Applications of multifunctional metal–organic frameworks in perovskite photovoltaics: roles, advantages and prospects
Ming-Wu
Liu†
a,
Gengling
Liu†
b,
Yu-Fen
Wang
*a,
Bing-Xin
Lei
*c and
Wu-Qiang
Wu
 *b
*b
aEnergy & Materials Engineering Center, College of Physics and Materials Science, Tianjin Normal University, Tianjin 300387, P. R. China. E-mail: yfwang@tjnu.edu.cn
bKey Laboratory of Bioinorganic and Synthetic Chemistry (MoE), Lehn Institute of Functional Materials, School of Chemistry, Sun Yat-sen University, Guangzhou 510006, P. R. China. E-mail: wuwq36@mail.sysu.edu.cn
cSchool of Materials and Environment, Guangxi Key Laboratory of Advanced Structural Materials and Carbon Neutralization, Guangxi Colleges and Universities Key Laboratory of Environmental-friendly Materials and New Technology for Carbon Neutralization, Guangxi Minzu University, Nanning 530105, P. R. China. E-mail: leibx@gxmzu.edu.cn
First published on 10th November 2023
Abstract
Metal–organic frameworks (MOFs) are considered to be highly fascinating materials due to their excellent photoelectric properties, tunable porous nanostructures and desirable chemical absorption characteristics, which play beneficial roles in constructing high-performance and eco-friendly perovskite solar cells (PSCs). Herein, the recent advancements in applying MOFs in perovskite photovoltaics have been reviewed, with a particular focus on emphasizing the multiple roles (i.e., serving as the carrier transport layer, interfacial modifier, crystallization regulator, defect passivator and Pb leakage inhibitor) and multifarious advantages of MOFs that enable efficient, durable and eco-friendly PSCs to be achieved. The limitations and challenges associated with currently employed MOFs materials in PSCs are discussed. An insightful perspective regarding the optimal molecular configuration design of advanced MOFs for fabricating highly efficient, sustainable, flexible and wearable MOFs-based perovskite optoelectronic devices is provided.
1. Introduction
Perovskite solar cells (PSCs) have been identified as the most popular photovoltaic devices due to their simple fabrication process and the superior photoelectric properties of perovskite materials including high light absorption coefficient, high carrier mobility, wide light absorption range and long carrier diffusion length.1,2 As of now, the efficiency of PSCs has been boosted to 26.1%, which shows their great application prospects in the photovoltaic industry.3 However, the instability, as well as potential lead leakage and related toxicity issues of PSCs still largely impede their practical deployment and large-scale commercialization. In addition, the inherent polycrystalline nature of ionic-type perovskites normally features abundant defects, which further limits the further improvement of the efficiency and stability of PSCs.4Over the past few decades, a number of efforts have been devoted to improve the stability of PSCs, such as composition engineering, additive engineering, encapsulation optimization, etc.5 Among these, the additive strategy has been recognized as the most facile and feasible approach to revolutionize PSCs. Specifically, intensive research efforts have been devoted to the design of more advanced additive molecules, which should ideally possess superior passivation potentiality, photothermal stability, as well as the ability to inhibit ion migration and facilitate charge transport. Among various kinds of utilized additives, the metal–organic frameworks (MOFs) materials have emerged as one of the most promising additives/ingredients in the field of PSCs. MOFs are usually composed of metal-containing nodes with organic ligands linked by coordinate bonds, which facilitate infinite structural diversity and functional tunability. Inspired from the applications of MOFs in a variety of applications such as chemical sensing and photocatalysis,6,7 very recently, a series of MOFs materials (i.e., Zn-TTB, PCN-224, ZIF-8, POM@Cu-BTC, ZrL3, etc.) with excellent properties have been likewise introduced to develop efficient and stable PSCs. Encouragingly, the perovskites intermarriage intimately with MOFs, which play multifunctional roles as the carrier transport layers (CTLs), interfacial modifier, crystallization regulator, defect passivator and lead leakage inhibitor8 (Fig. 1). Several successful examples have demonstrated that modulating the molecular structures of MOFs materials could afford comprehensive improvement of device performances while significantly inhibit the Pb leakage of PSCs, thus warranting practical application and real commercialization of perovskite photovoltaics.9 Generally, the well-designed MOFs materials with abundant functional groups could strongly bond with perovskites via robust chemical interactions, which inhibits the ion-migration and increases the water resistance of the resultant perovskite films, thus enhancing the inherent stability of relevant devices.10 Moreover, the relatively low-cost and ease of industrial-scale fabrication of MOFs materials further strengthens the feasibility of their integration and applications in the realm of PSCs.11
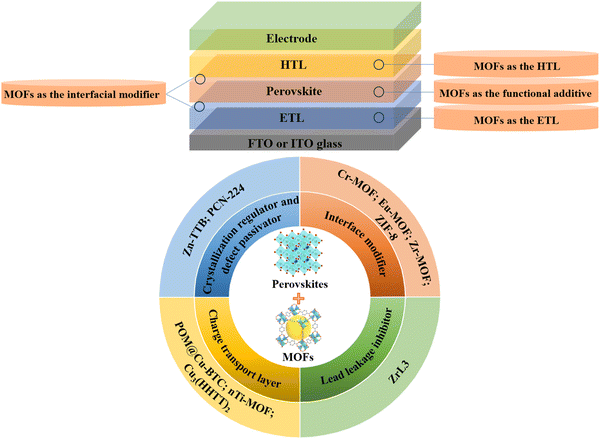 | ||
| Fig. 1 Schematic illustration of the applications of MOFs materials in PSCs (HTL: hole transport layer; ETL: electron transport layer). | ||
In this review, we have summarized the recent advances in applying MOFs in PSCs and have elaborated the multifunctional roles, unique advantages and vast potential of MOFs for simultaneously enhancing the device performances and mitigating Pb leakage of PSCs. The structure–property–performance relationship has been studied and elucidated when the MOFs materials were used as the additives/surface reagents/carrier transport layers in PSCs. For the future prospects, rational design of more advanced MOFs with optimized molecular structures for more efficient, durable and eco-friendly perovskite-based optoelectronic devices is proposed.
