Walters, B. R. Sutherland, S. Hoogland, D. Shi, R. Comin, D. P. Sellan, O. M. Bakr and E. H. Sargent, ACS Nano, 2015, 9, 9340–9346 CrossRef CAS PubMed.
- X. Mo, X. Li, G. Dai, P. He, J. Sun, H. Huang and J. Yang, Nanoscale, 2019, 11, 21386–21393 RSC.
- Y. Zhou, Z. Zhou, M. Chen, Y. Zong, J. Huang, S. Pang and N. P. Padture, J. Mater. Chem. A, 2016, 4, 17623–17635 RSC.
- H. Zhang, X. Fu, Y. Tang, H. Wang, C. Zhang, W. W. Yu, Y. Zhang, M. Xiao and X. Wang, Nat. Commun., 2019, 10, 1088 CrossRef CAS PubMed.
- X. H. Wang, J. Q. Ning, C. C. Zheng, B. R. Zhu, L. Xie, H. S. Wu and S. J. Xu, J. Mater. Chem. C, 2015, 3, 2589–2592 RSC.
- J. Xu, X. Li, J. Xiong, C. Yuan, S. Semin, T. Rasing and X. H. Bu, Adv. Mater., 2020, 32, 1806736 CrossRef CAS PubMed.
- Y. Xu, Q. Chen, C. Zhang, R. Wang, H. Wu, X. Zhang, G. Xing, W. W. Yu, X. Wang, Y. Zhang and M. Xiao, J. Am. Chem. Soc., 2016, 138, 3761–3768 CrossRef CAS PubMed.
- Y. Yamada, T. Yamada, L. Q. Phuong, N. Maruyama, H. Nishimura, A. Wakamiya, Y. Murata and Y. Kanemitsu, J. Am. Chem. Soc., 2015, 137, 10456–10459 CrossRef CAS PubMed.
- V. D’Innocenzo, A. R. Srimath Kandada, M. De Bastiani, M. Gandini and A. Petrozza, J. Am. Chem. Soc., 2014, 136, 17730–17733 CrossRef PubMed.
- B. Zhou, M. Jiang, H. Dong, W. Zheng, Y. Huang, J. Han, A. Pan and L. Zhang, ACS Photonics, 2019, 6, 793–801 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3tc03166g |
| This journal is © The Royal Society of Chemistry 2024 |