Recent progress on the synthesis of metal alloy nanowires as electrocatalysts
Shumin
Li
,
Hui
Jin
and
Yawen
Wang
 *
*
Institute of Advanced Synthesis (IAS), Chemistry and Molecular Engineering, Nanjing Tech University, Nanjing 211816, P.R. China. E-mail: ias_ywwang@njtech.edu.cn
First published on 30th December 2022
Abstract
Benefiting from both one-dimensional (1D) morphology and alloy composition, metal alloy nanowires have been exploited as advanced electrocatalysts in various electrochemical processes. In this review, the synthesis approaches for metal alloy nanowires are classified into two categories: direct syntheses and syntheses based on preformed 1D nanostructures. Ligand systems that are of critical importance to the formation of alloy nanowires are summarized and reviewed, together with the strategies imposed to achieve the co-reduction of different metals. Meanwhile, different scenarios that form alloy nanowires from pre-synthesized 1D nanostructures are compared and contrasted. In addition, the characterization and electrocatalytic applications of metal alloy nanowires are briefly discussed.
1. Introduction
Developing sustainable energy harvesting and storage methods to reduce or replace fossil fuel usage has become one of the most focused-on research areas in recent years.1–5 Overcoming the low kinetics of many important electrochemical processes is of critical importance for the application and commercialization of future techniques, such as fuel cells that directly convert chemical energy to electrical energy, the water-splitting process that produces H2 as clean energy sources, and the CO2 reduction and conversion devices that help to reduce the greenhouse gas level in the atmosphere and produce functional carbonaceous products.6–13 Catalysts are indispensable for achieving this, and thus significant amounts of research have been devoted to developing catalysts with high activity and durability.14–24The performances of the catalysts can be improved by optimizing the morphology, composition, and other structural properties of the materials.18,25,26 The adsorption and desorption of the reactants and intermediates on the surface of the catalysts depend directly on the amount of active sites and the adsorption energy at these sites, which is in turn determined by the shapes and surface structures of the catalysts.15
The unique structural characteristics of one-dimensional (1D) nanostructures, or nanowires, give rise to multiple advantages in catalytic reactions, as recently summarized in several comprehensive review works.25–34 Firstly, the nanowires with high aspect ratio have enormous surface areas that can provide a large number of active sites for electrochemical reactions.27,32,33 Although geometrically, the surface area of the nanowires is smaller than the same volume of nanoparticles with the same diameters, in practice they may still have more exposed surface areas during the electrochemical processes, as nanoparticles are more vulnerable to aggregation during the catalyst loading and cycling processes.30,31 This also give rise to the second advantage of nanowires: they usually have better structural stability, being more resistant to dissolution, aggregation and Ostwald ripening, and thus show improved durability in catalytic reactions.30 The linear elongation of nanowires is also considered to provide electron highways for charge transportation and diffusion tunnels for mass transportation, and both are beneficial for catalytic activity.27,29 Lastly, nanowires are readily assembled into free-standing 2D films or 3D networks, which are more easily processed into electrodes in devices.31
On the other hand, the formation of the metal alloy provides an additional dimension to modify the chemical and physical properties of catalytic nanomaterials. Recently, the exploration of 1D nanostructure catalysts has gradually extended from pristine metal nanowires to multi-metal alloys.25,32
One major benefit of forming an alloy is to provide adjustment of the surface electronic structures of the catalysts, leading to optimized binding between the catalyst surfaces and key intermediates of the electrocatalytic reactions.35–38 For example, metals have different adsorption energies towards the *OH species, which is the key intermediate during oxygen reduction reactions (ORRs).36,39 And although Pt and Pd are the best ORR catalyst candidates, the adsorption energies of *OH on these metals are still not optimized according to the volcano plot.36,40 Forming alloys inevitably leads to electron transfer between the different metals. In addition, the introduction of foreign metal would alter the bond lengths of the metallic crystals. These effects would cause change in the electron density on both elements, causing variation of the adsorption energies of the reaction intermediates.
Moreover, there are also synergistic ensemble effects between the metal compositions. For example, the AuCu surface would have increased selectivity for C–C formation in the CO2 reduction reaction (CO2RR), as the Au atoms around the Cu could provide addition binding sites for the *COOH intermediates.41 Also, the Pd-M alloy has a lower risk of being poisoned by CO, as the COad only adsorbs on the adjacent Pd atoms.42 The Pd-M alloy surface, with Pd more diluted on the surface compared with pure Pd, is thus more preferred in processes such as formic acid oxidation.
Combining both the advantages of 1D nanostructures and alloy materials, metal alloy nanowires are thus promising electrocatalyst candidates (Fig. 1). On one hand, this group of material inherits the structural advantage of 1D nanostructures, possessing a large number of surface active sites, high structural stability, and high electron mobility during electrochemical processes.34,43 On the other hand, the intrinsic catalytic activity of the material could be further enhanced by adjustment of the compositions.44,45 For instance, Ni and/or Co were often incorporated into Pt and Pd to from alloy nanowires with improved ORR performances. Other metals, such as Au, Ag, Cu, Rh, Ru, Zn, Ir, Pb and Fe etc. are also common metal compositions in alloy nanowires.
Synthesis is of fundamental importance in the exploration of advanced functional materials. Many works about alloy nanowires have emerged recently, and the electrocatalytic performances of these materials have been studied thoroughly. In this work, the synthetic approaches, mostly solution-based chemical methods, for different metal alloy nanowires are reviewed and categorized. 1D nanostructures with imperfect wire-like morphologies, such as chain-like nanostructures and branched nanowire networks, are also included as they are sometimes considered as nanowires and possess similar structural advantages. While some of the syntheses and applications of Pt and Pd-based nanowires have been separately reviewed in recent review works, this work aims to systematically discuss the mechanism for obtaining 1D structures, the various synthetic approaches that are used to enable the formation of the alloys, and the recently developed strategies that advance the synthetic capabilities. To better illustrate syntheses, the common characterizations for metal alloy nanowires are first introduced. In addition, the applications of metal alloy nanowires as electrocatalysts are also briefly summarized to demonstrate the advantage of this group of materials.
2. Characterization of metal alloy nanowires
Characterizations are vital for synthetic works, and they also provide clues for mechanism studies and discussions about structure–property co-relationships. For metal alloy nanowires, both the structures and the compositions of the synthetic products need to be well characterized, and the structural aspect includes both the morphological shapes and crystalline structures of the materials.The morphology of the 1D nanostructures can be confirmed easily with microscopic methods, including most commonly transmission electron microscopy (TEM) and scanning electron microscopy (SEM). Often, either TEM or SEM would be necessary to indicate the morphology of the nanowires, yet both can be used to give better illustrations, including the topology of nanowires with rough surfaces as well as possible porous structures. However, although almost all syntheses would allow microscopic characterizations, it should be noted that these methods can only provide information for a tiny portion of the product obtained from the synthesis. Low magnification images that contain sufficient numbers of the nanowires should be provided to verify the uniformity of the synthesis.
Another key issue that has often been overlooked is that microscopic methods are off-line characterizations that show only the appearance of the species on the specimen. The dried sample may undergo transformations when loaded to the sample holder and subjected to vacuum for electron microscopic characterizations. Therefore, although most works have interpreted growth mechanisms based on TEM images of the intermediates at various reaction stages, the possibility of deviation of the characterization results from the solution situations in reality needs to be considered when constructing the synthesis mechanisms. Control experiments are highly desirable to confirm the hypotheses in addition to the characterization results.
The crystalline structures of metal alloy nanowires are usually characterized with high-resolution TEM techniques (HRTEM) and diffraction techniques such as X-ray diffraction (XRD) and selected area electron diffraction (SAED). The former provides the d spaces value of some low index facets of a small portion of the samples, while the diffraction methods give the ensemble properties. It should be noted that ultrathin metal nanowire may suffer beam damage and undergo lattice transformations when under high energy electron beams during the HRTEM characterizations,46 bringing ambiguities to the structure discussions. Additionally, surface organic ligands may blur the images during the characterization after being reduced by the electron beam and accumulated on the surface of the nanowires. Therefore, the acceleration voltage of the electron beam may need to be lowered and the nanowires’ surface may need to be cleaned to remove organic ligands before HRTEM characterizations.
The composition of metal alloy nanowires takes much more effort to be characterized. Given the small diameters of most nanowires, the distribution of the metals is usually characterized with energy-dispersive X-ray spectroscopy (EDS) coupled with high-angle annular dark field scanning transmission electron microscopy (HAADF-STEM). EDS mapping can give the distribution of the various elements across the nanowires. Again, for ultrathin nanowires, the mapping process would be much more difficult, as the low quantity of metal materials would require repeated scans and thus prolonged scanning times, adding to the risk of being affected by image shifting, vibration and possible damage from the beam. Even with prolonged scan durations, EDS mapping may not provide atomic resolution. Therefore, characterization of the atomic distribution of the different elements remains a great challenge. On the other hand, HAADF-STEM, which characterizes the Z contrast of the samples, can provide certain information about the nanowires’ composition.47 It generates intensity profiles that can be used to establish the features of the element distributions, especially with the aberration-corrected high-resolution HAADF-STEM which can provide atomic-scale images.
Apart from the microscopic methods, powder XRD results are often used as evidence to illustrate the formation of alloy. Deviations of the XRD peaks from the pristine metal phases due to shrinking or expansion of the lattice structures is usually attributed to the incorporation of a foreign metal with different crystalline constant.48,49 A typical bimetallic alloy would have peaks occurring in between those of the two pristine metals, unless a certain alloy phase was generated. In addition, the width of the XRD peaks can be used to estimate the grain sizes in the nanowires.
The electronic structures of the metals in an alloy are often characterized with X-ray photoelectron spectroscopy (XPS) methods, which also help to confirm the existence of certain elements. The change of binding energies in XPS spectra can be explained by electron transfer between the metals in the alloy. Thus the XPS results are strong evidence for the formation of the alloy structures, and also can provide clues to explain the change of catalytic activity of the alloy nanowires.
Apart from the usual characterizations, recently the X-ray absorption spectroscopy (XAS) methods X-ray absorption near-edge structure (XANES) and extended X-ray absorption fine structure (EXAFS) have been employed to investigate the atomic and electronic structures of the metals in alloy nanowires.50 XANES can precisely characterize the oxidation state of the metals. The XANES edges change in intensity and position if the oxidation state of the target metal varies. And the oxidation state of the sample can be confirmed by comparing the spectra with those of compounds with known oxidation states. For instance, the XANES of the three elements in the PtNiMo nanowires were compared and contrasted with pristine metal foils and a few common compounds (Fig. 2a, c and e).51 In particular, the shoulder peak in the XANES feature of Mo suggests that some of the Mo in the alloy nanowires is similar to that in MoO3. The EXAFS method provides complementary information about the local atomic structures. The EXAFS signals are oscillations of the wave of the ejected photoelectron scattered by the surrounding atoms. Both the distances and types of the surrounding atoms affect the EXAFS spectrum, therefore it can also give the fine structures of the alloy nanowires. Again by comparing the EXAFS spectra with references, typical interactions can be assigned (Fig. 2b, d, and f). For instance, the EXAFS spectra of Ni indicate that there are two Ni species in PtNiMo alloy nanowires: NiO as indicated by the scattering between 1.5 and 2.0 Å, and metallic Ni in the PtNiMo alloy. The peak between 2.0 and 2.9 Å was probably the combination of both the Ni–Ni scattering in NiO and the Ni-metal scattering in the alloy.
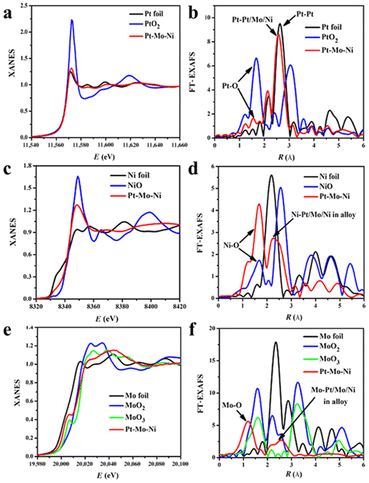 | ||
| Fig. 2 XAS analyses of the PtMoNi NWs. XANES (a) and EXAFS (b) analysis of Pt L3-edge (FT range, 4.3 to 14.5 Å−1). XANES (c) and EXAFS (d) analysis of Ni K-edge (FT range, 2.5 to 12.5 Å−1). XANES (e) and EXAFS (f) analysis of Mo K-edge (FT range, 2.0 to 10.5 Å−1). Reprinted with permission from ref. 51. Copyright 2017 from AAAS. | ||
Although the XAS methods can provide useful information on the fine structure of alloy nanowires, they have not been frequently used in the reported syntheses. One key issue is that they are synchrotron analyses that require the use of synchrotron radiation, which may not be available to many researchers. Similar issues also apply to the cutting-edge microscopic characterizations that provide atomic resolution on nanowire structures, as these methods require well-trained personnel to operate the instrument and interpret the results. Thus, interdisciplinary cooperation has become increasingly important and desirable in the related communities.
3. Direct synthesis of alloy nanowires
There are two major issues of concern in the synthesis of metal alloy nanowires: (1) how to induce the 1D growth of the metals, and (2) how to form the alloy with certain composition in a controlled manner.Unlike intrinsically linear materials such as carbon nanotubes, metals have highly symmetric crystalline structures. Instead of elongating in two directions, the equivalent facets in metal crystals grow simultaneously to give 3D structures, unless the symmetry is broken with certain mechanisms. In bottom-up approaches, the crystalline symmetry can be broken with either templates, the oriented attachment mechanism, or kinetic-controlled growth induced by either defects or ligands.
Direct synthesis of metal alloy nanowires usually follows similar mechanisms that form pristine metal nanowires, but with simultaneous co-precipitation of all alloy compositions (Fig. 3). The synthesis of nanowire can be realized via either templating methods or self-assembly processes. Tubular hard templates can be used coupled with the deposition process to generate 1D nanostructures. And in solution, one or multiple structure-directing reagents, such as CTAC, CO and PVP, are usually required to induce the 1D growth of the metal nanostructures. Some of the structure-directing reagents are considered to form micellar-structures to induce the linear growth of the metals, while in some cases the exact mechanisms of the 1D growth have not been completely established yet.
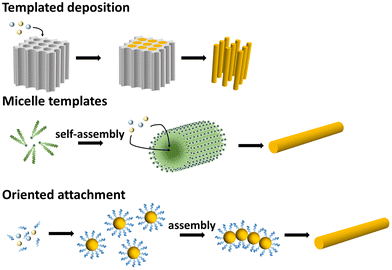 | ||
| Fig. 3 Schematics illustrating the formation mechanisms that forms the metal alloy nanowires directly with chemical methods. | ||
For templating methods, overcoming the differences in the reduction potential of various metal precursors is the key issue for a successful co-precipitation process. In solution-based synthesis, one composition of the alloy nanowire is usually responsible for the morphological construction, serving as the “nanowire template”. Pt or Pd is often the metal that plays this role, probably because the syntheses of the Pt and Pd nanowires are quite feasible compared with other nanowires. Nevertheless, those formed via a one-pot procedure and that have no obvious core–shell structures are still discussed in this section. Often, the chemical environments in direct syntheses allow the co-reduction or autocatalytic reduction of the precursors of the alloys, while sometimes synthetic adjustments are necessary to enable the co-precipitation processes. It is also noticed that in some rare cases nanowires can only be obtained when there are two or more metal precursors, and no single-metal nanowires can be obtained.
3.1 Electrodeposition with hard templates
Hard templates have often been combined with electrodeposition to synthesize nanowires. This approach has been frequently used to obtain nanowires made of the magnetic metals, for instance the super invar (Fe–CO–Ni) alloy nanowire; it is difficult to reduce these metals in a wet-chemical environment, probably due to their relatively low reduction potentials compared with the noble metals.52–56 One of the most widely applied templates for 1D electrodeposition is the anode-aluminum-oxide (AAO), which can be easily obtained by anodizing Al foil and removed after the synthesis with chemical etching. With the AAO template, a vertically aligned nanowire array can be electrodeposited, with the diameters of the nanowires determined by the pore sizes. Nanowires synthesized with this method usually have diameters of about the tens of nanometres.Recently, there have been several works reporting the syntheses of Pd-based nanowires with the AAO-templated electrodeposition methods, including pure Pd and PdBi,57 PdFe,58 PdCo59 and PdNi56 nanowire arrays. The applied potentials often significantly affect the composition of the PdM alloy nanowires. This is because Pd can be deposited at relatively low overpotential, while the other metals need much negative potentials. The overall Pd compositions usually decrease with the increase of the applied potentials. Alternatively, the ratio of the precursors in the electrolyte can be adjusted to control the composition of the final nanowires. The removal step of the hard AAO template can offer an additional chance to tune the nanowire composition and modify the surface topology of the nanowires. For instance, PdBi nanowires with a rough surface can be generated during the etching of the AAO templates after depositing smooth PdBi nanowires in the template.57 Meanwhile, the composition of Bi in the alloy also dropped from 15% to 3% due to the dissolution of the surface Bi (Fig. 4).
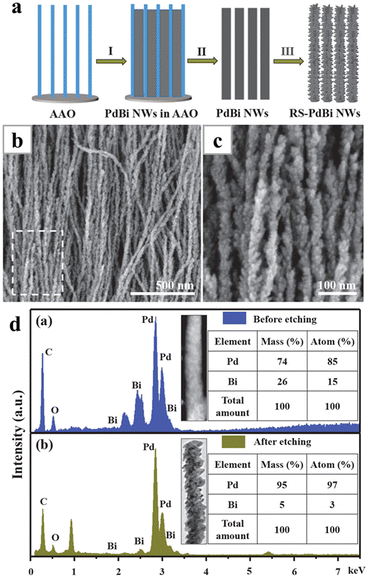 | ||
| Fig. 4 (a) Schematics illustrating the electrodeposition process with AAO template; (b) SEM and (c) magnified SEM images of PdBi nanowires with rough surfaces. (d) EDS analysis results showing the compositions of PdBi nanowires before and after being etched in 4 M NaOH. Reprinted with permission from ref. 57. Copyright 2019 from American Chemical Society. | ||
The templates in electrodeposition restrict the outlines of the nanowires, and the deposition conditions can provide extra controls on the morphologies of the alloy nanowires. A recent synthesis of FeCoNi nanowires carried out the deposition at low pH condition with polycarbonate as the template, and produced porous nanowires.60 The H2 bubbles formed during the electrodeposition were utilized as the dynamic templates of the pores in the alloy nanowires. Moreover, post-synthetic processing such as surface etching can be conducted to produce PdM nanowire arrays with rough or porous surfaces, and to further adjust the composition of the nanowires.57,59
Templated electrodeposition is a feasible method to synthesize metal alloy nanowires with both noble and non-noble metal compositions. The composition of the alloy nanowires is usually controlled by the reduction potentials. Both the surface morphology and compositions of the obtained nanowires can be further adjusted with post-synthetic treatments.
