Developing a gradient titanium dioxide/amorphous tantalum nitride electron transporting layer for efficient and stable perovskite solar cells†
Yue
Gou
a,
Haoyan
Wang
a,
Yutao
Li
a,
Chenyu
Zhao
a,
Lin
Fan
 ab,
Maobin
Wei
ab,
Huilian
Liu
ab,
Jinghai
Yang
ab,
Maobin
Wei
ab,
Huilian
Liu
ab,
Jinghai
Yang
 ab,
Fengyou
Wang
ab,
Fengyou
Wang
 *ab and
Lili
Yang
*ab and
Lili
Yang
 *ab
*ab
aKey Laboratory of Functional Materials Physics and Chemistry of the Ministry of Education, Jilin Normal University, Changchun 130103, China. E-mail: wfy@jlnu.edu.cn; llyang1980@163.com
bNational Demonstration Center for Experimental Physics Education, Jilin Normal University, Siping 136000, China
First published on 14th September 2023
Abstract
Metal oxides are extensively applied as one of the most potential electron transport layers (ETLs) in perovskite solar cells (PSCs). However, their inherent surface oxygen vacancies and imperfect energy level alignment with the perovskite layer usually result in photogenerated charge recombination at the ETL/perovskite heterointerface. Managing the interface defects and band alignment is significant to decrease energy loss and improve the power conversion efficiency of PSCs. Herein, we develop a gradient TiO2/a-TaNx (TOAN) ETL, which not only eliminates interfacial defects and improves the crystallization of perovskite films, but also displays good energy level alignment and charge transport characteristics. The MAPbI3 solar cells based on TOAN ETLs achieve an efficiency of 21.41% with an open-circuit voltage of 1.20 V and a short-circuit current density of 22.87 mA cm−2. In addition, the device with a TOAN ETL maintains 95% initial efficiency after 30 days in N2 without encapsulation and retains 91% of its initial efficiency after storing for 30 days in an atmospheric environment. This result demonstrates the effectiveness of an inorganic metal nitride as an interface modification layer and provides a reference for the further development of metal nitride electron transport layers.
Introduction
Organic–inorganic halide perovskite solar cells (PSCs) have rapidly evolved over the past few decades as a promising next-generation photovoltaic technology, owing to their benefits including small exciton binding energy, long carrier diffusion length, high absorption coefficient, ambipolar charge transport, and strong defect tolerance.1–5 The power conversion efficiency (PCE) of PSCs has risen from 3.8% (in 2009) to 26.1% (certified in 2023) via the strategies of thin film crystallization regulation, interface engineering, effective light management, and so on.6–12The electron transfer layer (ETL)/perovskite interface of PSCs plays a key role in tuning the crystallinity of the perovskite layer and promoting the overall charge transport efficiency.13,14 However, the widely used metal oxide ETLs often have surface oxygen vacancies and undesirable conduction band offsets with the perovskite, resulting in interfacial charge recombination loss. Meanwhile, the hydroxyl group introduced during the ETL fabrication process is easily absorbed into the surface oxygen vacancies, which may trigger the deprotonation reaction with the perovskite layer and degrade the long-term stability of the device. Many strategies are proposed to eliminate the oxygen vacancies and rearrange the energy levels at the ETL/perovskite interface for achieving better cell performance and stability. For instance, several electron-donor groups such as amino (–NH2), carboxyl (–COOH), and carbon–oxygen double bonds (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) have been utilized to reduce the uncoordinated dangling bonds at the ETL/perovskite interface,15–17 which inhibit the non-radiative recombination and cation migration, improving the PCE and retarding the decomposition of the devices. Notably, Sonmezoglu et al. adopted a series of modifiers, including 2-methylbenzimidazole, acetylacetone, biopolymer Caf1, and DNA molecules, to modify the ETL surface. These modifiers suppressed the interface-dependent nonradiative recombination and aligned the interface energy levels, leading to a remarkable improvement of the solar cells.18–21 Furthermore, a common method to strengthen the charge transfer at the ETL/perovskite interface is adjusting the energy level alignment, which could enhance the PCE by reducing the energy loss.22–24 For example, doping with metals ions, such as lithium, sodium, potassium, and tungsten, can change the bandgap alignment of ETL/perovskite,25,26 thus improving the conductivity and short-circuit current (Jsc) of the devices. The interfacial bandgap alignment can also be adjusted by constructing hybrid ETLs. Chen et al. developed a SnO2/2D-Bi2O2Se hybrid electron transporting layer, which possesses a better cascade band alignment with the perovskite layer compared to a single SnO2, resulting in low-hysteresis and high-performance PSCs.27 Zhang et al. strengthened the SnO2–perovskite buried interface by a strategy involving pre-grafted halides to manipulate perovskite defects and carrier dynamics, thereby resulting in both favorable perovskite crystallization and minimized interfacial carrier losses.28 Ghosh et al. demonstrated a low temperature solution-processed TiO2/AZO bilayer thin film as an ETL in a planar PSC configuration. The corresponding efficiency is improved owing to the reduction of trap-assisted recombination at the interface and better energy level alignment.29 Previously, we used an a-WOx/SnO2 ETL to adjust the energy level of the ETL/perovskite interface, which improves the free energy of the ETL/perovskite interface from 0.34 to 0.6 eV and enhances the driving force of charge transfer, yielding a PCE of 20.52%.30 All the above interface modulators contribute to the development of PSCs from different aspects, but some shortcomings are gradually exposed along with thorough research. For example, the organic modulators are usually subjected to solvent erosion from perovskite precursors and face the thermal instability issue, destroying the interface connection; moreover, the inorganic modulators are more stable but have no ideal passivation capability due to the lack of functionalized groups. Exploring a stable material that can not only reduce interface defects but also accelerate charge extraction at the ETL/perovskite interface is very significant for raising the PSC efficiency.
