Open Access Article
Open Access ArticleThymoquinone-loaded lipid nanocapsules with promising anticancer activity for colorectal cancer†
Mouna
Selmi
a,
Abir
Salek
a,
Mahassen
Barboura
a,
Leila
Njim
b,
Amine
Trabelsi
ac,
Aida
Lahmar
a,
Nolwenn
Lautram
d,
Emilie
Roger
d,
Tarek
Baati
 *e and
Leila chekir
Ghedira
a
*e and
Leila chekir
Ghedira
a
aLaboratoire des Substances Naturelles Bioactives et Biotechnologie UR17ES49, Faculté de Médecine Dentaire, Université de Monastir, Tunisia
bService d'Anatomie Pathologique, CHU de Monastir, Université de Monastir, Tunisia
cLaboratoire de Pharmacognosie, Faculté de Pharmacie, Université de Monastir, Tunisia
dUniversité d’Angers, INSERM, CNRS, MINT, SFR-ICAT, F-49000 Angers, France
eLaboratoire des Substances Naturelles, Institut National de Recherche et d’Analyse Physico-chimique, Biotechpôle Sidi Thabet, 2020 Tunisia. E-mail: tarek.baati@gmail.com; Fax: +216 71 537 688; Tel: +216 71 537 666
First published on 22nd August 2023
Abstract
Colorectal cancer (CRC) is the third most common worldwide. Depending on its stage, chemotherapy is usually given after surgery when CRC has already metastasized to other organs like the liver or lungs. Unfortunately, the current antineoplastics used for CRC therapies involve toxicity and side effects due to their lack of site-specificity. To overcome the drawbacks of heavy chemotherapy, this study proposes to assess the efficacy of thymoquinone (TQ), a bioactive constituent of black seeds (Nigella sativa), as an antiproliferative and pro-apoptotic agent on an experimental CRC model in mice. TQ was encapsulated in lipid nanocapsules (LNCs), used as nanocarriers, in order to increase its specificity and cell absorption. TQ-loaded LNCs (TQ-LNCs) have a diameter of 58.3 ± 3.7 nm and 87.7 ± 4.5% TQ encapsulation efficiency. In turn, in vivo studies showed that the intratumoral administration of TQ-LNCs decreased the tumor size in colorectal cancer bearing mice compared to the control group. TQ-LNCs were more effective than free TQ for inducing tumor cell death. These results highlight the potential of TQ entrapped in LNCs as an anticancer agent for CRC treatment.
1. Introduction
Colorectal cancer (CRC) is the third most common cancer after breast and lung cancer leading to mortality with around 1.93 million cases in 2020.1 To fight this cancer, chemotherapy drugs, such as 5-fluorouracil, cisplatin, and oxaliplatin, are used in order to destroy fast-growing cancer cells and prevent them from multiplying by interfering with their cell division mechanisms.2 Despite their anti-cancer efficacy, chemotherapy causes long-term side effects, such as hair loss, nausea, and vomiting,3 due to their lack of specificity to the site of drug action. Indeed, these medicines kill cancer cells as well as regular cells and consequently cause health complications, which are often very severe for the patient. Hence, there is an ongoing need for new anti-cancer agents exhibiting better toleration, specificity and efficiency in order to overcome the drawbacks of classical chemotherapy. Natural products can be a perfect alternative for the substitution of chemotherapy agents since they exhibit an acceptable therapeutic approach owing to their accessibility, applicability, and reduced cytotoxicity. Several anti-tumor agents have been found in plants, such as thymoquinone (TQ), which is a potent orally active phytochemical obtained from Nigella sativa plant. Thymoquinone is commonly used in traditional medicine for its biological activities, such as anti-oxidant, anti-inflammatory, anti-diabetic, gastric ulcer, and cardio protective activities.4,5 Besides these activities, TQ has been reported to have a potentiel anticancer effect when tested on a range of cancer cell lines, including breast and ovarian adenocarcinoma, lung carcinoma, human pancreatic adenocarcinoma, and CRC.6–13 Despite its great potency in killing cancer cells, TQ application has often raised several concerns related to its relatively low solubility in water and its rapid elimination after serum opsonin binding, leading to a considerable decease of its bioavailability and pharmacological activity.14–16 To deal successfully with these drawbacks, numerous studies have tried to encapsulate TQ into organic nanocarriers, used as delivery systems, like lipid-coated mesoporous silica nanoparticles,17 chitosan-solid lipid nanoparticles,18 and lipid nanocapsules.19 Indeed, owing to their high surface and volume ratio, nanocarriers are able to modify the basic properties and to enhance both drug solubility and stability20,21 and improve drug pharmacokinetics and biodistribution. In addition, nanocarriers significantly decrease drug toxicity through a controlled release mechanism.20–24 The lipid nanocapsules (LNCs) have gained considerable interest as drug nanocarrier systems (paclitaxel, ferrocifen, and etoposide) as they are highly biodegradable and devoid of toxicity compared to other lipid-based nanocarriers.25–27 Recently LNCs were successfully synthesized by a high-pressure homogenization method and tested for TQ loading, showing remarkable physiochemical properties, and encapsulation efficiency with stability up to 24 months of storage.19 Moreover, LNCs were suitable to enhance the pharmacokinetics and biodistribution of TQ after oral and intravenous administration into rats.19 However, the protocol of LNC synthesis contains oil palm and Tween 80, which could unlikely induce toxicity and health complication. For that we propose in this study to use a safety method to produce ultrapure LNCs without any trace of toxic organic solvent28 consisting of low-energy emulsification with GRAS (generally recognized as safe) excipients. Following that we determine their loading capacity of thymoquinone and for the first time this new formulation (TQ-LNCs) will be evaluated for intratumoral therapy on a colorectal cancer model subcutaneously developed in mice.2. Materials and methods