3.2 Solution-based synthesis with molecular ligands
Many metal-based nanowires have been synthesized via solution-based chemical methods. The homogeneous environment in wet-chemical synthesis can offer good structural uniformity and afford potentially massive production. During the syntheses, apart from the metal precursors and the reducing systems, one or more molecular structure-directing reagents or ligands, such as the organoammonium halides, organoamines, metal carbonyls which provide CO and/or non-ionic surfactants, are usually added as vital ingredients to induce the 1D growth of the metals. Normally, the ligands were considered as either promoting the oriented attachment of particle intermediates to form 1D assemblies, or forming micelle templates facilitating the 1D growth of the metal crystals. The exact mechanism of the 1D growth induced by some of the structure-directing reagents had been thoroughly studied, yet for some other ligands, it remains unclear how these species direct the linear growth of metal nanostructures.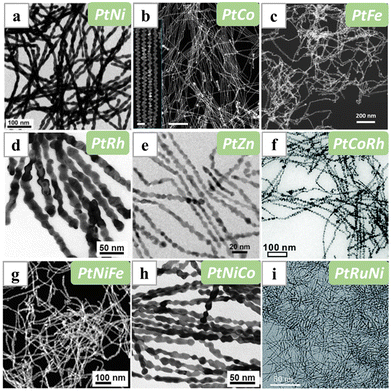 | ||
| Fig. 5 TEM/STEM/HAADF-STEM images of (a) PtNi, (b) PtCo, (c) PtFe, (d) PtRh, (e) PtZn, (f) PtCoRh, (g) PtNiFe, (h) PtNiCo and (i) PtRuNi nanowires synthesized with CTAC as the structure-directing reagent. Reprinted with permission from ref. 65, 71–75. Copyright 2015 and 2018 from WILEY-VCH GmbH, 2016 and 2019 from Springer Nature, 2020 and 2021 from the Royal Society of Chemistry. | ||
Mechanistic investigation of the formation of PtNi alloy nanowires indicated that during synthesis, Pt was first reduced and formed ultrathin nanowires, followed by the reduction of Ni and the formation of a core–shell intermediate.65 As the reduction progressed further, an alloy process occurred and the interdiffusion of the metal atoms led to the formation of the alloy nanowires (Fig. 6). Moreover, the surface layer of the ultrathin nanowires was usually considered Pt-rich, as compared with Ni the Pt atoms would be chemically more stable and thus concentrated on the surface as the result of both the interdiffusion and ripening processes. Overall, this method relies greatly on the formation of the 1D Pt nanostructure. Without Pt, Ni alone could not have linear growth. Nevertheless, the interdiffusion of metal atoms at high temperatures is also of critical importance to the formation of uniform alloy phases, such as the Pt3Ni alloy phase in PtNi alloy nanowires.
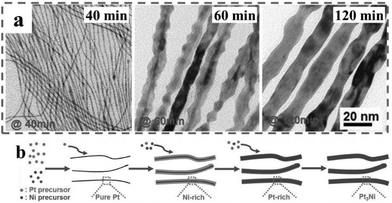 | ||
| Fig. 6 (a) TEM images of 1D PtNi intermediates collected from the reactions at different reaction times during the CTAC-mediated synthesis of PtNi alloy nanowires. (b) Schematics illustrating the growth mechanism of 1D PtNi nanowires. Reprinted with permission from ref. 65. Copyright 2015 from WILEY-VCH GmbH. | ||
The sequential reduction of Pt and Ni suggests that the synthesis can be modified to two-step procedures, through which the phase and composition of the PtNi alloy nanowires can be adjusted more precisely. It was reported that varying the quantity of the Ni(acac)2 added in the second step could lead to the formation of a series of Pt3Ni, Pt3Ni3, Pt3Ni4.5 and Pt3Ni6 alloy nanowires, with the excess Ni forming a segregated Ni phase.66 Further etching of the Ni phases with acetic acid led to the formation of a porous Pt3Ni nanowire with improved electrocatalytic performance. Another recent work indicates that apart from crystallization during the synthesis process, post-synthetic thermal treatment under NH3 atmosphere can also induce Ni segregation on the surface of the PtNi nanowires, as the Ni has stronger affinity towards NH3 than Pt.67
The two-step procedure also provides means to form alloys with metals having large potential differences or reducing kinetics with Pt, such as PtCu and PtCuIr, PtCuRh, PtCuPd nanowires.81,82 Typically, the reaction mixture was first heated with solely the Pt precursor to generate ultrathin Pt nanowires, and then the precursors of the other metals were added together with additional Pt precursor to induce the formation of the alloy nanowires. The reduction temperature of the second stage can be increased to facilitate the reduction of the second metal. For instance, PtCoRh spiral nanowires could be obtained by first forming PtCo alloy nanowires at 130 °C, and then raising the reaction temperature to 170 °C to achieve the reduction and deposition of Rh.74
In this CTAX (X = Br or Cl)-induced synthesis, although the solvent oleylamine is reductive, additional reductant glucose was also added to promote the reduction of the metal with low reduction potential. In the absence of glucose, pure Pt nanowires were usually obtained. An exception is the formation of chain-like PtNi nanostructures under the solvothermal condition at 180 °C.83 The high-temperature solvothermal condition probably maintained a highly reductive environment that allowed the reduction of Ni and the formation of Ni-rich nanospheres along the Pt nanowires. Other reductants have also been introduced. Replacing the glucose with ascorbic acid or citric acid during the synthesis of Pt3Ni nanowires yielded sinuous nanowires or short nanorods, repectively.84 These results verified that the reduction system also played an important role in the 1D growth, as the deposition of the metal atoms on certain surfaces of the nuclei was related closely to the adsorption/desorption kinetics of the ligand and the deposition kinetics of the metal. Alternatively, PtNiMo ultrathin nanowires were produced by purging pressurized H2 (30 bar) into the nanowire growth system.51 H2 was considered as both controlling the slow kinetics for the growth of the nanowires and providing structural regulation for nanowire formation.
Carbonyl reagents, typically Mo(CO)6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 51 and W(CO)6,85,86 have been introduced into some of the syntheses, and their addition can cause the transformation of the nanowire morphology from chain-like to ultrathin. Upon heating, metal carbonyl decomposes to release CO, which adsorbs strongly on the Pt surface and provides restriction to the lateral growth of the nanowires. As a result, ultrathin Pt-based nanowires were obtained with the metal carbonyl additives. For instance, the length of the Pt3Ni nanowire increased from 9 nm to about 35 nm when the mass of Mo(CO)6 added into the system was doubled from 2 mg to 4 mg.87 Moreover, the presence of CO was found to be critical for the smoothening of the nanowires after the oriented attachment process. Chain-like assembly of nanoparticles was obtained if there were no metal carbonyl additives during the synthesis (Fig. 7).82 Alternatively, CO could be introduced to the system by directly blowing the reaction mixture with gaseous CO. After that, the autoclave containing Pt(acac)2 and Fe(acac)2 was sealed and heated under solvothermal condition to generate ultrathin PtFe nanowires.88 Apart from morphological control, the introduction of CO also generates a highly reductive environment. Even without glucose, PtRu nanowires could still form in the presence of W(CO)6, with dimethyldioctadecylammonium chloride as the ligand.89
51 and W(CO)6,85,86 have been introduced into some of the syntheses, and their addition can cause the transformation of the nanowire morphology from chain-like to ultrathin. Upon heating, metal carbonyl decomposes to release CO, which adsorbs strongly on the Pt surface and provides restriction to the lateral growth of the nanowires. As a result, ultrathin Pt-based nanowires were obtained with the metal carbonyl additives. For instance, the length of the Pt3Ni nanowire increased from 9 nm to about 35 nm when the mass of Mo(CO)6 added into the system was doubled from 2 mg to 4 mg.87 Moreover, the presence of CO was found to be critical for the smoothening of the nanowires after the oriented attachment process. Chain-like assembly of nanoparticles was obtained if there were no metal carbonyl additives during the synthesis (Fig. 7).82 Alternatively, CO could be introduced to the system by directly blowing the reaction mixture with gaseous CO. After that, the autoclave containing Pt(acac)2 and Fe(acac)2 was sealed and heated under solvothermal condition to generate ultrathin PtFe nanowires.88 Apart from morphological control, the introduction of CO also generates a highly reductive environment. Even without glucose, PtRu nanowires could still form in the presence of W(CO)6, with dimethyldioctadecylammonium chloride as the ligand.89
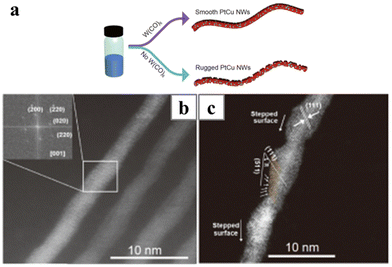 | ||
| Fig. 7 (a) Schematics illustrating the effect on the W(CO)6 on the surface of the PtCu nanowires and (b and c) HAADF-STEM images of (b) the smooth PtCu nanowires (inset showing the corresponding FFT pattern) and (c) the rugged PtCu nanowires. Reprinted with permission from ref. 82. Copyright 2020 from Springer Nature. | ||
Another type of double-chain organo-ammonium halide has been employed in the growth of Pd-based alloy nanowires in aqueous solutions. A typical system carries the reaction in aqueous solution, with dioctadecyldimethylammonium chloride (DODAC)90,91 as the structure-directing reagent and L-ascorbic acid as the reductant. The aqueous solution favours the usage of the L-ascorbic acid, while the hydrophobic carbonyl reagents are not compatible for this synthesis. More importantly, in aqueous solution the double-chain DODAC was considered to be a better ligand compared with the more commonly used CTAB and CTAC: the double-chain organo-ammonium halide can form stable tubular mesophases in the aqueous solution, providing stable soft templates for the formation of single-crystalline Pd nanowires (Fig. 8).92 Simply by reducing AgNO3 or H2PtCl6 together with H2PdCl4, alloys PdAg and PdPt nanowires can be obtained. One variation of this system was the introduction of NaH2PO2 as the reductant to replace the ascorbic acid. NaH2PO2 also provides P during the reduction, leading to the formation of PdCuP alloy nanowires.93 Recently, a similar molecule, dihexadecyldimethylammonium chloride (DHDAC) has been employed as the structure-directing reagent to synthesize ultrathin PdAg nanowires.94 The ratio of Pd and Ag in the alloy nanowire was consistent with the ratio of the metal precursors. Interestingly, this system is incapable of forming pure metal nanowires. When solely Pd or Ag precursor was used, Pd or Ag nanoparticles were obtained.
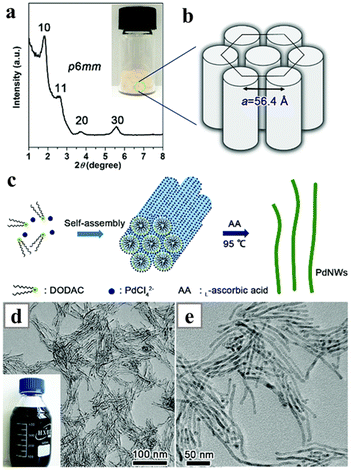 | ||
| Fig. 8 Synthesis of the Pd nanowires with DODAC. (a) Small-angle XRD patterns of the powder mainly consisting of DODAC and H2PdCl4, with the inset showing the photograph of the spongy powder in a glass bottle. (b) Structural representation of a characteristic p6mm mesostructure. (c) Proposed formation mechanism of Pd nanowires based on a self-assembly process. (d and e) TEM images of the Pd nanowires observed at different magnifications. Reprinted with permission from ref. 92. Copyright 2016 from the Royal Society of Chemistry. | ||
Besides the ammonium halides, hexadecylpyridinium chloride (C16-py) was also recently discovered to be capable of forming micelle-like soft templates for the synthesis of 1D Pd-based nanostructures. The amphiphilic ligand first assembled with the Pd precursor and formed columnar structures. After adding H3BO3 and dimethylamine borane (DMAB), PdB nanoparticles nucleated inside the columnar structures. Finally, the nanoparticles attached together to generate wavy PdB and PdMB (M = Cu, Pt) nanowires.95
In a recent work, PtIrFe alloy nanowires with face-centred cubic (fcc) lattice were first synthesized via the CO procedures, and then the lattice structure was transformed to the more active face-centred tetragonal phase with post-synthetic treatment (Fig. 9). The strong reducing ability of CO enabled the reduction of Pt(acac)2 and Ir(acac)2, and together with Fe that originated from Fe(CO)5, trimetallic nanowires with classic fcc lattice phase and random atomic distribution could be obtained.100 The presence of the high-melting-point metal Ir was vital to the following thermal treatment and lattice transformations. After being deposited on carbon support and encapsulated by silica shell, the nanowires were subjected to thermal treatment at 690 °C and underwent phase transition without changing in morphology. The obtained PtIrFe nanowires became an ordered alloy with fct phase, and exhibited improved catalytic performances.
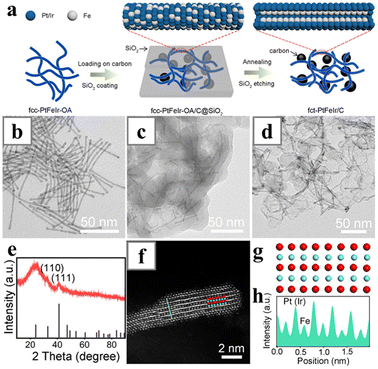 | ||
| Fig. 9 Synthesis of fct-PtFeIr/C via a silica-protected annealing strategy. (a) Schematics illustrating the synthesis process. (b–d) TEM images of (b) fcc-phase PtFeIr nanowires, (c) fcc-PtFeIr-OA/C@SiO2, and (d) fct-PtFeIr/C. (e) XRD pattern of the fct-PtFeIr/C. (f) HAADF-STEM image of a nanowire in fct-PtFeIr/C viewed along the [100] axis. Red and cyan spheres represent Pt (Ir) and Fe, respectively. (g) Simulated atomic arrangement for fct-phase PtFe viewed along the [100] axis. (h) Z-Contrast intensity profile taken along the cyan line in (f). Reprinted with permission from ref. 100. Copyright 2021 from Wiley-VCH GmbH. | ||
CO species normally induce the formation of 2D Pd-based materials, such as nanosheets and nanoplates.43,101–103 They were considered to bind strongly on the {111} facets of the Pd structures, preventing deposition on the basal surface. Thus, the nanostructures would expand only at the {111} twinning planes, leading to the formation of nanoplates and nanosheets. Recently, there was also work employing CO as ligands for the formation of Pd-based nanowires. PdBi and PdBiM (M = Cu, Co and Mn) nanowires were synthesized in the presence of the carbonyl reagent Mo(CO)6.104 The complex system contained another two capping agents, tetrabutylammonium bromide (TBAB) and PVP. Also, Mo(CO)6, ascorbic acid and the solvent formamide were all reductive. An orientated attachment mechanism was proposed to indicate that the twisty nanowires were formed via the assembly of PdBiCu nanoparticles (Fig. 10). The relatively complex ligand system probably weakened the strong effect of CO on the Pd-based materials. It was found that the presence of the Bi precursor was critical for the growth of the 1D nanostructures, while the CO helped the coalescence of the oriented particles.
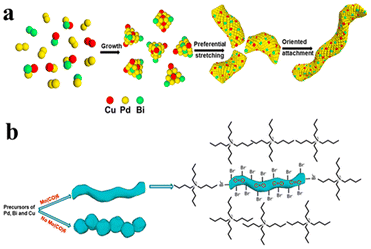 | ||
| Fig. 10 Schematics illustrating the growth mechanism of PdBiCu nanowires, (a) morphology evolution of nanowires from the precursor nuclei, and (b) effect of Mo(CO)6 presence on the morphology. Reprinted with permission from ref. 104. Copyright 2021 from the American Chemical Society. | ||
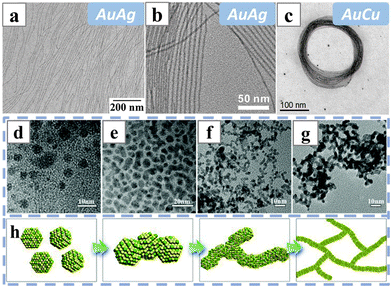 | ||
| Fig. 11 TEM images of (a) AuAg nanowires synthesized in oleylamine and oleic acid; (b) AuAg nanowires and (c) AuCu nanocoils synthesized with octadecylamine as the structure-directing reagent. (d–g) Pt3.5Pb nanowire intermediates taken at (d) 30 min, (e) 1 h, (f) 2 h and (g) 4 h of reaction times with oleylamine as the structure-directing reagent. (h) Schematics illustrating the formation mechanism of the Pt3.5Pb nanowires, with yellow balls and green balls representing Pt and Pb atoms. Reprinted with permission from ref. 108–111. Copyright 2016 from the American Chemical Society, 2013 from Wiley-VCH GmbH, 2018 from Elsevier. | ||
Recently, there was a report employing oleylamine as the structure-directing reagent to form Pt-based nanowires. The synthesis generated nerve-like PtPb alloy nanowires with various compositions under solvothermal conditions, with toluene as the solvent and borane tert-butylamine complex (BTBA) as the reducing agent (Fig. 11d–h).111 The obtained nanowires did not have well-defined linear or ultrathin morphologies. Their formation was attributed to an oriented attachment process induced by oleylamine, while the metal precursors, the acetylacetonate salts, were also of critical importance for co-reduction and the formation of uniform PtPb grains. The nanowire morphology would cease when the Pb(acac)2 precursor was absent.