O) have been utilized to reduce the uncoordinated dangling bonds at the ETL/perovskite interface,15–17 which inhibit the non-radiative recombination and cation migration, improving the PCE and retarding the decomposition of the devices. Notably, Sonmezoglu et al. adopted a series of modifiers, including 2-methylbenzimidazole, acetylacetone, biopolymer Caf1, and DNA molecules, to modify the ETL surface. These modifiers suppressed the interface-dependent nonradiative recombination and aligned the interface energy levels, leading to a remarkable improvement of the solar cells.18–21 Furthermore, a common method to strengthen the charge transfer at the ETL/perovskite interface is adjusting the energy level alignment, which could enhance the PCE by reducing the energy loss.22–24 For example, doping with metals ions, such as lithium, sodium, potassium, and tungsten, can change the bandgap alignment of ETL/perovskite,25,26 thus improving the conductivity and short-circuit current (Jsc) of the devices. The interfacial bandgap alignment can also be adjusted by constructing hybrid ETLs. Chen et al. developed a SnO2/2D-Bi2O2Se hybrid electron transporting layer, which possesses a better cascade band alignment with the perovskite layer compared to a single SnO2, resulting in low-hysteresis and high-performance PSCs.27 Zhang et al. strengthened the SnO2–perovskite buried interface by a strategy involving pre-grafted halides to manipulate perovskite defects and carrier dynamics, thereby resulting in both favorable perovskite crystallization and minimized interfacial carrier losses.28 Ghosh et al. demonstrated a low temperature solution-processed TiO2/AZO bilayer thin film as an ETL in a planar PSC configuration. The corresponding efficiency is improved owing to the reduction of trap-assisted recombination at the interface and better energy level alignment.29 Previously, we used an a-WOx/SnO2 ETL to adjust the energy level of the ETL/perovskite interface, which improves the free energy of the ETL/perovskite interface from 0.34 to 0.6 eV and enhances the driving force of charge transfer, yielding a PCE of 20.52%.30 All the above interface modulators contribute to the development of PSCs from different aspects, but some shortcomings are gradually exposed along with thorough research. For example, the organic modulators are usually subjected to solvent erosion from perovskite precursors and face the thermal instability issue, destroying the interface connection; moreover, the inorganic modulators are more stable but have no ideal passivation capability due to the lack of functionalized groups. Exploring a stable material that can not only reduce interface defects but also accelerate charge extraction at the ETL/perovskite interface is very significant for raising the PSC efficiency.
Transition metal nitrides, as stable inorganic compounds,31 with abundant N atoms and a tunable bandgap, may satisfy the above requirements simultaneously by donating lone pair electrons as well as forming gradient energy alignment with the perovskite layer. Herein, we developed a gradient titanium dioxide/amorphous tantalum nitride (TiO2/a-TaNx, TOAN) hybrid ETL to suppress ETL/perovskite interfacial energy loss and improve the photovoltaic performance of the PSCs. The TOAN possesses higher conductivity and a more suitable interface energy-level cascade compared to traditional TiO2which contributes to reducing the ETL/perovskite interfacial charge accumulation and the electron transfer barrier. Meanwhile, the N-rich surface of the TOAN ETL can eliminate the ETL/perovskite interfacial defects by forming N–Pb electron transfer channels with the perovskite layer, thus decreasing the non-radiative recombination loss. Concomitantly, the Gibbs free energy of the TOAN substrate is elevated compared with that of the TiO2 ETL, promoting the crystallization and growth of perovskite films. Pinhole-free and compact perovskite films could enhance the environmental stability and photostability of the devices. Consequently, the PSC based on a TOAN ETL showed a higher PCE of 21.47% with negligible hysteresis compared with the TiO2 device that had a PCE of 19.42%. In addition, the PSC based on the TOAN ETL retained 91% of its original efficiency after aging for 30 days under an ambient atmosphere.
Results and discussion
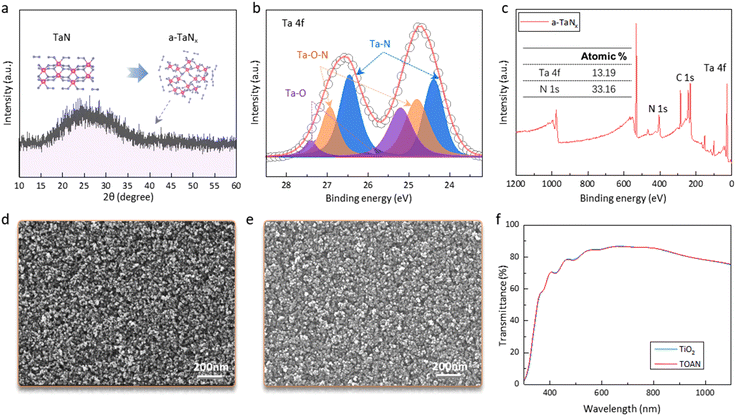
An ∼80 nm tantalum nitride film was prepared at room temperature by radio-frequency magnetron sputtering. Transmission electron microscopy (TEM) and X-ray diffraction (XRD) were conducted to explore the film microstructure. The TEM image shows no microcrystallite, and the diffusion rings are weak and broad, implying the characteristics of an amorphous metallic compound (Fig. S1†).32 Meanwhile, we only find a wide and degenerated peak of glass at 24.42° from the XRD patterns, which further proves the amorphous nature of the TaNx (a-TaNx) (Fig. 1a). To study the surface chemical states of a-TaNx film, X-ray photoelectron spectroscopy (XPS) was performed. The Ta 4f peak can be divided into three doublets, belonging to the Ta 4f7/2 (24.7, 25.5, and 26.2 eV) and the Ta 4f5/2 (26.5, 27.4, and 28.2 eV) orbits (Fig. 1b). The dominant Ta 4f7/2 peak and Ta 4f5/2 peak at binding energies of 24.7 and 26.5 eV are associated with Ta–N bonding, respectively. The Ta 4f7/2 and Ta 4f5/2 subcomponents centered at 25.5 and 27.4 eV are attributed to the Ta-ON species, and the remaining pair of peaks at 26.2 and 28.2 eV represents the Ta–O.33 These different chemical bonds and valence states of Ta imply that the a-TaNx films are non-stoichiometric. The reason for the formation of amorphous and non-stoichiometric TaNx films is that the sputtered TaNx precursor radicals have a low migration energy on the room-temperature substrate, making them only able to stay in the position where they hit the substrate and leading to a disordered network matrix. Moreover, XPS shows that the atomic ratio of Ta–N is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5, which is higher than the usual 1
2.5, which is higher than the usual 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 and 1
0.5 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.6 (Fig. 1c).34,35 This N-rich surface is beneficial to eliminate the trap states at the ETL/perovskite interface and will be discussed later.
