Reticular chemistry of uranyl phosphonates: synthesis, design, and beyond†
Ziwei
Liu
ab,
Chuang
Han
ab,
Wenzhuo
Tan
ab,
Jinyan
Ji
ac and
Tao
Zheng
 *ab
*ab
aYangtze River Delta Research Institute, Northwestern Polytechnical University, Suzhou 215400, People's Republic of China. E-mail: zhengtao@nwpu.edu.cn
bSchool of Materials Science and Engineering, Northwestern Polytechnical University, Xi'an 710072, People's Republic of China
cSchool of Environmental and Biological Engineering, Nanjing University of Science and Technology, Nanjing 210094, China
First published on 1st December 2022
Abstract
Compared to the uranium-based metal–organic framework (UMOF), achieving reticular chemistry in uranyl phosphonate MOFs is still challenging. In this review, three-dimensional (3D) uranyl phosphonates are collected and concluded for building blocks based on reticular chemistry. Among several design strategies, the sterically hindered phosphonate ligand (SHPL) strategy is introduced in detail due to its success in constructing porous uranyl phosphonate frameworks (UPFs) and their applications.
Design, System, ApplicationThe uranyl phosphonate framework (UPF) is essential for understanding the relationships between the structures and the properties/applications and further for improving the nuclear fuel cycle system. Inspired by reticular chemistry, developing an effective strategy for the target multifunctional materials design based on the inorganic and organic parts to demonstrate physical- and chemical properties for multi applications in the radioactive waste management system is urgent. The sterically hindered phosphonate ligand (SHPL) is one of the most successful design strategies for porous UPFs construction, providing more space for functionalization by grafting the organic group to the carbon backbone of the ligand, introducing guest molecules/ions, deprotonation of phosphonate group, or hybrid metal ions. Integrating the unique characteristics of different parts of UPFs for the reuse of depleted uranium, uranyl leaking prevention, uranyl concentration fluorescent sensing, and so on based on one compound makes cyclic utilization (CU@one) more efficient; for example, UPF-105 is an excellent case to make CU@one into practice. CU@one may provide a tip for the structure design, system integration, and practical applications of waste management, not just for uranium but also those related to inorganic and organic components. |
1. Introduction
Reticular chemistry inspires the construction of novel materials featured with crystallinity, porosity, functions, and beyond by connecting building blocks with strong interactions (coordinate and covalent bonds) to form organized and uniform structures and has been an attractive area of research in the last three decades.1 As one of the essential components, metal–organic frameworks (MOFs) consist of inorganic metal units and organic ligands that have been investigated for a variety of applications such as catalysis, proton conductivity, drug delivery, gas adsorption, separation, etc.2 Understanding and exploiting the relationships between structures and physical/chemical properties contribute significantly to the target-material design.3 For example, the optical properties of MOFs can be regulated by employing different lanthanide metal ions for specific colours, aromatic systems of organic ligands, guest molecules/ions filled in the void, or the interactions between these building blocks mentioned above.4 MOFs containing carboxylates and pyridyl ligands are prevalent because the coordination modes and topology of the structure are easily predicted from the ligands and transition metal ions.5 The development of MOFs broadens the concept of reticular chemistry and provides more strategies to the design toolbox, such as multi-directional bridging ligands, second ligands, multi-nuclear metal clusters, the introduction of heterometallic, heteroatom