The in situ formed dimethylamine was employed as the structure-directing reagent in a widely applied synthesis of Pt-based nanowires. It was initially developed to synthesize Pt nanowire bundles, and was later extended to a variety of Pt-based alloy nanowires (bundles), including bimetallic PtAu (PtPd),112,113 PtNi,114 PtCo,115,116 PtRu,117 and ternary PtNiCu118 and PtNiAu119 alloy nanowires (Fig. 12). Typically, Pt precursor H2PtCl6 and KOH were dissolved in a mixture of ethylene glycol (EG) and N,N-dimethylformamide (DMF) and heated under solvothermal conditions to induce the formation of Pt nanowire bundles. Although the decomposition of DMF released CO, spectroscopic results suggested that there was no CO binding on the nanowire bundles. Therefore, the other decomposition product of DMF, dimethylamine, was deduced to be the structure-directing reagent in this synthesis. In addition, the mechanism study suggested that the Pt nanowire bundles were formed via the coalescence of the 1D aligned nanoparticles.120
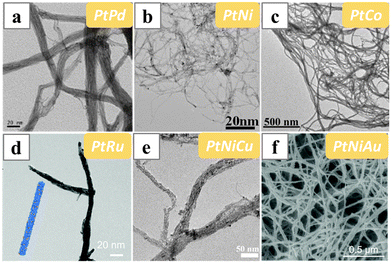 | ||
| Fig. 12 TEM and SEM images of (a) PtPd, (b) PtNi, (c) PtCo, (d) PtRu, (e) PtNiCu and (f) PtNiAu nanowire bundles synthesized by heating the precursors in the mixed solution of DMF and EG at solvothermal condition. Reprinted with permission from ref. 112, 114, 116–119. Copyright 2019 and 2020 from the American Chemical Society, 2018 and 2021 from Springer Nature, 2020 from the Royal Society of Chemistry. | ||
In order to achieve the co-precipitation of the different metals and the formation of alloys, the reaction parameters such as the type and ratio of the metal precursors, the solvent ratio and the amount of KOH, might need further modification, as the co-reduction of metals is sensitive towards the reaction conditions. For instance, strained PtPd nanowires were obtained with Pt(acac)2 and Pd(acac)2 as the metal precursors, and the composition of the alloy could be adjusted by varying the feeding ratio of the metal precursors.112 PtFe nanowires of various compositions were also obtained with Pt(acac)2 and Fe(acac)2, and by adding oleylamine as the solvent ingradient.121
Additional reducing reagent could be introduced into this system to promote the co-precipitation of Pt and metals with low reduction potentials. It was found that the addition of strong reducing agent ascorbic acid was vital for the formation of PtRu alloy nanowires.117 Without ascorbic acid, only Pt@Ru nanowires with the Ru islands attached to the Pt nanowires were obtained.
In terms of the formation mechanism, the autocatalytic effect was found to play a vital role in forming the alloy structures in this ligand system as well. For instance, Pt and Ni were co-precipitated in the solvothermal condition, yet the reduction system was not capable of reducing Ni2+ in the absence of Pt,114 indicating that Ni2+ was reduced via an autocatalytic mechanism. It is noted that although the Pt4+ was reduced prior to Ni2+, the product obtained was not the core–shell material. Interdiffusion between the two metals led to the formation of Pt3Ni alloy nanowires. In the same work the authors also believed that the growth of the Pt3Ni nanowires followed the seed-mediated mechanism, in contrast to the well-accepted oriented attachment mechanism, as no nanoparticle intermediates were observed.
Recently, another amino ligand, 4-aminopyridine, was exploited as a structure-directing reagent for alloy nanowires in aqueous solution. The anisotropic epitaxial growth yielded AuPd alloy nanowires and AuCu alloy nanowire bundles, at different temperature and reaction times (Fig. 13a–d).122–124 For the synthesis of the AuPd alloy nanowires, nanowires were formed by reducing Au and Pd with ascorbic acid at 80 °C for just 2 min. The nanowire structure cannot form in the absence of either Au, Pd precursors or the 4-aminopyridine. On the other hand, to form AuCu nanowires, the reaction only needed to be reacted at 20 °C for 10 min. Replacing the ascorbic acid with a stronger reducing agent such as NaBH4 compromised the linear structure of the nanowires. Au nanowires could be obtained at this condition with only the Au precursors. Thus, the reaction kinetics is also of critical importance for many ligand systems, and variation of the precursor concentration would cause the change of reduction kinetics. Amine-terminated poly(N-isopropylacrylamide) was employed to synthesize wavy AgPt nanowires (Fig. 13e).125 The amine-terminated polymer was believed to preferentially adsorb onto the {110} and {100} facets of the AgPt nanoparticles, facilitating the oriented attachment of the nanoparticles along the [111] direction.
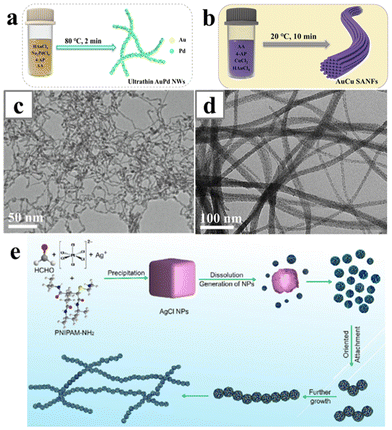 | ||
| Fig. 13 (a and b) Schematics illustrating the synthetic processes of AuPd nanowires and AuCu nanowire bundles with 4-aminopyridine as the structure-directing reagent. (c and d) TEM image of (c) AuPd nanowires and (d) AuCu nanowire bundles. (e) Schematics illustrating the formation mechanism of the ultrathin AgPt alloy nanowires with PNIPAM-NH2 as the structure-directing reagent. Reprinted with permission from ref. 122, 123 and 125. Copyright 2022 from Elsevier and 2017 from Springer Nature. | ||
Au-based AuAg126 and AuAgPd48 alloy nanowires with ultrathin morphology have been reported synthesized with PVP as the structure-directing ligands in DMF solution (Fig. 14). Both works obtained alloy nanowires with unusual fan-like twisted structures or the Boerdijk–Coxeter–Bernal (BCB) lattice. The AuAg alloy nanowires were considered strained, and the nanowire would undergo chiral transformation and twist into double helices upon Pd deposition and the formation of core–shell structures. Similarly, the AuAgPd alloy nanowires were found to be highly strained as well. The mechanism study indicated that during synthesis, single-crystalline AuAg nanowires would first form at the initial stage, followed by the deposition of the Pd sheath, which would cause the lattice structure of the nanowires to transform into fan-like structures.
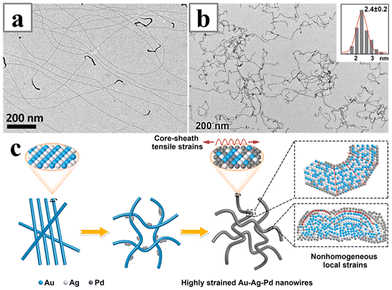 | ||
| Fig. 14 TEM images of (a) AuAg and (b) AuAgPd nanowires synthesized with PVP as ligand in DMF solution. (c) Schematics illustrating the formation of the highly strained and bent AuAgPd alloy nanowires. Reprinted with permission from ref. 126 and 48. Copyright 2011 and 2021 from the American Chemical Society. | ||
When PVP was employed as the structure-directing reagent in the syntheses of Pt and Pd-based alloy nanowires, wavy or chain-like networks were usually obtained, suggesting relatively weak control of PVP on the morphology of the nanowires (Fig. 15a–f). For instance, wavy Pt3Ag nanowires have been formed under solvothermal conditions with PVP as the capping agent.127 And Pd and PdM (M = Ag, Ni, Cu, Y) nanowire networks have recently been synthesized in a wet-chemical system with DMF and EG as the solvent and reducing agent, and also PVP as the capping agent.128 PdSn nanowire networks could be formed by employing SnC2O4 as the Sn source and adding NH4Cl as additional capping agent.129 Alternatively, PdSn nanochains were formed with (NH4)2PdCl4 and SnCl2 as precursors, and EG and citric acid as solvent and reductant in solvothermal conditions.130 PdCu nanowires have also been reported to be obtained with similar reagents in the solvothermal condition.131 In this case, Cu was believed to be crucial for the formation of the nanowire networks, and in the absence of Cu, only Pd nanoparticles were formed. Alternatively, pure Cu particles with sizes in the hundreds of nanometres were obtained if there was no Pd precursor. Almost all the Pd and Pd-based nanowires synthesized with PVP resemble chain-like networks, and mechanisms involving particle attachment were usually proposed to explain the assembly behaviour of the particles.
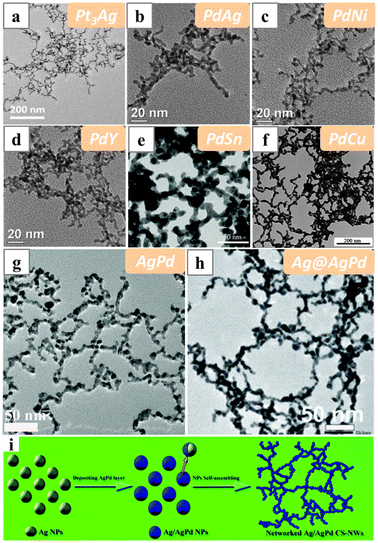 | ||
| Fig. 15 TEM images of (a) Pt3Ag, (b) PdAg, (c) PdNi, (d) PdY, (e) PdSn, (f) PdCu, (g) AgPd and (h) Ag@AgPd nanowires synthesized with PVP as the structure-directing reagent in aqueous solutions. (i) Schematics illustrating the oriented attachment of Ag@AgPd nanoparticles into networked Ag@AgPd nanowires. Reprinted with permission from ref. 127–129, 131, 133 and 134. Copyright 2020 from Springer Nature, 2018 and 2021 from the Royal Society of Chemistry, 2017 from Elsevier. | ||
There was also an unusual PVP-involved synthesis. Ru–Pd (Rh and Ag) alloy nanowires were generated by first synthesizing Ru nanoparticles with diameters of 2–3 nm with a polyol process, and then reducing the second metal together with the Ru nanoparticles in the presence of PVP under a solvothermal condition.132 The formation of the nanowire structure was attributed to an attachment process assisted by the heterogeneous nucleation of Pd, and the linear structure was obtained as a balance between the van der Waal's interaction and electrostatic repulsion between the particles. PVP in this work was believed to function as a stabilizer to assist the attachment process.
The PVP derivative polyvinylpyrrolidone imine was employed to induce the formation of AgPd nanowires in polyol solutions (Fig. 15g). Again an assembly mechanism was proposed: the two metals were first reduced into AgPd nanoparticles and then assembled into nanowire networks.133 And interestingly, Ag@AgPd core–shell nanowires could also be obtained by the assembly of Ag@AgPd core–shell nanoparticles (Fig. 15h and i).134
Sometimes, additional reagents need to be introduced in order to assist the co-reduction of metals during one-pot synthesis. With the presence of sulphite (SO32−) and proton (H+), twisty PdNi nanowires can be synthesized in PVP-containing aqueous solution under hydrothermal conditions.135 The effect of sulphite was found to be crucial for the co-precipitation process, and pure Pd nanowires could be obtained with it. H+ was found necessary for the formation of 1D morphology: increasing the pH would lead to the emergence of nanoparticles. While the HCl that was used to dissolve PdCl2 was considered as the proton source, supplying extra protons with H2SO4 can also lead to the formation of twisty nanowires, and it is noted that HCOOH was also involved in the system. A similar synthesis was developed to grow the ternary PtCuNi nanowires, with the solvent water replaced by EG.136 Sulphite also served as the key reagent for this electroless-plating process that enabled the co-precipitation of the metals, especially the reduction of Ni2+. Hence, sulphite is one useful reagent that helps the co-reduction and precipitation of metals, and induces alloy formation in the solution-based synthesis of alloy nanowires.
Additionally, there have also been reports about using other commercial surfactants, such as NP-40 and Pluronic F127, to synthesize Pd-based nanowires. Nanowires formed with these reagents had morphologies similar to those formed with PVP. Reducing K2PtCl4 and K2PdCl4 with ascorbic acid in the presence of F127 under hydrothermal conditions can yield PdPt nanowires.137 Introducing H3BO3/DMAB as the B source and NaH2PO3 as the P source during the synthesis can lead to the formation of PdBP nanowires.138 Chain-like PdBi and PdBiAu structures can be synthesized via a hydrothermal method, with DMF and oleylamine as the solvent and F127 as the additive.139 Alternatively, NP-40 as the structure-directing agent can be used to produce PdCu as well as PdFePb (and PdPb or PdFe) alloy nanowires.140,141 Both reports employ the borohydride reducing system, with either NaBH4 or KBH4 as the reducing agent in ice-cold aqueous solution. Formation of the nanowire structure was believed to be an anisotropic epitaxial growth guided by NP-40.
Apart from the abovementioned families of ligands, there are also syntheses of alloy nanowires or chain-like structures employing uncommon ligands or even without molecular ligands. For instance, using N,N-dimethylacetamide as the solvent and formamide as the ligand, binary PtCu, PtCo and ternary PtCuCo nanochains could be synthesized in solvothermal conditions.142
Overall, solution-based methods can be highly diverse in terms of the metal precursors, structure-directing reagent, reduction systems and the reaction conditions. Single or combinations of molecular and polymeric morphological directing agents or ligands are necessary during the reduction and deposition of metal to induce 1D growth. The most widely used ligands are CTAC (or CTAB), CO, various amine species and polymeric surfactants such as PVP. Micelle-templates or oriented attachment are the commonly proposed mechanisms in the solution-based synthesis. It is clear that the solvent and the reduction system need to be carefully matched with the ligand to enable the anisotropic growth of metal crystals.
3.3 Other methods
Besides the abovementioned template-based or solution chemical syntheses, several uncommon methods have also been developed to obtain alloy nanowires, and each method is specific to a certain type of alloy nanowire.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and deviation from that ratio would lead to failure of the formation of the PdPt nanowires: no nanowires could be obtained with pure K2PtCl4 or H2PdCl2 as the precursor. And the composition of the final PdPt nanowires was not controlled by the feeding ratio of the precursors, but rather by the upper potential limit of the square-wave potential applied during growth.
1, and deviation from that ratio would lead to failure of the formation of the PdPt nanowires: no nanowires could be obtained with pure K2PtCl4 or H2PdCl2 as the precursor. And the composition of the final PdPt nanowires was not controlled by the feeding ratio of the precursors, but rather by the upper potential limit of the square-wave potential applied during growth.
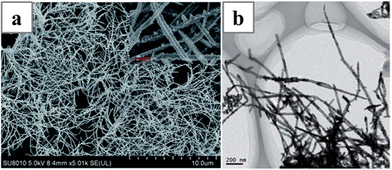 | ||
| Fig. 16 (a) SEM and (b) TEM image of the PdPt nanowires synthesized with template-less electrodeposition. Reprinted with permission from ref. 144. Copyright 2018 from the Royal Society of Chemistry. | ||
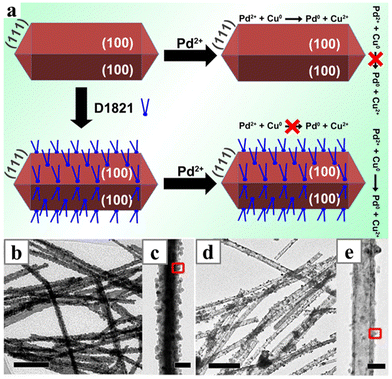
4. Forming alloy on existing nanowires
Compared with the direct formation approaches, synthesizing alloy nanowires based on existing nanowire structures circumvents the challenge of inducing 1D growth in metallic crystals. In cases of forming ternary or more alloys, the ingredients of the alloys can be introduced simultaneously or sequentially. Depending on the reactivity of the templating nanowires and the metal precursors, either replacement reactions, heterogeneous doping or heterogeneous nucleation would occur when the templating nanowires were mixed with the metal precursors (Fig. 17). The heterogeneous doping process was guided by the spontaneous interdiffusion between the metals, and a thermal annealing process could be employed to mix the metals in the core and shell layers if a core–shell structure was initially obtained.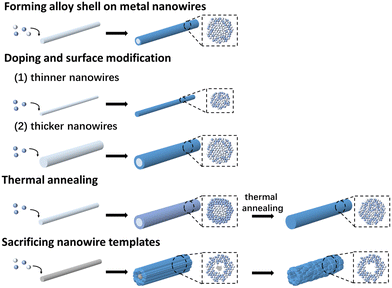 | ||
| Fig. 17 Schematics showing the different scenarios of forming alloy (core–shell) nanowires from existing 1D nanostructures. | ||
4.1 Forming alloy shell on metal nanowires
Synthesis of the core–shell nanowires with shell made of the alloy of the core metal can often be achieved simply with one-pot procedures. Due to the difference in the reduction potential, the second metal only starts to be reduced after the reduction and formation of the core nanowire, even if all the metal precursors are added together at the beginning of the reduction process. For instance, Au@PtAu nanowires could be obtained based on an α-naphthol-induced synthesis of Au nanowires (Fig. 18a).146 Pt was only present in the shell layer as its reduction only started after the formation of the core Au nanowires.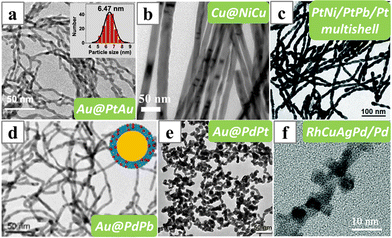 | ||
| Fig. 18 TEM images of (a) Au@PtAu, (b) Cu@NiCu, (c) PtNi/PtPb/Pt, (d) Au@PdPb, (e) Au@PdPt core–shell or multishell nanowires and (f) RhCuAgPd/Pd nanowires synthesized depositing the alloy shell on the preformed metal nanowires. Reprinted with permission from ref. 146–151. Copyright 2021 from Science Press and Elsevier, 2017 from the Royal Society of Chemistry, 2020 from Springer Nature, 2017 from the American Chemical Society. | ||
Besides, reaction temperature can be used to trigger the formation of the alloy shell. Cu@NiCu core–shell nanowire can be formed with a method derived from the synthesis of the Cu nanowires (Fig. 18b).147 Although both the Cu(acac)2 and NiCl2 were added at the beginning of the synthesis, the Ni only exists in the surface alloy layer as its reduction only occurred after the temperature was raised from 185 °C to 220 °C.