2.1. Materials
Thymoquinone (99%) and DMSO were purchased from Sigma-Aldrich (Saint-Quentin Fallavier, France). Captex® 8000 was provided by Abitec Corp (Saint-Quentin Fallavier, France). Lipoid® S100 and Kolliphor® HS15 were supplied by Lipoid Gmbh (Steinhausen, Switzerland) and BASF (Ludwigshafen, Germany), respectively. Sodium chloride was purchased from Prolabo VWR International (Fontenay-sous-Bois, France). All the other reactants, including HPLC grade acetic acid, acetonitrile, methanol, and water, were purchased from Fisher Scientific (Loughborough, United Kingdom).2.2. Preparation of thymoquinone-loaded lipid nanocapsules
Thymoquinone-loaded lipid nanocapsules (TQ-LNCs) were prepared according to the original protocol described by Heurtault et al.28 Briefly, 10 mg of TQ were solubilized within Captex® 8000 (13.12% w/w) under magnetic stirring, and then Lipoid® S100 (0.73% w/w) and Kolliphor® HS15 (10.94% w/w) were added. The mixture was then homogenized and supplemented with NaCl (0.8% w/w) and Milli-Q water (19.73% w/w). Next, the preparation was heated to 90 °C, followed by cooling it to 60 °C. Three heating–cooling cycles were applied, followed by a rapid addition at 70 °C of cold water of 2 °C (54.68% w/w). This irreversible thermal shock induces the spontaneous formation of LNCs, which were maintained under moderate stirring for 5 min at room temperature before filtration through a 0.22 μM membrane filter (Merck KGaA, Darmstadt, Germany) in order to remove potential residual components and free TQ. Unloaded LNCs were prepared in a similar manner as describe above without the addition of TQ. All formulations were stored at 4 °C.2.3 Particle size and zeta potential measurements
The mean particle size, polydispersity index (PDI), and zeta potential measurements of unloaded and TQ-loaded LNCs were determined using a Malvern Zetasizer Nano ZS setup (Malvern Instruments S.A., UK). Before measurement, unloaded LNCs or TQ-LNCs were diluted in deionized water at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 (v/v). All measurements were performed at room temperature after equilibration for 2 min. The data were obtained with the average of three measurements.
400 (v/v). All measurements were performed at room temperature after equilibration for 2 min. The data were obtained with the average of three measurements.
2.4 Determination of thymoquinone-LNC loading
The encapsulated thymoquinone amount was quantified using ultra performance liquid chromatography (UPLC). The analytical method was developed and validated as described previously with some modifications.29 Briefly, the analysis was performed using a UPLC-UV Acquity® H-Class Bio separation module (Waters, Saint-Quentin-en-Yvelines, France) equipped with a photodiode array detector (PDA) (Waters) and controlled by Empower software. The analytical column was ACQUITY UPLC BEH C18 1.7 μm, 2.1 × 50 mm I.D. The mobile phase composition consisted of phase A (2% acetic acid in water, pH 2.5) and phase B (methanol), and the separation of TQ was optimized using an isocratic mode (A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B 30
B 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 (v/v)). The flow rate was fixed at 0.2 mL min−1 and the run time was 5 min. The retention time of TQ was 1.57 min. The column was operated at 25 °C and TQ was monitored and quantified at 254 nm. The calibration curve was prepared from a stock solution of TQ in methanol at 10 mg mL−1, followed by appropriate dilutions to form concentrations ranging from 5 to 50 μg mL−1. Samples of filtrated TQ-LNCs were diluted by 200 in methanol before the injection of 1 μL. The LNC drug loading capacity (%) was calculated as the amount of total entrapped TQ divided by the total nanoparticle weight. Then the drug loading efficiency (%) was calculated as the ratio of the amount of TQ in the nanoparticles to the total amount of TQ applied in the formulation of the nanoparticles.
70 (v/v)). The flow rate was fixed at 0.2 mL min−1 and the run time was 5 min. The retention time of TQ was 1.57 min. The column was operated at 25 °C and TQ was monitored and quantified at 254 nm. The calibration curve was prepared from a stock solution of TQ in methanol at 10 mg mL−1, followed by appropriate dilutions to form concentrations ranging from 5 to 50 μg mL−1. Samples of filtrated TQ-LNCs were diluted by 200 in methanol before the injection of 1 μL. The LNC drug loading capacity (%) was calculated as the amount of total entrapped TQ divided by the total nanoparticle weight. Then the drug loading efficiency (%) was calculated as the ratio of the amount of TQ in the nanoparticles to the total amount of TQ applied in the formulation of the nanoparticles.