1.6 (Fig. 1c).34,35 This N-rich surface is beneficial to eliminate the trap states at the ETL/perovskite interface and will be discussed later.
We then constructed the TiO2/a-TaNx (TOAN) film by depositing 5 nm a-TaNx on the TiO2 surface. The uniform distribution of Ta and N elements indicates that the a-TaNx is finely distributed on the TiO2 surface (Fig. S2†). The surface morphologies of TiO2 and TOAN films were studied by scanning electron microscopy (SEM, Fig. 1d and e) and atomic force microscopy (AFM, Fig. S3†). The images show that the TOAN film is slightly smoother than the TiO2 film, with the root-mean-square (RMS) roughness reduced from 23.4 nm to 21.7 nm. In addition, since the Ta of a-TaNx has many more valence electrons compared with the Ti of TiO2, Ta has extra electronic donor centers, which may lead to higher conductivity of TOAN.36 The electrical properties of TiO2 and TOAN films were characterized in the dark using the vertical current density–voltage (J–V) test with an FTO/TiO2 (or TOAN)/Ag structure. As shown in Fig. S4,† the increased slope of the TOAN films shows that the resistance values are reduced compared with those of TiO2 for a similar thickness. The conductivity of the films can be calculated using the following equation: σ = d/(AR), where σ is the conductivity, A is the active area (0.09 cm2), R is the resistance calculated from the dark I–V curves, and d is the thickness of the films (∼200 nm).37 The values of σ are ∼1.2 × 10−2 mS cm−1, ∼2.04 × 10−2 mS cm−1 and ∼3.8 × 10−2 mS cm−1 for TiO2, TOAN and a-TaNx films, respectively, which show that the TOAN film has a better charge transport capability than the TiO2 film. Besides that, the efficiency of PSCs is affected by the generation of photogenerated carriers, which is associated with the ETL optical properties. Fig. 1f shows the optical transmittance spectra of TiO2 and TOAN films, both of which exhibit high transmittance in the visible region. The high conductivity, together with the excellent optical transmittance, makes the TOAN a suitable material for thin-film photovoltaic devices.
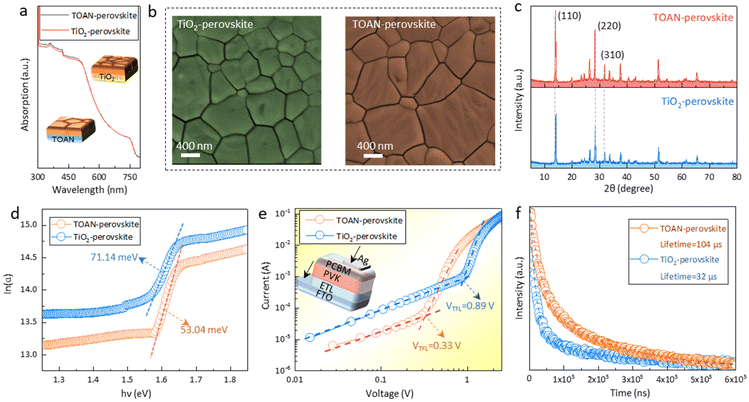
Ultraviolet–visible (UV–vis) spectroscopy was performed to further study the optical absorption of perovskite films grown on different substrates. Fig. 2a shows that the optical absorption of the two perovskite films is analogous, indicating that the TOAN layer has no additional parasitic absorption, which matches the results in Fig. 1f. The perovskite crystallization is also a key factor influencing the photovoltaic performance of PSCs. Fig. 2b and Fig. S5† present the SEM and AFM images of perovskite films based on TiO2 (TiO2-perovskite) and TOAN (TOAN-perovskite). Interestingly, the TOAN-perovskite shows a uniform surface and lower roughness in contrast to the TiO2-perovskite. Fig. S6† shows that TOAN-perovskite possesses a larger grain size (∼800 nm) than TiO2-perovskite (∼450 nm). The X-ray diffraction (XRD) peak of TOAN-perovskite is stronger and sharper than that of TiO2-perovskite, and the corresponding full width at half-maximum (FWHM) reduces from 0.228° to 0.182° at the main (110) crystallographic plane, consistent with the increased grain size shown by SEM (Fig. 2c). The favorable crystallization of perovskite films can help reduce their bulk defects. We then measured the Urbach tail energy (Eu) of the two perovskite films. A reduced Eu of the TOAN-perovskite film is shown in Fig. 2d, indicating a smaller energetic disorder within the bulk of the film.38 In order to quantitatively clarify the defect density of the perovskite films, the space-charge limited current (SCLC) was assessed with the device structure FTO/TiO2 (or TOAN)/perovskite/PCBM/Ag (Fig. 2e). The defect density can be calculated using the following equation: Ntrap = (2εε0VTFL)/(eL2), where ε is the MAPbI3 permittivity (ε = 32 F cm−1), ε0 is the vacuum permittivity, VTFL is the trap-filled voltage, e is the unit charge, and L is the thickness of the perovskite.39 As expected, compared with the TiO2 device (VTFL = 0.89 V, Ntrap = 2.57 × 1016 cm−3), the TOAN device shows smaller VTFL (0.33 V) and Ntrap (8.8 × 1015 cm−3). Moreover, as shown in Fig. 2f, the photovoltage decay lifetime of TOAN-perovskite (104 μs) is also higher than that of TiO2-perovskite (32 μs), confirming a decreased non-radiative recombination at the ETL/perovskite interface.40
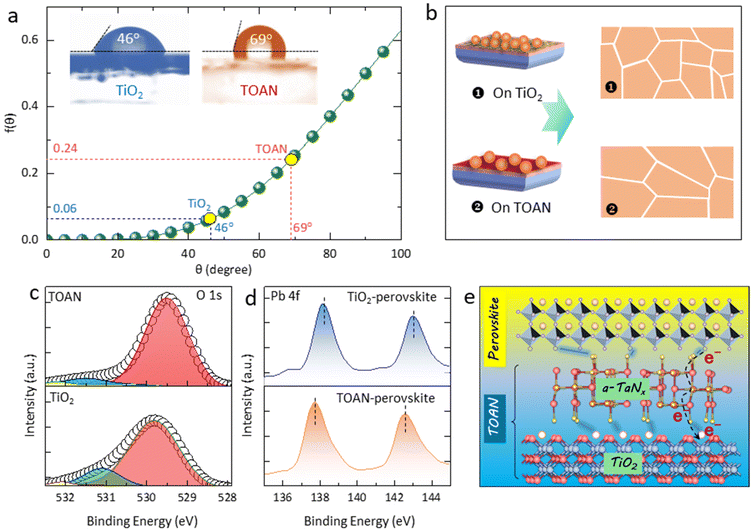