Nevertheless, a multi-step procedure is also quite plausible for the synthesis of core–shell alloy nanowires. PtNi/PtPb/Pt multishell nanowires were obtained by first synthesizing the PtNi nanowires in oleylamine solvent with CTAB as the structure-directing reagent, and then the reaction mixture was cooled slightly followed by the addition of Pb(acac)2 to induce the formation of the PtPb shell (Fig. 18c).148
Less commonly, alloy shells can be deposited on nanowires made of a foreign metal. The core and the shell materials must be carefully considered to allow the epitaxial growth of the shell on the core nanowires. PdPb (or Pd) shell can grow epitaxially on Au nanowires to form Au@PdPb nanowires, due to the small lattice mismatch between Au and Pd (Fig. 18d).149 Also, networks of Au@PdPt core–shell nanowires were synthesized by first reducing the HAuCl4 with sodium citrate to form in situ Au nanowires, and then adding the Na2PdCl4 and K2PtCl6 sequentially, and lastly another reducing agent ascorbic acid (Fig. 18e).150 Interestingly, the residue Au ions must be kept low during the reduction of Pd and Pt, as the ternary alloy Au–Pd–Pt would have a larger lattice mismatch with the Au nanowires, which would lead to the formation of isolated nanoparticles instead of coaxial nanowires.
When the interfacial energy between the alloy materials and the seed nanowires is too high to form uniform core–shell structures, nanowires with surface alloy islands are obtained. RhCuAgPd/Pd nanowires were synthesized with Pd nanowires as the seeds (Fig. 18f).151 The metal precursors were added after the formation of the Pt nanowires, and were reduced by the residual ascorbic acid left in the reaction mixture during the formation of the Pd nanowires.
4.2 Doping and surface modification
Post-synthetic doping and surface modification generate alloy nanowires based on the interdiffusion of metals, an entropy-driven process that occurs when atoms of different metals are brought together. Also, the diffusion kinetics of the metals must be sufficient to allow the spontaneous dispersion of the second metal into the lattice of the original nanowire and form solid solutions after being deposited onto the existing nanowires. For ultrathin nanowires, the interdiffusion process usually occurs easily; and in some cases, the formation of the alloy would even cause change in the lattice structure, inducing strain in the lattice and change in the morphologies of the nanowires. If the core nanowires were too thick to achieve homogeneous mixing of the different metals, core–shell nanowires with an alloy shell would be obtained.Strictly speaking, many of the abovementioned direct syntheses of Pt-based alloy nanowires also followed the heterogeneous doping mechanism. Although all metal precursors were added simultaneously during the one-pot syntheses, Pt with the highest reduction potential would be reduced first and form nanowires with the assistance of the structure-directing reagent, and the second metal was then deposited on the Pt nanowires to form the alloy nanowires. Alternatively, heterogeneous doping could also be carried out as a separated step, with the second metal being reduced in a different environment. In a recent work, Ga(acac)2 was reduced with ascorbic acid in the presence of preformed Pt nanowires to form ultrathin PtGa nanowires (Fig. 19a–d).152 A more obvious procedure was the formation of PtSn nanowires on carbon support.153 Pt precursors were first mixed with the carbon support and reduced by HCOOH for different times to obtain nanowires, followed by the addition and reduction of SnCl2 with HCOOH for 48 h to form the alloy nanowires.
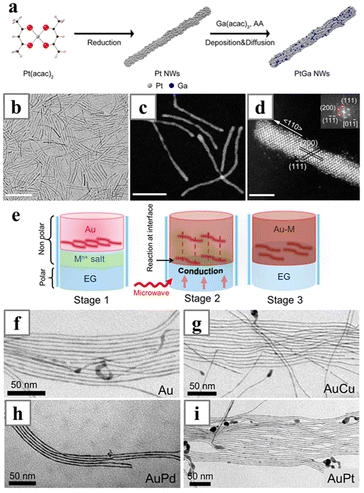 | ||
| Fig. 19 (a) Schematics illustrating the two-step synthesis of PtGa nanowires. (b) TEM, (c) HAADF-STEM and (d) atomic-resolution aberration-corrected HAADF-STEM image (with the corresponding FFT pattern) of the PtGa nanowires. (e) Schematics illustrating the formation of Au–M nanowires at the liquid–liquid interface. (f–i) The resulting TEM images of (f) Au, (g) AuCu, (h) AuPd, and (i) AuPt nanowire arrays. Reprinted with permission from ref. 152 and 155. Copyright 2019 and 2018 from the American Chemical Society. | ||
In a recent work, the early transition metal W was introduced into the pre-formed Pt nanowire through an electrochemical etching process to form PtW alloy nanowires.154 An amorphous WOx shell was first grown onto the Pt nanowires by depositing W precursors at 300 °C, followed by etching of the oxide layer and doping of W in the nanowires. Similar to other alloys, the reduction of W was attributed to the autocatalytic effect on the Pt surface, where the surface Pt–H acts as a strong reducing agent to reduce W. After that, the interdiffusion between the W and Pt in this electrochemical environment would allow the formation of the PtW nanowires.
Oleylamine-stabilized ultrathin Au nanowires have been employed as precursors for Au-based alloy nanowires. AuM (M = Pt, Cu and Pd) was obtained via heterogeneous doping processes occurring at the liquid–liquid interface of EG and hexane (Fig. 19e–i).155 The as-synthesized Au nanowires were first dissolved in the non-polar phase, while the metal precursors were in the polar phases and brought to the interface by the oleic acid additive. Glucose was employed as the reducing agent, and the reduction was carried out with microwave heating.
Alternatively, double helical AuAg and AuPd nanowires/nanoropes have been shown to be formed by directly reducing Ag or Pd in the presence of oleylamine-stabilized ultrathin Au nanowires (Fig. 20).156,157 The double helical AuAg nanowires were formed in the non-polar phase while the AuPd nanoropes were synthesized in aqueous solution. Either way, the interdiffusion of the metals can occur at room temperature and simultaneously with reduction of the second metal. The ultrathin Au nanowires were believed to be single crystalline and elongating along the [111] direction of the fcc lattice, and the alloying process altered the lattice structures of the ultrathin nanowires. The resulting lattice structures contained a lot of fan-like segments. While the BCB lattices should be straight in outline, the disordered lattice structure in the double helical nanowires may be the complex intermediates which were kinetically trapped half-way in between the transformation from fcc lattice to BCB lattices. The alloying process also imposed strain in the nanowires and twisted the straight nanowires into double helices. The strain and the lattice structure in the double helical alloy nanowires can be further adjusted with a H2O2-assisted fusion process, producing solid AuPd nanowires with optimized internal strain for catalytic applications.158
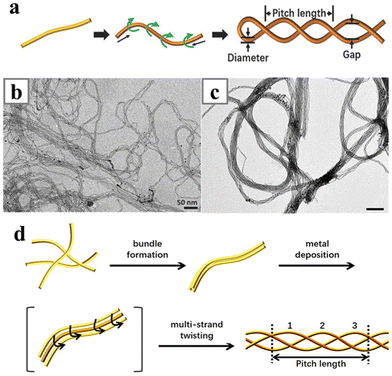 | ||
| Fig. 20 (a) Schematics illustrating the twisting of Au–Ag double helix nanowires. (b) TEM image of ultrathin Au–Ag alloy helices. (c) TEM image of AuPd nanoropes. (d) Schematics illustrating the braiding of AuPd nanoropes. Reprinted with permission from ref. 156 and 157. Copyright 2018 from WILEY-VCH GmbH and 2020 from the American Chemical Society. | ||
In contrast, surface deposition would not have a significant effect on the morphologies of nanowires with large diameters, but would form an alloy surface layer that greatly changes the physical and chemical properties of 1D nanostructures. Ag nanowires (or nanotubes) with improved chemical stability and mechanical strength can be obtained with surface-doping of Pd via galvanic replacement in the presence of the reducing agent ascorbic acid.159 The morphology and composition of the final structure can be adjusted by pH, which directly affects the reducing ability of ascorbic acid. Similarly, an Ir layer can be formed on the Ag nanowire surface and form Ag@AgIr core–shell nanowires with a two-step heating procedure.160 The Ag core could then be etched with HNO3 to yield the AgIr alloy nanotubes.
4.3 Thermal annealing
Thermal annealing is one conventional method to produce alloy materials.37,161 However, applying thermal annealing in the synthesis of alloy nanowires can be quite challenging. The nanowires, especially those that are ultrathin in nature, might not be able to maintain 1D morphology at elevated temperatures, as surface diffusion and atom migration would inevitably happen in company with the interdiffusion process. On the other hand, spontaneous interdiffusion of metal atoms would occur in ultrathin nanowires at room temperature, generating alloy nanowires directly during synthesis or via the post-synthetic deposition process. Thus, thermal annealing can only be applied to the thick core–shell 1D nanostructures to generate alloys with ordered crystalline phase.One typical demonstration of this approach was the synthesis of Cu3Au alloy nanowires with precise composition via the thermal annealing of the core–shell Cu@Au nanowires (Fig. 21).162 The annealing process was performed under the forming gas atmosphere so as to suppress the wire sintering during the high-temperature process. The Cu–Au forms a series of solid solutions at different annealing temperatures, and intermediate CuAu alloy nanowires with L10 phase were obtained when the temperature was below 200 °C. The L12 Cu3Au phase would emerge above 220 °C, and complete conversion to the uniform Cu3Au L12 phase was realized at 320 °C. Since the alloy process occurs by the interdiffusion of the core and shell materials, both raising the temperature and lengthening the annealing time would favour the formation of the alloy Cu3Au L12 phase. Compared with the pristine Cu nanowires and the Cu@Au core–shell nanowires, the final Cu3Au alloy nanowires exhibit improved thermal stability. When annealed in air, the Cu3Au only undergoes phase transition and morphology change above 250 °C, whereas phase transition in the other two nanowires starts at only 150 °C (Cu) and 200 °C (Cu@Au).
 | ||
| Fig. 21 (a–c) HAADF-STEM images and EDS elemental mappings of (a) pristine Cu@Au core–shell nanowire (b) an intermediate obtained after annealing the Cu@Au nanowires at 200 °C and (c) CuAu alloy nanowires obtained after annealing the Cu@Au nanowires at 320 °C. XRD patterns of (d) Cu3Au, (e) Cu@Au core–shell prepared at 140 °C and (f) Cu nanowires after annealing in air for 1 hour at elevated temperatures. Reprinted with permission from ref. 162. Copyright 2020 from Springer Nature. | ||
4.4 Sacrificing nanowire templates
When nanowires of the more active metal were employed as template to synthesize alloy nanowires, the alloying process would happen simultaneously with the galvanic replacement process. Sometimes, extra reducing agents were added to prevent overreaction between the metal nanowires and salt precursors. Often, tubular and porous structures with alloy shells were obtained, as the reduction and deposition always start from the surface of the template nanowires.PdCu–SnO2 nanowires could be formed by direct galvanic replacement between Na2PdCl4 and the Cu–SnO2 nanowires,163 while the porous PdAg nanowires need the presence of ascorbic acid in the galvanic replacement process between the Ag nanowires and Pd precursors at 80 °C.164 An opposite case is the formation of PtCu nanowires via the post-synthetic alloying of Cu nanowires. The Cu nanowires can directly react with H2PtCl4. However, in the absence of the reducing agent ascorbic acid, only surface-decorated Pt nanoparticles were obtained.165 In another work, the formation of either solid PdCu nanowires or hollow PdCu nanotubes was determined by a ligand, dimethyl distearylammonium chloride,166 which was believed to adsorb strongly on the (100) facets of the Cu nanowires (Fig. 22). In the presence of the ligand, the galvanic replacement could only occur at the ends of the Cu nanowires, leading to the formation of the tubular structure (Fig. 22d and e).
 | ||
| Fig. 22 (a) Schematics illustrating the selective galvanic displacement reaction on different facet of Cu nanowires; and the resulting TEM images of PdCu (b and c) nanowires and (d and e) nanotubes. Reprinted with permission from ref. 166. Copyright 2020 from Elsevier. | ||
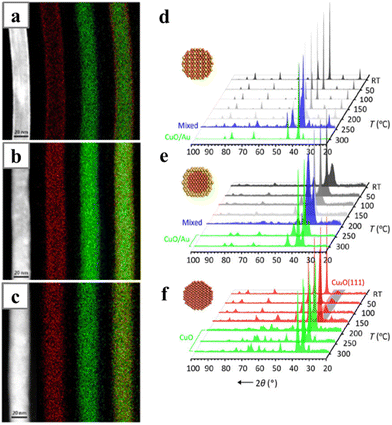
Te nanowires have been employed as a hard nanoscale template for the synthesis of various metal and metal alloy nanowires, such as Pd, PdPt and PdAu nanowires (Fig. 23a–e).167,168 The Te nanowires were oxidized with metal salt precursors. Therefore, the formation of the nanowires was achieved by galvanic replacement between the metals and the sacrificing Te template. Additionally, the sequence of adding metal precursors is crucial for the composition of the alloy nanowires. For instance, in order to form the quaternary PtPdRuTe alloy,169 the RuCl3 needed to be mixed first with the Te nanowires to initiate the galvanic replacement and yield the RuTe2 nanowire, followed by the addition of the Pt and Pd precursors. If all the metal precursors were added together, only ternary PtPdTe nanowires could form, as Pt and Pd possess high reduction potential, and they would deposit on the surface of Te nanowire first, inhibiting the galvanic replacement reaction between Te and Ru3+. Similarly, PtAgTe and PtRuAgTe nanowires could be obtained at solvothermal condition, with the stepwise transformation from Te nanowires to AgTe2 nanowires and finally the PGM-containing alloy nanowires.170 This templating method also offered a simple route to produce porous alloy nanowires, such as AuPt and PdPt porous nanowires. Typically, the Te-containing alloy nanowires were firstly formed via galvanic replacement between the Te nanowires and the metal precursors, and the residue Te in the alloy nanowires was then etched with NaClO to produce the porous morphology (Fig. 23f and g).171,172
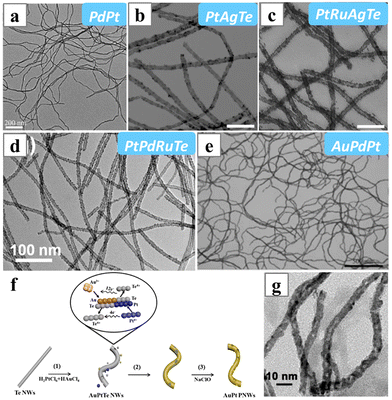 | ||
| Fig. 23 TEM images of (a) PdPt, (b) PtAgTe, (c) PtRuAgTe, (d) PtPdRuTe and (e) AuPdPt nanowiress synthesized via replacing the Te nanowires with metal precursors. (f) Schematics illustrating the formation mechanism of AuPt porous nanowires and (g) the resulting TEM image of AuPt porous nanowires. Reprinted with permission from ref. 167, 169–171. Copyright 2019 from the Royal Society of Chemistry, copyright 2017, 2019 and 2022 from the American Chemical Society. | ||
Metal ions and polymeric fibres, including biological macromolecules that form linear assemblies in solution and electrospun nanofibers,173,174 could assemble into 1D nanostructures and act as self-sacrificing templates to synthesize 1D alloy nanowires.175–179 For instance, insulin amyloid fibrils (INSAFs) have been exploited to synthesize PtRh180 and PtRhPd181 nanowires (Fig. 24a and b). The mixture of the metal precursors was first adsorbed onto the bio-templates, and then reduced with the strong reducing agent NaBH4 to yield alloy nanowires, or more specifically, nanochains made of small alloy nanoparticles. Afterwards, the biological template INSAF could be removed by washing with ammonia.
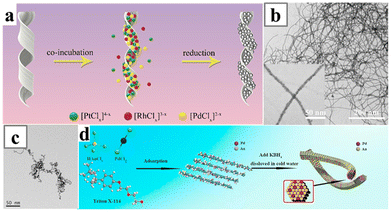 | ||
| Fig. 24 (a) Schematics illustrating the formation of PtRhPd nanocoils and (b) TEM image of the PtRhPd nanocoils. (c) TEM image of AuPd nanowires synthesized with Triton X-114 as the structure-directing reagent and (d) the schematics illustrating the formation mechanism of the Triton X-114 induced synthesis. Reprinted with permission from ref. 181 and 182. Copyright 2020 from the American Chemical Society and 2020 from Elsevier. | ||
Sometimes, co-assembly of metal ions with organic surfactants could form insoluble fabric nanostructures and act as self-templates for the formation of metal nanowires; for instance, incubating HAuCl4 and PdCl2 with the commercial surfactant Triton X-114 to form fabric assemblies (Fig. 24c and d).182 Reducing the intermediates with KBH4 yielded AuPd ultrathin nanowires. Similar procedures have also been employed to produce PdCu alloy nanowires.183
5. Metal alloy nanowires as electrocatalysts
Like the pure metal nanowires, most of the noble metal-based alloy nanowires are excellent electrocatalysts, as normally at least one of the nanowire compositions is an active catalyst for one or more electrochemical reactions.5.1 Oxygen reduction reactions
The oxygen reduction reaction (ORR) is the key process that happens at the cathode of fuel cells. Its slow kinetics remains the most challenging problem for the fuel cell industry.184 To date, platinum (Pt) remains the most promising and applicable commercial ORR catalyst, and replacing the benchmark Pt/C with Pt-based alloy nanowires with superior activity and stability is one meaningful way to reduce the usage of this expensive noble metal.185 Moreover, forming PtM alloys with non-noble metals can further cut the cost of the catalysts.While Pt is considered the most reliable metal catalyst for oxygen reduction, the binding energies of the oxygen species are still too strong on the Pt surface (typically the (111) facets) according to the volcano plot and calculations (Fig. 25a–c).36 Formation of PtM alloy is believed to decrease the binding energies of O2 and oxygen species (ΔEO) on the Pt surface, leading to enhancement of the ORR activities. Conventionally, the d-band centre, which is the average energy of d states that bind to the oxygen on the catalyst surface, is often evaluated as it is a direct indication of the ΔEO. A decrease in the d-band centre would lead to a less-filled d-band and thus weaker binding towards the surface oxide. Incorporating another transition metal, such as Ni and Co, would cause shrinkage of the skin Pt–Pt distance (the strain effect) as well as transfer of charge from Pt to the doping metal via the d-d interaction (the ligand effect), decrease of the d-band centre of the Pt and enhancement of the ORR activities (Fig. 25d).39 Based on this prediction, various PtNi, PtCo, PtFe, PtSn, PtCu, and PtPb alloy nanowires have been developed and demonstrated as superior ORR catalysts.