2.5 In vitro TQ release in an acidic environment from TQ-loaded LNCs
TQ-loaded LNCs were diluted at a final concentration of 10% (v/v) in an acidic solution (pH 4.5) and incubated at 37 °C under continuous shaking at 120 rpm (incubator Multitron Ortibale 50 mm, HT Infors, France). Samples were collected at 0, 0.25, 0.5, 1, 2, 3, 6, 12, and 24 h. Samples were filtered using a 0.2 μm filter to remove free precipitated TQ. All samples were characterized in terms of drug loading using the UPLC method in order to determine the kinetics of TQ release.2.6 In vivo experiments
After two weeks, all animals were anesthetized under isoflurane and sacrificed. Solid tumors from each mouse and organs, including the liver, spleen, heart, lungs, kidneys, and brain, were collected, washed with cooled 0.9% NaCl, weighed and then stored under −20 °C until analysis. The solid tumors were then weighed for tumor inhibitory rate (TIR) determination, which was calculated using the following equation: TIR = ((1 − tumor weight experiment)/tumor weight control) × 100.
2.7 Histology
For histological evaluation, collected organs and solid tumors were immediately fixed in 10% neutral buffered formalin and embedded in a paraffin wax. Sections of 5 μm were cut from each sample and stained with hematoxylin-eosin as reported previously.312.8 Genotoxicity
The genotoxicity effect of free TQ versus TQ-LNCs was assessed by the quantification of DNA damage induced in tumor cells using the comet assay in neutral and alkali conditions as reported previously.32 Briefly, 10 mg of solid tumor were accurately weighed and then homogenized with 1 mL of PBS. After shaking for 5 min, the homogenate was mixed gently with low melting agarose (1.2%) for cell adhesion and then placed on comet slides for 10 min at 4 °C. Subsequently, cells were incubated in lysis buffer (pH 10) for 1 h at 4 °C in darkness. Next, electrophoresis was performed under neutral condition at 25 V and 300 mA for 15 min and then embedded cells were fixed with ethanol (70%). At the end, comet slides were stained with diluted ethidium bromide (0.02 mg mL−1) and then observed by fluorescence microscopy (Olympus B×51 TRF, USA) using an FITC filter. Finally, the DNA damage percentage was determined according to Eke and Celik 2016, using the following formula: genotoxicity (%) = (total DNA damage of sample − total DNA damage of control/total DNA damage of control) × 100.2.9 Biochemical parameters
Tumor cytokine levels were measured in a homogenate of 50 mg of tumor mass in 1 mL of PBS using a commercial ELISA kit for GM-CSF (Abcam, ab174448) and TNF-α (Abcam, ab208348).2.10 Statistical analysis
The data are expressed as mean ± SD. Statistical comparisons among groups were analyzed using one-way and two-way analysis of variance (ANOVA), followed by Tukey's multiple comparison test. Statistical significance was considered for p-value < 0.01.3. Results
3.1. Nanoparticle characterization
The particles size distribution measured by dynamic light scattering (DLS) shows a mean hydrodynamic size ranging from 56.5 ± 4.7 nm for unloaded LNCs to 58.3 ± 3.7 for TQ-LNCs, indicating no significant difference even when TQ is loaded within nanoparticles (Table 1). Likewise, unloaded LNC and TQ-LNC aqueous suspensions showed a homogeneous monodisperse nanoparticle system according to the polydispersity index, which corresponded to 0.04 ± 0.01 and 0.05 ± 0.02 for unloaded and TQ-loaded nanoparticles, respectively. Moreover, a similar neutral zeta potential (∼3.8 mV) was measured for both formulations, indicating that TQ loading did not influence the surface charge of the nanoparticles. Finally, a nanoparticle drug loading up to 8.8 ± 0.5 mg mL−1 of TQ per LNC suspension was obtained, corresponding to a high drug loading efficiency of 87.7 ± 4.9%.| Size (nm) | PDI | Zeta potential (mV) | Drug loading capacity (%) | Drug loading efficiency (%) | |