2. The multifunctional roles and unique advantages of MOFs in PSCs
The device performance of PSCs will be fundamentally affected by the quality of perovskite films and the relevant contact interfaces between CTLs and the perovskite layer. A series of effective strategies have been developed to improve the efficiency and stability of the devices via regulating the crystallization of perovskite films and manipulating interfacial carrier transport throughout the devices. Notably, owing to the diverse framework structures, abundant binding sites and tunable porosity, MOFs are considered to be promising materials to be integrated in PSCs.12 According to previous research, MOF materials could assist in achieving high-performance PSCs via the following aspects: (i) modulating the nucleation and crystallization processes towards high-quality perovskite films with few defects, and the multifunctional groups of MOFs multifocally interacting with perovskites which could thoroughly passivate the defects of perovskites; (ii) modifying interface imperfection via alleviating perovskite lattice stress, promoting interfacial charge transport and improving UV stability of PSCs; (iii) serving as independent CTLs or dopant of CTLs to promote charge extraction and collection within PSCs; and (iv) inhibiting lead leakage for promoting sustainable deployment of PSCs. The following sections will elaborate each aspect in detail.2.1. MOF-assisted crystallization regulation and defect passivation
The MOF functional materials possess abundant active sites and long-range ordered molecular structures, which could effectively regulate the crystallization kinetics and passivate multi-type defects of perovskite films. Wang and co-workers employed a small molecule additive 1-(triazol-1-ly)-4-(tetrazol-5-ylmethyl) benzene (TTB) with terminal triazole and tetrazole groups as the ligand to construct a highly stable one-dimensional Zn-based MOFs, namely Zn-TTB. Subsequently, Zn-TTB was incorporated into the perovskite precursor, in which Zn-TTB could serve as the porous scaffold for perovskite penetration, thus realizing well-organized growth of the perovskite film (Fig. 2a). In addition, a macromolecular mesophase could be generated through the strong chemical interaction between MOFs and perovskites (i.e., coordination bonding, hydrogen bonding, etc.), thus leading to the formation of high-quality perovskite films with enlarged grain sizes and preferable grain orientation. Consequently, the Zn-TTB-modified device exhibited a champion PCE of 23.14%, which is nearly 7.9% higher than that of the control device (Fig. 2b).13 Liu and co-workers developed a versatile strategy to improve the device performances of PSCs via incorporating miraculous Zr-based porphyrinic MOF quantum dots (QDs), namely PCN-224, into the perovskite precursor and subsequently fabricating high-quality perovskite films via one-step spin-coating deposition technology (Fig. 2c). Particularly, PCN-224 QDs could favorably interact with under-coordinated Pb2+via the Lewis acid–base interaction effect, which positively regulated the crystallization kinetics and resulted in the formation of a high-quality perovskite film with reduced defect density. Notably, the multiple Lewis-base groups (i.e., C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C
O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, and C–N) in PCN-224 QDs could effectively coordinate with PbI2, as portrayed in Fig. 2d, which is beneficial to fully passivate the deep energy-level defects of perovskite films. Interestingly, PCN-224 QDs could also alleviate the migration of Li+ from the hole transport layer (HTL) to the perovskite layer, which further avoided the formation of deleterious defects during long-term storage of devices. As a consequence, a PCN-224 QDs-modified device (denoted as PVK(QDs)) afforded the champion PCE of 21.59%, which was over 13% higher than that of the control device. More interestingly, PCN-224 QDs have been also employed in the HTL (denoted as PVK(QDs)/HTL(QDs)) which could further increase the PCE of the corresponding device to 22.51% (Fig. 2e), which could be ascribed to the excellent hole extraction and transport as well as the abovementioned effective suppression of Li+ ion migration. Overall, an intelligent approach via integrating MOFs materials as additives has been demonstrated that it could effectively passivate the Pb-related and I-related defects, optimize crystallization, and improve homogeneity of the perovskite films, thus achieving suppressed charge recombination within the devices. Another promise of this strategy is to improve the stability of perovskite films and devices under the stress of humidity, heat and/or light.14