doping, and templating agents.6Uranium is one of the most extensively studied actinide elements, as it is the essential nuclear fuel used for the nuclear industry.7 Understanding the physical/chemical properties of uranium in novel materials provides more information and tips for solving the issues involving the storage and reuse of depleted uranium in the process of the nuclear fuel cycle.8 Tetra- and hexa-valent uranium are commonly found, and hexavalent uranium is present in chemicals and crystal structures in the form of uranyl units (UO22+) (Fig. 1a).9 Coordination compounds of uranyl have been studied with lots of effort since it is an excellent inorganic building block of uranium-based metal–organic frameworks (UMOFs) with fluorescence properties and unique coordination modes for topology design. Like other MOFs, the number of UMOFs is also dominated by carboxylate-based compounds.7 However, uranyl carboxylates are unstable under harsh conditions, limiting their practical applications. Uranyl phosphonates are more robust and maybe a promising future for UMOFs.10
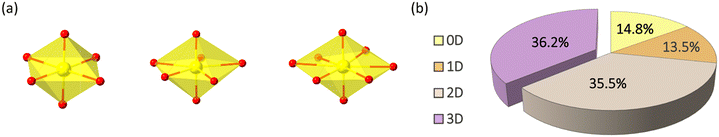 | ||
| Fig. 1 (a) UO6, UO7, and UO8 coordination polyhedrons in hexavalent uranium phosphonates. (b) Distribution of 0D–3D uranyl phosphonate coordination polymers. | ||
Different modifications on carboxylate ligands impact the structural changes of UMOFs. For instance, semi-rigid carboxylic ligands and polycarboxylates have been successfully applied in the synthesis of UMOFs.11 Weak interactions are considered critical factors in constructing UMOFs with extended structures. Therefore, organic ligands provide covalent (backbone), coordination bonds, and weak interactions to build UMOFs with high-dimensional structures.11 Compared to the carboxylate system, achieving the reticular chemistry of uranyl phosphonates is extremely challenging because both metal phosphonates and uranyl units are prone to expand at 2D topologic directions forming layered structures.12,13 Some cases show 3D extended structures. At the same time, there is still no effective design strategy for constructing uranyl phosphonate frameworks (UPFs). Herein, we would like to discuss an effective strategy for developing 3D reticular structures of uranyl phosphonates, as some other excellent reviews have discussed the structures and applications in detail.12–14
2. Structure, design strategy, and applications
For uranyl phosphonates, hexavalent uranyl units are dominated and typically found as tetragonal, pentagonal, or hexagonal bipyramidal (Fig. 1a).7 Uranyl polyhedron presents as a secondary building unit (SBU) solely or aggregated by edging or corner-sharing to form di- to multi-cluster or even a one-dimensional chain. A survey of uranyl phosphonate crystal structures (Nov. 2022) based on the Cambridge Crystal Database and literature search shows that the low-dimensional structures are dominant. In contrast, 3D structure accounts for 36.2%. Most 3D structures are nonporous with pillared layer structure of uranyl phosphonate, with only a few net-like extended structures (Tables S1–S9,†Fig. 1b).Several strategies have been concluded to achieve 3D structures of uranyl phosphonates among the compounds mentioned above: a) utilizing phosphonate ligands grafted with other functional groups to avoid forming layer structures. For example, in Cs3(UO2)(4-cpp)3(H2O)3·4H2O (4-cpp = 4-carboxy-phenylphosphonic acid), carboxylate and organic moieties in the ligand act as the bridge to form 3D framework.15 b) Introducing secondary ligands, including carboxylates, imidazole, and multi-topic pyridines. The 3D heterometallic compounds, [Cu(H2O)]2{(UO2)4F2[(PO3C6H4)(C6H4PO3H)3]2(bipy-m)}·6H2O utilizing Cu2+ with 2,2-bipyridyl as a bridge to form an extended structure.16 c) Incorporating transition metal ions as a ‘glue’ to connect the low-dimensional parts to construct a 3D network. For [Mn3(UO2)6(2-pmb)6(H2O)·10H2O (2-pmbH3 = 2-(phosphonomethyl)benzoic acid), the MnO6 inorganic chains help to enhance the dimension.17 d) The introduction of uncoordinated template reagents, such as ammonium cations, aromatic amines, and other organic cations, which serve as structure-guiding reagents in building the 3D framework. For instance, [N(C2H5)4]K{(UO2)3[CH2(PO3)2]2(H2O)2}·1.5H2O employs the tetraethylammonium cation as the template reagent.18 The examples of those strategies will not show in detail as Yang and Sun's review collects and classifies the structures very clearly.12