|---|---|---|---|---|---|
| Unloaded LNCs | 56.5 ± 4.7 | 0.04 ± 0.01 | 3.8 ± 0.5 | — | — |
| TQ-LNCs | 58.3 ± 3.7 | 0.05 ± 0.02 | 3.8 ± 0.6 | 7.9 ± 0.8 | 87.7 ± 4.9 |
3.2. Thymoquinone-LNC release
The TQ release profile in acidic medium (pH 4.5) at 37 °C (Fig. 1) showed a burst release of 2.2% of the total encapsulated TQ in LNCs after 0.25 h, followed by an increase to reach 6.4% release after 24 h.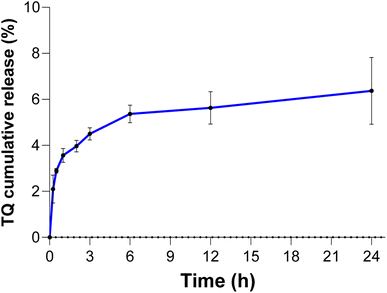 | ||
| Fig. 1 Cumulative release of TQ in acidic medium (pH 4.5) at 37 °C under continuous stirring. All experiments were carried out in triplicate (n = 3). | ||
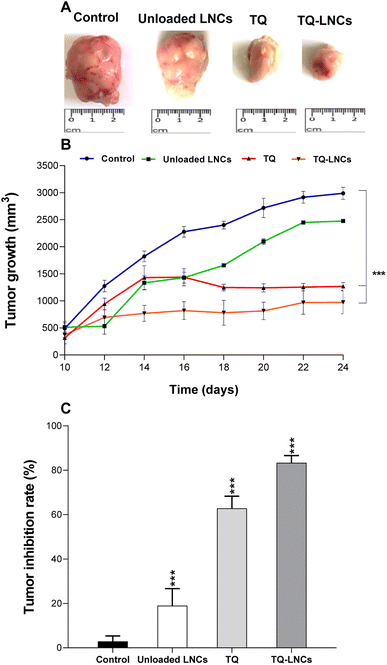
3.3. Antitumor activity of TQ versus TQ-LNCs in a subcutaneous tumor xenograft model
The mice treated with free TQ and TQ-LNCs had a significantly high relative weight compared to the control group (Fig. S1†). The anticancer activity of TQ-LNCs was tested on a xenograft model of murine colorectal cancer developed by the injection of CT26 murine colorectal carcinoma cells in the flank of BALB/c mice. Tumor growth was controlled for 15 days following the intratumoral injection of thymoquinone at 5 mg kg−1 in solution or encapsulated in LNCs. For the control group (treated with PBS) the average tumor volume increased exponentially from 150 ± 45 mm3 to 2988.7 ± 99.3 mm3 at day 15 (Fig. 2B and C).After the administration of free TQ three times per week, the tumor volume significantly reduced to an average of 1268.3 ± 65.2, corresponding to a substantial tumor growth inhibition by 62.8% (p < 0.01) compared to the control group. Moreover, a more drastic tumor regression has occurred after TQ-LNC treatment, resulting in lower tumor volume ranging from 715.1 to 840.3 mm3, indicating a tumor growth inhibition by 83.4% (p < 0.01) compared to the control group. Meanwhile, the tumor growth of unloaded LNC treated mice increased similarly to that of the control group, reaching an average tumor volume of 2600 mm3, which was not significantly different from that of the control group (3000 mm3).
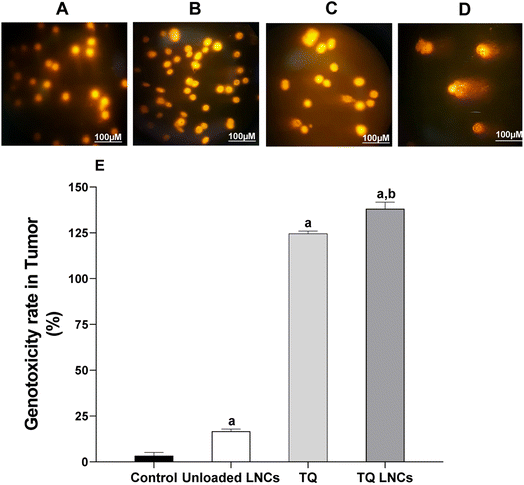
3.4. Genotoxicity effect of TQ versus TQ-LNCs
DNA damage measurement was conducted using the comet assay to analyze tail parameters in cells isolated from the solid tumors of the control group, unloaded LNCs and mice treated with free TQ and TQ-LNCs at 5 mg kg−1. The treatment with free TQ and TQ-LNCs (Fig. 3A and B) showed a high positive response in the comet assay, indicating the maximum genotoxicity with relative tail moment values of 124.6 ± 1.3 and 138.2 ± 3.6 (p < 0.01), respectively. This effect is higher (up to 40-fold) than that of the control group treated with PBS (3.4 ± 1.8) (p < 0.01) and unloaded LNC treatment (16.7 ± 1.3). Moreover, the relative tail moment value in the TQ-LNC treated group is almost 1.1-fold (p < 0.05) higher than that of the free TQ treated group, evidencing enhanced DNA damage in cancer cells upon LNC mediated drug delivery.3.5. Effect of free TQ versus TQ-LNCs on cytokine levels in tumor tissues
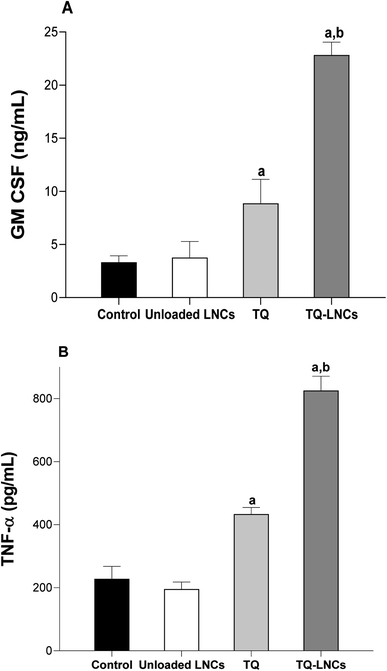
The effect of free TQ and TQ-LNC treatment on the immune system was investigated through the assessment of characteristic cytokine levels, such as GM-CSF and TFN-α, in tumor tissues (Fig. 4A and B). While the unloaded LNCs administered alone did not cause any modification of the GM-CSF and TFN-α levels as compared with the control group, the intratumoral injection of free TQ or TQ-LNCs led to a significant increase in the level of these cytokines, indicating a chronic inflammation. In addition, the levels of cytokines determined in tumors of mice treated with TQ-LNCs are significantly higher than that of mice treated with free TQ (p < 0.05).3.6. Histology
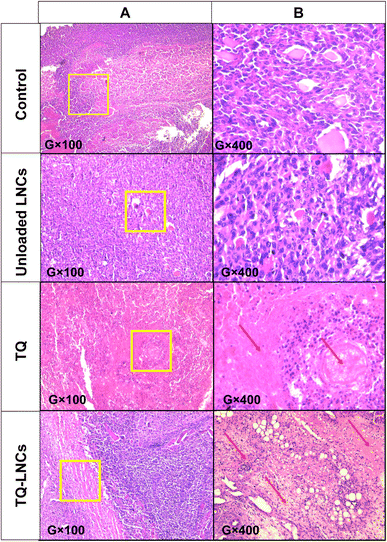