N, and C–N) in PCN-224 QDs could effectively coordinate with PbI2, as portrayed in Fig. 2d, which is beneficial to fully passivate the deep energy-level defects of perovskite films. Interestingly, PCN-224 QDs could also alleviate the migration of Li+ from the hole transport layer (HTL) to the perovskite layer, which further avoided the formation of deleterious defects during long-term storage of devices. As a consequence, a PCN-224 QDs-modified device (denoted as PVK(QDs)) afforded the champion PCE of 21.59%, which was over 13% higher than that of the control device. More interestingly, PCN-224 QDs have been also employed in the HTL (denoted as PVK(QDs)/HTL(QDs)) which could further increase the PCE of the corresponding device to 22.51% (Fig. 2e), which could be ascribed to the excellent hole extraction and transport as well as the abovementioned effective suppression of Li+ ion migration. Overall, an intelligent approach via integrating MOFs materials as additives has been demonstrated that it could effectively passivate the Pb-related and I-related defects, optimize crystallization, and improve homogeneity of the perovskite films, thus achieving suppressed charge recombination within the devices. Another promise of this strategy is to improve the stability of perovskite films and devices under the stress of humidity, heat and/or light.14
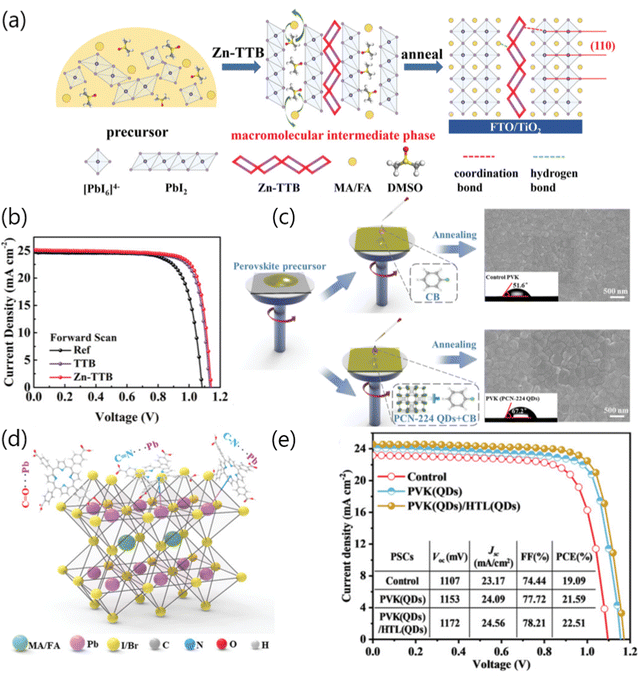 | ||
| Fig. 2 (a) Schematic illustration of crystallization behavior evolution of the perovskite film via incorporating Zn-TTB. (b) The J–V curves of the reference, TTB-based and Zn-TTB based devices. Reproduced with permission.13 Copyright 2022 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. (c) Schematic diagram of control (without PCN-224 QDs) or PCN-224 QD-assisted crystallization of perovskite films and the corresponding top-view scanning electron microscopy (SEM) images of the perovskite films. (d) Schematic diagram of chemical interactions between PCN-224 QDs and perovskites. (e) The J–V curves of control, PVK (QDs)-based and PVK (QDs)/HTL(QDs)-based PSCs. Reproduced with permission.14 Copyright 2022 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
2.2. MOFs-assisted interface modification
An ideal interface modifier should normally enable intimate chemical bonding with adjacent perovskites, which could simultaneously assist in managing the growth of the perovskite, regulating the lattice strain, promoting the charge transport and reducing the non-radiative recombination. In this section, we have reviewed recent progress of MOFs-based interface modifiers in PSCs.The insufficient solid–liquid reaction between lead halide and organic ammonium halide salt occurring in the conventional two-step sequential deposition technique usually results in poor-quality of the perovskite film with numerous defects. To address this issue, Sheng and co-workers developed a miraculous strategy by using the thermally stable zeolitic imidazolate framework-8 (ZIF-8) wrapped formamidinium iodide (FAI) (ZIF-8@FAI) capsule as a multifunctional interface modifier between SnO2 and PbI2. As shown in Fig. 3a, the incorporation of the ZIF-8@FAI capsule interlayer successfully induced the appearance of porous channels in the PbI2 layer, which could promote the penetration of the organic ammonium cation solution, thus enabling the complete conversion from PbI2 to the photovoltaic-active perovskite phase. Besides, the residual tensile strain of the perovskite film has also been greatly alleviated and the voltage loss of relevant PSCs has been further minimized due to the robust coordination between imidazole N and uncoordinated Pb2+ ions. As a result, the PCE of PSCs was successfully increased from 21.63% to 24.08% upon ZIF-8@FAI capsule-assisted interface modification (Fig. 3b). Another promise of inserting the ZIF-8@FAI capsule interlayer is to improve the light utilization efficiency and improve the UV stability of perovskite films benefited from the down-conversion effect of ZIF-8, which could adsorb the UV light and re-emit low-energy visible light. In this case, the undesirable decomposition of perovskites under UV illumination can be effectively mitigated (Fig. 3c). The PCE evolution under 365 nm UV illumination (20 mW cm−2) has been further traced, as depicted in Fig. 3d. The PCE of the control device drops significantly, retaining only 15% of its original value, while the ZIF-8-modified and ZIF-8@FAI-modified devices could maintain 75% and 83% of their initial PCEs over 300 hours, respectively. Additionally, ZIF-8 could be also used as an interlayer between mesoporous TiO2 and perovskite. The ZIF-8 modification could effectively improve the crystallinity and grain size, and enhance the UV-visible absorption of the perovskite film, thus enabling the improvement of device efficiency by 25%.15
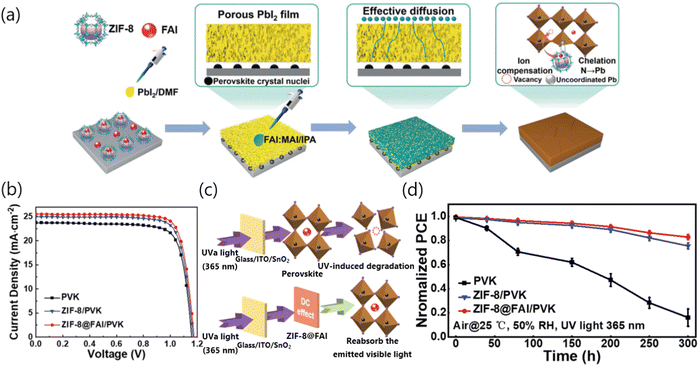 | ||
| Fig. 3 (a) Schematic of the two-step sequential deposition procedure for fabricating perovskite films assisted by the ZIF-8@FAI interface modifier. (b) J–V curves of control, ZIF-8 and ZIF-8@FAI based devices. (c) Schematic illustration of inhibiting the UV-induced degradation process via applying the ZIF-8@FAI down-conversion layer. Readapted with permission.15 (d) The PCE evolution behavior of the PSCs exposed to continuous 365 nm UV irradiation. Reproduced with permission.15 Copyright 2023, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