Considering that the preparation process of all of the perovskite films is identical, we deduce that the enhanced crystallization of the perovskite is probably correlated with the surface properties of the substrate. Generally, perovskite nucleation and growth largely depend on the Gibbs free energy (ΔG) of the substrate surface, and the heterogeneous nucleation barrier ΔGhe can be represented by the following equation:41
| ΔGhe = ΔGh0·f(θ) | (1) |
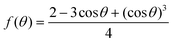 | (2) |
The change in ΔGhe may be attributed to the difference in dangling bonds on the substrate surface.43Fig. 3c shows that the TiO2 film has three O 1s peaks at 529.79, 531.18, and 531.9 eV, which belong to the lattice oxygen, oxygen deficiency, and O–H bonding, respectively.44 However, for the TOAN film, the O 1s peak of the oxygen deficiency is apparently weakened, suggesting that the oxygen deficiency defects are passivated by the surface N atoms. We have conducted electron spin resonance (EPR) measurements on both the TiO2 and TOAN films (Fig. S7†). The g-value calculated from the resonance magnetic field is 2.006, which is similar to the g-value of the free electron (2.008) that corresponds to an oxygen vacancy. The higher peak intensity observed in the TOAN film compared to that in the TiO2 film indicates that the oxygen vacancies on the TiO2 surface are effectively passivated by the a-TaNx layer.45–47 This result demonstrates that the redistribution of surface bonds affects the electron configuration of the substrate surface, thereby changing the ΔGhe and the substrate wettability.48
Apart from modulating perovskite crystallization, the N-rich surface of the TOAN may also passivate the uncoordinated Pb2+ at the perovskite buried interface, reducing the interfacial recombination loss. Fourier transform infrared (FTIR) spectra show that the stretching vibration of the N–H bond in the TiO2-perovskite located at 1499 and 3492 cm−1 is obviously shifted to 1491 and 3468 cm−1 for the TOAN-perovskite (Fig. S8†). XPS was also conducted for the TOAN-perovskite and TiO2-perovskite. In order to accurately reflect the bonding situation at the ETL/perovskite interface, ion beams were utilized to etch the perovskite films (from the top surface) to ∼10 nm before the measurement (Fig. 3d). The Pb 4f7/2 and Pb 4f5/2 peaks of the TOAN-perovskite film show a large shift towards lower binding energies compared to those of the TiO2-perovskite. This downshift in peaks in the TOAN-perovskite implies Pb–N coordination between TOAN and perovskite because N can donate electrons to Pb2+ dangling bonds, weakening the binding energy of electrons outside the Pb nucleus.49,50 Therefore, from the overall analysis of the bonding situation, it can be seen that the N in TOAN can not only effectively eliminate the oxygen vacancy defects on the ETL surface, but also passivate the dangling bonds of the perovskite buried interface, polishing the microstructure of the ETL/perovskite interface (Fig. 3e).
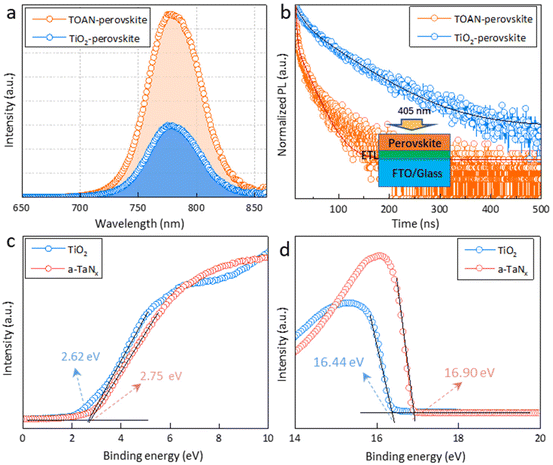
The dynamics of charge transfer at the ETL/perovskite interface was measured and is shown in Fig. 4a and b. The photoluminescence (PL) intensity of the TOAN-perovskite film was suppressed compared with that of the TiO2-perovskite film, which is attributed to the more efficient extraction of photogenerated charges from the perovskite to the TOAN layer. The time-resolved PL (TRPL) shows that the decay time of the TOAN-perovskite film is smaller than that of the TiO2-perovskite film (Table S1†), implying that the TOAN film accelerates the charge extraction at the ETL/perovskite interface. The rapid transfer of charges is closely linked with the improved contact properties and the well-matched energy level at the ETL/perovskite interface. We explored the band alignment between the different ETLs and the perovskite layer by UV-vis absorption spectroscopy and ultraviolet photoelectron spectroscopy (UPS). The bandgaps of a-TaNx and TiO2 calculated by UV-vis absorption spectroscopy are 2.93 and 3.15 eV, respectively (Fig. S9†). The calculated conduction band minima are −4.14 eV for a-TaNx and −4.25 eV for TiO2 (Fig. 4c and d). Therefore, we can conclude that the TOAN can establish a gradient energy level arrangement with the perovskite photo-absorber layer, promoting charge transport and maintaining a high built-in field for PSCs (Fig. S10†).
