Open Access Article
Open Access ArticleElectrochemical CO2 reduction with ionic liquids: review and evaluation†
Yangshuo
Li
 a,
Fangfang
Li
a,
Aatto
Laaksonen
abcde,
Chuan
Wang
f,
Paul
Cobden
a,
Fangfang
Li
a,
Aatto
Laaksonen
abcde,
Chuan
Wang
f,
Paul
Cobden
 f,
Per
Boden
g,
Yanrong
Liu
h,
Xiangping
Zhang
f,
Per
Boden
g,
Yanrong
Liu
h,
Xiangping
Zhang
 h and
Xiaoyan
Ji
*a
h and
Xiaoyan
Ji
*a
aEnergy Engineering, Division of Energy Science, Luleå University of Technology, Luleå 97187, Sweden. E-mail: xiaoyan.ji@ltu.se
bDepartment of Materials and Environmental Chemistry, Arrhenius Laboratory, Stockholm University, 106 91 Stockholm, Sweden
cCentre of Advanced Research in Bionanoconjugates and Biopolymers, Petru Poni Institute of Macromolecular Chemistry, Aleea Grigore Ghica-Voda, 41A, 700487 Iasi, Romania
dState Key Laboratory of Materials-Oriented and Chemical Engineering, Nanjing Tech University, Nanjing, 210009, P. R. China
eUniversity of Cagliari, Department of Chemical and Geological Sciences, Campus Monserrato, SS 554 bivio per Sestu, 09042, Monserrato, Italy
fMetallurgy Department, Swerim AB, 97125, Luleå, Sweden
gSMA Mineral AB, 68227, Filipstad, Sweden
hCAS Key Laboratory of Green Process and Engineering, Beijing Key Laboratory of Ionic Liquids Clean Process, State Key Laboratory of Multiphase Complex Systems, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China
First published on 1st February 2023
Abstract
The increasing CO2 emission, as the chief culprit causing numerous environmental problems, could be addressed by the electrochemical CO2 reduction (CO2R) to the added-value carbon-based chemicals. Ionic liquids (ILs) as electrolytes and co-catalysts have been widely studied to promote CO2R owing to their unique advantages. Among the potential products of CO2R, those only containing one carbon atom, named C1 products, including CO, CH3OH, CH4, and syngas, are easier to achieve than others. In this study, we first summarized the research status on CO2R to these C1 products, and then, the state-of-the-art experimental results were used to evaluate the economic potential and environmental impact. Considering the rapid development in CO2R, future scenarios with better CO2R performances were reasonably assumed to predict the future business for each product. Among the studied C1 products, the research focuses on CO, where satisfactory results have been achieved. The evaluation shows that producing CO via CO2R is the only profitable route at present. CH3OH and syngas of H2/CO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the targeted products can become profitable in the foreseen future. In addition, the life cycle assessment (LCA) was used to evaluate the environmental impact, showing that CO2R to CH4 is the most environmentally friendly pathway, followed by the syngas of H2/CO (2
1) as the targeted products can become profitable in the foreseen future. In addition, the life cycle assessment (LCA) was used to evaluate the environmental impact, showing that CO2R to CH4 is the most environmentally friendly pathway, followed by the syngas of H2/CO (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and CO, and the further improvement of the CO2R performance can make all the studied C1 products more environmentally friendly. Overall, CO is the most promising product from both economic and environmental impact aspects.
1) and CO, and the further improvement of the CO2R performance can make all the studied C1 products more environmentally friendly. Overall, CO is the most promising product from both economic and environmental impact aspects.
Keywords: Electrochemical-CO2-reduction; Ionic-liquids; C1-product; Economic-evaluation; Environmental-impact.
1. Introduction
On the one hand, the increasing anthropogenic CO2 emissions due to the long and extensive burning of fossil fuels have caused severe environmental problems, such as the greenhouse effect leading to extreme weather events due to the rise of Earth's surface temperature.1 On the other hand, CO2 itself is an abundant carbon resource that can be converted to chemicals and fuels. Therefore, carbon capture and utilization via conversion (CCU) have become an important strategy to mitigate CO2 emissions and produce carbon-based chemicals and fuels.2–4 Several methods have been proposed to convert CO2, including thermal,5 chemical,6 and bio-conversion7 as well as electrocatalytic,8–10 photocatalytic,11 and photoelectric12 conversions and co-conversions. Among them, electrochemical CO2 reduction (CO2R) is among the most promising owing to the mild reaction conditions, as well as easy and flexible controllability. Also, its driving force, electricity, can be potentially integrated with renewable energy sources, for example, solar and wind power.13–15The performance of CO2R is mainly characterized by the faradaic efficiency (FE), current density, and cell voltage, reflecting product selectivity, reaction rate, and energy usage.16 Therefore, higher FE and current density as well as lower cell voltage are being pursued in the performance of CO2R, where the suitable electrocatalyst, electrolyte, electrolyzer, and applied potential, as well as reaction temperature and pressure, can all contribute to the performance. Currently, the research on electrocatalysts has focused on noble metals (Au, Ag, Pt),17–22 transition metal disulfide compounds (MoS2, MoSe2, WS2),23–26 metal organic frames,27 graphene-based synthetic materials,28–30 and molecular and single-atom catalysts31,32 for their desirable catalytic performances.33 While in CO2R, the electrolyte not only enriches the dissolved CO2 (as the carbon source) but also provides protons for the reduction, so that their concentration, pH, and buffer capacity can affect the local reaction conditions, thereby a variety of products.10,34–36 Thus, it is desirable to have an electrolyte having the ability to dissolve CO2 and then stabilize it as the reaction intermediate. Previously, the aqueous solutions of sodium and potassium salts have been widely used as electrolytes, but their solubility of CO2 is limited.33,37–40 Recently, it has been discovered that ionic liquids (ILs) can greatly promote the performance of CO2R because IL is a powerful CO2 absorbent, and additionally, ILs can also activate CO2 to facilitate the further conversion (co-catalyst).41–45 ILs themselves are electrolytes, which can also be readily mixed with other electrolytes. Besides, ILs, with their tunable structures and properties, wide electrochemical windows, and high electrical conductivities, can provide a lower overpotential, a higher current density, and improved product selectivity for CO2R.46–49 Significantly, ILs can effectively inhibit the hydrogen evolution reaction (HER), which is a competitive reaction with CO2R.
Developing IL-based CO2R has been studied. It was found that, when ILs were immobilized into the cathode catalyst, the cell operating current was increased by a factor of two or more, and FE was enhanced by 20–30%, indicating that ILs could effectively promote CO2R.42 Rosen et al.50 were the first to report that the use of 1-ethyl-3-methylimidazolium tetrafluoroborate ([EMIM][BF4]) increased the selectivity for CO and lowered the overpotential. Since then, developing ILs as electrolytes have been intensively studied, and their efficiency has grown tremendously. Kumar et al.51 performed CO2R to CO in the [BMIM][BF4] electrolyte, showing an extremely low overpotential of 0.17 V over the metal-free carbon nanofiber electrode. Choi et al.52 demonstrated that the addition of [EMIM][BF4] into an aprotic electrolyte could reduce the overpotential and enhance the kinetics of electron transfer for CO2R, and the turnover frequency (TOF) in the IL-based systems was 4 times higher than without ILs. Min et al.53 reported that the current density of syngas could reach up to 644.7 mA cm−2 in the gas diffusion electrode (GDE) electrolyzer with the imidazolium-based ILs as the electrolytes. Furthermore, the product of CO2R was changed into CO from syngas by replacing the C2–H of the imidazolium cation with methyl, and the current density could reach up to 528.3 mA cm−2, which has already achieved the industrial standard, indicating that the IL-based electrolytes are efficient in GDE. All these studies indicate that ILs can play an important role in CO2R.
Among the potential products that can be obtained from CO2R with ILs, the ones containing just one carbon atom, named C1 products, including CO, CH3OH, CH4, or syngas, are easier to achieve. Much research work has already been conducted and even summarized in review articles.46,54–57 However, we feel that the recent studies now lead to a rapidly improving performance call for a fresh update. Also, such constant improvements in technological development attract great attention and interest toward novel implementations. To understand the economic feasibility of CO2R with ILs as electrolytes, Chang et al.58 evaluated CO2R to CO, pointing out that it could be competitive with fossil fuels when the current density reached 200 mA cm−2 at the condition of 99% FE. Rumayor et al.59 analyzed the competitiveness of CHOOH generation from CO2R in comparison to traditional methods, concluding that the CO2R pathway was more economically feasible. On the other hand, Jouny et al.60 evaluated the commercial value for CO2R without limiting the types of systems, including non-IL-based electrolytes, and products, including syngas, CO, CHOOH, CH3OH, CH4, C2H4, C2H5OH, and n-C3H8O by calculating the end-of-life net present value (NPV) using the state-of-the-art indicators in 2018. However, IL, as an efficient and green solvent that can replace toxic organic solvents and conventional aqueous carbonate solutions with limited CO2 solubility, has always been sufficiently interested and concerned as novel electrolytes for CO2R, and the research on IL-based electrolytes is extensively booming in recent years due to the unique advantages of ILs in CO2R. To the best of our knowledge, no work has been conducted to discuss and systematically compare the economic benefits of different C1 products focusing on IL-based electrolyte systems, as well as analyzing environmental impacts.
Therefore, in this work, to evaluate and compare the economic potential and environmental impact of CO2R to C1 with ILs as electrolytes, a thorough literature survey was conducted to update the research progress and provide state-of-the-art research achievements for different target C1 products, including CO, syngas, CH4, and CH3OH, which was performed in H-cell. Subsequently, the economic performance was evaluated, and the environmental impact was analyzed based on the state-of-the-art research achievement. Considering the rapid development in CO2R, several future scenarios with improved FE and current density, as well as decreased cell voltage for each target C1 product, were created, and their performances from both economic and environmental aspects were predicted. Additionally, a sensitivity analysis was conducted to provide a comprehensive understanding of how the main parameters would affect TPC for each product. Finally, the traditional and IL-based electrolytes were compared from economic and environmental aspects.