Similarly, Jin and co-workers explored a host–guest system consisting of methylammonium chloride confined by ZIF-8 nanosheets (MACl@ZIF-8) to modify the SnO2/perovskite interface. As shown in Fig. 4a, MACl@ZIF-8 acted as an interface molecular bridge that could simultaneously reduce the oxygen vacancies of SnO2 and optimize the crystallization quality of perovskite films via the strong chemical interactions with uncoordinated Pb2+ and halide ions of perovskites, thus resulting in a substantial PCE inreament from 19.61% to 22.10%. In particular, the UV shielding properties of the imidazole ligand in ZIF-8 could help to prevent potential perovskite decomposition induced by UV radiation (Fig. 4b), thus enabling durable perovskite photovoltaics to be realized.16 Subsequently, Dou and co-workers employed Eu-MOF as an interface modifier that was sandwiched between the SnO2 layer and the perovskite layer to improve device performance (Fig. 4c). Specifically, the metal ions and/or functional groups of Eu-MOF could chemically interact with perovskites, which eliminated the generation of Pb0 cluster and effectively reduced the deep-level defect states (Fig. 4d).17 In addition, the residual tensile strain of the perovskite film could transform into favorable compressive strain via the incorporation of Eu-MOF, which is beneficial to improve the stability of perovskite films and devices. Yuan and co-workers synthesized a specific MOF material with a π-conjugated aromatic structure, namely terpyridyl chromium (Cr-MOF). When used as a perovskite surface modifier, Cr-MOF could serve as an A-site cation to construct a new-type of 2D perovskite layer on the surface of the 3D perovskite layer (Fig. 4e). Interestingly, the π-conjugated terpyridyl could delocalize the excited valence electrons of Cr3+, thereby endowing the CsPbI2Br perovskites with multi-interactive charge-carrier transport channels. Compared with the control devices, the Cr-MOF-modified inverted PSCs based on CsPbI2Br perovskites yielded a high PCE of 17.02% (Fig. 4f), along with dramatically enhanced stability in air.18 Similarly, Lee and co-workers explored the effectiveness of two types of Zr-MOFs (UiO-66 and MOF-808) as interfacial modifiers at the NiOx HTL/perovskite interface. The results showcased that the PCE of the UiO-66/MOF-808-hybrid PSCs improved from 15.79% (control) to 18.01%, which can be attributed to the beneficial roles of Zr-MOF in passivating the defects and enhancing the robustness of the perovskite lattice.19
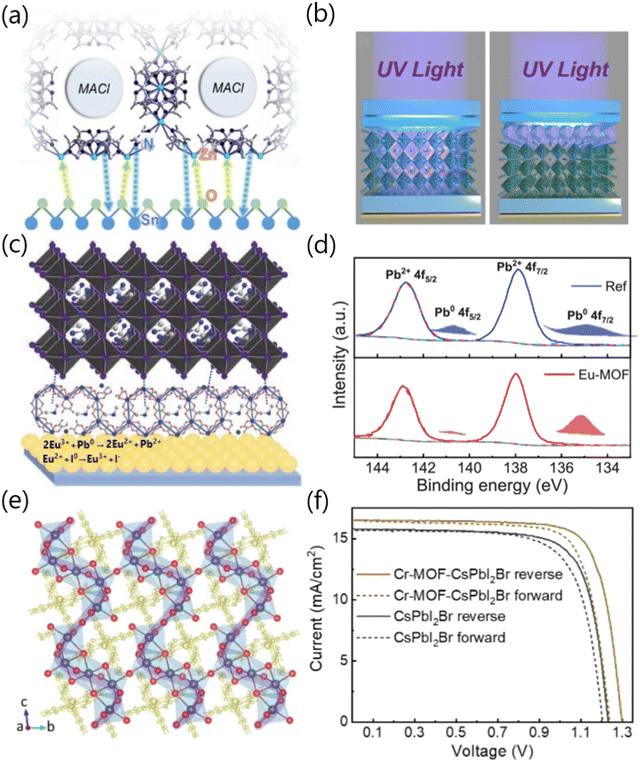 | ||
| Fig. 4 (a) Schematic diagram of the interaction between MACl@ZIF-8 and SnO2. (b) Schematic diagram of the UV shielding effect of PSCs induced by MACl@ZIF-8 interface modification (the left graph demonstrates the perovskite device without ZIF-8, while the right one demonstrates the perovskite device with ZIF-8 incorporated. ZIF-8 greatly reduces the impact of ultraviolet radiation on the perovskite layer due to its UV absorption properties). Reproduced with permission.16 Copyright 2023, The Royal Society of Chemistry. (c) Schematic illustration of the coordination between Eu-MOF and perovskite. Readapted with permission.17 Copyright 2021, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. (d) The high-resolution XPS spectra of Pb 4f showing the chemical interaction between Eu-MOF and perovskite. Reproduced with permission.17 Copyright 2021, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. (e) Crystal structure of the Cr-MOF-modified perovskite along the (100) lattice plane. (f) Reverse and forward scanning J–V curves of pristine CsPbI2Br and Cr-MOF-CsPbI2Br-based PSCs. Reproduced with permission.18 Copyright 2021 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
2.3. MOF-based charge transport layers
In addition to serving as the crystallization regulator, defect passivator or interfacial modifier for perovskites, MOFs materials are also competent enough to perform the role of the charge transport layer or chemically dope the charge transport layer through designing the molecular structure and modulating the chemical–physical properties, which could be significant for the improvement of light absorption, charge extraction and stability of the relevant devices.It is well-known that the application of Spiro-OMeTAD ([2,2′,7,7′-tetrakis(N,N-di(4-methoxyphenyl)amino)-9,9-spirobifluorene]) as the HTL for PSCs usually requires the introduction of hygroscopic lithium bis(trifluoromethanesulfonyl)imide (Li-TFSI) salts. However, the aggregation of Li salts and uncontrolled oxidation of Spiro-OMeTAD HTL severely affect the efficiency, stability and reproducibility of the corresponding devices. To address this issue, Dong and co-workers successfully designed and synthesized a novel MOF material, namely, POM@Cu-BTC, which could chemically dope Spiro-OMeTAD and exhibit multi-advantages. The presence of POM@Cu-BTC could accelerate the oxidation of Spiro-OMeTAD and simultaneously facilitate hole extraction and suppress charge recombination at the perovskite/HTL interface (Fig. 5a), thus yielding a PCE of 21.44% for the relevant PSCs, outperforming those of pristine PSCs (PCE = 20.21%) (Fig. 5b). Meanwhile, the long-term stability of PSCs under humid conditions could be significantly reinforced due to the incorporation of the solid-state POM@Cu-BTC dopant with the robust framework.20 Interestingly, the functional groups in MOFs could effectively interact with metal and halogen ions of perovskites and further inhibit ion migration. It is reported that the degradation of PSCs caused by external environmental factors such as oxygen and