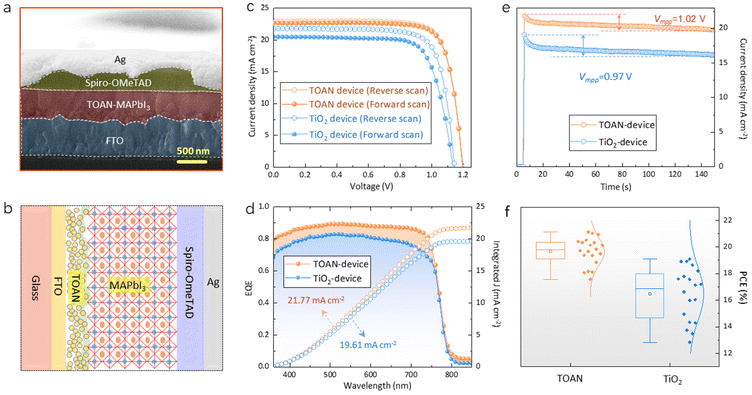
Devices with a structure of FTO/TiO2 (or TOAN)/perovskite/spiro-OMeTAD/Ag were fabricated. The cross-sectional SEM image and the structure diagram of the complete device are shown in Fig. 5a and b. Fig. 5c shows the reverse and forward J–V curves of the TiO2- and TOAN-PSCs. The TOAN device displays an open circuit voltage (Voc) of 1.20 V, a short-circuit current density (Jsc) of 22.87 mA cm−2, a fill factor (FF) of 78.03%, and a champion PCE of 21.41%. In contrast, the device based on the TiO2 ETL yields a Voc of 1.14 V, a Jsc of 21.75 mA cm−2, an FF of 76.74%, and a PCE of 19.02%. The detailed photovoltaic parameters are summarized in Table S2.† The credibility of Jsc was confirmed by external quantum efficiency (EQE) tests (Fig. 5d). The integrated Jsc values (21.77 mA cm−2 for the TOAN device and 19.61 mA cm−2 for the TiO2 device) obtained from EQE curves were found to exhibit the same trend as the Jsc values gained from the J–V measurement. The output photocurrent is also taken from the maximum power point of the champion devices (Fig. 5e). The TOAN device shows a higher current density and a smaller attenuation compared with the TiO2 device. The stabilized PCE at the maximum power point voltage (Vmpp) of the champion PSCs was also tested. The TOAN device achieved a steady-state PCE of 19.88% at a Vmpp of 1.02 V, whereas the TiO2 device reached 16.05% at 0.97 V. The statistical distributions of 20 TiO2 and 20 TOAN devices are shown in Fig. 5f (Tables S3 and S4†), which shows that the TOAN devices have a tighter distribution with better repeatability than the TiO2 devices. We also analyzed the reasons and physical mechanisms behind the device performance improvement. The improvement of Jsc can be attributed to the enhanced ETL/perovskite interface charge transfer. Also, the high electrical conductivity of a-TaNx facilitates efficient electron transfer from the perovskite layer to the ETL, thereby increasing Jsc. With regard to Voc, the use of TOAN as the ETL contributes to a reduction in interface defects between the TiO2 and perovskite layers. By minimizing the energy barriers and charge trapping sites at this interface, TOAN promotes more effective charge separation and reduces non-radiative recombination, resulting in an enhanced Voc. TOAN assists in improving the FF of the device because the superior electrical conductivity of TOAN helps reduce charge accumulation and resistive losses within the device, leading to improved charge extraction and reduced series resistance. These factors collectively contribute to improving the charge collection efficiency, leading to a reduction in hysteresis behavior.
The built-in potential (Vbi) of both cells was studied using capacitance–voltage (C–V) measurements to analyze the possible mechanism for the improvement in Voc (Fig. S11†). The TOAN device has a larger Vbi (1.08 V) than the TiO2 device (Vbi = 1.02 V). A higher Vbi of the TOAN device indicates a stronger electric field in PSCs, which contributes to the charge separation and collection, thus enabling a larger Voc.51 Meanwhile, the relationship between light intensity and Voc was studied to illustrate the enhancement of the FF. The slope of the fitting line was minimized from 1.53kT/q for the TiO2 device to 1.37kT/q for the TOAN device, which implies that the trap-assisted surface recombination obviously decreased in the TOAN device (Fig. S12†).52 We further performed electrochemical impedance spectroscopy (EIS) for both devices to investigate the charge-transfer dynamics under dark conditions. The TOAN device holds a smaller transport resistance (Rtr, high-frequency component) and a larger recombination resistance (Rrec, low-frequency component) than the TiO2 device, which demonstrates that the carrier extraction and transport at the interface are enhanced while the interfacial recombination is suppressed by applying the TOAN ETL (Fig. S13†).53,54 Furthermore, the capacitance–frequency (C–f) curves of PSCs were drawn to evaluate the defect-induced capacitance value. Fig. S14† shows that the TOAN device has smaller values in the low-frequency region, which means a lower series resistance at the TOAN/perovskite interface than that at the TiO2/perovskite interface, consistent with the reduced interface defects.55 Therefore, as shown in Fig. S15,† the improved performance of the TOAN-based devices can be attributed to two aspects: (1) the N in TOAN reduces the defect density at the ETL/perovskite interface and regulates the crystallization of the perovskite film, thereby ultimately inhibiting the non-radiative recombination of photogenerated charges; and (2) the TOAN device forms a gradient energy alignment at the ETL/perovskite interface, which promotes charge transfer and strengthens the built-in field of the device.
The PSC stability was also assessed at a relative humidity (RH) of ∼35% and a temperature of 25 °C (Fig. S16a†). After 30 days of aging, both the TiO2 device and the TOAN device displayed good stability under a nitrogen atmosphere, which could retain 93% and 95% of the initial PCE, respectively. However, for devices in ambient environment, the TOAN cell shows a higher stability than the TiO2 cell. Moreover, we also studied the operational stability of the PSCs under MPP tracking with continuous light irradiation (Fig. S16b†). The control device shows a downward PCE to 51.5% of the initial PCE after being irradiated for 12 h, and the TOAN-based device presents better long-term stability, keeping more than 80.7% of the original PCE after 12 h of irradiation. To figure out the reason for this improved stability, the XRD patterns of TiO2- and TOAN-perovskite films were further recorded (Fig. S17†). We can observe that none of the fresh perovskite films showed the PbI2 peak (Fig. 2c), but after placing in an atmospheric environment for 3 days at a temperature of 25 °C and a humidity of ∼42%, the PbI2 phase was observed in the TiO2-perovskite film. Moreover, Fig. S18† shows the UV-vis absorption spectra of the unencapsulated perovskite film deposited on TOAN and TiO2 substrates. After storing for 15 days in the ambient environment with ∼42% humidity at room temperature, the aged TOAN-perovskite film closely resembles the pristine TOAN-perovskite film. However, the absorption of the TiO2-perovskite decreases significantly after 15 days of aging under the same conditions, indicating that this film starts to decompose. The improved stability may be related to the favorable crystallization and the ordered structure of perovskite films, which resist the invasion of oxygen and water molecules, delaying the degradation of the devices. In addition, the reduction of dangling bonds could weaken the deprotonation effect at the ETL/perovskite interface, thus stabilizing the structure of perovskite films.