2. IL-based electrochemical CO2 reduction to C1 products
In general, aqueous solutions containing carbonate sodium and potassium salts are used as the electrolyte for CO2R, but the product selectivity and current density are often unsatisfactory. To address these problems, IL-based electrolytes can be used to improve the absorption capacity of CO2 and stabilize the intermediate, and reconstruct the electrode interface.61–63 Herein, the achievements of CO2R to CO, CH3OH, CH4, and syngas in IL-based electrolytes are summarized in the following subsections, and the best performance for each target product was identified for further evaluation.2.1. CO as the target product
The electrochemical conversion of CO2 to CO is the most promising one among the various CO2R pathways due to the lower electron demand (2e−) for the formation of CO and the pivotal role of CO in the industry as the primary ingredient for the synthesis of hydrocarbons and alcohols.64–66 Recently, relevant research is growing fast, and previous important research work has been summarized in review articles published in the past few years.67 Therefore, we focused only on the latest research, reported mainly since 2020, as summarized in Fig. 1a.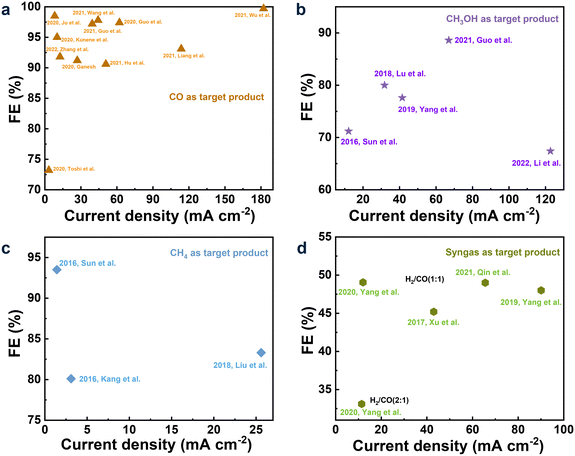 | ||
| Fig. 1 Performance, including FE and current density, of CO2R to CO (a); CH3OH (b); CH4 (c); and syngas (d) in the IL-base electrolytes. | ||
For the IL-based CO2R to CO, Liang et al.68 reported that the FE of CO and current density could be up to 93.1% and 122.0 mA cm−2, respectively, in the 1-butyl-3-methylimidazolium hexafluorophosphate ([BMIM][PF6])/acetonitrile (MeCN) electrolyte. In the work of Oguma and Azumi,69 1-ethyl-3-methylimidazolium ethyl sulfate ([EMIM][EtSO4]) was added into the 0.1 mol dm−3 K2CO3 aqueous solution for CO2R on the Ag electrode, and observable improvements in the FE of CO and current density were obtained, owing to the high complexation ability of EMIM with CO2 that was intensively adsorbed on the electrode surface. Guo et al.70 studied CO2R in 0.5 mol L−1 (M) [BMIM][PF6]/MeCN on the Cu–Co bimetallic electrode, and the FE of CO and the current density could reach 97.4% and 62.1 mA cm−2, respectively. Zhang et al.71 designed a catalyst with ZIF-8 films on Zn foils (ZIF-8/Zn) to promote CO2R, and FECO of 91.8% along with the current density of 12.6 mA cm−2 was obtained at −1.9 V in the electrolyte with (30 wt% [BMIM][PF6] + 65 wt% MeCN + 5 wt% water). Kunene and co-workers72 explored the CO2R to CO in the MeCN/100 mmol L−1 (mM) tetrabutylammonium hexafluorophosphate ([TBA][PF6]) electrolyte with 1-butyl-3-methylimidazolium trifluoromethanesulfonate ([BMIM][OTf]) as the additive on the soldering alloy Bi50Sn22Pb28 as the anode, where the influences of [BMIM][Otf] concentrations (50, 100, and 200 mM) and the applied potentials (−1.85, −1.95, and −2.05 V) on CO2R were investigated. The result showed that the FE of 95% and current density of 10.2 mA cm−2 were obtained when the concentration of [BMIM][Otf] was 100 mM at −2.05 V. To make the CO2R to CO more economical with ILs as the electrolytes, Ganesh73 synthesized the low-cost highly pure 1-butyl-3-methylimidazolium tetrafluoroborate ([BMIM][BF4]) using the inexpensively accessible raw materials (1-bromobutane (BB) and 1-methylimidazole (MI)). The 20 mM synthesized [BMIM][BF4] was mixed with 0.1 M [TBA][PF6] and MeCN and then used as the electrolyte to perform CO2R on the Sn and MoSi2 electrodes, obtaining FE of 91.2% and the current density of 27 mA cm−2 at 622 mV potential.
The linear CO2 being very thermodynamically stable and kinetically inert is difficult to be reduced, and the formation of CO˙− radical in the first-step one-electron reduction of CO2 is the major obstacle, which requires a high potential.74 It was reported that the formation of the CO2-imidazolium intermediate compound could help to reduce the overpotential of CO2R. Ju and co-workers75 discovered that the imidazolium ILs could be adsorbed on the electrode and form a film layer during CO2R, which can help CO2 to contact the catalyst and stabilize the generated CO2˙− after the CO molecule obtains an electron according to the comparison of six imidazolium-ILs, consisting of the same anion [BF4]− and different cations ([EMIM]+, [BMIM]+, 1-hexyl-3-methyl-imidazolium ([HMIM]+), 1-methyl-3-octyl-imidazolium ([OMIM]+) and 1,3-dimethyl-imidazolium ([DMIM]+)), with [TBA][BF4] in propylene-carbonate (PC) as the electrolytes. Among them, [BMIM][BF4] was identified as the most efficient electrolyte, which may be attributed to its suitable chain length at the N1-position of the imidazolium cation. Hu et al.76 found that, in the system of N-octyltrimethyl ammonium 1,2,4-triazole ([N1118][TRIZ])/MeCN electrolyte, the linear CO2 molecule could change into a bend because the extra electron was filled in the lowest unoccupied molecular orbital (LUMO) from [TRIZ]−. The formation of the [TRIZ–CO2] complex was emphasized and demonstrated via Fourier transform infrared (FTIR), which could result in the high solubility of CO2 and low energy barrier for CO2 activation.
To confirm the role of imidazolium-2-carboxylate species, Ratschmeier and Braunschweig77 detected the produced CO molecule adsorbed on the Pt electrode in the 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ([BMIM][NTf2]) with 0.5 M H2O electrolyte via the in situ vibrational sum-frequency generation (SFG). Also, the in operando Fourier transform infrared reflection absorption spectroscopy (FT-IRAS) was used to determine the formation of imidazolium-2-carboxylic acid species. The investigation revealed that the mechanism strongly depended on the types of ILs. For [BMIM][NTf2], providing an active C2 position of the imidazolium ring, the [BMIM]-2-carboxylic acid species could be generated through the carbene intermediate. While for 1-butyl-2,3-dimethylimidazolium bis(trifluoromethylsulfonyl)imide ([BMMIM][NTf2]) with protected C2 position by the methyl group and 1-butyl-1-methyl pyrrolidinium bis(trifluoromethylsulfonyl)imide ([BMPyrr][NTf2]) without the active C2 position (their structures were shown in Fig. 2), when 0.5 M H2O was used as the electrolyte, the stabilization of CO2˙− was attributed to the formation of bicarbonate and the Coulomb interactions between CO2˙− and the IL-cations. Meanwhile, the anion can also effectively facilitate CO2R to CO. For example, Hu and co-workers76 developed a novel IL [N1118][TRIZ] and used it as the electrolyte to explore the effect on CO2R, where the electrochemical methods, FTIR spectroscopy, and the density functional theory (DFT) were combined to analyze the mechanism. It showed that the 1,2,4-triazole ([TRIZ]) anion was equally effective for facilitating CO2R to CO on the Ag electrode.
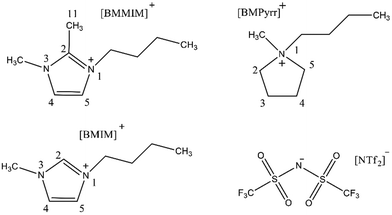 | ||
| Fig. 2 The chemical structures of [BMIM]+, [BMMM]+, [BMPyrr]+, and [NTf2]−.77 Copyright 2021 American Chemical Society. | ||
To overcome the high viscosity of ILs, adding H2O or organic solvent is an efficient way, and their content plays an important role. In the work of Wang et al.,78 CO2R to CO was explored with various contents of [BMIM][PF6] and H2O in the MeCN electrolyte, where a series of porous zinc oxide nanosheets grafted with the hydroxyl groups on the carbon paper substrates were used as the catalyst. They found that the current density first raised and then dropped with increasing H2O concentrations. This observation was caused by the appropriate electrostatic attraction between the anions and cations, availing the ion movement and charge transport and thereby promoting CO2R. In this work, the best performance was obtained at a low overpotential of 340 mV when 5 wt% H2O was added into 30 wt% [BMIM][PF6]-based electrolytes, where the addition of H2O might increase the electrolyte conductivity and the CO2 solubility by changing the microstructure of the electrolyte. Similarly, Hu et al.76 explored the influence of the H2O contents on the current density, drawing analogical conclusions. With increasing water content from 0 to 7.5 wt%, the onset potential of CO2R was changed from −1.93 to −1.75 V vs. Ag/Ag+, indicating that the addition of water reduced the initial reduction energy barrier.