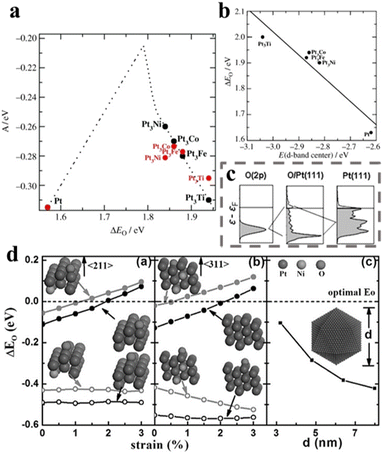 | ||
| Fig. 25 (a) Model of the activity (A) at a cell potential of 0.9 V as a function of the adsorption energy of oxygen, ΔEO. The predictions of the model for Pt3M (M = Ni, Co, Fe, Ti) alloys are shown relative to the predictions for Pt at the DFT-calculated values of ΔEO. (b) The correlation between the d-band center and ΔEO (c) sp-broadened 2p orbital for O(g), projected p density of states of oxygen atoms on Pt(111), and projected d density of states of Pt(111). (d) DFT calculations of oxygen adsorption energy. ΔEO as a function of compressive strain on (d(a)) (211) and (d(b)) (311) surfaces. The filled circles represent ΔEO values on the hollow sites while the open circles represent the bridge sites. The black and grey curves correspond to ΔEO values on pure Pt and Pt3Ni surface with top-layer Ni atoms completely removed. (d(c)) ΔEO on the (111) facet of the Pt nanoparticles as a function of the particle size. Reprinted with permission from ref. 36 and 39. Copyright 2006 and 2015 from Wiley-VCH GmbH. | ||
Besides Pt, Pd alloy is also an emerging material for ORR, owing to the similar physical and chemical properties of Pt and Pd. Many Pd-based alloy nanowires have also been applied as ORR catalysts, such as PdAu, PdNi, PdCu and PdBi nanowires. Similar to Pt, formation of PdM alloy also offers the ligand and strain effect on the electronic structure of Pd, lowing the d-band centre of Pd and offering much improved performances.
While the PtNi alloy has a more optimized electronic structure than the pristine Pt, a NH3 treatment was developed recently to further fine-tune the ΔEO on the PtNi nanowire surface.67 The as-synthesized PtNi nanowires were first washed with acetic acid to obtain a Pt-rich skin, followed by the NH3 treatment. NH3 has a slightly stronger adsorption to Ni than Pt, and this would promote the spillover of Ni and increase the Ni composition at the surface, decreasing the d-band centre and ΔEO (Fig. 26).67 The PtNi-C/NH3 nanowires exhibited specific (SA) and mass activities (MA) of 3.86 mA cm−2 and 1.02 A mgPt−1 respectively, much better than the benchmark Pt/C and the untreated PtNi/C nanowires.
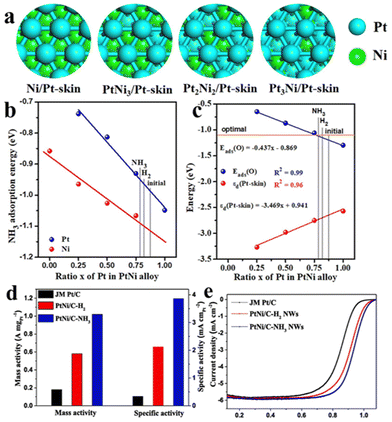 | ||
Fig. 26 (a) Models of Pt-skin covered PtNi alloys with different Pt![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni ratio. (b) NH3 adsorption on Pt or Ni atoms of PtNi alloy with different Pt Ni ratio. (b) NH3 adsorption on Pt or Ni atoms of PtNi alloy with different Pt![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni ratio. (c) d-band center (εd, red line) of Pt-skin covered on PtNi alloy with different Pt Ni ratio. (c) d-band center (εd, red line) of Pt-skin covered on PtNi alloy with different Pt![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni ratio and corresponding O adsorption energy (blue line). (d) mass and specific activities of Pt/C, PtNi/C–H2 NWs and PtNi/C–NH3 NWs at 0.9 V (vs. RHE). (e) ORR Polarization curves of PtNi/C–H2 NWs, PtNi/C–NH3 NWs and Pt/C. Reprinted with permission from ref. 67. Copyright 2021 from Elsevier. Ni ratio and corresponding O adsorption energy (blue line). (d) mass and specific activities of Pt/C, PtNi/C–H2 NWs and PtNi/C–NH3 NWs at 0.9 V (vs. RHE). (e) ORR Polarization curves of PtNi/C–H2 NWs, PtNi/C–NH3 NWs and Pt/C. Reprinted with permission from ref. 67. Copyright 2021 from Elsevier. | ||
On the other hand, the strain effect of alloying was also recently shown to be quite sensitive to the alloy compositions.112 A series of strain-modulated PtPd nanowires was synthesized via the solvothermal process in the mixed solvent of DMF and EG. The nanowires have the BCB lattice with mostly (111) facets, which are favourable for ORR. Moreover, XRD characterizations indicated that the alloying process caused either shrinkage or expansion of the lattice in the nanowires (Fig. 27). Lattice shrinking would lead to compressive Pt–Pt distance and weakening of the adsorption energy of oxygen species (*OH and *O) on the nanowire surface. The DFT calculation indicated that nanoclusters with 75%Pt in the alloy can bind more O2 species and less OH and O. Thus, the nanowires with the optimized composition of Pt78Pd22 had a subtle shrinking in lattice and exhibited the best catalytic activity among the series.
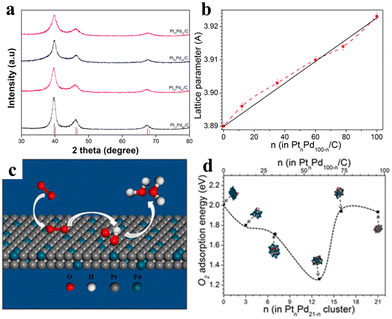 | ||
| Fig. 27 (a) XRD patterns of Pt12Pd88/C, Pt35Pd65/C, Pt60Pd40/C, and Pt78Pd22/C nanowires (b) lattice parameters for PtnPd100−n NWs/C on the relative composition of Pt%, the solid black line is according to Vegard's law based on the lattice parameters of Pt and Pd. (c) Schematic illustration of the catalytic synergy for the ORR over PtPd nanowires featuring the (111) facet. (d) Adsorption energy for molecularly adsorbed O on PtnPd21−n clusters (n = 1–21) obtained by DFT calculations. Reprinted with permission from ref. 112. Copyright 2020 from the American Chemical Society. | ||
Although the d-block transition metals are usually used for alloying with Pt and Pd, main group metals such as Ga can also be incorporated into Pt to form alloy nanowires with improved ORR performance.152 Instead of the d–d interaction, the Ga varied the electronic structure of Pt via the p–d hybridization interaction, causing redistribution of electron density on the skin Pt(111) surface and the downshift of the d band centre. This led to the boosting of ORR activities. Another advantage of forming PtGa alloy is the increase of the surface stability. The p–d interaction caused the Pt to be more resistant to oxidation, and the Ga to be less prone to leaching. The stability of both Pt and Ga contributed to the overall stability of the catalysts.
In terms of stability, alloy nanowires benefit from both the anisotropic morphology and the alloy nature. It is well accepted that the 1D morphology can provide more interaction points between the metal catalyst and the carbon support, helping to prevent the dissolution of the nanowires and increase the charge transfer capabilities. The alloy contributes to the chemical stability of the catalysts. For instance, it was calculated that PtML/Pt3Co had a decreased surface energy compared with pristine Pt, and addition of Ga would further decrease the surface energy, leading to an increase in the stability of the surface and the stability of the PtCoGa catalyst during catalytic cycles (Fig. 28).76
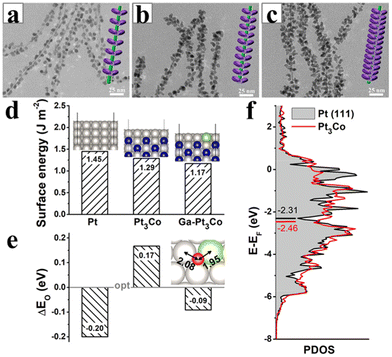 | ||
| Fig. 28 (a) TEM images and schematic illustrations of (a) 2% Ga–Pt3Co, (b) 4% Ga–Pt3Co, and (c) 8% Ga–Pt3Co nanowires. (d) Surface energy and (e) ΔEO of the pure Pt, Pt3Co, and Ga-doped Pt3Co (111) surfaces. The optimal EO is set to 0. The computational models are shown in insets: gray, blue, green, and red spheres represent Pt, Co, Ga, and O atoms, respectively. (f) The partial density of states (PDOS) for the 3d band of the Pt atoms in the pure Pt and Pt3Co surfaces, respectively. The horizontal lines indicate the calculated d-band centres. Reprinted with permission from ref. 76. Copyright 2020 from the American Chemical Society. | ||
In a recent work, a leaching mechanism was proposed to explain the ultrahigh stability of twisty PtNi nanowires.135 The nanowires would first benefit from the twisting morphology, which formed a self-tangled morphology on the electrodes, and thus made the nanowires more resistant to coalescence and detachment. Moreover, the continuous leaching of Ni in the alloy would constantly replenish the surface active sites during the catalytic process. As a result, the E1/2 of the twisty PtNi can be maintained for almost 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ORR cycles, much longer than those in normal ORR catalytic systems.
000 ORR cycles, much longer than those in normal ORR catalytic systems.
5.2 Alcohol oxidation reactions
Many Pt- and Pd-based alloys are bi-functional catalysts. They also exhibit satisfying activities for alcohol oxidation reactions (AORs), which are the important anode reactions in direct alcohol fuel cells (DAFCs). This is because the AOR catalyst needs to have a certain affinity for the oxygen species, as the catalytic AORs involve the surface dissociation of H2O and formation of oxide species on the catalysts as the oxygen sources. Typical alcohol oxidation reactions include the methanol oxidation (MOR), ethanol oxidation (EOR), and sometimes formic acid oxidation (FAOR), ethylene glycol oxidation (EGOR) and glycerol oxidation (GOR) reactions. Among them, MOR is the most studied catalytic process. Many Pt-based alloy nanowires, including PtPd, PtPb, PtCu, PtCo, PtRu, PtIr, and ternary PtCuNi nanowires have been demonstrated as having excellent performances as MOR catalysts. It is also reported that some Pd-based nanowires and the NiCu alloy nanowires are good MOR catalysts. As the anode reaction for direct ethanol fuel cells (DEFCs), EOR reactions are also widely studied. PtAg, PtZn, PtFe and PtCuNi, PtMoNi, and the Pd-based PdAu, PdAg and PdCu alloy nanowires have been shown to have improved EOR performances.One key concern in MOR (and also other AORs) is the elimination of the carbonaceous intermediates, typically COad, during the oxidation reaction. CO binds strongly on the pristine Pt surface, blocking the surface active sites and deactivating the catalysts. The catalytic activity is thus compromised, as extra overpotential is required to remove the intermediates. Owing to the ligand effect of alloying, many metal alloy nanowires benefit from the reduced binding energy for the carbonaceous intermediates. For instance, the onset oxidation potential for COad would continuously shift to the cathodic side as the Pd percentage in the AuAgPd nanowire decreased gradually, indicating the decrease of the CO adsorption energy on the catalyst surface (Fig. 29d).48 This could be attributed to the electron donation from Au and Ag to Pd. A similar effect was observed in Pt3Ag nanowires, and the weak affinity of Ag was also believed contribute to the weak adsorption of CO on the Pt3Ag nanowires.127
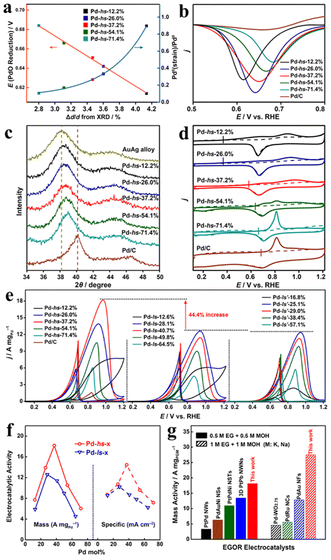 | ||
| Fig. 29 Properties of the highly strained AuAgPd alloy nanowires (Pd-hs-x,x = 12.2%–71.4%). (a) The potentials of the PdO reduction peaks and the XPS area ratios of Pd0(strain)/Pd0 as functions of the tensile strains (Δd/d from XRD) in the AuAgPd alloy nanowires. (b) XRD patterns of the Pd-hs-x nanowires. (c) PdO reduction peaks of the Pd-hs-x nanowires in N2-saturated 0.5 M KOH (scan range: 0.1–1.2 V). (d) CO stripping voltammograms of the nanowires in 0.5 M KOH. Vertical bars indicate the onset potentials of the CO oxidation peaks. (e) CV curves of the Pd-hs-x, Pd-ls-x, Pd-ls′-x nanowires and the commercial Pd/C in 0.5 M EG + 0.5 M KOH (scan rate: 50 mV s−1). (f) SA and MA of the Pd-hs-x and Pd-ls-x catalysts in the EGOR. (g) Comparison of the EGOR activity of the Pd-hs-37.2% nanowires with previously reported values. Reprinted with permission from ref. 48. Copyright 2021 from the American Chemical Society. | ||
In addition to lowering the adsorption of COad, the performance of alcohol oxidation catalysts can be enhanced by promoting the adsorption of OHad, which would accelerate the oxidation kinetics and the elimination of the carbonaceous species. A strain effect was recently introduced to elevate the affinity of surface Pd towards OHad. The tensile strain in Pd, which is caused by the lattice mismatch between the AuAg and sheath Pd in the ultrathin nanowires, raised the d-band centre of Pd and increased the adsorption of OH species.48 As a result, the highly strained AuAgPd showed superior performances in EGOR and other biomass-derived alcohol oxidations (Fig. 29).
Alternatively, some oxophilic metals can be incorporated into the metal nanowires to alter the surface affinity for OHad. In a recent work, PtCoRh ternary nanowires were reported to possess outstanding MOR activities.74 Typically, Rh is more oxophilic than Pt, and the formation of PtRh nanowires could increase the surface adsorption of OH species. Moreover, COad bonds to the PtRh surface in a bridge manner and was thus more easily removed.
Oxophilic main group metals can also be incorporated to tune CO adsorption and the performance of the alcohol oxidation on the Pd and Pt surface. For example, Pb can be oxidized at much lower potential. Therefore, on the surface of PtPb alloy, water dissociation occurs more easily on the Pb sites, and the resulting Pb–OHad could help to oxidize the COad on adjacent Pt atoms. As a result, the nerve-like Pt3.5Pb nanowires exhibited improved MOR activity in both acidic and alkaline conditions: the SA and MA are 4.10 (1.18 A mgPt−1) times and 5.24 (2.78 mA cm−2) times higher than those of Pt/C in the acidic condition, and 3.45 (2.84 A mgPt−1) and 4.12(6.51 mA cm−2) times higher in the alkaline condition.111 This strategy was also employed in ultrathin PdB nanowires.95 The incorporation of B into the nanowires was believed not only to weaken the adsorption of CO, but also facilitate the adsorption of OH.
Besides enhancing the activities of the nanowires, alloying also contributes to the stability of the catalysts. One typical case is the ultrathin PtNiMo nanowires synthesized via the thermal decomposition of Mo(CO)6 and the H2-assisted reduction of Pt(acac)2 and Ni(acac)2.51 The ternary nanowires exhibited superior activity and durability in MOR, and the enhanced durability of the PtNiMo nanowires was attributed to the Mo incorporation. The author carried out DFT calculations and showed that Mo can stabilize the uncoordinated sites via the strong Mo–Pt and Mo–Ni bonds, preventing the PtNi nanowires from dissolution and diffusion. As a result, the PtNiMo alloy nanowires exhibited much better activities and stabilities compared with Pt black and Pt/C (Fig. 30).
 | ||
| Fig. 30 Morphology and the electrochemical performance of PtNiMo alloy nanowires. (a) CVs of Pt black, Pt/C and as-prepared PtMoNi nanowires recorded in 0.5 M H2SO4 + 2 M CH3CH2OH solution at a scan rate of 50 mV s−1. (b) Comparison of SAs and MAs of the three catalysts. (c) chronoamperometric curves for the three catalysts recorded at 0.5 V versus SCE. (d) HAADF-STEM image (inset: enlarged image) of the PtMoNi nanowires. Reprinted with permission from ref. 51. Copyright 2017 from AAAS. | ||
Being a type of 1D nanostructure, many works about alloy nanowires emphasized the structural advantage of these catalysts, such as the pathway of electron and mass transport and the increased number of surface defects as active sites. Recently, further optimization of the structure factor was performed in a type of PtNiCu alloy nanowire. The exposed (110) and (100) facets in the PtNiCu nanowires increased from 36% to 88.3% after washing the as-synthesized nanowires with acetic acid.118 Compared with (111) facets, the (110) facets possess more abundant active catalytic sites and the (100) facets have lower surface energy, which favours the desorption of COad. The synergistic effect of the two facets led to lower onset potentials for COad and easier oxidation of the COad on the catalyst surface. Thus, the PtNiCu alloy nanowires after acid treatment exhibited a specific activity of 3.25 mA cm−2, 2.2 and 4.5 times higher than those of PtNiCu nanowires and Pt/C.