The histopathological sections of the tumor revealed a significant reduction of viable cells in TQ and TQ-LNC groups compared with the unloaded LNCs and control group (Fig. 5). Interestingly, TQ and its nano delivery systems revealed the presence of necrotic areas in tumor sections, evidencing the antitumor potential of TQ and TQ-loaded LNCs. On the other hand, the histopathological examination of liver tissues revealed normal parenchyma regardless of the accumulation of polynuclear neutrophiles as well as in the control group, suggesting a chronic inflammation (Fig. S2†). Furthermore, the histopathological examination of all other organs, including the spleen, kidneys, heart, lungs, and brain, revealed a classical architecture without any apparent change or necrosis in the cellular structures in all treated groups as compared to the control group (Fig. S3–S5†).4. Discussion
In this study, lipid nanocapsules were synthesized by a phase-inversion process for thymoquinone loading and delivery in order to increase its intratumoral therapy in a colorectal cancer model subcutaneously developed in BALB/C mice. LNCs showed a great drug loading efficiency close to 90% and a drug loading capacity of about 8%. Prior to the in vivo experiments, both the unloaded and TQ-loaded LNC suspensions were characterized for their size distribution and surface charge, which play a crucial role in the tumoral cell uptake process. As shown in our experiment, no significant changes were recorded in the hydrodynamic diameter of LNCs due to TQ loading compared to that of the unloaded LNCs. Anyway, TQ-LNCs have been successfully formulated with a mean size less than 100 nm, promising a high tumor cell uptake since the nanoscale size (150–200 nm) decreases the recognition of the phagocytic system and consequently the destruction of nanocarriers.33,34 Besides the nanoparticle size, the structural abnormalities of the tumor microenvironment contribute to increase the retention of nanocarriers through the enhanced permeability and retention (EPR) phenomenon.34–38 Indeed, defective angiogenesis develops a high vessel fenestration (150–200 nm) in comparison with the normal vessels, allowing hyperpermeability to the passage of nanoparticles generally with a size lower than 200 nm.39,40 Moreover, the poor lymphatic drainage of the tumor microenvironment associated with cancer cell proliferation allows not only the accumulation of nanoparticles but also the release of their contents to tumor cells as described for other types of nanoparticles.40–42The acidic tumor microenvironment can modulate the drug release of TQ. In vitro release study demonstrated that after incubation under acidic conditions for 24 h, only 6.4% of TQ was released from LNCs, which could be explained by TQ entrapment into the lipid core of the nanocapsule and its protection by the shell composed by Kolliphor®HS15 and Lipoid®S100. Several studies reported the high stability of LNCs loading paclitaxel and Sn38 under acidic condition incubation without drug burst effect.43–46 Moreover, the TQ loading process did not influence the surface charge of TQ-LNCs as compared with the unloaded LNCs. Indeed, the zeta potential of both nanoparticles' suspensions did not shift significantly and took a relatively neutral charge.47–49 Interestingly, several researches using nanoparticles for drug delivery reported that relatively neutral surface charge enhanced significantly the nanoparticle tumor uptake and reduced their interaction with biological compounds and proteins. Consequently, neutral charge allows nanoparticles to cross easily the cell membranes without the requirement of specific bindings through internalization pathways.47–49 Thus, these properties of TQ-LNCs enable them to be suitable for application in the treatment of cancer diseases.