Apart from the co-catalysis concept of ILs as the electrolyte, the coupling of electrocatalyst and IL-based electrolyte can be another important accelerant for CO2R. To further provide a comprehensive insight into the synergistic effect of catalysts and IL-based electrolytes, Rudnev and co-workers79 explored the performance of thirteen catalyst materials, including Pt, Ag, Bi, Sn, Mo, Zn, Pd, Cu, Au, Pb, Ni, Fe, and glassy carbon (GC), in three IL-based electrolytes ([BMIM][BF4], [BMIM][NTf2] and [BMPyrr][NTf2]). It was found that the co-catalyst effect required balanced interactions between the IL and the electrode surface, depending on the chemical properties of both the IL and catalyst material. Only the proper combination of ILs and electrode materials could enhance the performance of CO2R. Besides, IL-anions significantly influenced the CO2R peak current density in the order of [BMIM][BF4] ≤ [BMIM][NTf2] ≪ [BMPyrr][NTf2], being consistent with the decreasing trend of their viscosities. This observation indicates that low viscosity leads to a high diffusion rate and then a high current density. Among their studied cases, the best performance (99.7% FE of CO) was obtained at −1.84 V when Ag was used as the electrode in the [BMIM][BF4] electrolyte. Zeng et al.80 also found that the combination of a nanoporous Au film electrode with [EMIM][BF4]/H2O as the electrolyte could efficiently perform electrocatalytic CO2 to CO with high selectivity of 92.5% at a low overpotential of 440 mV. In this work, the CO2 reduction rate was strongly affected by the transport rate of CO2 within the nanopores of the Au film. The adsorption of [EMIM][BF4] on the nanopore, confirmed by X-ray photoelectron spectroscopy (XPS), led to the high solubility of CO2, improving the driving force in the mass transfer of CO2 and thereby promoting CO2 conversion. They also found that the performance of CO2R in [EMIM][BF4]/H2O was superior compared to that in 1 M NaHCO3 aqueous electrolyte, indicating the important role of IL.
Additionally, the catalyst itself is of importance in CO2R. The research group of Han81 conducted several studies on the catalysts. They found that the catalyst of the atomic anchored on the N-doped carbon (InA/NC) could significantly promote CO2R to CO in 0.5 M [BMIM][PF6]/MeCN. As a result, the FECO of 97.2%, a total current density of 39.4 mA cm−2, and a TOF of 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1 were obtained. Comparatively, FECO and current density decreased to 53.3% and 0.9 mA cm−2, respectively, when the electrolyte was replaced by 0.5 M KHCO3 aqueous solution, also indicating the indispensable role of [BMIM][PF6]. Later, Han's group82 further improved the performance greatly by synthesizing a novel Cd single-atom catalyst (SACs) CdN4S1/CN, where 0.5 M [BMIM][PF6]/MeCN was used as the electrolyte. The FE of CO could reach up to 99.7% along with the high current density of 182.2 mA cm−2 and the TOF of 73
000 h−1 were obtained. Comparatively, FECO and current density decreased to 53.3% and 0.9 mA cm−2, respectively, when the electrolyte was replaced by 0.5 M KHCO3 aqueous solution, also indicating the indispensable role of [BMIM][PF6]. Later, Han's group82 further improved the performance greatly by synthesizing a novel Cd single-atom catalyst (SACs) CdN4S1/CN, where 0.5 M [BMIM][PF6]/MeCN was used as the electrolyte. The FE of CO could reach up to 99.7% along with the high current density of 182.2 mA cm−2 and the TOF of 73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1 at a low overpotential of 0.6 V. This is the most outstanding performance of CO2R to CO in the H-type electrolysis cell so far. During this process, both the introduction of an axial coordination structure to the Cd SACs and the formation of [BMIM–CO2]+ in [BMIM][PF6]/MeCN electrolyte could reduce the reaction barrier of CO2R and suppress the competitive HER in the aprotic solvent.
000 h−1 at a low overpotential of 0.6 V. This is the most outstanding performance of CO2R to CO in the H-type electrolysis cell so far. During this process, both the introduction of an axial coordination structure to the Cd SACs and the formation of [BMIM–CO2]+ in [BMIM][PF6]/MeCN electrolyte could reduce the reaction barrier of CO2R and suppress the competitive HER in the aprotic solvent.
2.2. CH3OH as the target product
CH3OH as the important platform molecule with high energy density can be converted to different chemicals and fuels.35,83 The formation of one CH3OH via CO2R needs a transfer of six electrons (6e−), making it more difficult to perform the reduction compared to CO2R to CO.56,57 Only a few related articles from Han's group reported improved performance by designing and synthesizing efficient electrocatalysts in the IL-based electrolytes. These achievements are summarized in Fig. 1b.Initially, Sun et al.84 searched for efficient IL-based electrolytes among [BMIM][BF4], [BMIM][PF6], [BMIM][ClO4], and [BMIM][NTf2] in MeCN for electrocatalytic CO2 conversion to CH3OH over a Mo–Bi bimetallic chalcogenide on the carbon paper (CP) (Mo–Bi BMC/CP) electrocatalyst. As a result, the highest selectivity of CH3OH was obtained with an FE of 71.2% and a current density of 12.1 mA cm−2 at −0.7 V (vs. the standard hydrogen electrode (SHE)) when [BMIM][BF4] was the supporting electrolyte. On the contrary, CH3OH was not detected in the tetrabutylammonium/tetraethylammonium salts as the supporting electrolytes, suggesting the important role of ILs in the electrolyte. Additionally, different concentrations of [BMIM][BF4] led to different FEs of CH3OH and current density, further indicating the vital role of the IL in CO2R. Similarly, Yang et al.85 reported that [BMIM][BF4] was the most efficient supporting electrolyte in the MeCN/H2O solution for CO2R to CH3OH among the [BMIM]-based ILs with various anions (PF6−, NTf2−, OAC−, NO3−, and ClO4−). Over the Cu1.63Se electrode, the highest FE of CH3OH and current density were 77.6% and 41.5 mA cm−2, respectively.
Hereafter, Han's group further explored the electrocatalytic CO2 conversion to CH3OH in the [BMIM][BF4] aqueous solution by designing and synthesizing efficient electrocatalysts. Lu et al.86 synthesized a kind of bimetallic catalyst of Pd83Cu17 on CP as the electrode, which successfully enhanced the FE of CH3OH and current density up to 80.0% and 31.8 mA cm−2, respectively. Guo et al.87 designed the atomically dispersed Sn site anchored on the defective CuO catalysts (Sn1/V0–CuO) with high conductivity in the [BMIM][BF4]/H2O electrolyte. The high selectivity of CH3OH (FE of 88.6%) and current density (67.0 mA cm−2) were obtained. In the work of Li et al.,88 the current density surpassed 100 mA cm−2 for the first time with a value of 122.7 mA cm−2 by developing the Ag, S–Cu2O/Cu electrocatalyst combined with [BMIM][BF4]/H2O as the electrolyte. During this process, the S anion could regulate the morphology and electron structure of the electrocatalyst, making it more efficient for CO2R to CH3OH, and, at the same time, the Ag cation could suppress the competing HER. The synergistic effect of the Ag and S heteroatoms and the Cu2O/Cu host greatly contributed to the dramatic enhancement of the current density.
Sun et al.84 studied CO2R to CH3OH using Mo–Bi BMC as the electrocatalyst in the [BMIM][BF4]/H2O electrolyte. They proposed that, in this system, firstly, the [BMIM–CO2]+ complex was quickly formed, reducing the reaction barrier. Furthermore, the synergistic effect of Mo and Bi atoms in Mo–Bi BCM/CP electrocatalyst greatly promoted the CO2 reduction to CH3OH. The Bi sites favored the formation of CO, and the Mo sites were for the generation of H2, conducive to the further hydrogenation of CO to form CH3OH. By using Sn1/V0–CuO as the catalyst, Guo et al.87 found that the formation of Lewis acid–base interaction between the Sn single atom and the oxygen vacancy of CuO reduced the energy barrier for the dissociation of *COOH and conversion to *CO, which was confirmed via in situ X-ray absorption (XAS) spectra, in situ Raman spectra, and DFT. Subsequently, the formed *CO free radical was combined with Cu to generate *CHO, and the moderate binding energy of *CHO with the electrocatalysts promoted the production of *OCH2 and further conversion to CH3OH.
2.3. CH4 as the target product
CH4 is more difficult to produce via CO2R compared to the other C1 products due to the high C–H bond energy (434 kJ mol−1) and the 8e−-transfer demand.33,40,89–93 Therefore, it is a challenge to achieve high FE and current density. To the best of our knowledge, only three articles have reported satisfactory FE and current density in the IL-based electrolytes, as summarized in Fig. 1c.Kang et al.94 deposited Zn–1,3,5-benzenetricarboxylic acid metal–organic frameworks on carbon paper (Zn–BTC-MOFs/CP) as the electrocatalyst, and relatively high product selectivity (FE of 80.1%) and current density (3.1 mA cm−2) in the pure [BMIM][BF4] electrolyte at a low overpotential of 0.25 V were obtained. In this work, the combinations of different catalysts (Au, Ag, Pt, Fe, Zn) and electrolytes ([TBA][BF4]/dimethylformamide (DMF), [TBA][PF6]/MeCN, [BMIM][BF4]/MeCN) were conducted, and the desired performance was attributed to the optimal compatibility between Zn–BTC-MOFs/CP and [BMIM][BF4] as Zn–BTC-MOFs/CP was synthesized in the imidazolium-based ILs.
Sun et al.95 reported an efficient catalyst, the metal-free electrode N-doped graphene-like materials (NGMs), for CO2R to CH4 in the [BMIM][BF4]/H2O electrolyte. It was found that FE was improved from 20.8 to 93.5% by increasing the content of the doped N from 1.8 to 4.8%, implying the vital importance of the active-doped N for the selective production of CH4. A possible pathway (shown in Fig. 3) was proposed as follows: (1) CO2˙− was firstly generated via CO2 adsorbed on the active N sites of the electrode. (2) CO2˙− was coupled with the CO2 molecule dissolved in the electrolyte and then reduced to CO2–CO2˙−. (3) after obtaining the second electron, the adsorbed CO was formed, i.e., COads. (4) The formed COads was converted to CHOads by accepting the proton. (5) the formed CHOads was further transformed to CH4 after obtaining six electrons and protons. During this pathway, the strong interaction between COads and electrons favored the hydrogenation of COads to generate CHOads, an important intermediate of CO2R to CH4. On the other hand, [BMIM][BF4]/H2O as the electrolyte improved the solubility of CO2, driving the transformation of CO2 to CO2˙−.