Apart from the AORs, there were reports about applying metal alloy nanowires, such as the high-entropy PtRuNiCoFeMo nanowires, as catalysts for the hydrogen oxidation reaction (HOR), which is also an important anode reaction for fuel cells. A recent work investigated in detail the effect of Ni in the PtNi alloy nanowires for alkaline HOR.139,186 PtNi alloy nanowires with the Ni presented either at the core or the surface of the nanowires were compared, and it was found that the surface Ni could enhance the HOR activity more significantly by increasing the adsorption of both *H and *OH species.
5.3 Water-splitting and CO2 reduction reactions
The electrocatalytic water-splitting process produces H2 as chemical fuel. The process involves the hydrogen evolution reaction (HER) and oxygen evolution reaction (OER). As a 1 − e− process, the kinetics for HER is relatively fast on a Pt catalyst surface. Still, many Pt- and Pd-based nanowires have been demonstrated to be capable of further accelerating the HER kinetics.187 For instance, the PtM–S alloy nanowires formed with SO32− as the additive in aqueous condition had ultrathin morphology and an optimized number of surface Pt/M–S(OH) interfaces. The Pt/M–S(OH) interfaces were composed of both the oxide/sulphide sites for effective water cleavage and the Pt surface for effective formation of H2 from the Had. Thus, the alloy nanowires showed superior activity for the HER in alkaline conditions. The MA and SA of the alloy nanowires were more than 5 times higher than that of the Pt/C.Pt and Pd-based metal alloy nanowires have seldom been applied as OER catalysts, probably because they were out-competed by RuO2 and IrO2 in catalytic OER reactions. Nevertheless, the OER activity of the Ru-containing high-entropy AlNiCoRuX alloy nanowires had been demonstrated besides their HER and ORR activity.143 It was found that the OER activities decreased when Co was added into the AlNiRu alloy, while the further incorporation of Mo yielded the AlNiRuCoMo alloy with the best OER performance. The author investigated the effect of Mo using calculation methods. By calculating the occupancies of the eg orbital of the octahedral sites in the surface oxide with inverse spinel structure, it was concluded that Mo doping would make the eg occupancies in both Co and Ru close to unity, leading to optimized ORR and OER activities in the quinary alloy.
Electrocatalytic reduction of CO2 (CO2RR) has become an emerging field in recent years, as it is one potential solution for slowing down and stopping the increase of the level of CO2 in the atmosphere. However, CO2RR is a complex reaction with multiple pathways and products, and must compete with HER in aqueous systems.188–191 Thus, a catalyst is of critical importance for electrocatalytic CO2RR, and a variety of metal-based catalysts have been developed for effective and selective CO2RR.
On the other hand, there are only a few reports about alloy nanowires for CO2RR applications, as Pt-based materials are more suited to the competitive HER.192 In one recent work, AgPd (Pd4Ag) nanowires were designed as an efficient CO2RR catalyst, as Pd can reduce CO2 to formate with low overpotential and high selectivity (Fig. 31).193 Since Ag has much weaker affinity for CO, the formation of an Ag alloy would weaken the CO binding on the catalyst surface sites, therefore inhibiting the CO poisoning that usually happens in Pd-based catalysts (Fig. 31e–g). As a result, the AgPd alloy nanowire can maintain more than 90% formate selectivity until the reduction potential reaches −0.3 V (vs. RHE), and also exhibits long-term stability for formate production at −0.43 V (vs. RHE).
 | ||
Fig. 31 CO2RR performance of AgPd and Pd nanowires. (a) Polarization curves of Pd4Ag in CO2- or N2-saturated 0.1 M KHCO3. (b) Total current density of AgPd at different working potentials. (c) Potential-dependent formate selectivity of AgPd in comparison with those of pure Pd and typical Pd catalyst from the literature. (d) Long-term chronoamperometric curves of AgPd and pure Pd for 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 s at a few selected potentials as indicated. (e–g) Analysis of the CO accumulation on AgPd and pure Pd during CO2RR. Positive going polarization curves on (e) AgPd and (f) pure Pd immediately after 1 h CO2RR electrocatalysis at different working potentials as indicated; (g) integrated CO oxidation charge and corresponding CO surface coverage on AgPd and pure Pd at different working potentials. Reprinted with permission from ref. 194. Copyright 2020 from Wiley-VCH GmbH. 000 s at a few selected potentials as indicated. (e–g) Analysis of the CO accumulation on AgPd and pure Pd during CO2RR. Positive going polarization curves on (e) AgPd and (f) pure Pd immediately after 1 h CO2RR electrocatalysis at different working potentials as indicated; (g) integrated CO oxidation charge and corresponding CO surface coverage on AgPd and pure Pd at different working potentials. Reprinted with permission from ref. 194. Copyright 2020 from Wiley-VCH GmbH. | ||
5.4 Other applications
Besides functioning directly as electrocatalysts, metal nanowires have been utilized in electrochemical analysis, catalytic reactions and other applications. For instance, PtNi nanowires have been assembled on reduced graphene oxide and applied as H2O2 sensors;194 ultrathin PdAg nanowires were applied as glucose sensors;195 and PdPt nanowires with excellent oxidation ability can be used for the detection of acid phosphatase in acidic conditions.168PtCoFe alloy nanowires were applied as the counter electrode and boosted the efficiency of dye-sensitized solar cells.98 Compared with pure Pt nanowires, the PtCoFe nanowires catalysed the conversion of I3− to I− more efficiently. The DFT calculation showed that the (111) facets of the ternary alloy could adsorb the I2 intermediates after the initial decomposition of the I3−, and facilitate the dissociation of I2 into I−. The theoretical result also further specified that the more active ternary alloy was Pt49Co23Fe28 from the adsorption and desorption energies of I2 on different (111) surfaces.
PdM (M = Ag, Y, Ni, Cu) nanowires were recently loaded into a polybenzimidazole (PBI) membrane to form a mixed matrix membrane (MMM) with improved H2 transportation and separation abilities (Fig. 32).128 Since H2 dissolves and diffuses through the metallic Pd in the form of H atoms, the PdM nanowires in the membrane can form continuous highways for the transportation of H2 through the membrane. The addition of alloy metal would further increase the permeability of H and in the meantime reduce consumption of the expensive Pd. On the other hand, CO2, the other product of steam reforming, can only diffuse through the PBI blocks. As a result, the MMM showed an impressive separation ability compared with the polymer membranes.
 | ||
| Fig. 32 (a) XRD patterns and (b) FTIR spectra of PBI and PdAg NW/PBI-MMM; SEM images of (c) bottom (d) top section of PBI-PdAg NW/PBI-MMM. Inset shows a photograph of a PdAg NW/PBI-MMM. (e) Schematic illustrating the hydrogen separation mechanism in a PdM NW-PBI MMM. Reprinted with permission from ref. 128. Copyright 2021 from the Royal Society of Chemistry. | ||
Some alloy nanowires were shown to be highly active in certain organic reactions. For example, PtFe nanowires can facilitate the selective and efficient hydrogenation of acetophenone and yield more than 90% of 1-phenylethanol at 99.3% conversion at 200 min.196 Both the activity and the selectivity of the alloy nanowires were much better than that of the pure Pt nanowires as well as the PtFe nanocubes. The high activity and selectivity of the alloy were attributed to the electron transfer from Pt and Fe, which produced electron-deficient surface Pt sites that are more favourable to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups. Moreover, the nanowire morphology leads to various high-index surface facets, as compared with the cubic PtFe. The stepped sites on the high-index facets would prefer the adsorption of C
O groups. Moreover, the nanowire morphology leads to various high-index surface facets, as compared with the cubic PtFe. The stepped sites on the high-index facets would prefer the adsorption of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O over the aromatic rings, while both groups would be adsorbed on the cubic facets, leading to the improved selectivity on the nanowires.
O over the aromatic rings, while both groups would be adsorbed on the cubic facets, leading to the improved selectivity on the nanowires.
In summary, metal alloy nanowires have diverse applications in electrocatalysis owing to the numerous metal combinations. Pt- and Pd-based nanowires have been investigated as advanced ORR, AOR and HER catalysts, as alloying could further optimize the electronic structures of the Pt and Pd and improve the catalytic performances. Nanowires containing Ru or Ir may be applied as OER catalyst, and the non-noble metals like Co and Ni are also active for this reaction. The electrocatalytic CO2 reduction, on the other hand, is to some extent underexplored at this moment. The Au and Ag-based materials usually have high selectivity for CO, and Cu is current the only metal that can facilitate the formation of the C–C bond in CO2RR. Therefore, it is desirable to synthesize and investigate the CO2RR properties of nanowires made of these metals.
6. Conclusions and outlook
This work focuses mainly on the chemical syntheses of alloy, mostly noble metal-based, nanowires in recent years. Both the morphological control and the composition adjustment need to be considered in the synthesis of metal alloy nanowires. In order to achieve growth of the 1D nanostructure, the alloy nanowires can either be formed directly or via the post-synthetic processing of pristine metal nanowires. The latter can also be considered as a type of templating method, with the metal nanowires as the templating precursors, while the former method involves the co-reduction and precipitation of multiple precursors under certain chemical conditions.Many methods that directly grow alloy nanowires have been developed from the syntheses of the pristine metal nanowires. Despite various compositions, alloy nanowires and the corresponding synthesis of the pristine nanowires share the same or similar mechanism for 1D growth, such as templating methods, ligand-direct deposition and elongation, and oriented attachment. Nevertheless, given the various reduction potentials of the metal ions, modifications to the syntheses need to be imposed to enable the co-reduction of the metal precursors. The autocatalytic effect of Pt plays an important role in many Pt-based syntheses, leading to the formation of a large variety of Pt-based nanowires. Furthermore, additional reducing agents with strong reducing abilities are required to create a more reductive environment for non-noble metals. Sometimes, less reactive precursors or additional coordination ligands were introduced to overcome the differences in the reduction potentials.
The methods based on existing nanowires can be divided into two categories: displacement-based methods and those without displacement reactions. The latter rely largely on the interdiffusion of the atoms to form the alloy nanowires. Typically, the interdiffusion can occur easily in ultrathin nanowires, resulting in a thorough mixing of the metal atoms and homogeneous phases in the alloy nanowires. In the relatively thicker nanowires, interdiffusion would encounter a kinetics problem, resulting in only surface doping. The thermal annealing process can help to initiate the interdiffusion and formation of homogeneous crystalline phases, while protection layers may be required for ultrathin nanowires to maintain the morphology during the high-temperature processes. In contrast, syntheses with template nanowires made of metals with low reduction potentials usually involve displacement reactions between the templates and the growth materials. These methods often produce tubular structures, as the displacement is always initiated from the nanowire surfaces.
Applications of metal alloy nanowires were also briefly reviewed. The metal alloy nanowires all exhibited improved catalytic properties, demonstrating the advantages of this group of materials. Many works discussed the compositional advantages of the alloys: the strain effect and the ligand effect of the alloy formation alter (or optimize) the electronic structure of the nanomaterials, making the surface more favourable to heterogeneous catalytic processes. Computational works, such as DFT calculations, were sometimes employed to articulate the effect in detail. Generally, the Pt or Pd composition in the alloy is still considered as the active site for catalytic processes, and the d band centre of the surface Pt/Pd and the adsorption energies on the surface Pt/Pd are varied by the alloying metals.
Although a variety of metal alloy nanowires have been synthesized and investigated, there remain plenty of challenges in this field. First, exploration of new routes or approaches to the synthesis of nanowires remains highly desirable. Synthesis is the foundation of all applications. Increase of the composition and structural diversities in the alloy nanowires would provide larger material reservoirs for customized applications. On the other hand, although a variety of synthetic protocols have been developed in the past several decades, ligand systems that are compatible with both inducing 1D growth and accommodating mixing of different metals are still quite limited.
In terms of understanding the syntheses, although the majority of the reported works provided studies on the growth processes, the underlying formation mechanisms for many 1D structures remain largely unclear. For example, many works attributed the formation of the 1D nanostructures to the oriented attachment mechanism, while the motivation as well as the step-by-step procedures for the oriented attachment process are to some extent underexplored: metal crystals are highly symmetric, and a symmetry-breaking mechanism would be required to drive the 1D assembly. Furthermore, the exact roles of the structural-directing reagents in many solution-based chemical processes were not specified. For instance, both CO and PVP have been reported as being able to induce the formation of Pt-based nanowires, yet the morphologies of the nanowires obtained with CO reagents are distinctively different from those induced by PVP. Therefore, it is quite unlikely that the two species take effect in a similar way. Cutting-edge characterization techniques such as in situ TEM might be able to provide additional insights into the growth mechanism and properties,197 while systematic and ingenious design of the control experiments would also be helpful for logical deductions.
There is still plenty of room for new alloy materials. Bimetallic alloy nanowires remain to be the most commonly synthesized 1D nanostructures. Forming alloys with more metals would definitely create materials with more diverse properties. And, of course, this would add to the complexities of the synthesis. Also, the compositions of the alloy nanowires need to be better controlled and characterized. Currently, the compositions of many metal alloy nanowires are adjusted by varying the feeding ratios of the metal precursors, and sometimes the metal ratio in the final nanowires deviates from the feeding ratios. Although characterizations such as EDS mapping were provided in almost all works, the microscopic distribution of the metal atoms remains largely unclear, except for those forming alloy crystal with determining phases. This uncertainty would compromise the validity of the theoretical calculations, and also cause difficulties in identifying the structure–property correlations.
Finally, being an additional dimension to tuning the chemical and physical properties of nanostructures, phase engineering has drawn increasing attention in recent years. A series of nanostructures containing metastable phases have been synthesized and demonstrated to have excellent electrocatalytic activities.198,199 Nanowires with high strain phases have been reported in some works, and the investigation of phase-dependent activities in alloy nanowires has just began.
Author contributions
S. Li, H. Jin and Y. Wang conceived the idea. Y. Wang wrote the original draft, S. Li and H. Jin completed the reproduction of figures. S. Li and Y. Wang revised the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge financial support from the National Natural Science Foundation of China (21703104), Jiangsu Science and Technology Plan (BK20170980), and Nanjing Tech University (39837131).References
- M. Aneke and M. Wang, Appl. Energy, 2016, 179, 350–377 CrossRef.
- S. Koohi-Fayegh and M. A. Rosen, J. Energy Storage, 2020, 27, 101047 CrossRef.
- A. Evans, V. Strezov and T. J. Evans, Renewable Sustainable Energy Rev., 2012, 16, 4141–4147 CrossRef.
- T. M. I. Mahlia, T. J. Saktisahdan, A. Jannifar, M. H. Hasan and H. S. C. Matseelar, Renewable Sustainable Energy Rev., 2014, 33, 532–545 CrossRef.
- T. Kousksou, P. Bruel, A. Jamil, T. El Rhafiki and Y. Zeraouli, Sol. Energy Mater. Sol. Cells, 2014, 120, 59–80 CrossRef CAS.
- O. Z. Sharaf and M. F. Orhan, Renewable Sustainable Energy Rev., 2014, 32, 810–853 CrossRef CAS.
- H. Xie, T. Wang, J. Liang, Q. Li and S. Sun, Nano Today, 2018, 21, 41–54 CrossRef CAS.
- P. E. Dodds, I. Staffell, A. D. Hawkes, F. Li, P. Grünewald, W. McDowall and P. Ekins, Int. J. Hydrogen Energy, 2015, 40, 2065–2083 CrossRef CAS.
- R. Sharifian, R. M. Wagterveld, I. A. Digdaya, C. Xiang and D. A. Vermaas, Energy Environ. Sci., 2021, 14, 781–814 RSC.
- B. Zhang, Y. Jiang, M. Gao, T. Ma, W. Sun and H. Pan, Nano Energy, 2021, 80, 105504 CrossRef CAS.
- A. Eftekhari and B. Fang, Int. J. Hydrogen Energy, 2017, 42, 25143–25165 CrossRef CAS.
- H. Chen, Y. S. Zhou, W. Guo and B. Y. Xia, Chin. Chem. Lett., 2022, 33, 1831–1840 CrossRef CAS.
- F. Y. Shi, L. L. Zhai, Q. Q. Liu, J. Y. Yu, S. P. Lau, B. Y. Xia and Z. L. Xu, J. Energy Chem., 2023, 76, 127–145 CrossRef CAS.
- H. Shen, T. Peppel, J. Strunk and Z. Sun, Sol. RRL, 2020, 4, 1900546 CrossRef CAS.
- L. Sun, V. Reddu, A. C. Fisher and X. Wang, Energy Environ. Sci., 2020, 13, 374–403 RSC.
- J. Mohammed-Ibrahim and X. Sun, J. Energy Chem., 2019, 34, 111–160 CrossRef.
- M.-I. Jamesh and X. Sun, J. Power Sources, 2018, 400, 31–68 CrossRef CAS.
- X. Li, X. Hao, A. Abudula and G. Guan, J. Mater. Chem. A, 2016, 4, 11973–12000 RSC.
- H. Sun, Z. Yan, F. Liu, W. Xu, F. Cheng and J. Chen, Adv. Mater., 2020, 32, 1806326 CrossRef CAS PubMed.
- T. K. Kormilina, E. A. Stepanidenko, S. A. Cherevkov, A. Dubavik, M. A. Baranov, A. V. Fedorov, A. V. Baranov, Y. K. Gun'ko and E. V. Ushakova, J. Mater. Chem. C, 2018, 6, 5278–5285 RSC.
- H. M. An, Z. L. Zhao, L. Y. Zhang, Y. Chen, Y. Y. Chang and C. M. Li, ACS Appl. Mater. Interfaces, 2018, 10, 41293–41298 CrossRef CAS PubMed.
- L. Jin, H. Xu, C. Chen, H. Shang, Y. Wang, C. Wang and Y. Du, ACS Appl. Mater. Interfaces, 2019, 11, 42123–42130 CrossRef CAS PubMed.
- H. Xu, F. Ren, B. Yan, J. Wang, S. Li and Y. Du, J. Electroanal. Chem., 2018, 811, 37–45 CrossRef CAS.