moisture can be suppressed through either the internal or external encapsulation of devices.21 Wang and co-workers synthesized a novel Li-TFSI endohedral MOFs dopant (namely Li-TFSI@NH2-MIL-101), which could provide steric hindrance for weakening Li+ migration and H2O molecule penetration to the perovskite film, thus significantly improving the long-term stability of the devices (Fig. 5c). Furthermore, the strong interaction between ammonium groups (–NH2) and uncoordinated Pb2+ ions would passivate the trap states, which further improved device performance.22 Benefitting from the above advantages, the Li-TFSI@NH2-MIL-101 doped-HTL based PSCs showed enhanced long-term stability, relative to the conventional Li-TFSI-based PSCs (Fig. 5d). Cao and co-workers successfully synthesized a two-dimensional conjugated MOF (2D c-MOF) material, namely, Cu3(HHTT)2 (HHTT: 2,3,7,8,12,13-hexahydroxytetraazanaphthotetraphene), which has been demonstrated as a new, but more well-matched HTL for Sn–Pb mixed PSCs, as compared to the commonly used HTLs such as NiOx or PEDOT:PSS.23 On the one hand, the HHTT ligand with expanded π-conjugation and embedded heteroatoms features ordered π–π stacking and strong inter-ligand interactions. On the other hand, the orbitals of square-planar Cu2+ ions were well-matched to the radical-state ligands, which could realize in-plane π conjugation (Fig. 5e). All these characteristics endowed superior carrier transport capability for the 2D c-MOF. Besides, the ultra-smooth surface of the Cu3(HHTT)2 film could be in favor of perovskite growth, enabling the formation of a high-quality, less-defect perovskite film, thus realizing an efficiency over 22.0% for the ideal-bandgap mixed Sn–Pb PSCs. Ryu and co-workers successfully synthesized a nanocrystalline Ti-based MOF (nTi-MOF) material and demonstrated that it could be perfectly adapted as an ETL in PSCs (Fig. 5f). The electronic structure of nTi-MOF was found to be suitable for charge injection and transfer from the perovskite layer to the front electrodes. Mixing [6,6]-phenyl-C61-butyric acid (PCBM) with nTi-MOF for constructing the hybrid ETL could not only enable efficient electron transfer, but also inhibit direct contact between the perovskite and the front electrode, which greatly reduced the carrier recombination, thus realizing good device performance for both rigid (PCE = 18.94%) and flexible (PCE = 17.43%) PSCs.24
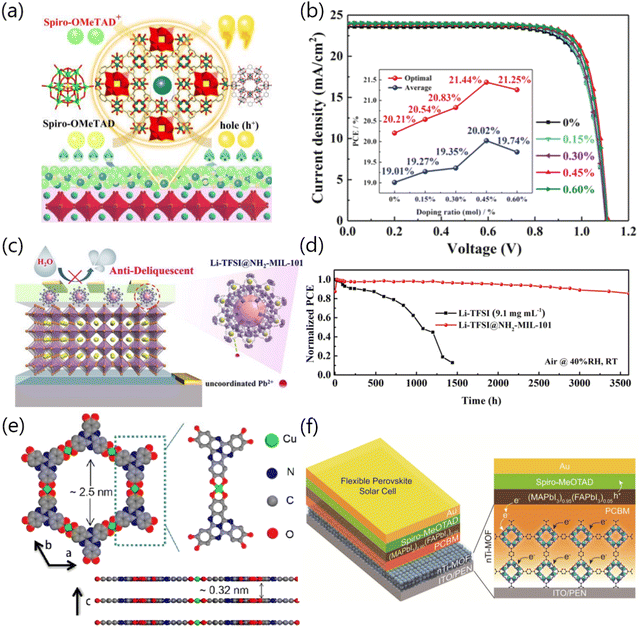 | ||
| Fig. 5 (a) Schematic diagram of the facilitated hole extraction and transfer via the incorporation of POM@Cu-BTC into Spiro-OMeTAD. (b) J–V curves of PSCs with different concentrations of POM@Cu-BTC (the inset figure shows the optimal doping ratio of POM@Cu-BTC and the average PCE value obtained from about 50 devices). Reproduced with permission.20 Copyright 2019, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. (c) Schematic diagram of the structure of Li-TFSI@NH2-MIL-101 and the corresponding interaction mechanism between Li-TFSI@NH2-MIL-101 and perovskite. Reproduced with permission.22 Copyright 2022 Elsevier B. V. (d) The long-term stability of unencapsulated devices stored in the surrounding environment (RH ≈ 40%, room temperature). Reproduced with permission.23 Copyright 2022, Elsevier B.V. (e) Schematic diagram of the crystal structure of the 2D c-MOF material. (f) Structure diagram of flexible PSCs based on the nTi-MOF ETL and related carrier transport dynamics within the devices. Reproduced with permission.24 Copyright 2018 American Chemical Society. | ||
2.4. MOF-assisted lead leakage inhibition
Generally, a mature commercial photovoltaic product needs to be non-toxic and harmless for human health and the ecological environment. However, the virulent lead-containing ingredients from perovskite photovoltaics seem to be a huge hidden danger, which overshadows the commercialization value of PSCs.25 Specially, Kwak and co-workers investigated the embryonic toxicity of PbI2 toward two kinds of fish species (i.e., zebrafish and Japanese medaka) and subsequently tracked the chemical speciation of PbI2 in the embryonic rearing medium. As shown in Fig. 6a, the fish exposed to PbI2 surroundings showed a dramatic increase in mortality, deformity, hatching failure, growth inhibition, and other pathologies when compared with the control counterparts.26 These results suggested the sublethal toxic effects of Pb2+, which, in turn, indicates that the development of lead adsorption strategies and the optimization design of device configurations to minimize the lead leakage from PSCs are extremely urgent. To solve the significant environmental risks and safety concerns caused by lead toxicity, an increasing level of enthusiasm to inhibit the lead leakage has been seen in the past few decades.27 To this end, the porous MOFs materials with tunable pore sizes, shapes as well as functions endow themselves with peculiar and selective adsorption properties for the dissociative metal ions, which are deemed to be promising lead sequestration scaffolds and thus have attracted widespread attention in recent years.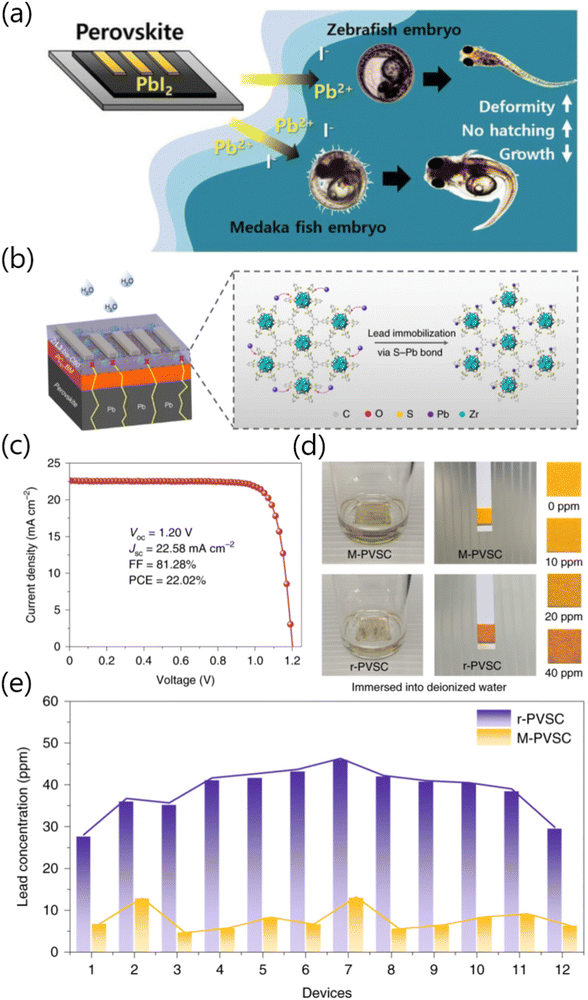 | ||