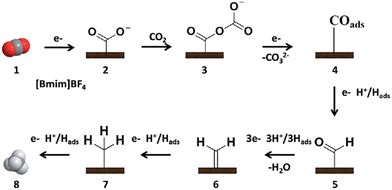 | ||
| Fig. 3 Mechanism schematic diagram of CO2 reduction to CH4 at the NGM/CP electrode.95 Copyright 2016 Royal Society of Chemistry. | ||
In the same electrolyte, [BMIM][BF4]/H2O, by using MoTe2 nanoflakes as the catalyst, Liu et al.96 greatly improved the current density to 25.6 mA cm−2 along with the FE of 83%. Considering both the current density and FE of CH4, the performance in this work was the best. Here, the MoTe2 nanosheets provided more active sites and stronger adsorption capacity of CO2 compared to the bulk materials, being beneficial for the CO2 conversion, and the Tafel slope was very close to the theoretical value (68 mV dec−1), indicating the ultrathin MoTe2 to be an ideal catalyst. Additionally, the DFT calculations also suggested that the interaction between the MoTe2 nanosheets and CO2 molecules might be formed during CO2R through the formation of intermediates.
2.4. Syngas as the target product
Syngas compositing of H2 and CO, as an important feedstock in industry, is mainly derived from coal or natural gas, which are non-renewable resources.97–99 CO2R potentially provided a more energy-efficient method to produce syngas with different ratios by adjusting the reaction environment, including the electrolyte and catalyst.100–106 Among them, H2/CO with a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, i.e., H2/CO (1
1, i.e., H2/CO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) is suitable for the Fischer–Tropsch hydrocarbon synthesis, while H2/CO (2
1) is suitable for the Fischer–Tropsch hydrocarbon synthesis, while H2/CO (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) can be used to synthesize formaldehyde via hydroformylation.107–111 Herein, H2/CO (1
1) can be used to synthesize formaldehyde via hydroformylation.107–111 Herein, H2/CO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and H2/CO (2
1) and H2/CO (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were mainly discussed. The related available results are summarized in Fig. 1d.
1) were mainly discussed. The related available results are summarized in Fig. 1d.
The structures and properties of the catalysts greatly affect the ratio of syngas. Xu et al.112 compared the efficiency of CO2R to syngas by using the MoSeS alloy as well as the MoSe2 and MoS2 monolayers in the [EMIM][BF4]/H2O electrolyte. The MoSeS alloy monolayer exhibited the best catalytic effect, leading to the current density of 43 mA cm−2 along with the FE for CO of 45.2% at −1.15 V vs. the reversible hydrogen electrode (RHE). For comparison, the FE of CO for the MoS2 and MoSe2 catalysts was 16.6% and 30.5%, respectively. The high catalytic activities of the MoSeS alloy monolayer were attributed to its strong absorption ability of CO2, high intrinsic conductivity, and low charge transfer resistance. Moreover, the desorption of CO* from the electrode was also an important influencing factor, and the MoSeS alloy monolayer exhibited low CO onset desorption temperature.
As reported, the synergistic effect of Au and Ni could be in favor of syngas generation under suitable conditions.113–115 Inspired by this, Yang et al.116 designed a kind of catalyst where the Au nanowires (Au NWs) were grown on the porous nickel foam. They found that the porous nickel foam was mainly responsible for the electrocatalytic HER, while the Au NWs with large active surface areas and abundant edge sites were beneficial to the CO2 conversion to CO. The longer and thinner the Au NW was, the higher selectivity of CO was obtained. On the contrary, when Au NWs were grown tightly on the nickel foam, the active sites would be covered, reducing the selectivity of CO and the overall catalytic performance.
Apart from the catalyst, the ratio of syngas also strongly depends on the applied potential and electrolytes. Yang et al.117 synthesized a nanoflower-like catalyst γ-In2Se3 (F-γ-In2Se3/CP) for CO2R in different IL-based electrolytes. Within the applied potential (−1.8 to 2.3 V), the ratio of H2/CO was changed from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 to 3
24 to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. For the electrolyte with ([BMIM][PF6], H2O, and MeCN), upon increasing the [BMIM][PF6] content from 5 to 70 wt%, the ratio of H2/CO was changed from 1
1. For the electrolyte with ([BMIM][PF6], H2O, and MeCN), upon increasing the [BMIM][PF6] content from 5 to 70 wt%, the ratio of H2/CO was changed from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 to 16
24 to 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9; upon increasing the H2O content from 0 to 20 wt%, the ratio was varied from 1
9; upon increasing the H2O content from 0 to 20 wt%, the ratio was varied from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 to 3
24 to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. Significantly, H2/CO (1
2. Significantly, H2/CO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was obtained with a current density of 90.1 mA cm−2 at the applied potential of −2.3 V in the electrolyte composed of (30 wt% [BMIM][PF6] + 65 wt% MeCN + 5 wt% H2O).
1) was obtained with a current density of 90.1 mA cm−2 at the applied potential of −2.3 V in the electrolyte composed of (30 wt% [BMIM][PF6] + 65 wt% MeCN + 5 wt% H2O).
Similarly, Yang and co-workers116 showed that the addition of H2O into the electrolyte composed of [TBA]Br and DMF would greatly affect the ratio of syngas. This can be explained as follows. Without the addition of water, DMF could be oxidized to reduce protons in the anode chamber, thereby producing abundant H2. After the addition of H2O, the oxygenolysis of DMF could be suppressed, stabilizing the DMF. Besides, the addition of H2O could also generate proton-coupled electron transfer (PCET) in favor of CO2 activation, and enhance HER, which would have a great influence on the ratio of H2/CO. The applied potential was also found to take an important role. H2/CO (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and H2/CO (1
1) and H2/CO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were obtained at −1.6 and −1.8 V, respectively, when the concentration of H2O was 1 M. Qin et al.118 also tuned the ratio of H2/CO from 0.15
1) were obtained at −1.6 and −1.8 V, respectively, when the concentration of H2O was 1 M. Qin et al.118 also tuned the ratio of H2/CO from 0.15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 4
1 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by controlling the applied potential in the [BMIM][PF6]/MeCN electrolyte, where H2/CO (1
1 by controlling the applied potential in the [BMIM][PF6]/MeCN electrolyte, where H2/CO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was obtained with the current density of 65.6 mA cm−2 at −1.395 V vs. RHE.
1) was obtained with the current density of 65.6 mA cm−2 at −1.395 V vs. RHE.
2.5. Identification of the state-of-the-art research results
According to the reviews, when CO, CH4, and syngas were chosen as the target products, their state-of-the-art research results can be identified according to their FE and current density. The corresponding results, together with the cell voltages, are listed in Table 1. While for CO2R to CH3OH, there were two potential cases, of which one is with an FE of 88.6% along with a current density of 67.0 mA cm−2, the other showed a current density of 122.7 mA cm−2 along with an FE of 67.4%. Thus, a preliminary study on economic benefit was conducted (the detailed comparison can be found in the ESI†), showing the case with the FE of 88.6% along with the current density of 67.0 mA cm−2 as included in Table 1 was more promising and thus was chosen for further study.| FE (%) | Current density (mA cm−2) | Cell voltage (V) | |
|---|---|---|---|
| a The value was calculated on the basis of ref. 82 and experimental data; b The value was assumed based on ref. 96, 116 and 117. | |||
| CO | 99.7 | 182.2 | −3.29a |
| CH3OH | 88.6 | 67 | −3.28 |
| CH4 | 83 | 25.6 | −1.37b |
Syngas (H2/CO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)) 1)) |
47 (CO) | 90.1 (CO) | −3.14b |
Syngas (H2/CO (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)) 1)) |
33.1 (CO) | 11.4 (CO) | −2.19b |
According to the study conducted, the recent research is more on CO, followed by methanol, syngas, and methane (Fig. 1). The performance of CO2R to CO is much better compared to targeting other C1 products (Table 1), especially, the current density for CO2R to CO is approaching 200 mA cm−2. However, for CO2R to CH4, the FE value is lower than 85%, and the current density is only 25.6 mA cm−2, calling for more research concerns. For the syngas of H2/CO (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the current density is the lowest among the studied C1 product, which is as low as 11.4 mA cm−2.
1), the current density is the lowest among the studied C1 product, which is as low as 11.4 mA cm−2.
IL-based electrolytes have been developed for CO2 to C1. Currently, the ILs with imidazolium cation are the most commonly used electrolytes and are usually beneficial to the improvement of CO2R performance through the formation of the CO2-imidazolium intermediate compound, which can help to reduce the overpotential of CO2R. The viscosity, conductivity, and interaction between cations and anions of ILs can all influence the efficiency, which can be optimized by adding water or organic solvents, changing the carbon chain length of the imidazole cation, and replacing the suitable anions. Regrettably, most studied IL-based electrolytes are concentrated on the imidazolium-based ones, and the ILs other than those of imidazolium-based as well as the effect of IL-anions have not been studied sufficiently, calling for more research. Also, the mechanism of ILs as the electrolyte in CO2R should be deeply explored, and novel, clean, and highly efficient IL-based electrolytes with functional cations and anions need to be developed to improve the performance of CO2R.