Conclusion
In summary, we have developed a gradient TOAN ETL for achieving efficient and stable PSCs. TOAN can not only effectively eliminate the oxygen vacancy defects on the ETL surface, but also passivate the dangling bonds of the perovskite buried interface, polishing the microstructure of the ETL/perovskite interface. Moreover, TOAN can form a gradient bandgap alignment with the perovskite, thereby enhancing the built-in field and interfacial charge transfer. These benefits result in a high PCE of 21.41% and negligible hysteresis for MAPbI3 solar cells. Furthermore, the device based on the TOAN ETL retains 91% of its initial PCE after 30 days under an ambient atmosphere. We believe that this work not only presents the potential of an inorganic metal nitride as an interface modification layer for PSCs, but also provides a reference for the further development of metal nitride electron transport layers for other optoelectronic devices.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge the support from the National Natural Science Foundation of China (grant no. 62275101, 11904127, 22075101), the Program for the Development of Science and Technology of Jilin Province (item no. YDZJ202201ZYTS300), and the Program for the Science and Technology of Education Department of Jilin Province (item no. JJKH20220440KJ).References
- V. V. Satale, N. Kumar, H. B. Lee, M. M. Ovhal, S. Chowdhury, B. Tyagi, A. Mohamed and J.-W. Kang, Spray-assisted deposition of a SnO2 electron transport bilayer for efficient inkjet-printed perovskite solar cells, Inorg. Chem. Front., 2023, 10, 3558–3567 RSC.
- W. Cao, Z. Hu, Z. Lin, X. Guo, J. Su, J. Chang and Y. Hao, Defects and doping engineering towards high performance lead-free or lead-less perovskite solar cells, J. Energy Chem., 2022, 68, 420–438 CrossRef CAS.
- H. Wu, B. Murti, J. Singh, P.-K. Yang and M.-L. Tsai, Prospects of metal-free perovskites for piezoelectric applications, Adv. Sci., 2022, 9, 2104703 CrossRef CAS PubMed.
- X. Yang, Q. Li, Y. Zheng, D. Luo, Y. Zhang, Y. Tu, L. Zhao, Y. Wang, F. Xu and Q. Gong, Perovskite hetero-bilayer for efficient charge-transport-layer-free solar cells, Joule, 2022, 6, 1277–1289 CrossRef CAS.
- L. Kong, X. Zhang, C. Zhang, L. Wang, S. Wang, F. Cao, D. Zhao, A. Rogach and X. Yang, Stability of perovskite light-emitting diodes: existing issues and mitigation strategies related to both material and device aspects, Adv. Mater., 2022, 34, 2270300 CrossRef.
- M. Kim, J. Jeong, H. Lu, T. Lee, F. Eickemeyer, Y. Liu, I. Choi, Y. Jo and H.-B. Kim, Conformal quantum dot-SnO2 layers as electron transporters for efficient perovskite solar cells, Science, 2022, 375, 6578 CrossRef PubMed.
- Q. Dou, T. Whatley, T. Syed, W. Wei and H. Wang, Carbon nanomaterials-polymer composites for perovskite solar cells: Preparation, properties and applications, J. Mater. Chem. A, 2022, 10, 19211–19230 RSC.
- T. Zhou, Z. Xu, R. Wang, X. Dong, Q. Fu and Y. Liu, Crystal growth regulation of 2D/3D perovskite films for solar cells with both high efficiency and stability, Adv. Mater., 2022, 34, 2200705 CrossRef CAS PubMed.
- Best Research-Cell Efficiency Chart, Photovolataic Research NREL, https://ww.nrel.gov/pv/cell-efficency.html.
- F. Berry, R. Mermet-Lyaudoz, J. Davila, D. Djemmah, H. Nguyen, C. Seassal, E. Fourmond, C. Chevalier, M. Amara and E. Drouard, Light management in perovskite photovoltaic solar cells: A perspective, Adv. Energy Mater., 2022, 12, 2200505 CrossRef CAS.
- J. Wawrzyniak, K. Grochowska, J. Karczewski, P. Kupracz, J. Ryl, A. Dolega and K. Siuzdak, The geometry of free-standing titania nanotubes as a critical factor controlling their optical and photoelectrochemical performance, Surf. Coat. Technol., 2020, 389, 125628 CrossRef CAS.
- Y. Chao, J. Chen, D. Yang, Y. Tseng, C.-H. Hsu and J. Chen, High-performance non-volatile flash photomemory via highly oriented quasi-2D perovskite, Adv. Funct. Mater., 2022, 32, 2112521 CrossRef CAS.
- Z. Lin, W. Zhang, Q. Cai, X. Xu, H. Dong, C. Mu and J. Zhang, Precursor engineering of the electron transport layer for application in high-performance perovskite solar cells, Adv. Sci., 2021, 8, 2102845 CrossRef CAS.
- H. Wang, Z. Zhang, J. Milic, L. Tan, Z. Wang, R. Chen, X. Jing, C. Yi, Y. Ding and Y. Li, Water stable haloplumbate modulation for efficient and stable hybrid perovskite photovoltaics, Adv. Energy Mater., 2021, 11, 2101082 CrossRef CAS.
- B. Chen, H. Chen, Y. Hou, J. Xu, S. Teale, K. Bertens, H. Chen, A. Proppe, Q. Zhou and D. Yu, Passivation of the buried interface via preferential crystallization of 2D perovskite on metal oxide transport layers, Adv. Mater., 2021, 33, 2103394 CrossRef CAS PubMed.
- W. Pan, J. Lin, J. Wu, B. Rong, X. Zhang, Q. Chen, M. Zhang, S. Wang, W. Sun and X. Wang, Interface modification by formamidine acetate for efficient perovskite solar cells, Sol. Energy, 2022, 232, 304–311 CrossRef CAS.