| Fig. 6 (a) Schematic illustration of embryonic toxicity of the PbI2 leaked from degraded PSCs and its sublethal effects in fish (zebrafish and medaka). Reproduced with permission.26 Copyright 2016, Springer Nature Limited. (b) Schematic showing the PSC device architecture and the corresponding lead immobilization caused by chemical interactions between the perovskites and the 2D ZrL3 MOFs. (c) The J–V curve of the champion PSC with ZrL3:bis-C60 ETL. (d) Pb concentration in the contaminated water roughly detected by the Pb testing paper. (e) Pb concentration in contaminated water accurately detected by ICP-OES measurements. Reproduced with permission.28 Copyright 2020, Springer Nature Limited. | ||
Recently, Wu and co-workers applied a 2D conjugated MOF material, namely, ZrL3, as the electrical-extraction layer (EEL) in PSCs (Fig. 6b). Zr ions could selectively bind carboxyl groups and created dense arrays of individual thiol groups around Zr oxygen clusters, which endowed the MOF material with steric shielding properties. In addition, the electron extraction and hole-blocking ability of relevant devices was effectively enhanced. As expected, the device with a ZrL3:bis-C60 ETL (denoted as M-PSCs) realized a PCE of 22.02%, which was significantly higher than that of the reference device (denoted as r-PSCs, 21.32%) (Fig. 6c). Particularly, ZrL3 showcased the capabilities in trapping heavy metal ions due to the networks with crosslinked disulfide linkages, thus simultaneously reducing the leakage of Pb2+ and improving the long-term operational stability of PSCs. To specifically quantify the Pb leakage that occurs in degraded PSCs, the simulation of the rainwater washout from PSCs by immersing PSCs in deionized water (acid-rain pattern, pH ∼ 5.6) has been further conducted. As shown in Fig. 6d, the Pb2+ concentration of water extract from the r-PSC sample is much higher than that of the M-PSC counterpart, as is clearly indicated by the Pb2+ test paper. Furthermore, the Pb2+ concentration has been precisely quantified using the inductively coupled plasma optical emission spectroscopic (ICP-OES) technology. Compared with the r-PSC counterparts, the average Pb2+ concentration of M-PSCs dropped sharply from 38.4 ppm to 7.6 ppm (Fig. 6e), indicating the efficient suppression of Pb leakage due to the chelation between thiol-functionalized ZrL3 and Pb2+,28 thus minimizing the adverse impacts of PSC deployment on human health and ecosystems.
3. Conclusions and outlook
In summary, MOFs materials have demonstrated a variety of excellent functionalities, which endow the great promise for applications in perovskite photovoltaics. The recent advancement of the MOFs-tailored PSCs has been summarized. Encouragingly, the best-performing PSCs fabricated via MOFs modification showed a champion PCE of 24.08% (Table 1). The MOFs materials featuring high porosity could serve as a scaffold for perovskite growth, which promotes the crystallization of perovskites and facilitates the formation of high-quality perovskite films. Obviously, the abundant coordination sites of MOFs play the role of multidentate claws that could jointly passivate a variety of deep-level defects of perovskite films, reinforce interfacial contact junctions, accelerate charge extraction and suppress the interfacial charge recombination within PSCs. The incorporation of MOFs could effectively improve the humidity, heat and/or light stability of perovskite films and devices. In detail, when serving as a CTL or an interfacial modifier, the MOFs molecules could favorably interact with perovskites and inhibit the ion migration within the PSCs, thus remarkably improving the light/operational stability of the resultant devices. Upon being incorporated as additives in perovskite precursors for crystallization regulation and defect passivation, the MOFs molecules primarily play critical roles in achieving high-quality perovskite films with enhanced moisture resistance, thus endowing relevant PSCs with resilience under humid conditions. Most importantly, the value-added advantage in terms of Pb leakage mitigation in MOFs-modified PSCs is extremely prominent, which concurrently addresses the most challenging lead contamination issues. Apparently, this is in line with the objective of realizing a balanced trade-off in terms of the efficiency–stability–environmental friendliness relationship towards practical application and real commercialization. To maximize the superiority of MOFs materials applied in PSCs, some future research directions of merit should be considered, which are proposed as follows.| MOFs materials | Roles | V oc (V) | J sc (mA cm−2) | PCE (%) | FF | Ref. |
|---|---|---|---|---|---|---|
| Zn-TTB | Crystallization regulator | 1.148 | 25.16 | 23.14 | 0.801 | 13 |
| PCN-224 | Crystallization regulator | 1.153 | 24.09 | 21.59 | 0.777 | 14 |
| Carrier transport layer | 1.172 | 24.56 | 22.51 | 0.782 | ||
| ZIF-8@FAI | Interface modifier | 1.176 | 25.55 | 24.08 | 0.801 | 15 |
| MACl@ZIF-8 | Interface modifier | 1.160 | 24.07 | 22.10 | 0.791 | 16 |
| Eu-MOF | Interface modifier | 1.140 | 23.71 | 22.16 | 0.820 | 17 |
| Cr-MOF | Interface modifier | 1.300 | 16.51 | 17.02 | 0.790 | 18 |
| UiO-66 | Interface modifier | 1.072 | 21.85 | 18.01 | 0.769 | 19 |
| MOF-808 | Interface modifier | 1.062 | 21.01 | 17.81 | 0.798 | |
| POM@Cu-BTC | Interface modifier | 1.110 | 23.90 | 21.44 | 0.800 | 20 |