- F. Gao, Y. Zhang, H. You, Z. Li, B. Zou and Y. Du, Small, 2021, 17, e2101428 CrossRef PubMed.
- D. Huo, M. J. Kim, Z. H. Lyu, Y. F. Shi, B. J. Wiley and Y. N. Xia, Chem. Rev., 2019, 119, 8972–9073 CrossRef CAS PubMed.
- R. S. Devan, R. A. Patil, J. H. Lin and Y. R. Ma, Adv. Funct. Mater., 2012, 22, 3326–3370 CrossRef CAS.
- H. Xu, H. Y. Shang, C. Wang and Y. K. Du, Adv. Funct. Mater., 2020, 30, 2000793 CrossRef CAS.
- A. Kumar, M. M. Mohammadi and M. T. Swihart, Nanoscale, 2019, 11, 19058–19085 RSC.
- Y. Zhang, F. Gao, H. You, Z. Li, B. Zou and Y. Du, Coord. Chem. Rev., 2022, 450, 214244 CrossRef CAS.
- Y. Wang, Y. Yuan and H. Huang, Chin. J. Chem., 2021, 39, 1389–1396 CrossRef CAS.
- J. Lai and S. Guo, Small, 2017, 13, 1702156 CrossRef PubMed.
- Q. Shao, K. Lu and X. Huang, Small Methods, 2019, 3, 1800545 CrossRef.
- F. Gao, Y. Zhang, Z. Wu, H. You and Y. Du, Coord. Chem. Rev., 2021, 436, 213825 CrossRef CAS.
- P. Li and W. Chen, Chin. J. Catal., 2019, 40, 4–22 CrossRef CAS.
- L. Zhang, Z. Xie and J. Gong, Chem. Soc. Rev., 2016, 45, 3916–3934 RSC.
- V. Stamenkovic, B. S. Mun, K. J. J. Mayrhofer, P. N. Ross, N. M. Markovic, J. Rossmeisl, J. Greeley and J. K. Norskov, Angew. Chem., Int. Ed., 2006, 45, 2897–2901 CrossRef CAS PubMed.
- M. Zhou, C. Li and J. Y. Fang, Chem. Rev., 2021, 121, 736–795 CrossRef CAS PubMed.
- Y. Nakaya and S. Furukawa, Chem. Rev., 2022, 89, Article ASAP, DOI:10.1021/acs.chemrev.2c00356.
- L. Z. Bu, J. B. Ding, S. J. Guo, X. Zhang, D. Su, X. Zhu, J. L. Yao, J. Guo, G. Lu and X. Q. Huang, Adv. Mater., 2015, 27, 7204–7202 CrossRef CAS PubMed.
- T. Wang, A. Chutia, D. J. L. Brett, P. R. Shearing, G. He, G. Chai and I. P. Parkin, Energy Environ. Sci., 2021, 14, 2639–2669 RSC.
- D. Kim, J. Resasco, Y. Yu, A. M. Asiri and P. Yang, Nat. Commun., 2014, 5, 4948 CrossRef CAS PubMed.
- J. Fan, H. Du, Y. Zhao, Q. Wang, Y. Liu, D. Li and J. Feng, ACS Catal., 2020, 10, 13560–13583 CrossRef CAS.
- L. Y. Zhang, C. X. Guo, H. J. Cao, S. Wang, Y. R. Ouyang, B. H. Xu, P. Z. Guo and C. M. Li, Chem. Eng. J., 2022, 431, 133237 CrossRef CAS.
- J. W. M. Crawley, I. E. Gow, N. Lawes, I. Kowalec, L. Kabalan, C. R. A. Catlow, A. J. Logsdail, S. H. Taylor, N. F. Dummer and G. J. Hutchings, Chem. Rev., 2022, 122, 6795–6849 CrossRef CAS PubMed.
- F. Gao, Y. Zhang, F. Ren, Y. Shiraishi and Y. Du, Adv. Funct. Mater., 2020, 30, 2000255 CrossRef CAS.
- X. H. Song, X. Y. Zhang, Q. Chang, X. Yao, M. F. Li, R. P. Zhang, X. T. Liu, C. Y. Song, Y. X. A. Ng, E. H. Ang and Z. H. Ou, Small, 2022, 18, 2203310 CrossRef CAS PubMed.
- D. B. Williams and C. B. Carter, Transmission Electron Microscopy A Textbook for Materials Science, Springer, Springer New York, New York, 2009 Search PubMed.
- S. Zhang, K. Liu, Z. Liu, M. Liu, Z. Zhang, Z. Qiao, L. Ming and C. Gao, Nano Lett., 2021, 21, 1074–1082 CrossRef CAS PubMed.
- W. Zhang, Y. Yang, B. Huang, F. Lv, K. Wang, N. Li, M. Luo, Y. Chao, Y. Li, Y. Sun, Z. Xu, Y. Qin, W. Yang, J. Zhou, Y. Du, D. Su and S. Guo, Adv. Mater., 2019, 31, e1805833 CrossRef PubMed.
- D. E. Ramaker and C. Roth, X-ray absorption near edge structure (Δμ XANES) techniques for low temperature fuel cell characterization, in Polymer Electrolyte Membrane and Direct Methanol Fuel Cell Technology, 2012, pp. 120–145 Search PubMed.
- J. J. Mao, W. X. Chen, D. S. He, J. W. Wan, J. J. Pei, J. C. Dong, Y. Wang, P. F. An, Z. Jin, W. Xing, H. L. Tang, Z. B. Zhuang, X. Liang, Y. Huang, G. Zhou, L. Y. Wang, D. S. Wang and Y. D. Li, Sci. Adv., 2017, 3, 323–324 Search PubMed.
- D. Shore, A. Ghemes, O. Dragos-Pinzaru, Z. Gao, Q. Shao, A. Sharma, J. Um, I. Tabakovic, J. C. Bischof and B. J. H. Stadler, Nanoscale, 2019, 11, 14607–14615 RSC.
- S. Khan, N. Ahmad, N. Ahmed and X. F. Han, J. Magn. Magn. Mater., 2018, 460, 120–127 CrossRef CAS.
- J. C. Xu, B. Hong, X. L. Peng, X. Q. Wang, H. L. Ge and J. Hu, Chem. Phys. Lett., 2021, 767, 138368 CrossRef CAS.
- S. Samanifar, M. A. Kashi and A. Ramazani, Phys. C, 2018, 548, 72–74 CrossRef CAS.
- L. Du, D. Feng, X. Xing, C. Wang, G. S. Armatas and D. Yang, Chem. Eng. J., 2020, 400, 125864 CrossRef CAS.
- L. Du, L. Zheng, H. Wei, S. Zheng, Z. Zhu, J. Chen and D. Yang, ACS Appl. Nano Mater., 2019, 2, 1178–1184 CrossRef CAS.
- J. S. Riva, A. V. Juárez, S. E. Urreta and L. M. Yudi, Electrochim. Acta, 2019, 298, 379–388 CrossRef CAS.
- C. Wang, L. Zheng, R. Chang, L. Du, C. Zhu, D. Geng and D. Yang, ACS Appl. Mater. Interfaces, 2018, 10, 29965–29971 CrossRef CAS PubMed.
- D. Li and E. J. Podlaha, Nano Lett., 2019, 19, 3569–3574 CrossRef CAS PubMed.
- P. Zhang, I. Wyman, J. Hu, S. Lin, Z. Zhong, Y. Tu, Z. Huang and Y. Wei, Mater. Sci. Eng., B, 2017, 223, 1–23 CrossRef CAS.
- X. Li, Y. Wang, C. Yin and Z. Yin, J. Mater. Chem. C, 2020, 8, 849–872 RSC.
- S. Fahad, H. Yu, L. Wang, Zain-ul-Abdin, M. Haroon, R. S. Ullah, A. Nazir, K.-u.-R. Naveed, T. Elshaarani and A. Khan, J. Mater. Sci., 2018, 54, 997–1035 CrossRef.
- N. A. C. Lah and S. Trigueros, Sci. Technol. Adv. Mater., 2019, 20, 225–261 CrossRef CAS PubMed.
- L. Bu, J. Ding, S. Guo, X. Zhang, D. Su, X. Zhu, J. Yao, J. Guo, G. Lu and X. Huang, Adv. Mater., 2015, 27, 7204–7212 CrossRef CAS PubMed.
- K. Jiang, Q. Shao, D. Zhao, L. Bu, J. Guo and X. Huang, Adv. Funct. Mater., 2017, 27, 1700830 CrossRef.
- H. Tang, Y. Su, B. Chi, J. Zhao, D. Dang, X. Tian, S. Liao and G.-R. Li, Chem. Eng. J., 2021, 418, 129322 CrossRef CAS.
- P. Lv, B. Jin, C. Mei, H. Zhou, T. Yuan, Z. Li, H. He, X. Shen and X. Hong, Chemistry, 2018, 24, 14636–14638 CrossRef CAS PubMed.
- P. Wang, X. Zhang, J. Zhang, S. Wan, S. Guo, G. Lu, J. Yao and X. Huang, Nat. Commun., 2017, 8, 14580 CrossRef CAS PubMed.
- M. Luo, Y. Sun, Y. Qin, S. Chen, Y. Li, C. Li, Y. Yang, D. Wu, N. Xu, Y. Xing, L. Wang, P. Gao and S. Guo, Chem. Mater., 2018, 30, 6660–6667 CrossRef CAS.
- L. Bu, S. Guo, X. Zhang, X. Shen, D. Su, G. Lu, X. Zhu, J. Yao, J. Guo and X. Huang, Nat. Commun., 2016, 7, 11850 CrossRef CAS PubMed.
- M. Luo, Y. Sun, X. Zhang, Y. Qin, M. Li, Y. Li, C. Li, Y. Yang, L. Wang, P. Gao, G. Lu and S. Guo, Adv. Mater., 2018, 30, 1705515 CrossRef PubMed.
- Y. Xu, X. Cui, S. Wei, Q. Zhang, L. Gu, F. Meng, J. Fan and W. Zheng, Nano Res., 2019, 12, 1173–1179 CrossRef CAS.
- X. Chen, W. Wang, X. Chen, X. Liao, Z. Lyu, K. Liu and S. Xie, Nanoscale, 2021, 13, 2632–2638 RSC.
- H. Li, Y. Pan, D. Zhang, Y. Han, Z. Wang, Y. Qin, S. Lin, X. Wu, H. Zhao, J. Lai, B. Huang and L. Wang, J. Mater. Chem. A, 2020, 8, 2323–2330 RSC.
- M. Li, Z. Zhao, Z. Xia, Y. Yang, M. Luo, Y. Huang, Y. Sun, Y. Chao, W. Yang, W. Yang, Y. Yu, G. Lu and S. Guo, ACS Catal., 2020, 10, 3018–3026 CrossRef CAS.
- P. T. Wang, Q. Shao, X. N. Cui, X. Zhu and X. Q. Huang, Adv. Funct. Mater., 2018, 28, 1705918 CrossRef.
- C. Zhan, Y. Xu, L. Bu, H. Zhu, Y. Feng, T. Yang, Y. Zhang, Z. Yang, B. Huang, Q. Shao and X. Huang, Nat. Commun., 2021, 12, 6261 CrossRef CAS PubMed.
- Y. Xin, S. H. Li, Y. Y. Qian, W. K. Zhu, H. B. Yuan, P. Y. Jiang, R. H. Guo and L. B. Wang, ACS Catal., 2020, 10, 11280–11306 CrossRef CAS.
- X. Wang, W. Guo and Y. Z. Fu, J. Mater. Chem. A, 2021, 9, 663–701 RSC.
- C. Chen, H. Xu, H. Shang, L. Jin, T. Song, C. Wang, F. Gao, Y. Zhang and Y. Du, Nanoscale, 2019, 11, 20090–20095 RSC.
- L. Huang, W. Zhang, Y. Zhong, P. Li, D. Xiang, W. Uddin, X. Li, Y.-G. Wang, X. Yuan, D. Wang and M. Zhu, Sci. China Mater., 2020, 64, 601–610 CrossRef.
- X. L. Tian, X. Zhao, Y. Q. Su, L. J. Wang, H. M. Wang, D. Dang, B. Chi, H. F. Liu, E. J. M. Hensen, X. W. Lou and B. Y. Xia, Science, 2019, 366, 850–856 CrossRef CAS PubMed.
- F. Gao, Y. Zhang, P. Song, J. Wang, B. Yan, Q. Sun, L. Li, X. Zhu and Y. Du, Nanoscale, 2019, 11, 4831–4836 RSC.
- Y. Mo, S. Feng, T. Yu, J. Chen, G. Qian, L. Luo and S. Yin, J. Colloid Interface Sci., 2022, 607, 1928–1935 CrossRef CAS PubMed.
- P. Song, H. Xu, J. Wang, Y. Zhang, F. Gao, J. Guo, Y. Shiraishi and Y. Du, Nanoscale, 2018, 10, 16468–16473 RSC.
- K. Z. Jiang, D. D. Zhao, S. J. Guo, X. Zhang, X. Zhu, J. Guo, G. Lu and X. Q. Huang, Sci. Adv., 2017, 3, 1601705 CrossRef PubMed.
- N. Cheng, L. Zhang, Y. Zhou, S. Yu, L. Chen, H. Jiang and C. Li, J. Colloid Interface Sci., 2020, 572, 170–178 CrossRef CAS PubMed.
- L. Huang, X. Zhang, Q. Wang, Y. Han, Y. Fang and S. Dong, J. Am. Chem. Soc., 2018, 140, 1142–1147 CrossRef CAS PubMed.
- X. Xu, H. Lv, L. Sun, P. Song, B. Liu and X. Chen, ChemPlusChem, 2020, 85, 970–976 CrossRef CAS PubMed.
- H. Lv, X. Chen, D. Xu, Y. Hu, H. Zheng, S. L. Suib and B. Liu, Appl. Catal., B, 2018, 238, 525–532 CrossRef CAS.
- D. Xu, X. Liu, M. Han and J. Bao, Chem. Commun., 2016, 52, 12996–12999 RSC.
- H. Lv, L. Sun, D. Xu, Y. Ma and B. Liu, Appl. Catal., B, 2019, 253, 271–277 CrossRef CAS.
- N. Han, M. Sun, Y. Zhou, J. Xu, C. Cheng, R. Zhou, L. Zhang, J. Luo, B. Huang and Y. Li, Adv. Mater., 2021, 33, 2005821 CrossRef CAS PubMed.
- Y. Wang, H. Lv, L. Sun, X. Guo, D. Xu and B. Liu, ACS Appl. Mater. Interfaces, 2021, 13, 17599–17607 CrossRef CAS PubMed.
- W. L. Pei, C. Wu, Y. Zhang, Q. Wang, C. S. He, J. J. Wang, K. H. Zhang and X. Zhao, Int. J. Nanotechnol., 2016, 13, 801–808 CrossRef CAS.
- S. Guo, D. Li, H. Zhu, S. Zhang, N. M. Markovic, V. R. Stamenkovic and S. Sun, Angew. Chem., Int. Ed., 2013, 52, 3465–3468 CrossRef CAS PubMed.
- C.-C. P. Chiang, C.-Y. Hung, S.-W. Chou, J.-J. Shyue, K.-Y. Cheng, P.-J. Chang, Y.-Y. Yang, C.-Y. Lin, T.-K. Chang, Y. Chi, H.-L. Chou and P.-T. Chou, Adv. Funct. Mater., 2018, 28, 1703282 CrossRef.
- H. Zhu, S. Zhang, D. Su, G. Jiang and S. Sun, Small, 2015, 11, 3545–3549 CrossRef CAS PubMed.
- Z. Yang, H. Yang, L. Shang and T. Zhang, Angew. Chem., Int. Ed., 2022, 61, e202113278 CAS.
- H. J. Yu, T. Q. Zhou, Z. Q. Wang, Y. Xu, X. N. Li, L. Wang and H. J. Wang, Angew. Chem., Int. Ed., 2021, 60, 12027–12031 CrossRef CAS PubMed.
- T. T. Zeng, X. M. Meng, H. W. Huang, L. W. Zheng, H. B. Chen, Y. Zhang, W. Y. Yuan and L. Y. Zhang, Small, 2022, 18, 8 Search PubMed.
- J. Li, Z. Y. Zhou, H. Xu, C. Wang, S. Hata, Z. X. Dai, Y. Shiraishi and Y. K. Du, J. Colloid Interface Sci., 2022, 611, 523–532 CrossRef CAS PubMed.
- H. Ali, H. Lin, Q. Lu and X. Wang, J. Phys. Chem. C, 2021, 125, 14646–14655 CrossRef CAS.
- S. Mourdikoudis and L. M. Liz-Marzán, Chem. Mater., 2013, 25, 1465–1476 CrossRef CAS.
- Z. Y. Huo, C. K. Tsung, W. Y. Huang, X. F. Zhang and P. D. Yang, Nano Lett., 2008, 8, 2041–2044 CrossRef CAS PubMed.
- C. Wang, Y. J. Hu, C. M. Lieber and S. H. Sun, J. Am. Chem. Soc., 2008, 130, 8902–8903 CrossRef CAS PubMed.
- X. Hong, Z. Yin, Z. Fan, Y. Y. Tay, J. Chen, Y. Du, C. Xue, H. Chen and H. Zhang, Small, 2014, 10, 479–482 CrossRef CAS PubMed.
- R. Mendoza-Cruz, L. Bazán-Díaz, J. Jesús Velázquez-Salazar, G. Plascencia-Villa, D. Bahena-Uribe, J. Reyes-Gasga, D. Romeu, G. Guisbiers, R. Herrera-Becerra and M. José-Yacamán, Nano Lett., 2016, 16, 1568–1573 CrossRef CAS PubMed.
- Y. Imura, T. Mori, C. Morita-Imura, H. Kataoka, R. Akiyama, H. Kurata and T. Kawai, Colloids Surf., A, 2018, 543, 9–14 CrossRef CAS.
- L. Huang, Y. Han, X. Zhang, Y. Fang and S. Dong, Nanoscale, 2017, 9, 201–207 RSC.
- F. Chang, Z. Bai, M. Li, M. Ren, T. Liu, L. Yang, C. J. Zhong and J. Lu, Nano Lett., 2020, 20, 2416–2422 CrossRef CAS PubMed.