- H. Ban, T. Nakajima, Z. Liu, H. Yu, Q. Sun, L. Dai, Y. Shen, X. Zhang, J. Zhu and P. Chen, Over 8% efficient CsSnI3-based mesoporous perovskite solar cells enabled by two-step thermal annealing and surface cationic coordination dual treatment, J. Mater. Chem. A, 2022, 10, 3642–3649 RSC.
- S. Sonmezoglu and S. Akin, Suppression of the interface-dependent nonradiative recombination by using 2-methylbenzimidazole as interlayer for highly efficient and stable perovskite solar cells, Nano Energy, 2020, 76, 105127 CrossRef CAS.
- H. J. Lee, J. K. Park, J. H. Heo and S. H. Im, Acetylacetone-TiO2 promoted large area compatible cascade electron transport bilayer for efficient perovskite solar cells, Energy Environ. Mater., 2023, e12582 CrossRef.
- S. Akin, Y. Ulusu, H. Waller, J. H. Lakey and S. Sonmezoglu, Insight into interface engineering at TiO2/Dye through molecularly functionalized Caf1 biopolymer, ACS Sustainable Chem. Eng., 2018, 6, 1825–1836 CrossRef CAS.
- Ö. Ateş Sönmezoğlu, S. Akın, B. Terzi, S. Mutlu and S. Sönmezoğlu, An effective approach for high-efficiency photoelectrochemical solar cells by using bifunctional DNA molecules modified photoanode, Adv. Funct. Mater., 2016, 26, 8776–8783 CrossRef.
- J. Zhang, J. Fu, Q. Chen, H. Ma, Z. Jiang, Z. Zhang, Y. Zhou and B. Song, 3,5-Difluorophenylboronic acid-modified SnO2 as ETLs for perovskite solar cells: PCE > 22.3%, T-82 > 3000 h, Chem. Eng. J., 2022, 433, 133744 CrossRef CAS.
- D. Saranin, S. Pescetelli, A. Pazniak, D. Rossi, A. Liedl, A. Yakusheva, L. Luchnikov, D. Podgorny, P. Gostischev and S. Didenko, Transition metal carbides (MXenes) for efficient NiO-based inverted perovskite solar cells, Nano Energy, 2021, 82, 105771 CrossRef CAS.
- Q. Guo, J. Wu, Y. Yang, X. Liu, W. Sun, Y. Wei, Z. Lan, J. Lin, M. Huang and H. Chen, Low-temperature processed rare-earth doped brookite TiO2 scaffold for UV stable, hysteresis-free and high-performance perovskite solar cells, Nano Energy, 2020, 77, 105183 CrossRef CAS.
- R. Azmi, S. Hwang, W. Yin, T.-W. Kim, T. Ahn and S.-Y. Jang, High efficiency low-temperature processed perovskite solar cells integrated with alkali metal doped ZnO electron transport layers, ACS Energy Lett., 2018, 3, 1241–1246 CrossRef CAS.
- H. Wang, C. Zhao, L. Yin, X. Li, X. Tu, E. Lim, Y. Liu and C. Zhao, W-doped TiO2 as electron transport layer for high performance solution-processed perovskite solar cells, Appl. Surf. Sci., 2021, 563, 150298 CrossRef CAS.
- J. Chen, J. Zhang, C. Huang, Z. Bi, X. Xu and H. Yu, SnO2/2D-Bi2O2Se new hybrid electron transporting layer for efficient and stable perovskite solar cells, Chem. Eng. J., 2021, 410, 128436 CrossRef CAS.
- J. Deng, K. Wei, L. Yang, L. Lin, Y. Xiao, X. Cai, C. Zhang, D. Wu, X. Zhang and J. Zhang, Halides-enhanced buried interfaces for stable and extremely low-voltage-deficit perovskite solar cells, Adv. Mater., 2023, 35, 2300233 CrossRef CAS PubMed.
- N. K. Rana, A. Kumar, N. Chander and D. S. Ghosh, Fabrication of a highly functional TiO2/AZO bilayer electron transport layer for planar perovskite solar cells, ACS Appl. Electron. Mater., 2023, 5, 1050–1056 CrossRef CAS.
- F. Wang, Y. Zhang, M. Yang, J. Du, L. Xue, L. Yang, L. Fan, Y. Sui, J. Yang and X. Zhang, Exploring low-temperature processed a-WOx/SnO2 hybrid electron transporting layer for perovskite solar cells with efficiency > 20.5%, Nano Energy, 2019, 63, 103825 CrossRef CAS.
- K. Chaudhuri, A. Shaltout, D. Shah, U. Guler, A. Dutta, V. Shalaev and A. Boltasseva, Photonic spin Hall effect in robust phase gradient metasurfaces utilizing transition metal nitrides, ACS Photonics, 2019, 6, 99–106 CrossRef CAS.
- Y. Shen and Y. Mai, Structural studies of amorphous and crystallized tungsten nitride thin films by EFED, XRD and TEM, Appl. Surf. Sci., 2000, 16, 59–68 CrossRef.
- X. Yang, E. Aydin, H. Xu, J. Kang, M. Hedhili, W. Liu, Y. Wan, J. Peng, C. Samundsett and A. Cuevas, Tantalum nitride electron-selective contact for crystalline silicon solar cells, Adv. Energy Mater., 2018, 8, 1800608 CrossRef.
- H. Jiang, L. Feng, S. Zhu, S. Li and S. Zang, Hydrous RuO2 and CoxNy difunction-modified Ta3N5@Ta2N multi-heterojunction nanoplates for efficient visible-light-driven photocatalytic hydrogen production, Int. J. Hydrogen Energy, 2021, 46, 39855–39867 CrossRef CAS.
- C. Wang, T. Hisatomi, T. Minegishi, M. Nakabayashi, N. Shibata, M. Katayama and K. Domen, Effects of interfacial layers on the photoelectrochemical properties of tantalum nitride photoanodes for solar water splitting, J. Mater. Chem. A, 2016, 4, 13837–13843 RSC.
- A. Culu, I. Kaya and S. Sonmezoglu, Spray-pyrolyzed tantalium-doped TiO2 compact electron transport layer for UV-photostable planar perovskite solar cells exceeding 20% efficiency, ACS Appl. Energy Mater., 2022, 5, 3454–3462 CrossRef CAS.