Then, the antitumor efficacy of TQ-LNCs has been investigated on mice bearing the colorectal cancer CT26 cell line following intratumoral injection of a relatively high dose of TQ-LNCs (at 5 mg kg−1 dose). Fifteen days following the treatment, mice treated with unloaded LNCs showed tumor regression may be attributed to the presence of Kolliphor® HS15 used for synthesis and well known to be toxic at high concentration notably after nanoparticles accumulation in the target tissue with a lethal concentration (LC50) reached 0.427 mg mL−1.50 Likewise the accumulation of Solutol® HS15 released from LNCs has the ability to inhibit P-gp pump activity and consequently inhibit tumor cell growth.51,52 On the other hand, mice treated with TQ-LNCs showed a more drastic tumor-growth inhibition of up to 86% without any body-weight loss, evidencing their anticancer efficacy. According to the histopathological assessment, cell necrotic areas decrease significantly in tumor tissues of mice treated with TQ-LNCs compared to those treated with unloaded LNCs and the control group. Meanwhile treatment with free TQ induces less necrosis cells as compared to TQ-LNCs, suggesting lower pharmacological effectiveness probably due to its lipophilicity, which limits its affinity for the tumoral microenvironment and favored its rapid degradation and systemic elimination.49,50
The ameliorated anticancer activity observed with TQ upon its entrapment into the LNCs indicates that the lipid nanocapsules improved the drug delivery into the tumor cells, and thus equivalent TQ anticancer activity could be obtained at lower concentrations. This can be explained by the increased number of TQ molecules that are internalized in tumor cells when present in LNCs. Collectively, TQ-LNCs appear to form a better delivery system than free TQ in colorectal cancer treatment without observable toxicity to the other organs, thus improving the efficacy and the therapeutic index of the drug. This proves how the design of TQ-LNCs has gained increasing interest as a means of improving the treatment of neoplastic diseases.14,15 Altogether, the LNC delivery system enhanced the therapeutic effect of TQ on colorectal cancer through cell apoptosis mechanism and genotoxic effect expressed by cell cycle blocking and DNA damage.51–53 This was also described for other types of cancers, including breast, ovarian, pancreatic, and lung cancers.10–12 Similarly, several studies have shown that TQ induces intrinsic apoptotic cell death via multiple mechanisms, including the down-regulation of BCL-2 family anti-apoptotic protein expression, activation of the mitochondrial pathway, and caspase expression.54–59 Commonly, the apoptosis form of death is more favorable than necrosis in eliminating cancer cells as far as it does not induce inflammatory response in the normal cells.60 Compared to free TQ or TQ-LNC treatment, the immune cytokine levels, such as GM-CSF and TFN-α, determined in tumor tissues increased significantly as compared with those of the unloaded LNCs and control group. These results suggest the induction of a chronic inflammation, which is more expressed in the TQ-LNC group. Thus, LNCs could enhance the resorption of TQ within tumor cells, improving its cytotoxic effect. These results are in accordance with the previous findings, where treatment with TQ increased cytokine levels in breast cancer cells.54 Regarding the toxicity, all studied parameters, including the healthy behavior of animals and absence of toxicity in the kidneys, spleen, and liver, confirm the biocompatibility of TQ-LNCs. These results clearly confirm the interest in the use of these non-toxic LNCs as a nanocarrier for drug delivery for biomedical applications, notably in cancer therapy.
5. Conclusion
Lipid nanocapsules were synthesized by a phase-inversion process for thymoquinone loading and delivery in order to increase its intratumoral therapy in a colorectal cancer model subcutaneously developed in mice. The main physical parameters (size, shape, and surface charge) that can influence the toxicity of TQ-LNCs were studied and found to be suitable for their bioapplication in cancer therapy. LNCs showed a great thymoquinone loading efficiency close to 90% with slow and sustained release inducing the progressive apoptosis of tumor cells and drastic colorectal tumor regression. This study clearly confirmed the interest in the use of TQ-LNCs in nano-oncology, notably in the treatment of colorectal cancer, and paves the way for the treatment of other types of cancer.Conflicts of interest
There are no conflicts to declare.References
- H. Sung, J. Ferlay, R. L. Siegel, M. Laversanne, I. Soerjomataram, A. Jemal, F. Bray and C. A. Cancer, J. Clin., 2021, 71, 209–249 Search PubMed.
- B. Viswanath, S. Kim and K. Lee, Int. J. Nanomed., 2016, 11, 2491 CAS.
- M. S. Aslam, S. Naveed, A. Ahmed, Z. Abbas, I. Gull and M. A. Athar, J. Cancer Ther., 2014, 5, 817–822 CrossRef.
- N. E. Abdelmeguid, R. Fakhoury, S. M. Kamal and R. J. Al Wafai, J. Diabetes, 2010, 2, 256–266 CrossRef CAS PubMed.
- S. Padhye, S. Banerjee, A. Ahmad, R. Mohammad and F. H. Sarkar, Cancer Ther., 2008, 6, 495 CAS.
- S. Darakhshan, A. B. Pour, A. H. Colagar and S. Sisakhtnezhad, Pharmacol. Res., 2015, 95, 138–158 CrossRef PubMed.
- S. N. Goyal, C. P. Prajapati, P. R. Gore, C. R. Patil, U. B. Mahajan, C. Sharma, S. P. Talla and S. K Ojha, Front. Pharmacol, 2017, 8, 656 CrossRef PubMed.
- M. Imran, R. Rauf, I. A. Khan, M. Shahbaz, T. B. Qaisrani, S. Fatmawati, T. A. Zneid, A. Imran, K. U. Rahman and T. A. Gondal, Biomed. Pharmacother., 2018, 106, 390–402 CrossRef CAS PubMed.
- S. Idris, B. Refaat, R. Almaimani, H. Ahmed, J. Ahmad and M. Alhadrami, Life Sci., 2022, 296, 120442 CrossRef CAS PubMed.
- S. H. Jafri, J. Glass, R. Shi, S. Zhang, M. Prince and H. K. Hancock, J. Exp. Clin. Cancer Res., 2010, 29, 1–11 CrossRef PubMed.