| Li-TFSI@NH2-MIL-101 | Interface modifier | 1.073 | 23.41 | 19.01 | 0.757 | 22 |
| Cu3(HHTT)2 | Carrier transport layer | 0.920 | 30.13 | 22.01 | 0.794 | 23 |
| nTi-MOF | Carrier transport layer | 1.066 | 22.47 | 16.41 | 0.682 | 24 |
| nTi-MOF/PCBM | Carrier transport layer | 1.082 | 23.18 | 18.94 | 0.755 | |
| ZrL3 | Lead leakage inhibitor | 1.200 | 22.58 | 22.02 | 0.813 | 28 |
Firstly, the more rational molecular configuration design of MOFs materials is necessary for their coordination strength, conductivity and conformational stability. Hence, one should consider developing more advanced MOFs-derived materials featuring: (i) abundant functional groups. This enables multiple chemical coordination with perovskites such as hydrogen bonds, ionic bonds, covalent bonds, etc., which could maximize the passivation efficacy and extend the passivation longevity. (ii) Suitable pore size and topology: the pore size and topology of MOFs could be tuned via modulating metal ions, organic ligands and synthetic solvents. MOFs with relatively large porous structures are beneficial to the perovskite penetration, thus facilitating the crystal growth and enabling the formation of high-quality, low-defect perovskite films. (iii) High electrical conductivity: usually, MOFs molecules exhibit poor conductivity owing to the relatively weak electronic interactions between metal nodes and conventional organic moieties. MOFs that afford the matchable energy level and good charge transport properties could efficaciously reduce the interfacial impedance and further restrain the non-recombination loss of PSCs. Through the rational design of MOFs materials, their conductivity should be unceasingly improved, thus further promoting the charge transport and the device efficiency of relevant devices. Notably, the accessible ligand selection of MOFs is crucial for their electrical conductivity. (iv) More multifarious and bendable frame structure: MOFs materials as additives or interfacial layers could serve as bifacial molecular bridges and/or grain-to-grain linkers through multiple interactions, thus further enhancing the interfacial mechanical toughness. This is expected to effectively improve the bending resistance of the devices, hence enabling the broader application potential of MOFs materials in constructing flexible and wearable PSCs. In addition, although the capability of MOFs in capturing Pb2+ ions from degraded devices has been demonstrated, the distinct anchoring behavior of metal ions determined by the diversity of porosity and ligand nanostructures of MOFs derivatives deserves to be further systematically investigated. Last but not least, some other unique advantages of MOFs materials are yet to be explored further, and their potential applications in other perovskite-based optoelectronic devices should also be explored, such as detectors or X-ray imagers.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was supported by Tianjin Normal University Research Innovation Project for post-graduate (2023KYCX071Y). W. Q. Wu acknowledges the financial support from Guangxi Key Laboratory of Advanced Structural Materials and Carbon Neutralization (NO. GXAMCN23-2).References
- N. Li, X. Niu, L. Li, H. Wang, Z. Huang, Y. Zhang, Y. Chen, X. Zhang, C. Zhu, H. Zai, Y. Bai, S. Ma, H. Liu, X. Liu, Z. Guo, G. Liu, R. Fan, H. Chen, J. Wang, Y. Lun, X. Wang, J. Hong, H. Xie, D. S. Jakob, X. G. Xu, Q. Chen and H. Zhou, Liquid medium annealing for fabricating durable perovskite solar cells with improved reproducibility, Science, 2021, 373, 561–567 CrossRef CAS PubMed.
- J. Chen, X. Zhao, K. Seulgi and N.-G. Park, Multifunctional chemical linker imidazoleacetic acid hydrochloride for 21% efficient and stable planer perovskite solar cells, Adv. Mater., 2019, 31, 1902902 CrossRef PubMed.
- J. Park, J. Kim, H. Yun, M. J. Paik, E. Noh, H. J. Mun, M. G. Kim, T. J. Shin and S. I. Seok, Controlled growth of perovskite layers with volatile alkylammonium chlorides, Nature, 2023, 616, 724–730 CrossRef CAS PubMed.
- J. Huang, S. Tan, P. Lund and H. Zhou, Impact of H2O on organic-inorganic hybrid perovskite solar cells, Energy Environ. Sci., 2017, 10, 2284–2311 RSC.
- A. K. Jena, A. Kulkarni and T. Miyasaka, Halide perovskite photovoltaics: background, status, and future prospects, Chem. Rev., 2019, 119, 3036–3103 CrossRef CAS PubMed.
- L. E. Kreno, K. Leong, O. K. Farha, M. Allendorf, R. P. Van Duyne and J. T. Hupp, Organic framework materials as chemical sensors, Chem. Rev., 2012, 112, 1105–1125 CrossRef CAS PubMed.
- L. Wen, F. Wang, J. Feng, K. Lv, C. Wang and D. Li, Structures, photoluminescence, and photocatalytic properties of six new metal-organic frameworks based on aromatic polycarboxylate acids and rigid imidazole-based synthons, Cryst. Growth Des., 2009, 9, 3581–3589 CrossRef CAS.
- D. T. Heo, H. H. Do, S. H. Ahn and S. Y. Kim, Metal-organic framework materials for perovskite solar cells, Polymers, 2020, 12, 2061 CrossRef CAS PubMed.
- S. K. Yadav, G. K. Grandhi, D. P. Dubal, J. C. de Mello, M. Otyepka, R. Zbořil, R. A. Fischer and K. Jayaramulu, Metal halide perovskite@metal-organic framework hybrids: synthesis, design, properties, and applications, Small, 2020, 16, 2004891 CrossRef CAS PubMed.