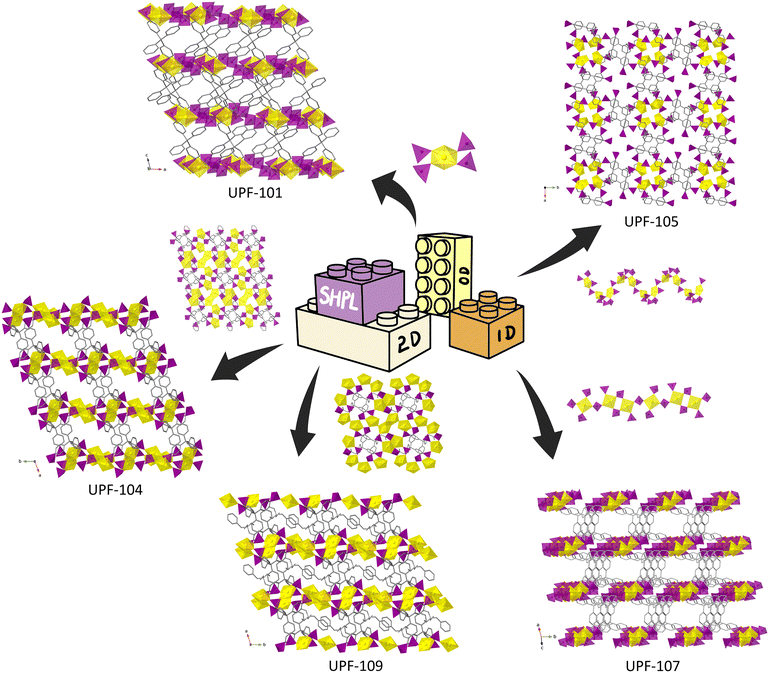
Among these strategies, the sterically hindered phosphonate ligand (SHPL) strategy is claimed and used to obtain a series of porous UPFs successfully. The 0D, 1D, and 2D SBUs of uranyl phosphonate corporate with tetrakis[4-(dihyroxyphosphoryl)phenyl]methane (TppmH8) and 1,3,5,7-tetrakis(4-phosphonophenyl)adamantine (TppaH8) to give 3D porous framework structures (Fig. S1†). 0D SBU found in UPFs is a tetragonal bipyramid in UPF-101 connected and isolated by –CPO3 groups from neighbour TppmH8 ligands (Fig. 2).19
The 1D chains are found in 3D structures as backbones, and they are all constructed by corner-sharing of uranyl units and phosphonate groups (Fig. S2†). The ligands further link the chains to give 3D porous structures. In UPF-105, UO7 units and tetrahedra –PO3 alternately connected to form a zig-zag along the c axis (Fig. 2).10 For UPF-102, UPF-106, and UPF-107, UO6 units are alternately connected with –CPO3 in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio to form wave-like chains (Fig. S2† and 2).19,20 The final structures are obtained by the connection of the 1D chain SBUs and the ligands, which provide more space for design.
2 ratio to form wave-like chains (Fig. S2† and 2).19,20 The final structures are obtained by the connection of the 1D chain SBUs and the ligands, which provide more space for design.
2D SBUs are found in UPF-104, UPF-108, and UPF-109, respectively.19–21 It is worth noting that the dimers of UO7 units are found in the wave-like layers of uranyl phosphonates, which play a critical role in extending the topology link, similar to other reported 2D structures of uranyl phosphonates (Fig. 2 and S3†). The edge-sharing UO7 dimers connect more phosphonate groups than monomers of the uranyl unit. UPF-109's layers are achieved by corner-sharing of dimers of UO7 units, UO7 monomers, and phosphonate groups (Fig. 2).21 The formed 2D SBUs are further connected by rigid phenyl spacers of the ligands to form a 3D structure. Therefore, the design of reticular chemistry can be relied on the topology extending of the phosphonate ligands.
The successful synthesis of the porous UPF series shows that the SHPL strategy enables a 3D framework structure constructed from low-dimensional SBU in the uranyl phosphonate system, which is vital for the reticular chemistry of uranyl phosphonates. In UPFs, both 0D and 1D SBUs are composed of mononuclear uranyl units, suggesting that discrete mononuclear clusters are more likely to form lower dimensional SBUs, which positively impacts the construction of porous uranyl phosphonate MOFs. The rigid backbone of ligands provides a large sterically hindrance and offers the possibility of forming diverse 3D porous uranyl phosphonate structures. Moreover, the porosity of the 3D porous uranyl phosphonates is collected or calculated by PLATON (Table S10†), and it shows that almost all UPF series compounds possess high porosity, which is outstanding in uranyl phosphonates.22 The porosity can be regulated by the template reagent in UPFs.20 Therefore, the SHPL strategy can also put designability into practice by modifying the rigid carbon backbone, achieving the concept of reticular chemistry in the uranyl phosphonate system. It is successfully in constructing 3D porous metal phosphonates not only for uranyl but also for transition, lanthanide, and actinide metal ions.