3. Evaluation of IL-based electrochemical CO2 reduction to C1 products
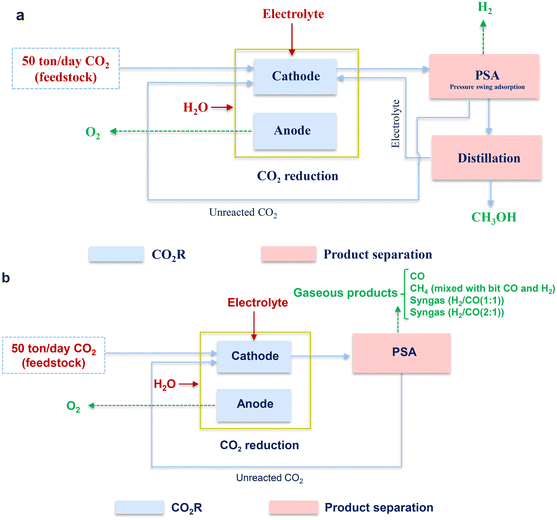
Both, economic analysis and environmental impact depend on the performance of CO2R and other related processes, such as the separation to obtain pure products. In this part, the identified parameters listed in Table 1 were used for evaluating CO2R, and the cost estimation was based on the specific process for a different target product, and then the energy demand and environmental impact were further estimated for comparison and discussion.3.1. Processes
As shown in Fig. 4, the whole process for all cases can be divided into two parts, namely, CO2R and product separation, which can be liquid or gas separation, depending on the target products.For CO2R, a CO2 gas stream was used as the feedstock and injected into the cathode chamber for the reduction to obtain the products. The unit of CO2R was assumed as a black-box model since it is still at the bench scale, in which the main and parasitic reaction equations are listed in Table 2. In general, by-products can be generated. In this work, when CH3OH was targeted, H2 was considered to be the only by-product. On the contrary, the production of H2 was ignored due to the extremely high CO selectivity when CO was the targeted product. For the case of CH4 production via CO2R, H2, and CO were considered as the by-products, while no other by-products were considered. As for syngas comprising CO and H2, there were no other gaseous and liquid products generated. For all the cases, oxygen evolution took place in the anode chamber.
| Products | Cathode | Anode |
|---|---|---|
| CO | CO2 + H+ + 2e → CO + H2O | 2H2O − 4e− → O2 + 4H+ |
| 2H+ + 2e → H2 | ||
| CH3OH | CO2 + 6H+ + 6e → CH3OH + H2O | |
| 2H+ + 2e → H2 | ||
| CH4 | CO2 + 8H+ + 8e → CH4 + 2H2O | |
| CO2 + H+ + 2e → CO + H2O | ||
| 2H+ + 2e → H2 | ||
| Syngas | CO2 + H+ + 2e → CO + H2O | |
| 2H+ + 2e → H2 |
After CO2R, the separation unit was followed for different purposes. When the gaseous product (CO, CH4, or syngas) was obtained as the products from CO2R, the unreacted CO2 was separated from the targeted product with pressure swing adsorption (PSA) and returned to the cathode compartment; while in terms of CH3OH as the targeted product, an additional unit of distillation was used to separate the liquid product (CH3OH) from the electrolyte. Notably, the further purification of CH4, i.e., the removal of the small amounts of H2 and CO was excluded as H2 and CO could also be considered as energy gases or reducing agents. For all the cases, it was assumed that the (regenerated) electrolyte was recycled back to the electrolyzer without any waste and loss.
3.2. Scenarios of CO2R
CO2R is a relatively new technology, which has been investigated intensively with great and rapid progress, as summarized in section 2. It is expected that more progress will be achieved in the coming years. To evaluate the process fairly, in this work, both the current status and future potential cases were considered, i.e., one base case using the identified parameters listed in Table 1, which are also summarized in Table 3, and three future cases with further development of CO2R, e.g., cell voltage, current density, and FE (shown in Table 3), which was based on the development in recent two years as well as the performance of CO2R with other electrolytes.119,120 The other used parameters and assumptions are listed in Table S1.†| Base case | Case 1 | Case 2 | Case 3 | |
|---|---|---|---|---|
| CO as the target product | ||||
| Current density (mA cm−2) | 182.2 | 200 | 400 | 600 |
| FE (%) | 99.7 | 99.7 | 99.7 | 99.7 |
| Cell voltage (V) | 3.29 | 3 | 2.5 | 2 |
| CH3OH as the target product | ||||
| Current density (mA cm−2) | 67 | 200 | 400 | 600 |
| FE (%) | 88.6 | 90 | 95 | 99 |
| Cell voltage (V) | 3.28 | 3 | 2.5 | 1.5 |
| CH4 as the target product | ||||
| Current density (mA cm−2) | 25.6 | 200 | 400 | 600 |
| FE (%) | 83 | 90 | 95 | 99 |
| Cell voltage (V) | 1.37 | 1.3 | 1.25 | 1.2 |
Syngas (H2/CO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)) as the target product 1)) as the target product |
||||
| Current density (mA cm−2), CO | 90.1 | 200 | 400 | 600 |
| FE (%), CO | 47 | 47 | 47 | 47 |
| Cell voltage (V) | 3.14 | 2.5 | 2 | 1.5 |
Syngas (H2/CO (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)) as target product 1)) as target product |
||||
| Current density (mA cm−2), CO | 11.4 | 200 | 400 | 600 |
| FE (%), CO | 33.1 | 33.1 | 33.1 | 33.1 |
| Cell voltage (V) | 2.19 | 2 | 1.5 | 1.34 |
3.3. Economic analysis
The total production cost (TPC) was the summation of the annual capital cost (ACC) and the total operating cost (TOC),121 which was used as the criterion to evaluate the economic benefit of CO2R by comparing it with the current market price for each case.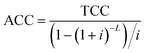 | (1) |
| Total capital cost (TCC) | Total operating cost (TOC) | ||
|---|---|---|---|
| Direct cost (DC)60 | Percentage of DC (%) | Variable operating cost value (VOC)58 | |
| Electrolyzer cost | 65 | CO2 feed cost | The amount of CO2 consumed × price |
| BoP cost | 35 | IL make-up | The amount of IL make-up × price |
| Indirect cost (IC)122 | Percentage of DC (%) | Organic solvent make-up | The amount of organic solvent make-up × price |
| Contingency | 15 | H2O cost | The amount of H2O consumed × price |
| Site preparation | 2 | Electricity cost | The power demand × electricity price |
| Engineering and design | 8 | ||
| Up-front permitting | 15 | ||
| Percentage of FCCa (%) | Fixed operating cost (FOC) | Percentage of DC (%) | |
| Working capital | 5 | Operation and maintenance | 3.2 |
| PSA | Calculated based on Table S2† | ||
| Distillation | Calculated based on Table S3† | PSA123 | Calculated based on Table S2† |
| Initial solvent cost | Initial input quantity × price | Distillation124 | Calculated based on Table S3† |
| Initial catalyst cost | Initial input quantity × price | ||
| TCC | Sum of the above costs | TOC | Sum of the above costs |
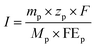 | (2) |
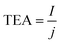 | (3) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 485 C mol−1), and I and j are the total current and current density through the electrolysis, respectively.
485 C mol−1), and I and j are the total current and current density through the electrolysis, respectively.
The equipment costs for PSA and distillation were calculated on the basis of ref. 123 and 124 according to eqn (4).128
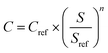 | (4) |
The expenditure of the initial electrolyte and cathode catalyst was considered in the capital cost, which was independently calculated by multiplying the univalence of the item and its mass consumption. The main parameters and the counting process are described in the ESI.†
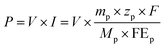 | (5) |
Additionally, the operating costs for PAS and distillation were calculated independently, as shown in the ESI.†
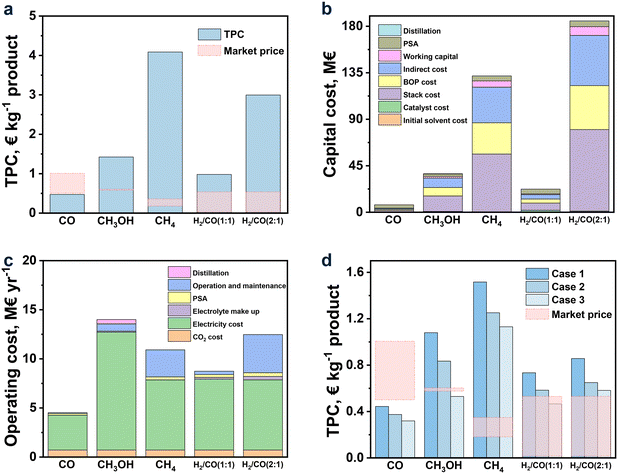 | ||
Fig. 5 (a) TPC; (b) capital cost and (c) operating cost for CO2R to CO, CH3OH, CH4, and syngas (H2/CO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and H2/CO (2 1) and H2/CO (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)) in the base case; (d) TPC for each product in the future cases. 1)) in the base case; (d) TPC for each product in the future cases. | ||
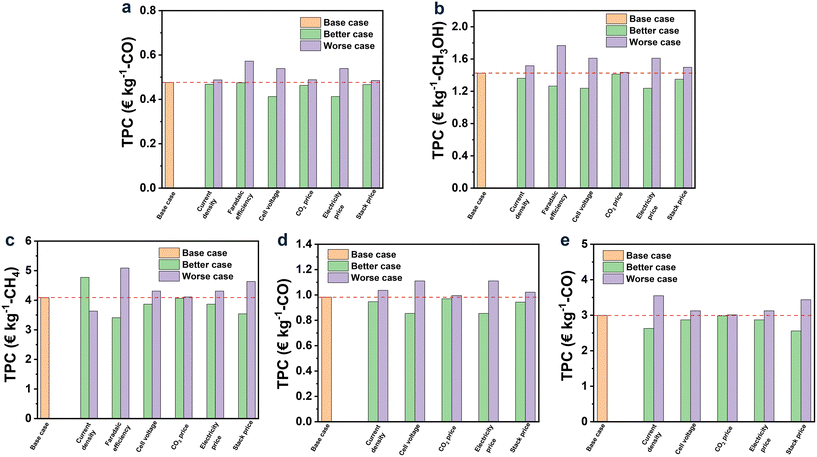
Among all the studied products, CO was found to be the only profitable product for CO2R with ILs as electrolytes at the present status. While for other products, their TPCs were still too high to be profitable, and especially for CH4 and H2/CO (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), their TPCs were 4.09 and 2.99 € per kg, respectively, far away from the market prices (0.18–0.35 and 0.03–0.54 € per kg, respectively). This observation was, on the one hand, in line with the achievement of CO2R, where the current density and FE for CO were already up to 182.2 mA cm−2 and 99.7%, respectively, while for CH4 and H2/CO (2
1), their TPCs were 4.09 and 2.99 € per kg, respectively, far away from the market prices (0.18–0.35 and 0.03–0.54 € per kg, respectively). This observation was, on the one hand, in line with the achievement of CO2R, where the current density and FE for CO were already up to 182.2 mA cm−2 and 99.7%, respectively, while for CH4 and H2/CO (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), their current densities were as low as 25.6 and 11.4 mA cm−2, respectively, together with low FE (Table 1). On the other hand, the relatively low market price of CH4 and H2/CO (2
1), their current densities were as low as 25.6 and 11.4 mA cm−2, respectively, together with low FE (Table 1). On the other hand, the relatively low market price of CH4 and H2/CO (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) can be another reason, which was further discussed later in this work.