- A. O. Kaseb, K. Chinnakannu, D. Chen, A. Sivanandam, S. Tejwani, M. Menon, Q. P. Dou and G. P. V. Reddy, Cancer Res., 2007, 67, 7782–7788 CrossRef CAS PubMed.
- A. H. Rahmani, M. A. Alzohairy, M. A. Khan and S. M. Aly, Evidence-Based Complementary Altern. Med., 2014, 10, 1–13 Search PubMed.
- M. Khan, S. K. Lam, S. Yan, Y. Feng, C. Chen and F. C. F. Ko, The anti-neoplastic impact of thymoquinone from Nigella sativa on small cell lung cancer: in vitro and in vivo investigations, Research square, 2023, DOI:10.21203/rs.3.rs-2434644/v1.
- S. A. Pathan, G. K. Jain, S. M. A. Zaidi, S. Akhter, D. Vohora, P. Chander, P. L. Kole, F. J. Ahmada and R. K. Khara, Biomed. Chromatogr., 2011, 25, 613–620 CrossRef CAS PubMed.
- J. M. M. Salmani, S. Asghar, H. Lv and J. Zhou, Molecules, 2014, 19, 5925–5939 CrossRef PubMed.
- M. A. Khan, M. Tania, S. Fu and J. Fu, Oncotarget, 2017, 8, 51907 CrossRef PubMed.
- I. Rahat, M. Rizwanullah, S. J. Gilani, M. N. Bin-Jummah, S. S. Imam and C. Kala, J. Drug Delivery Sci. Technol., 2021, 64, 102565 CrossRef CAS.
- H. M. Fahmy, M. M. Ahmed, A. S. Mohamed, E. S. Eldin and M. T. A. El-Daim, BMC Pharmacol. Toxicol., 2022, 23, 1–13 CrossRef PubMed.
- F. H. Zakarial Ansar, S. Y. Latifah, W. H. B. Wan Kamal, K. C. Khong, Y. Ng and J. N. Foong, Int. J. Nanomed., 2020, 15, 7703–7717 CrossRef PubMed.
- E. Cukierman and D. R. Khan, Biochem. Pharmacol., 2010, 80, 762–770 CrossRef CAS PubMed.
- A. Khan, M. A. Alsahli, M. A. Aljasir, H. Maswadeh, M. A. Mobark and F. Azam, Pharmaceutics, 2022, 14, 153 CrossRef CAS PubMed.
- H. Su, Z. Deng, Y. Liu, Y. Zhao, H. Liu and Z. Zhao, Mater. Chem. Front., 2022, 6, 1317–1323 RSC.
- A. Aziz, Y. Sefidbakht, S. Rezaei, H. Kouchakzadeh and V. Uskoković, J. Pharm. Sci., 2022, 111, 1187–1196 CrossRef CAS PubMed.
- L. Woythe, D. Porciani, T. Harzing, S. van Veen, D. H. Burke and L. Albertazzi, J. Controlled Release, 2023, 355, 228–237 CrossRef CAS PubMed.
- S. Peltier, J. M. Oger, F. Lagarce, W. Couet and J. P. Benoît, Pharm. Res., 2006, 23, 1243–1250 CrossRef CAS PubMed.
- P. Idlas, E. Lepeltier, G. Jaouen and C. Passirani, Cancers, 2021, 13, 2291 CrossRef CAS PubMed.
- J. A. Blanco, V. Sebastián, J. P. Benoit and A. I. T. Suárez, Eur. J. Pharm. Biopharm., 2019, 134, 126–137 CrossRef PubMed.
- B. Heurtault, P. Saulnier, B. Pech, J. E. Proust and J. P. Benoit, Pharm. Res., 2002, 19, 875–880 CrossRef CAS PubMed.
- N. Ahmad, R. Ahmad, S. Al Qatifi, M. Alessa, H. Alhajji and M. Saefaroz, BMC Chem., 2020, 14, 10–15 CrossRef CAS PubMed.
- J. Ma, X. Hu, J. Li, D. Wu, L. Q. Wu and Q. Wang, Oncotarget., 2017, 8, 85926 CrossRef CAS PubMed; Y. S. Ma, J. J. Lin, C. C. Lin, J. C. Lien, S. F. Peng, M. J. Fan, F. T. Hsu and J. G. Chung, Environ. Toxicol., 2018, 33, 1097–1104 CrossRef PubMed.
- T. Baati, A. A. Kattan, M. A. Esteve, L. Njim, Y. Ryabchikov, F. Chaspoul, M. Hammami, M. Sentis, A. V. Kabashin and D. Braguer, Sci. Rep., 2016, 6, 1–13 CrossRef PubMed.
- M. B. Imen, F. Chaabane, M. Nadia, K. Soumaya, G. Kamel and C. G. Leila, Life Sci., 2015, 135, 173–178 CrossRef CAS PubMed.
- J. A. Nagy, S. H. Chang, S. C. Shih, A. M. Dvorak and H. F. Dvorak, Semin. Thromb. Hemostasis, 2010, 36, 321–331 CrossRef CAS PubMed.