- M. Ren, F. Chang, R. Miao, X. He, L. Yang, X. Wang and Z. Bai, Inorg. Chem. Front., 2020, 7, 1713–1718 RSC.
- M. Gong, Z. Deng, D. Xiao, L. Han, T. Zhao, Y. Lu, T. Shen, X. Liu, R. Lin, T. Huang, G. Zhou, H. Xin and D. Wang, ACS Catal., 2019, 9, 4488–4494 CrossRef CAS.
- X. Zhai, P. Wang, K. Wang, J. Li, X. Pang, X. Wang and Z. Li, Ionics, 2020, 26, 3091–3097 CrossRef CAS.
- Q. Lu, L. Sun, X. Zhao, J. Huang, C. Han and X. Yang, Nano Res., 2018, 11, 2562–2572 CrossRef CAS.
- M. Li, Y. Wang, J. Cai, Y. Li, Y. Liu, Y. Dong, S. Li, X. Yuan, X. Zhang and X. Dai, Dalton Trans., 2020, 49, 13999–14008 RSC.
- S. Li, Y. Wang, Y. Li, X. Fang, Y. Liu, M. Li, Z. Wang, Y. Gao, H. Sun, F. Gao, X. Zhang and X. Dai, Nano Res., 2021, 15, 2877–2886 CrossRef.
- Z. Lin, Y. Sheng, J. Li, Z. Rui, Y. Liu, J. Liu and Z. Zou, Chem. Commun., 2020, 56, 4276–4279 RSC.
- B. Y. Xia, H. B. Wu, Y. Yan, X. W. Lou and X. Wang, J. Am. Chem. Soc., 2013, 135, 9480–9485 CrossRef CAS PubMed.
- F. Chang, J. Wei, Q. Zhang, Z. Jia, Y. Liu, L. Yang, X. Wang and Z. Bai, Mater. Chem. Front., 2021, 5, 8118–8126 RSC.
- S. Liu, H. Ren, S. Yin, H. Zhang, Z. Wang, Y. Xu, X. Li, L. Wang and H. Wang, Chem. Eng. J., 2022, 435, 134823 CrossRef CAS.
- S. Liu, S. Yin, Z. Wang, Y. Xu, X. Li, L. Wang and H. Wang, Cell Rep. Phys. Sci., 2022, 3, 100869 CrossRef CAS.
- Z.-J. Wang, X. Wang, J.-J. Lv, J.-J. Feng, X. Xu, A.-J. Wang and Z. Liang, New J. Chem., 2017, 41, 3894–3899 RSC.
- X. Jiang, G. T. Fu, X. Wu, Y. Liu, M. Y. Zhang, D. M. Sun, L. Xu and Y. W. Tang, Nano Res., 2018, 11, 499–510 CrossRef CAS.
- Y. Wang, Q. Wang, H. Sun, W. Zhang, G. Chen, Y. Wang, X. Shen, Y. Han, X. Lu and H. Chen, J. Am. Chem. Soc., 2011, 133, 20060–20063 CrossRef CAS PubMed.
- X. Fu, C. Wan, A. Zhang, Z. Zhao, H. Huyan, X. Pan, S. Du, X. Duan and Y. Huang, Nano Res., 2020, 13, 1472–1478 CrossRef CAS.
- A. Kumar, L. Huang, L. Hu, D. Yin, H. Lin and M. T. Swihart, J. Mater. Chem. A, 2021, 9, 12755–12762 RSC.
- H. You, F. Gao, C. Wang, J. Li, K. Zhang, Y. Zhang and Y. Du, Nanoscale, 2021, 13, 17939–17944 RSC.
- Y. Gong, X. Liu, Y. Gong, D. Wu, B. Xu, L. Bi, L. Y. Zhang and X. S. Zhao, J. Colloid Interface Sci., 2018, 530, 189–195 CrossRef CAS PubMed.
- B. Yan, H. Xu, K. Zhang, S. Li, J. Wang, Y. Shi and Y. Du, Appl. Surf. Sci., 2018, 434, 701–710 CrossRef CAS.
- D. Sun, H. Yu, H. Su, F. Jin, J. Liu and C. C. Li, ChemCatChem, 2017, 9, 347–353 CrossRef CAS.
- H. Liu, Y. S. Yu, W. W. Yang, W. J. Lei, M. Y. Gao and S. J. Guo, Nanoscale, 2017, 9, 9305–9309 RSC.
- H. Liu, X. Y. Liu, Y. S. Yu, W. W. Yang, J. Li, M. Feng and H. B. Li, J. Mater. Chem. A, 2018, 6, 4611–4616 RSC.
- L. Sahoo, R. Garg, K. Kaur, C. P. Vinod and U. K. Gautam, Nano Lett., 2022, 22, 246–254 CrossRef CAS PubMed.
- Z. Zhang, M. Xie, Z. Liu, Y. Lu, S. Zhang, M. Liu, K. Liu, T. Cheng and C. Gao, ACS Appl. Energy Mater., 2021, 4, 6824–6832 CrossRef CAS.
- L. Wu, Z. Liu, M. Xu, J. Zhang, X. Yang, Y. Huang, J. Lin, D. Sun, L. Xu and Y. Tang, Int. J. Hydrogen Energy, 2016, 41, 6805–6813 CrossRef CAS.
- H. Lv, L. Sun, D. Xu and B. Liu, Sci. Bull., 2020, 65, 1823–1831 CrossRef CAS PubMed.
- X. Li, K. X. Yao, F. Zhao, X. Yang, J. Li, Y. Li and Q. Yuan, Nano Res., 2022, 15, 6036–6044 CrossRef CAS.
- J. J. Lv, Z. J. Wang, J. J. Feng, R. h. Qiu, A. J. Wang and X. h. Xu, Appl. Catal., A, 2016, 522, 188–193 CrossRef CAS.
- R. L. Zhang, J. J. Feng, L. Zhang, C. G. Shi and A. J. Wang, J. Colloid Interface Sci., 2019, 555, 276–283 CrossRef CAS PubMed.
- B. Luo, F. Zhao, Z. Xie, Q. Yuan, F. Yang, X. Yang, C. Li and Z. Zhou, ACS Appl. Mater. Interfaces, 2019, 11, 32282–32290 CrossRef CAS PubMed.
- Z. Y. Jin, J. Lyu, Y. L. Zhao, H. L. Li, X. Lin, G. Q. Xie, X. J. Liu, J. J. Kai and H. J. Qiu, ACS Mater. Lett., 2020, 2, 1698–1706 CrossRef CAS.
- J. X. Tang, Q. S. Chen, L. X. You, H. G. Liao, S. G. Sun, S. G. Zhou, Z. N. Xu, Y. M. Chen and G. C. Guo, J. Mater. Chem. A, 2018, 6, 2327–2336 RSC.
- X. Yuan, M. S. Riaz, X. Wang, C. Dong, Z. Zhang and F. Huang, Chemistry, 2018, 24, 3707–3711 CrossRef CAS PubMed.
- Q. Xue, X. Y. Bai, Y. Zhao, Y. N. Li, T. J. Wang, H. Y. Sun, F. M. Li, P. Chen, P. Jin, S. B. Yin and Y. Chen, J. Energy Chem., 2022, 65, 94–102 CrossRef CAS.
- D. Wu, W. Zhang and D. Cheng, ACS Appl. Mater. Interfaces, 2017, 9, 19843–19851 CrossRef CAS PubMed.
- N. Zhang, Y. Zhu, Q. Shao, X. Zhu and X. Huang, J. Mater. Chem. A, 2017, 5, 18977–18983 RSC.
- X. Jiang, Y. Xiong, R. Zhao, J. Zhou, J.-M. Lee and Y. Tang, Nano Res., 2020, 13, 2691–2696 CrossRef CAS.
- J. Wang, X. Zhang, M. Zhang, P. Zhang, Y. Song and J. Zhang, Mater. Lett., 2021, 305, 130823 CrossRef CAS.
- D. Fan, K. Guo, Q. Hao, Y. Zhang and D. Xu, Chem. Commun., 2022, 58, 7773–7776 RSC.
- L. Gao, X. X. Li, Z. Y. Yao, H. J. Bai, Y. F. Lu, C. Ma, S. F. Lu, Z. M. Peng, J. L. Yang, A. L. Pan and H. W. Huang, J. Am. Chem. Soc., 2019, 141, 18083–18090 CrossRef CAS PubMed.
- Y. Z. Guo, S. Y. Yan, C. W. Liu, T. F. Chou, J. H. Wang and K. W. Wang, J. Mater. Chem. A, 2017, 5, 14355–14364 RSC.
- L. Gao, Z. Yang, T. Sun, X. Tan, W. Lai, M. Li, J. Kim, Y. F. Lu, S. I. Choi, W. Zhang, C. Ma, S. C. Smith, Y. G. Zhou and H. Huang, Adv. Energy Mater., 2022, 12, 2103943 CrossRef CAS.
- D. Chatterjee, S. Shetty, K. Muller-Caspary, T. Grieb, F. F. Krause, M. Schowalter, A. Rosenauer and N. Ravishankar, Nano Lett., 2018, 18, 1903–1907 CrossRef CAS PubMed.
- Y. Lu, S. Yang, J. Xu, Z. Liu, H. Wang, M. Lin, Y. Wang and H. Chen, Small, 2018, 14, 1801925 CrossRef PubMed.
- Y. Lu, X. Cheng, H. Li, J. Zhao, W. Wang, Y. Wang and H. Chen, J. Am. Chem. Soc., 2020, 142, 10629–10633 CrossRef CAS PubMed.
- J. L. Yu, H. Jin, Q. Wang, X. L. Wei, H. Y. Chen and Y. W. Wang, Small, 2022, 2203458 CrossRef CAS PubMed.
- J. H. Song, Y.-T. Kim, J.-B. Seol, C.-G. Park, J.-M. Myoung and U. Jeong, Adv. Mater. Interfaces, 2018, 5, 1800250 CrossRef.
- M. Zhu, Q. Shao, Y. Qian and X. Huang, Nano Energy, 2019, 56, 330–337 CrossRef CAS.
- B. Zhang, X. Zhang, Y. Wei, L. Xia, C. Pi, H. Song, Y. Zheng, B. Gao, J. Fu and P. K. Chu, J. Alloys Compd., 2019, 797, 1216–1223 CrossRef CAS.
- Z. Niu, S. Chen, Y. Yu, T. Lei, A. Dehestani, K. Schierle-Arndt and P. Yang, Nano Res., 2020, 13, 2564–2569 CrossRef CAS.
- T. Song, F. Gao, L. Jin, Y. Zhang, C. Wang, S. Li, C. Chen and Y. Du, J. Colloid Interface Sci., 2020, 560, 802–810 CrossRef CAS PubMed.
- Z. Li, Y. Zhang, B. Zou, Z. Wu, F. Gao and Y. Du, Inorg. Chem., 2022, 61, 9693–9701 CrossRef CAS PubMed.
- H. Xiang, Y. Zheng, Y. Sun, T. Guo, P. Zhang, W. Li, S. Kong, M. Ouzounian, H. Chen, H. Li, T. S. Hu, G. Yu, Y. Feng and S. Liu, Nanoscale Adv., 2020, 2, 1603–1612 RSC.
- D. Wu and D. Cheng, Appl. Surf. Sci., 2019, 493, 139–145 CrossRef CAS.
- C. Zhu, S. Guo and S. Dong, Adv. Mater., 2012, 24, 2326–2331 CrossRef CAS PubMed.
- L. Jin, Y. Sun, L. Shi, C. Li and Y. Shen, J. Mater. Chem. B, 2019, 7, 4561–4567 RSC.
- S. Y. Ma, H. H. Li, B. C. Hu, X. Cheng, Q. Q. Fu and S. H. Yu, J. Am. Chem. Soc., 2017, 139, 5890–5895 CrossRef CAS PubMed.
- Q. X. Chen, C. X. Yu, H. H. Li, Z. He and J. W. Liu, Inorg. Chem., 2020, 59, 1376–1382 CrossRef CAS PubMed.
- C. Li, T. Guo, M. Guo, Y. Song, Z. Nie, X. Li and G. Yu, J. Phys. Chem. C, 2022, 126, 12472–12479 CrossRef CAS.
- T. Guo, H. Xiang, W. Li, H. Li, H. Chen, S. Liu and G. Yu, ACS Sustainable Chem. Eng., 2020, 8, 2901–2909 CrossRef CAS.
- B. Yang, J. Fang, C. Xu, H. Cao, R. Zhang, B. Zhao, M. Huang, X. Wang, H. Lv and R. Che, Nanomicro Lett., 2022, 14, 170 CAS.
- Z. H. Zhai, Y. Wang, C. H. Si, P. Liu, W. F. Yang, G. H. Cheng and Z. H. Zhang, Nano Res., 2022 DOI:10.1007/s12274-022-4861-x.
- K. R. Sapkota, P. Gyawali, A. Forbes, I. L. Pegg and J. Philip, J. Appl. Phys., 2012, 111, 123906 CrossRef.
- Y. Huang, M. Garcia, S. Habib, J. L. Shui, F. T. Wagner, J. L. Zhang, J. Jorne and J. C. M. Li, J. Mater. Chem. A, 2014, 2, 16175–16180 RSC.
- S. N. Zhao, J. F. Huang, Y. Y. Liu, J. H. Shen, H. Wang, X. L. Yang, Y. H. Zhu and C. Z. Li, J. Mater. Chem. A, 2017, 5, 4207–4214 RSC.
- A. Costas, C. Florica, E. Matei, M. E. Toimil-Molares, I. Stavarache, A. Kuncser, V. Kuncser and I. Enculescu, Beilstein J. Nanotechnol., 2018, 9, 2345–2355 CrossRef CAS PubMed.
- X. T. Deng, S. F. Yin, X. B. Wu, M. Sun, Z. Y. Xie and Q. Z. Huang, Electrochim. Acta, 2018, 283, 987–996 CrossRef CAS.
- L. Hou, Y. Niu, Y. Jiang, T. Jiao, Y. Guo, Y. Zhou and F. Gao, Colloids Surf., A, 2019, 573, 6–13 CrossRef CAS.
- Y. Jiang, Y. Guo, Y. Zhou, S. Deng, L. Hou, Y. Niu and T. Jiao, ACS Omega, 2020, 5, 14805–14813 CrossRef CAS PubMed.
- L. Wang, Z. Liu, S. Zhang, M. Li, Y. Zhang, Z. Li and Z. Tang, Int. J. Hydrogen Energy, 2021, 46, 8549–8556 CrossRef CAS.
- D. Song, Y. Li, X. Lu, M. Sun, H. Liu, G. Yu and F. Gao, Anal. Chim. Acta, 2017, 982, 168–175 CrossRef CAS PubMed.
- C. Santoro, P. Bollella, B. Erable, P. Atanassov and D. Pant, Nat. Catal., 2022, 5, 473–484 CrossRef CAS.
- X. Q. Wang, Z. J. Li, Y. T. Qu, T. W. Yuan, W. Y. Wang, Y. Wu and Y. D. Li, Chem, 2019, 5, 1486–1511 CAS.
- K. Wang, H. Yang, J. Zhang, G. Ren, T. Cheng, Y. Xu and X. Huang, Nano Res., 2022, 15, 5865–5872 CrossRef CAS.
- Z. J. Liu, J. Qi, M. X. Liu, S. M. Zhang, Q. K. Fan, H. P. Liu, K. Liu, H. Q. Zheng, Y. D. Yin and C. B. Gao, Angew. Chem., Int. Ed., 2018, 57, 11678–11682 CrossRef CAS PubMed.
- J. J. Wang, X. P. Li, B. F. Cui, Z. Zhang, X. F. Hu, J. Ding, Y. D. Deng, X. P. Han and W. B. Hu, Rare Met., 2021, 40, 3019–3037 CrossRef CAS.
- H. Xie, T. Y. Wang, J. S. Liang, Q. Li and S. H. Sun, Nano Today, 2018, 21, 41–54 CrossRef CAS.
- P. Saha, S. Amanullah and A. Dey, Acc. Chem. Res., 2022, 55, 134–144 CrossRef CAS PubMed.
- J. Yu, W. Wang, S. Li, B. Yu, H. Chen and Y. Wang, Chem. Commun., 2022, 58, 989–992 RSC.
- S. Zhu, Q. Wang, X. Qin, M. Gu, R. Tao, B. P. Lee, L. Zhang, Y. Yao, T. Li and M. Shao, Adv. Energy Mater., 2018, 8, 1802238 CrossRef.
- N. Han, M. Z. Sun, Y. Zhou, J. Xu, C. Cheng, R. Zhou, L. Zhang, J. Luo, B. L. Huang and Y. G. Li, Adv. Mater., 2021, 33, 2005821 CrossRef CAS PubMed.
- Y. J. Sun, M. C. Luo, Y. N. Qin, S. H. Zhu, Y. J. Li, N. Y. Xu, X. X. Meng, Q. S. Ren, L. Wang and S. J. Guo, ACS Appl. Mater. Interfaces, 2017, 9, 34715–34721 CrossRef CAS PubMed.
- X. X. Xu, H. Lv, L. Z. Sun, P. Song, B. Liu and X. Chen, ChemPlusChem, 2020, 85, 970–976 CrossRef CAS PubMed.
- W. L. Wu, J. W. Li, Z. Y. Chen, W. Z. Chen, H. S. Pang, K. B. Ma and J. Zeng, J. Catal., 2019, 373, 209–214 CrossRef CAS.
- L. Fu, C. Yang, R. Wei, X. Pei, J. Teng, D. Kong, Y. Lu, Y. Guo, T. Liu, Y. Hu, B. Yin, Z. Zhang, A. Li, L. Wang and X. Han, Mater. Today Nano, 2021, 15, 9 Search PubMed.
- H. Cheng, N. Yang, Q. Lu, Z. Zhang and H. Zhang, Adv. Mater., 2018, 30, 1707189 CrossRef PubMed.
- Y. Chen, Z. Lai, X. Zhang, Z. Fan, Q. He, C. Tan and H. Zhang, Nat. Rev. Chem., 2020, 4, 243–256 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2023 |