1) can be another reason, which was further discussed later in this work.
To further analyze, the detailed capital cost is shown in Fig. 5b. As we can see, the TCC of CO was quite low, followed by H2/CO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and CH3OH, while those of CH4 and H2/CO (1
1) and CH3OH, while those of CH4 and H2/CO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) were much higher. Compared to CO, the BoP and stack costs for CH3OH, CH4, H2/CO (1
2) were much higher. Compared to CO, the BoP and stack costs for CH3OH, CH4, H2/CO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and H2/CO (2
1) and H2/CO (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were much higher, owing to the lower current density and thus higher electrolyzer area. The high costs of BoP and stack lead to high indirect costs. The summations of (indirect, BoP, and stack costs) for H2/CO (1
1) were much higher, owing to the lower current density and thus higher electrolyzer area. The high costs of BoP and stack lead to high indirect costs. The summations of (indirect, BoP, and stack costs) for H2/CO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), CH3OH, CH4, and H2/CO (2
1), CH3OH, CH4, and H2/CO (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) contribute to 67.6, 87.0, 91.7, and 91.8% in TCC, respectively, and this trend is opposite to that of their current densities in CO2R, further indicating the vital role of the current density on TCC. As the current densities for products other than CO are still low, it is important to further develop CO2R to improve the performance and then make it possible to achieve desirable results in the near future.
1) contribute to 67.6, 87.0, 91.7, and 91.8% in TCC, respectively, and this trend is opposite to that of their current densities in CO2R, further indicating the vital role of the current density on TCC. As the current densities for products other than CO are still low, it is important to further develop CO2R to improve the performance and then make it possible to achieve desirable results in the near future.
According to the detailed operating cost shown in Fig. 5c, the most intensive part was the electricity usage, which was closely linked to the cell voltage and FE. The yearly (operation and maintenance) cost was also the main expenditure for CH4 and H2/CO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) due to the high investment cost reflected by the direct capital cost. As to CH3OH, the capital cost for the distillation was also the obvious part due to the low concentration of CH3OH (30 wt%) in the electrolyte that requires high energy demand for its separation.134
1) due to the high investment cost reflected by the direct capital cost. As to CH3OH, the capital cost for the distillation was also the obvious part due to the low concentration of CH3OH (30 wt%) in the electrolyte that requires high energy demand for its separation.134
As discussed above, the performance of CO2R, including current density, cell voltage, and FE, had a great influence on TPC. This implied that the development status of CO2R was of importance in cost estimation. According to section 2, the development of CO2R was vital all these years, and performance improvement was rapid, making it essential to predict the cost based on the results that can be achieved in the near future. Hence, in this part, three future cases for each product, along with the improvement of current density and FE as well as the diminishing of cell voltage were assumed, according to the research results in recent years and those for the CO2R using electrolytes other than ILs.119,120 The results are shown in Fig. 5d.
Overall, TPCs evidently declined with the hypothetical augmented performance of CO2R. For CO, TPC was decreased to 0.32 € per kg, approaching half of the low limit market price (0.5 € per kg) when the current density was enhanced to 600 mA cm−2 and the cell voltage descended to 2 V, manifesting the perspective to set CO as the target product in CO2R. As for CH3OH, it can be profitable when the current density and FE reach 600 mA cm−2 and 99%, respectively, and the cell voltage drops to 1.5 V. For the syngas of H2/CO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), under the situation of case 3, its TPC would be lower to the price for that from the corn stover (0.54 € per kg). However, for CH4 and H2/CO (2
1), under the situation of case 3, its TPC would be lower to the price for that from the corn stover (0.54 € per kg). However, for CH4 and H2/CO (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), their TPCs were still much higher than the market price even though with a dramatic improvement in their performance of CO2R within the created scenarios.
1), their TPCs were still much higher than the market price even though with a dramatic improvement in their performance of CO2R within the created scenarios.
To find out how the CH4 and syngas (H2/CO (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)) could be profitable, the current density of 1000 mA cm−2 (the industrial performance of PEM for commercial water electrolysis),125,135 FE of 100%, and the cell voltage of 1.06 V for CH4 and 1.34 V for syngas (the standard cell voltage)136 were assumed, and the corresponding TPCs were estimated. As a result, the TPC of H2/CO (2
1)) could be profitable, the current density of 1000 mA cm−2 (the industrial performance of PEM for commercial water electrolysis),125,135 FE of 100%, and the cell voltage of 1.06 V for CH4 and 1.34 V for syngas (the standard cell voltage)136 were assumed, and the corresponding TPCs were estimated. As a result, the TPC of H2/CO (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) just reached the upper limit market price, but still, it remains unprofitable for CH4. This observation suggested that CH4 as the target product was an undesirable path of CO2R. There may be two reasons for the unprofitable of CO2R to CH4. One is the low market price, and the other is the highest electron demand (8e−) for the formation of CH4 among the different paths of CO2R to C1 products.9,38,89,95,96
1) just reached the upper limit market price, but still, it remains unprofitable for CH4. This observation suggested that CH4 as the target product was an undesirable path of CO2R. There may be two reasons for the unprofitable of CO2R to CH4. One is the low market price, and the other is the highest electron demand (8e−) for the formation of CH4 among the different paths of CO2R to C1 products.9,38,89,95,96
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and H2/CO (2
1) and H2/CO (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the target products, FE was not considered due to the fixed ratio.
1) as the target products, FE was not considered due to the fixed ratio.
In Fig. 6, the results of TPC for each product under the base, better and worse cases are exhibited via orange, green, and purple bars, respectively. Overall, FE was the most influential factor, while the effect of CO2 price can be negligible for all the products. The effect degrees of current density and cell voltage on the TPC of each product depended on the performance of CO2R itself. For example, when the current density improved by 20%, the TPCs of CO (the current density of 182.2 mA cm−2 in the base case) and syngas of H2/CO (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (the current density of 11.4 mA cm−2 in the base case) decreased by 1.6 and 12.3%, respectively. Similarly, when the cell voltage decreased by 20%, the TPCs of CO (the cell voltage of 3.29 V in the base case) and syngas of H2/CO (2
1) (the current density of 11.4 mA cm−2 in the base case) decreased by 1.6 and 12.3%, respectively. Similarly, when the cell voltage decreased by 20%, the TPCs of CO (the cell voltage of 3.29 V in the base case) and syngas of H2/CO (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (the cell voltage of 2.19 V in the base case) decreased by 13.3 and 4.2%, respectively. These results suggest that the slight change in the current density and cell voltage can lead to an obvious variation in TPCs when the performance of CO2R itself is relatively poor. Additionally, the 20% fluctuation in electricity and stack prices can cause an obvious change in TPC, as shown in Fig. 6 for all the studied products.
1) (the cell voltage of 2.19 V in the base case) decreased by 13.3 and 4.2%, respectively. These results suggest that the slight change in the current density and cell voltage can lead to an obvious variation in TPCs when the performance of CO2R itself is relatively poor. Additionally, the 20% fluctuation in electricity and stack prices can cause an obvious change in TPC, as shown in Fig. 6 for all the studied products.
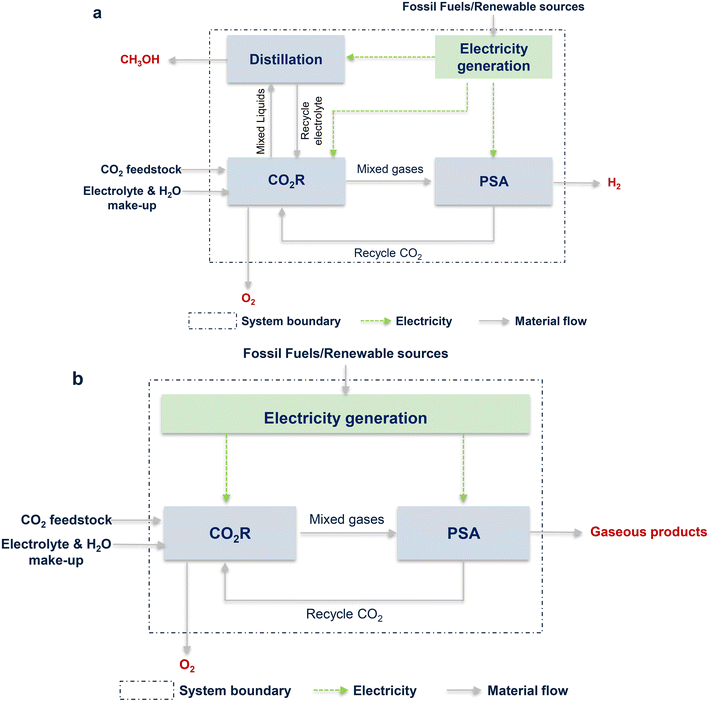
3.4. Environment assessment
The life cycle assessment (LCA) was used to assess the environmental impacts of “CO2R to product” based on the ISO 14040/14044 framework, and the global warming impact (GWI) was selected as the main indicator.134 Herein, the total amount of CO2 emitted from the CO2R process was evaluated via the cradle-to-gate LCA method to account for all sources of CO2 emissions in each unit. Finally, the amounts of CO2 emissions were obtained for the assessment. It should be noted that the environmental burdens of by-products (such as H2 and O2) were also considered. The system boundary is shown in Fig. 7. GWI was calculated based on the electricity generation intensity and electrical energy required in each unit. The calculation process is described in the ESI.†As described in the above paragraph, the calculation of GWI was based on electricity, i.e., LCA strongly depends on energy usage.137–139 Here the energy demands, including CO2R, BoP, and PSA units and the additional distillation unit for CH3OH, for producing one kg of CO, CH3OH, CH4, and syngas were calculated. The results are depicted in Fig. 8a. Obviously, the electricity usage of the CO2R unit was the major part for all the products, accounting for more than 80% when CO, CH4, or syngas was the product and more than 50% for CH3OH as the target product. Different from other products, the production of CH3OH requires separating the liquid product CH3OH from the electrolyte, and the corresponding energy demand was another main contributor. Comparatively, the energy usages for BoP and gas separation were insignificant for all the products.