- D. Fukumura and R. K. Jain, J. Cell. Biochem., 2007, 101, 937–949 CrossRef CAS PubMed.
- A. zur Mühlen, C. Schwarz and W. Mehnert, Eur. J. Pharm. Biopharm., 1998, 45, 149–155 CrossRef PubMed.
- J. S. Choi, C. Cao, M. Naeem, J. Noh, N. Hasan, H. K. Choi and J. W. Yoo, Colloids Surf., B, 2014, 122, 545–551 CrossRef CAS PubMed.
- L. Xu, M. Xu, X. Sun, N. Feliu, L. Feng, W. J. Parak and L. Sijin, ACS Nano, 2023, 17(3), 2039–2052 CrossRef CAS PubMed.
- S. K. Debnath, B. Singh, N. Agrawal and R. Srivastava, EPR-Selective Biodegradable Polymer-Based Nanoparticles for Modulating ROS in the Management of Cervical Cancer, in Handbook of Oxidative Stress in Cancer: Therapeutic Aspects, Springer, 2022, 1, p. 28 Search PubMed.
- C. Hoskins, P. K. Thoo-Lin and W. P. Cheng, Ther. Delivery, 2012, 3, 59–79 CrossRef CAS PubMed.
- V. P. Torchilin, AAPS J., 2007, 9, E128–E147 CrossRef CAS PubMed.
- P. Carmeliet and R. K. Jain, Nature, 2000, 407, 249–257 CrossRef CAS PubMed.
- J. Z. Du, T. M. Sun, W. J. Song, J. Wu and J. Wang, Angew. Chem., 2010, 122, 3703–3708 CrossRef.
- J. Liu, Y. Huang, A. Kumar, A. Tan, S. Jin, A. Mozhi and X. J. Liang, Biotechnol. Adv., 2014, 32, 693–710 CrossRef CAS PubMed.
- E. Roger, F. Lagarce, E. Garcion and J. P. Benoit, J. Controlled Release, 2009, 140, 174–181 CrossRef CAS PubMed.
- E. Roger, F. Lagarce and J. P. Benoit, Eur. J. Pharm. Biopharm., 2011, 79, 181–188 CrossRef CAS PubMed.
- A. Villanueva, M. Canete, A. G. Roca, M. Calero, S. V. Verdaguer, C. J. Serna, M. D. P. Morales and R. Miranda, Nanotechnology, 2009, 20, 115103 CrossRef PubMed.
- A. M. Bannunah, D. Vllasaliu, J. Lord and S. Stolnik, Mol. Pharm., 2014, 11, 4363–4373 CrossRef CAS PubMed.
- C. He, Y. Hu, L. Yin, C. Tang and C. Yin, Biomaterials, 2010, 31, 3657–3666 CrossRef CAS PubMed.
- B. M. Razavi and H. Hosseinzadeh, J. Endocrinol. Invest., 2014, 37, 1031–1040 CrossRef CAS PubMed.
- K. M. Alkharfy, F. A. Ali, M. A. Alkharfy, B. L. Jan, M. Raish, S. Alqahtani and A. Ahmad, Xenobiotica, 2020, 50, 858–862 CrossRef CAS PubMed.
- S. Al Bitar, F. Ballout, A. Monzer, M. Kanso, N. Saheb, D. Mukherji, W. Faraj, A. Tawil, S. Doughan, M. Hussein, W. Abou Kheir and H. G. Muhtasib, Cancers, 2022, 2022(14), 1363 CrossRef PubMed.
- F. Ballout, A. Monzer, M. Fatfat, H. E. Ouweini, M. A. Jaffa, R. A. Samad, N. Darwiche, W. A. Kheir and H. G. Muhtasib, Oncotarget, 2020, 11, 2959 CrossRef PubMed.
- H. G. Muhtasib, M. Diab assaf, C. Boltze, J. Alhmaira I, R. Hartig, A. Roessner and R. Schneider Stock, Int. J. Oncol., 2004, 25, 857–866 Search PubMed.
- O. H. Alobaedi, W. H. Talib and I. A. Basheti, Asian Pac. J. Trop. Med., 2017, 10, 400–408 CrossRef CAS PubMed.
- W. Asfour, S. Almadi and L. Haffar, Sci Reas, 2013, 4, 11 Search PubMed.
- C. C. Woo, A. Hsu, A. P. Kumar, G. Sethi and K. H. B. Tan, PLoS One, 2013, 8, e75356 CrossRef CAS PubMed.
- F. Alhmied, A. Alammar, B. Alsultan, M. Alshehri and F. H. Pottoo, Comb. Chem. High Throughput Screening, 2021, 24, 1644–1653 CrossRef CAS PubMed.
- R. Khalife, M. H. Hodroj, R. Fakhoury and S. Rizk, Planta Med., 2016, 82, 312–321 CrossRef CAS PubMed.
- R. Schneider-Stock, I. H. Fakhoury, A. M. Zaki, C. O. ElBaba and H. U. G. Muhtasib, Drug Discovery Today, 2014, 19, 18–30 CrossRef CAS PubMed.
- G. Kroemer, B. Dallaporta and M. R. Rigon, Annu. Rev. Physiol., 1998, 60, 619–642 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3na00445g |
| This journal is © The Royal Society of Chemistry 2023 |