- H. He, Y. Cui, B. Li, B. Wang, C. Jin, J. Yu, L. Yao, Y. Yang, B. Chen and G. Qian, Confinement of perovskite-QDs within a single MOF crystal for significantly enhanced multiphoton excited luminescence, Adv. Mater., 2018, 31, 1806897 CrossRef PubMed.
- J. Ren, T. Li, X. Zhou, X. Dong, A. V. Shorokhov, M. B. Semenov, V. D. Krevchik and Y. Wang, Encapsulating all-inorganic perovskite quantum dots into mesoporous metal organic frameworks with significantly enhanced stability for optoelectronic applications, Chem. Eng. J., 2019, 358, 30–39 CrossRef CAS.
- C. Zhang, W. Li and L. Li, Metal halide perovskite nanocrystals in metal–organic framework host: not merely enhanced stability, Angew. Chem., Int. Ed., 2020, 60, 7488–7501 CrossRef PubMed.
- J. Wang, J. Zhang, S. Gai, W. Wang, Y. Dong, B. Hu, J. Li, K. Lin, D. Xia, R. Fan and Y. Yang, Self-organized small molecules in robust MOFs for high-performance perovskite solar cells with enhanced degradation activation energy, Adv. Funct. Mater., 2022, 32, 2203898 CrossRef CAS.
- Y. Liu, T. Liu, X. Guo, M. Hou, Y. Yuan, S. Shi, H. Wang, R.-Z. Zhang, C. Galiotis and N. Wang, Porphyrinic metal–organic framework quantum dots for stable n–i–p perovskite solar cells, Adv. Funct. Mater., 2022, 33, 2210028 CrossRef.
- W. Sheng, J. He, J. Yang, Q. Cai, S. Xiao, Y. Zhong, L. Tan and Y. Chen, Multifunctional metal-organic frameworks capsules modulate reactivity of lead iodide toward efficient perovskite solar cells with ultraviolet resistance, Adv. Mater., 2023, 10, 202301852 Search PubMed.
- Z. Jin, B. Li, Y. Xu, B. Zhu, G. Ding, Y. Wang, J. Yang, Q. Zhang and Y. Rui, Confinement of MACl guest in 2D ZIF-8 triggers interface and bulk passivation for efficient and UV-stable perovskite solar cells, J. Mater. Chem. C, 2023, 11, 6730–6740 RSC.
- J. Dou, C. Zhu, H. Wang, Y. Han, S. Ma, X. Niu, N. Li, C. Shi, Z. Qiu, H. Zhou, Y. Bai and Q. Chen, Synergistic effects of Eu-MOF on perovskite solar cells with improved stability, Adv. Mater., 2021, 33, 2102947 CrossRef CAS PubMed.
- S. Yuan, Y. Xian, Y. Long, A. Cabot, W. Li and J. Fan, Chromium-based metal–organic framework as A-site cation in CsPbI2Br perovskite solar cells, Adv. Funct. Mater., 2021, 31, 2106233 CrossRef CAS.
- C. Lee, C. Chen, Y. Liao, K. Wu and C. Chueh, Enhancing efficiency and stability of photovoltaic cells by using perovskite/Zr-MOF heterojunction including bilayer and hybrid structures, Adv. Sci., 2018, 5, 1801715 Search PubMed.
- Y. Dong, J. Zhang, Y. Yang, L. Qiu, D. Xia, K. Lin, J. Wang, X. Fan and R. Fan, Self-assembly of hybrid oxidant POM@Cu-BTC for enhanced efficiency and long-time stability of perovskite solar cells, Angew. Chem., Int. Ed., 2019, 57, 2565–2571 Search PubMed.
- L. Duan and U. Ashraf, Defects and stability of perovskite solar cells: a critical analysis, Mater. Chem. Front., 2021, 6, 400–417 RSC.
- J. Wang, J. Zhang, Y. Yang, Y. Dong, W. Wang, B. Hu, J. Li, W. Cao, K. Lin, D. Xia and R. Fan, Li-TFSI endohedral metal-organic frameworks in stable perovskite solar cells for anti-deliquescent and restricting ion migration, Chem. Eng. J., 2022, 429, 132481 CrossRef CAS.
- J. Cao, C.-K. Liu, V. Piradi, H.-L. Loi, T. Wang, H. Cheng, X. Zhu and F. Yan, Metal−organic framework films as hole transport layers in ideal-bandgap perovskite solar cells, ACS Energy Lett., 2022, 7, 3362–3369 CrossRef CAS.
- U. Ryu, S. Jee, J.-S. Park, I.-K. Han, J.-H. Lee, M. Park and K.-M. Choi, Nanocrystalline titanium metal-organic frameworks for highly efficient and flexible perovskite solar cells, ACS Nano, 2018, 12, 4698–4975 Search PubMed.
- Y. Zhang, B. Li, L. Fu, Q. Li and L. Yin, MOF-derived ZnO as electron transport layer for improving light harvesting and electron extraction efficiency in perovskite solar cells, Electrochim. Acta, 2019, 330, 135280 CrossRef.
- A. Babayigit, D. Thanh, A. Ethirajan, J. Manca, M. Muller, H.-G. Boyen and B. Conings, Assessing the toxicity of Pb- and Sn-based perovskite solar cells in model organism Danio rerio, Sci. Rep., 2016, 6, 18721 CrossRef CAS PubMed.
- D. He, L. Shen, Y. Bai and L. Wang, Rational strategies toward efficient and stable lead-free tin halide perovskite solar cells, Mater. Chem. Front., 2021, 5, 4107–4127 RSC.
- S. Wu, Z. Li, M.-Q. Li, Y. Diao, F. Lin, T. Liu, J. Zhang, P. Tieu, W. Gao, F. Qi, X. Pan, Z. Xu, Z. Zhu and A. K.-Y. Jen, 2D metal–organic framework for stable perovskite solar cells with minimized lead leakage, Nat. Nanotechnol., 2020, 15, 934–940 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © the Partner Organisations 2024 |