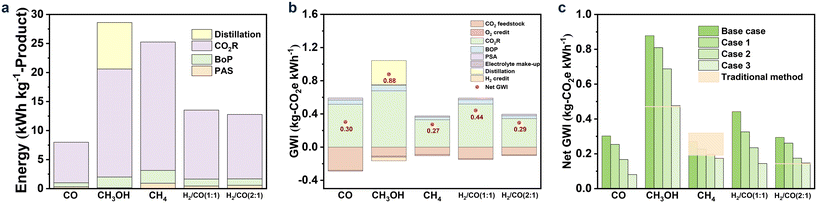 | ||
| Fig. 8 The demanded energy (a) and GWI breakdown (b) for each product under the base case; as well as net GWI (c) for each product under the base and future cases. | ||
Overall, the energy demand for producing one kg-CH3OH was higher than that of CO or syngas, which was ascribed to the additional energy usage from the distillation unit due to its low concentration (30 wt%).134 For CH4, the total energy usage was also higher than that for CO and syngas, and the corresponding energy demand of the CO2R unit was higher than that for CH3OH, which was attributed to its suboptimal performance, such as low current density, unsatisfactory FE, and high cell voltage.
The CO2 emissions per kW h, containing the CO2 footprint of all the co-product credits, energy usage, and raw materials, were calculated to appraise their GWI, thereby, their environmental impact, owing to its tight relationship with the global warming potential. Noteworthily, in this evaluation process, the GWI of CO2 feedstock was calculated based on the carbon footprint of −0.5 kg-CO2e per kg-captured-CO2 taken from the literature.140Fig. 8b shows the GWI breakdown for the five products in CO2 feedstock, CO2R, and separation under the base case, where the solid circle represents the net GWI. As indicated in Fig. 8b, the GWI for producing CH3OH was the highest (0.88 kg-CO2e per kW h) among the studied products, which was also higher than that of the coal-to-CH3OH process (0.47 kg-CO2e per kW h) due to the intensive energy usages for CO2R and liquid-separation. The GWI of CH4 was the lowest (0.27 kg-CO2e per kW h) among the five products, which was also comparable to that of the thermochemical CO2 conversion (0.19–0.32 kg-CO2e per kW h).134,141 This result suggested that CH4 was the most suitable product from the environment-friendly aspect. For the syngas of H2/CO (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the GWI (0.29 kg-CO2e per kW h) of the CO2R route was higher than that of the traditional method (0.14 kg-CO2e per kW h).142 As for CO and H2/CO (1
1), the GWI (0.29 kg-CO2e per kW h) of the CO2R route was higher than that of the traditional method (0.14 kg-CO2e per kW h).142 As for CO and H2/CO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), to the best of our knowledge, no GWI value has been reported. Since the GWI of CO (0.30 kg-CO2e per kW h) was similar to that of CH4 and syngas of H2/CO (2
1), to the best of our knowledge, no GWI value has been reported. Since the GWI of CO (0.30 kg-CO2e per kW h) was similar to that of CH4 and syngas of H2/CO (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), CO was a more attractive product than CH3OH and H2/CO (1
1), CO was a more attractive product than CH3OH and H2/CO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), indicating that the path of CO2R to CO was also beneficial for the environment.
1), indicating that the path of CO2R to CO was also beneficial for the environment.
For the future cases, with the hypothetically improved CO2R performance, the net GWI was distinctly decreased, as shown in Fig. 8c. For example, for CH4, when the FE and current density were increased to 99% and 600 mA cm−2, respectively, as well as the cell voltage was decreased to 1.2 V, the net GEI of CH4 was sharply declined from 0.27 to 0.17 kg-CO2e per kW h, which was lower than that of thermochemical CO2 conversion (0.19 kg-CO2e per kW h). When CH3OH and H2/CO (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were set as the target products, under the most desirable case (case 3), the net GWI of CO2R was almost comparable to the traditional method. The net GWIs of the CO2R to CO and H2/CO (1
1) were set as the target products, under the most desirable case (case 3), the net GWI of CO2R was almost comparable to the traditional method. The net GWIs of the CO2R to CO and H2/CO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were also greatly decreased from 0.3 and 0.44 kg-CO2e per kW h to 0.08 and 0.14 kg-CO2e per kW h, respectively. Therefore, CO2R is a promising path to reaching environment-friendly requirements.
1) were also greatly decreased from 0.3 and 0.44 kg-CO2e per kW h to 0.08 and 0.14 kg-CO2e per kW h, respectively. Therefore, CO2R is a promising path to reaching environment-friendly requirements.
Combining the environmental assessment with the economic analysis, CO2R to CO is a desirable pathway for CO2R, even in the current situation. In the future, with the further improvement of CO2R, CH3OH and H2/CO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) will be desirable routes. However, for CH4 and H2/CO (2
1) will be desirable routes. However, for CH4 and H2/CO (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the unfavorable economic results of CO2R make it challenging to compete with other routes. Adding H2 produced from other routes to H2/CO (1
1), the unfavorable economic results of CO2R make it challenging to compete with other routes. Adding H2 produced from other routes to H2/CO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and thus forming H2/CO (2
1) and thus forming H2/CO (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) can be an alternative way, which will be studied in our future work.
1) can be an alternative way, which will be studied in our future work.
This work summarized and evaluated the CO2R using IL-based electrolytes. There has been much research work conducted on other systems using electrolytes other than IL-based, and the performance can be better than that with ILs listed in this work. For example, for CO, the current density was over 300 mA cm−2.119,120 Such results further indicate the prospectives of CO2R and the reasonability of the assumed parameters for the future case. We expect that the development of CO2R, together with ILs, will greatly improve the performance in the foreseen future.
It should also be pointed out that for future cases (cases 1 to 3), in this work, only the parameters linked to the CO2R performance were adjusted, while in principle, other parameters, such as CO2 price, electricity, and product price also need to be considered. However, based on the sensitivity analysis, the CO2 price has a limited influence on the TPC for all studied products. Given the unstable variation tendency of electricity prices last year and other uncertainties linked to war and politics, it is difficult to make reasonable assumptions about electricity prices in the future. Similarly, reasonably assuming future product prices is another puzzle, which involves a deep knowledge of different industrial technologies. Therefore, it is reasonable to fix the parameters as the base case for the parameters of CO2 price, electricity, and product price in future cases.
Additionally, industrialization is mandatory for CO2R to achieve commercial value. The current density and product selectivity are required to be over 200 mA cm−2 and 90%, respectively, for industrial applications. This is in line with the results obtained from the section on economic analysis. Therefore, except for CO, the performance of CO2R to other products needs to be greatly improved. Besides, research on the stability of CO2R systems at the industrial level should be performed in future studies.143 Meanwhile, from the sensitivity analysis, the enhancement of CO2R performance, including current density, FE, and cell voltage, are the keys to reducing TPC, especially for CH3OH and syngas of H2/CO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). While it is difficult for CH4 and syngas H2/CO (2
1). While it is difficult for CH4 and syngas H2/CO (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to be profitable via only improving the performance of CO2R due to the low energy efficiency. Developing a novel electrolyzer might be a choice for CH4 to be profitable. As to syngas H2/CO (2
1) to be profitable via only improving the performance of CO2R due to the low energy efficiency. Developing a novel electrolyzer might be a choice for CH4 to be profitable. As to syngas H2/CO (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), integrating CO2R with commercial hydrogen production may maximize its economic benefits.
1), integrating CO2R with commercial hydrogen production may maximize its economic benefits.
4. Comparison of traditional and ionic liquids-based electrolytes
The electrolyte is a vital consideration apart from the extensively studied electrocatalysts to achieve the satisfactory performance of CO2R via controlling reduction surrounding at the interface between electrocatalyst and electrolyte, dissolving the reactant CO2, and stabilizing the reaction intermediates.10 Traditional electrolytes, including the aqueous of sodium or potassium salts and organic solvents (e.g., MeCN, DMF, DMSO, and acetone), have been widely used in CO2R. Potassium or sodium solutions have good electrical conductivity while a fairly limited ability to dissolve CO2 (<34 mM under the standard condition).144 Besides, the undesirable HER side reaction is easier to occur in these aqueous solutions. Normally, the solubility of CO2 in the organic solvent is higher than that in the aqueous electrolyte solutions. For example, the solubility of CO2 in MeCN (270 mM) is approximately 9 times as high as that in aqueous electrolyte solutions.145 However, the current density of CO2R with organic solvents is low, although they can effectively inhibit HER, which limits their further large-scale application.146 IL-based electrolytes with high CO2 solubility, as discussed above, not only have unique properties in promoting the performance of CO2R but also can be the activator of CO2 and stabilizer of CO2˙−, which have been increasingly studied and developed in these years, as summarized in this work and other review articles.13,26,42,46,67 Nevertheless, when IL-based electrolytes are used to replace traditional electrolytes, the economic (cost, stability) and environmental influence are usually questioned. Therefore, traditional and IL-based electrolytes were further analyzed and compared from economic and environmental aspects.The market price of traditional electrolytes is indeed lower than that of ILs, which is about 3400, 2876, and 205 € per ton to MeCN,147 DMF,148 and NaCO3,149 respectively, while it is 5800 € per ton to ILs.58 However, when CO is set as the target product of CO2R, the proportions of IL cost are as low as 0.3 and 0.02% in TCC and TOC, respectively. This situation is similar to the other products, suggesting that the market price of ILs will not hamper the use of ILs as electrolytes in CO2R. Additionally, the long-term stability of electrolytes is beneficial for cost saving. For industrialization, the stability of the CO2R system should reach at least 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h.143 Regrettably, there is no study on the stability of electrolytes at the industrial level. While CO2R with IL-based electrolytes shows comparable stability to that with traditional electrolytes in a large number of studies. Moreover, Yuan et al.150 found that the CO selectivity in the IL-based electrolytes is 51.3% higher than that in 0.1 M KHCO3 aqueous electrolytes after 10 h of continuous operation in an upscaling modified H-type cell, suggesting the higher stability of IL-based electrolytes. Therefore, IL-based electrolyte is not inferior to traditional electrolytes from the economic aspects.