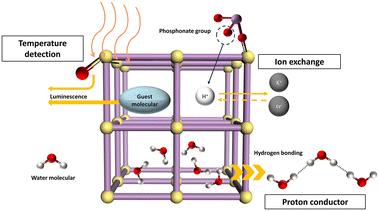
Several applications of uranyl phosphonates have been developed, as shown in Fig. 3. The fluorescence properties of uranyl phosphonates mainly originate from the luminescence of uranyl units, the coordination modes of phosphonate groups, and the guest molecules/ions in the framework. The visible yellow-green emission of the uranyl complexes is mainly due to the lowest electronically excited states of spin-triplet character, where the lowest unoccupied molecular orbital (LUMO) and highest occupied molecular orbital (HOMO) electronic transition is coupled with stretch vibrations of UO2 unit.23 The relation between the intensity and the temperature is generally negative, while positive in some rare cases because of the energy-delay in a different way.24–26 Therefore, UPFs are good candidates for temperature sensors and may also be possible for other physical- and chemical stimuli.
For UPFs, the proton or guest molecules/ions in channels are easily exchanged by particular ions with high selectivity, such as UO22+. UPF-105 not only adsorbs uranyl ions effectively but also probes the concentration of uranyl ions in the solution.10 Therefore, the utilization of depleted uranium, safe storage, uranyl leaking prevention, and probing the concentration of the uranyl ions can be achieved on a sole compound, UPF-105, which may reduce the cost of the whole process of uranium management. This also inspires the design of multifunctional compounds using radioactive elements and other so-called ‘waste’ materials. The excellent thermal and chemical stability and high density of proton in the framework make the UPFs excellent candidates for proton conductors, for maintaining a rich hydrogen bonding network at high temperatures by encapsulating LiBr in negatively charged UPF to keep the lattice water stable at low relative humidity, and the change of proton conductivity for UPFs may also provide a signal for sensoring.27
3. Summary and outlook
In conclusion, the reticular chemistry of uranyl phosphonates is developing, and more porous UPFs are needed to be created and concluded to give more effective strategies for further improvement. Among the survey of the reported cases of the crystal structures, the SHPL strategy proved successful for constructing porous UPFs. The porosity of UPFs is still calculated theoretically, rather than experimentally with the N2 adsorption and desorption, due to the difficulty for purging the guest molecule/ions filled in the channels. Enlarging the inside space of the UPFs would provide more possibilities for the design of novel multifunctional materials. It may also bring more solutions for the reclamation of actinide elements and reduce the risk of radioactive waste and provide tips for other porous metal phosphonates.Author contributions
Tao Zheng conceived the project and conceptualization. Ziwei Liu, Chuang Han, Wenzhuo Tan, and Jinyan Ji contributed to the investigation of crystal structure analysis. The manuscript was completed with the contributions of Ziwei Liu and Tao Zheng. All writers have proved the contributions.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by following fundings: the National Science Foundation of China (21976090), the Natural Science Foundation of Jiangsu Province (BK20191281), the Fundamental Research Funds for the Central Universities, and the Gusu Leading Talents Program (ZXL2021204).References
- O. M. Yaghi, ACS Cent. Sci., 2019, 5, 1295 CrossRef CAS.
- R. Freund, O. Zaremba, G. Arnauts, R. Ameloot, G. Skorupskii, M. Dincă, A. Bavykina, J. Gascon, A. Ejsmont, J. Goscianska, M. Kalmutzki, U. Lächelt, E. Ploetz, C. S. Diercks and S. Wuttke, Angew. Chem., 2021, 60, 23975 CrossRef CAS.
- O. M. Yaghi, M. O'Keeffe, N. W. Ockwig, H. K. Chae, M. Eddaoudi and J. Kim, Nature, 2003, 423, 705 CrossRef CAS.