- B. Jin, Y. Ming, Z. Wu, J. Cao, Y. Liu, Y. Zhu, S. Wang, Z. Liang and C. Wu, Silk fibroin induced homeotropic alignment of perovskite crystals toward high efficiency and stability, Nano Energy, 2022, 94, 106936 CrossRef CAS.
- J.-C. Hebig, I. Kuhn, J. Flohre and T. Kirchartz, Optoelectronic properties of (CH3NH3)3 Sb2I9 thin films for photovoltaic applications, ACS Energy Lett., 2016, 1, 309–314 CrossRef CAS.
- Y. Yun, D. Vidyasagar, M. Lee, O. Gong, J. Jung, H.-S. Jung, D.-H. Kim and S. Lee, Intermediate phase-free process for methylammonium lead iodide thin film for high-efficiency perovskite solar cells, Adv. Sci., 2021, 8, 2102492 CrossRef CAS PubMed.
- P. Wang, B. Chen, R. Li, S. Wang, Y. Li, X. Du, Y. Zhao and X. Zhang, 2D perovskite or organic material matter? Targeted growth for efficient perovskite solar cells with efficiency exceeding 24%, Nano Energy, 2022, 94, 106914 CrossRef CAS.
- B. Fan, J. Xiong, Y. Zhang, C. Gong, F. Li, X. Meng, X. Hu, Z. Yuan, F. Wang and Y. Chen, A bionic interface to suppress the coffee-ring effect for reliable and flexible perovskite modules with a near-90% yield rate, Adv. Mater., 2022, 34, 2201840 CrossRef CAS PubMed.
- H. Zhao, S. Wang, M. Sun, F. Zhang, X. Li and Y. Xiao, Enhanced stability and optoelectronic properties of MAPbl3 films by a cationic surface-active agent for perovskite solar cells, J. Mater. Chem. A, 2018, 6, 10825–10834 RSC.
- Q. Wang, M. Xue and Z. Zhang, Chemical synthesis of borophene: Progress and prospective, Acta Phys.-Chim. Sin., 2019, 35, 565–571 CAS.
- Q. Zhou, D. He, Q. Zhuang, B. Liu, R. Li, H. Li, Z. Zhang, H. Yang, P. Zhao and Y. He, Revealing steric-hindrance-dependent buried interface defect passivation mechanism in efficient and stable perovskite solar cells with mitigated tensile stress, Adv. Funct. Mater., 2022, 32, 2205507 CrossRef CAS.
- F. Ali, N. D. Pham, H. J. Bradford, N. Khoshsirat, K. Ostrikov, J. M. Bell, H. Wang and T. Tesfamichael, Tuning the amount of oxygen vacancies in sputter-deposited SnOx films for enhancing the performance of perovskite solar cells, ChemSusChem, 2018, 11, 3096–3103 CrossRef CAS PubMed.
- N. J. Jeon, T. Y. Yang, H. H. Park, J. Seo, D. Y. Nam, D. Jeong, S. Hong, S. H. Kim, J. M. Cho, J. J. Jang and J. K. Lee, Thermally activated, light-induced electron-spin-resonance spin density reflected by photocurrents in a perovskite solar cell, Appl. Phys. Lett., 2019, 114, 013903 CrossRef.
- F. Ali, N. D. Pham, L. Fan, V. Tiong, K. Ostrikov, J. M. Bell, H. Wang and T. Tesfamichael, Low hysteresis perovskite solar cells using an electron-beam evaporated WO3−x thin film as the electron transport layer, ACS Appl. Energy Mater., 2019, 2, 5456–5464 CrossRef CAS.
- H. Kim, J. Hong, C. Kim, E.-Y. Shin, M. Lee, Y.-Y. Noh, B. Park and I. Hwang, Impact of hydroxyl groups boosting heterogeneous nucleation on perovskite grains and photovoltaic performances, J. Phys. Chem. C, 2018, 122, 16630–16638 CrossRef CAS.
- S. Zhan, Y. Duan, Z. Liu, L. Yang, K. He, Y. Che, W. Zhao, Y. Han, S. Yang and G. Zhao, Stable 24.29%-efficiency FA0.85MA0.15PbI3 perovskite solar cells enabled by methyl haloacetate–lead dimer complex, Adv. Energy Mater., 2022, 12, 2200867 CrossRef CAS.
- J. Guo, J. Sun, L. Hu, S. Fang, X. Ling, X. Zhang, Y. Wang, H. Huang, C. Han and C. Cazorla, Indigo: A natural molecular passivator for efficient perovskite solar cells, Adv. Energy Mater., 2022, 12, 2200537 CrossRef CAS.
- X. Zhuang, D. Zhou, S. Liu, R. Sun, Z. Shi, L. Liu, T. Wang, B. Liu, D. Liu and H. Song, Learning from plants: Lycopene additive passivation toward efficient and “fresh” perovskite solar cells with oxygen and ultraviolet resistance, Adv. Energy Mater., 2022, 12, 2200614 CrossRef CAS.
- A. Wang, X. Deng, J. Wang, S. Wang, X. Niu, F. Hao and L. Ding, Ionic liquid reducing energy loss and stabilizing CsPbI2Br solar cells, Nano Energy, 2021, 81, 105631 CrossRef CAS.
- Y. Niu, Y. Peng, X. Zhang, Y. Ren, R. Ghadari, J. Zhu, G. Tulloch, H. Zhang, P. Falaras and L. Hu, Resonant molecular modification for energy level alignment in perovskite solar cells, ACS Energy Lett., 2022, 7, 3104–3111 CrossRef CAS.
- Y. Wang, Y. Yang, N. Li, M. Hu, S. Raga, Y. Jiang, C. Wang, X. Zhang, M. Lira-Cantu and F. Huang, Ionic liquid stabilized perovskite solar modules with power conversion efficiency exceeding 20%, Adv. Funct. Mater., 2022, 32, 2204396 CrossRef CAS.
- Y. Xiong, L. Xu, P. Wu, L. Sun, G. Xie and B. Hu, Bismuth doping-induced stable Seebeck effect based on MAPbI3 polycrystalline thin films, Adv. Funct. Mater., 2019, 29, 1900615 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3qi01178j |
| This journal is © the Partner Organisations 2023 |