- Y. Cui, Y. Yue, G. Qian and B. Chen, Chem. Rev., 2012, 112, 1126 CrossRef CAS PubMed.
- J.-P. Zhang, Y.-B. Zhang, J.-B. Lin and X.-M. Chen, Chem. Rev., 2012, 112, 1001 CrossRef CAS PubMed.
- Z. Chen, H. Jiang, M. Li, M. O'Keeffe and M. Eddaoudi, Chem. Rev., 2020, 120, 8039 CrossRef CAS PubMed.
- T. Loiseau, I. Mihalcea, N. Henry and C. Volkringer, Coord. Chem. Rev., 2014, 266, 69 CrossRef.
- R. J. Baker, Coord. Chem. Rev., 2014, 266–267, 123 CrossRef CAS.
- L. A. Borkowski and C. L. Cahill, Inorg. Chem., 2003, 42, 7041 CrossRef CAS PubMed.
- H. Zhao, C. Qi, X. Yan, J. Ji, Z. Chai, S. Wang and T. Zheng, ACS Appl. Mater. Interfaces, 2022, 14, 14380 CrossRef CAS.
- J. Su and J. Chen, Struct. Bonding, 2015, 163, 265 CrossRef CAS.
- W. Yang, T. G. Parker and Z.-M. Sun, Coord. Chem. Rev., 2015, 303, 86 CrossRef CAS.
- K. J. Gagnon, H. P. Perry and A. Clearfield, Chem. Rev., 2012, 112, 1034 CrossRef CAS PubMed.
- G. K. H. Shimizu, R. Vaidhyanathan and J. M. Taylor, Chem. Soc. Rev., 2009, 38, 1430 RSC.
- P. O. Adelani, A. G. Oliver and T. E. Albrecht-Schmitt, Cryst. Growth Des., 2011, 11, 3072 CrossRef CAS.
- P. O. Adelani, N. D. Cook, J.-M. Babo and P. C. Burns, Inorg. Chem., 2014, 53, 4169 CrossRef CAS PubMed.
- G.-H. Wen, Q. Zou, X.-D. Huang, K. Zhang, S.-S. Bao and L.-M. Zheng, Polyhedron, 2021, 205, 115327 CrossRef CAS.
- D. Juan and T. E. Albrecht-Schmitt, Chem. Commun., 2012, 48, 3827 RSC.
- Y. Wang, X. Wang, Y. Huang, F. Zhou, C. Qi, T. Zheng, J. Li, Z. Chai and S. Wang, Chem. – Eur. J., 2019, 25, 12567 CrossRef CAS PubMed.
- J. Ji, C. Qi, H. Zhao, X. Yan, Z. Chai, S. Wang and T. Zheng, Inorg. Chem., 2022, 61, 16794 CrossRef CAS.
- J. Ji, C. Qi, X. Yan and T. Zheng, J. Mol. Struct., 2022, 1269, 133814 CrossRef CAS.
- A. Spek, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 9 CrossRef CAS PubMed.
- J. Su, K. Zhang, W. H. E. Schwarz and J. Li, Inorg. Chem., 2011, 50, 2082 CrossRef CAS.
- T. Zheng, Y. Gao, D. Gui, L. Chen, D. Sheng, J. Diwu, Z. Chai, T. E. Albrecht-Schmitt and S. Wang, Dalton Trans., 2016, 45, 9031 RSC.
- T. Zheng, Q.-Y. Wu, Y. Gao, D. Gui, S. Qiu, L. Chen, D. Sheng, J. Diwu, W.-Q. Shi, Z. Chai, T. E. Albrecht-Schmitt and S. Wang, Inorg. Chem., 2015, 54, 3864 CrossRef CAS PubMed.
- Y. Wang, X. Wang, D. Zhang, F. Zhou, D. Gui, T. Zheng, J. Li, Z. Chai and S. Wang, CrystEngComm, 2018, 20, 3153 RSC.
- K. Zhang, G.-H. Wen, X.-J. Yang, D.-W. Lim, S.-S. Bao, M. Donoshita, L.-Q. Wu, H. Kitagawa and L.-M. Zheng, ACS Mater. Lett., 2021, 3, 744 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2me00217e |
| This journal is © The Royal Society of Chemistry 2023 |