000 h.143 Regrettably, there is no study on the stability of electrolytes at the industrial level. While CO2R with IL-based electrolytes shows comparable stability to that with traditional electrolytes in a large number of studies. Moreover, Yuan et al.150 found that the CO selectivity in the IL-based electrolytes is 51.3% higher than that in 0.1 M KHCO3 aqueous electrolytes after 10 h of continuous operation in an upscaling modified H-type cell, suggesting the higher stability of IL-based electrolytes. Therefore, IL-based electrolyte is not inferior to traditional electrolytes from the economic aspects.
As to the environment, ILs are considered “green solvents” because of their negligible volatility and flammability as well as high stability. However, the statement on “green solvents” for ILs has been questioned in recent years, because it lacks a comprehensive understanding of their toxicity and biodegradability. Given this, Costa and co-workers151 summarized and updated the relevant data on the toxicity and (bio)degradability of ILs. This work showed that the commonly used ILs with N-substitution cations or fluoride anions were resistant to biodegradation, which, however, can be improved through functionalizing the imidazole cations. Bystrzanowska et al.152 evaluated the greenness of more than 300 commercially available ILs and ranked them with organic solvents from the toxicity to organisms, biodegradability, hazard statements, and precautionary measures when handling them. As a result, the most commonly used ILs, including 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ([Emim][Tf2N]), [Bmim][BF4], and [Bmim]Br, were ranked between polar and nonpolar molecular solvents in terms of greenness. Comprehensively, ILs appear to be more resistant to biodegradation and toxicity than traditional electrolytes. Nevertheless, traditional electrolytes, such as DMF and MeCN, can cause direct harm to human beings through touch and breath.153 On the contrary, ILs have little impact on researchers, especially under normal pressure and temperature as those operated within CO2R. Moreover, ILs as the electrolyte in CO2R are usually used in small quantities and can be recycled. Therefore, devoting more efforts to designing and synthesizing novel effective ILs with low toxicity and high biodegradability as electrolytes are necessary as they will be meaningful for the future development of CO2R considering the unique superiority of ILs in promoting the performance of CO2R.
5. Conclusions
An instant literature survey on the CO2R to C1 products (CO, CH3OH, CH4, and syngas) with ILs-based electrolytes was conducted summarizing the most recent results from the past two years, and the best performance for each target product was identified. The literature survey shows that CO2R to CO was mostly studied in the recent two years, and desirable performance has been achieved (FE of 99.7% and current density of 182.2 mA cm−2); for CO2R to other studied C1 products, much fewer studies were conducted, and the performance needs to be further improved.The economic analysis and environmental assessment of CO2R to C1-product were estimated, where both the parameters of the state-of-the-art (base case) and future cases with improved performance (cases 1 to 3) were used. Currently, CO is the only profitable product, in the future, CH3OH and the syngas of H2/CO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) will achieve profitability, but CH4 and the syngas of H2/CO (2
1) will achieve profitability, but CH4 and the syngas of H2/CO (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) will always be unprofitable. For the environmental impact, CH4 is the most environmentally friendly product from CO2R, followed by the syngas of H2/CO (2
1) will always be unprofitable. For the environmental impact, CH4 is the most environmentally friendly product from CO2R, followed by the syngas of H2/CO (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and CO, and then CH3OH, and the desirable CO2R performance will make CO2R to C1-products an environmentally friendly pathway. Overall, CO2R-to-CO is the most profitable path considering both economic and environmental aspects.
1) and CO, and then CH3OH, and the desirable CO2R performance will make CO2R to C1-products an environmentally friendly pathway. Overall, CO2R-to-CO is the most profitable path considering both economic and environmental aspects.
Summarily, for CO2R with IL-based electrolytes, the pathway of CO2R to CO has shown commercial potential based on the state-of-the-art achievement at the laboratory level from both economic and environmental aspects. For the other products, more efforts are needed to be implemented to improve the CO2R performance or develop more advanced electrolyzers (e.g., GDE-type electrolyzer, membrane electrode assembly-type electrolyzer, microfluidic-type electrolyzer, and solid-state electrolyte-type electrolyzer). Furthermore, ILs should be further exploited in future CO2R as follows: (1) the adjustable feature of ILs in the structure and properties provides unique advantages and feasibilities for designing more efficient and suitable electrolytes of CO2R; (2) the capability of ILs to dissolve a variety of solvents and electrolytes can integrate other solvents and electrolytes, further improving the performance of CO2R; (3) the cleaner ILs can be designed and synthesized applying into CO2R to mitigate the environmental burden; (4) except as electrolytes, ILs can also be the co-catalyst or modifier for the catalyst exhibiting prominent performance.
Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was financially supported by the Swedish Energy Agency (51239-1 or P2020-90066). AL acknowledges the financial support from the Swedish Research Council, from Kempe Foundation, and from European Union's Horizon Europe research and innovation programme under grant agreement No. 101086667, project BioMat4CAST (BioMat4CAST – “Petru Poni” Institute of Macromolecular Chemistry Multi-Scale in silico Laboratory for Complex and Smart Biomaterials).References
- J. Lelieveld, K. Klingmuller, A. Pozzer, R. T. Burnett, A. Haines and V. Ramanathan, Effects of fossil fuel and total anthropogenic emission removal on public health and climate, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 7192–7197 CrossRef CAS PubMed.
- M. M. F. Hasan, L. M. Rossi, D. P. Debecker, K. C. Leonard, Z. Li, B. C. E. Makhubela, C. Zhao and A. Kleij, Can CO2 and renewable carbon be primary resources for sustainable fuels and chemicals?, ACS Sustainable Chem. Eng., 2021, 9, 12427–12430 CrossRef CAS.
- M. Takht Ravanchi and S. Sahebdelfar, Catalytic conversions of CO2 to help mitigate climate change: Recent process developments, Process Saf. Environ. Prot., 2021, 145, 172–194 CrossRef CAS.
- L. Desport and S. Selosse, An overview of CO2 capture and utilization in energy models, Resour., Conserv. Recycl., 2022, 180, 106150 CrossRef CAS.
- Z. Li, T. Yang, S. Yuan, Y. Yin, E. J. Devid, Q. Huang, D. Auerbach and A. W. Kleyn, Boudouard reaction driven by thermal plasma for efficient CO2 conversion and energy storage, J. Energy Chem., 2020, 45, 128–134 CrossRef.
- S. R. Sun, H. X. Wang, D. H. Mei, X. Tu and A. Bogaerts, CO2 conversion in a gliding arc plasma: Performance improvement based on chemical reaction modeling, J. CO2 Util., 2017, 17, 220–234 CrossRef CAS.
- S. Cantera, D. Tamarit, P. J. Strong, I. Sánchez-Andrea, T. J. G. Ettema and D. Z. Sousa, Prospective CO2 and CO bioconversion into ectoines using novel microbial platforms, Rev. Environ. Sci. Bio/Technol., 2022, 21, 571–581 CrossRef CAS.
- D. D. Ma, S. G. Han, C. Cao, X. Li, X. T. Wu and Q. L. Zhu, Remarkable electrocatalytic CO2 reduction with ultrahigh CO/H2 ratio over single-molecularly immobilized pyrrolidinonyl nickel phthalocyanine, Appl. Catal., B, 2020, 264, 118530 CrossRef.
- T. Zhang, W. Li, K. Huang, H. Guo, Z. Li, Y. Fang, R. M. Yadav, V. Shanov, P. M. Ajayan, L. Wang, C. Lian and J. Wu, Regulation of functional groups on graphene quantum dots directs selective CO2 to CH4 conversion, Nat. Commun., 2021, 12, 5265 CrossRef CAS PubMed.
- M. Mourade Salles Pupo and R. Kortlever, Electrolyte effects on the electrochemical reduction of CO2, ChemPhysChem, 2019, 20, 2926–2935 CrossRef CAS PubMed.
- G. Tang, J. Li, Y. Lu, T. Song, S. Yin, G. Mao, B. Long, A. Ali and G. J. Deng, Donor-acceptor organic polymer with sulfur bridge for superior photocatalytic CO2 reduction to CH4 under visible light illumination, Chem. Eng. J., 2023, 451, 138744 CrossRef CAS.
- A. Pan, X. Ma, S. Huang, Y. Wu, M. Jia, Y. Shi, Y. Liu, P. H. Wangyang, L. He and Y. Liu, CsPbBr3 perovskite nanocrystal grown on mxene nanosheets for enhanced photoelectric detection and photocatalytic CO2 reduction, J. Phys. Chem. Lett., 2019, 10, 6590–6597 CrossRef CAS PubMed.
- Q. Lu and F. Jiao, Electrochemical CO2 reduction: Electrocatalyst, reaction mechanism, and process engineering, Nano Energy, 2016, 29, 439–456 CrossRef CAS.
- B. V. Mathiesen, H. Lund and K. Karlsson, 100% renewable energy systems, climate mitigation and economic growth, Appl. Energy, 2011, 88, 488–501 CrossRef.
- N. L. Panwar, S. C. Kaushik and S. Kothari, Role of renewable energy sources in environmental protection: A review, Renewable Sustainable Energy Rev., 2011, 15, 1513–1524 CrossRef.
- W. Lai, Y. Qiao, J. Zhang, Z. Lin and H. Huang, Design strategies for markedly enhancing energy efficiency in the electrocatalytic CO2 reduction reaction, Energy Environ. Sci., 2022, 15, 3603–3629 RSC.
- H. Kim, H. S. Jeon, M. S. Jee, E. B. Nursanto, J. P. Singh, K. Chae, Y. J. Hwang and B. K. Min, Contributors to enhanced CO2 electroreduction activity and stability in a nanostructured Au electrocatalyst, ChemSusChem, 2016, 9, 2097–2102 CrossRef CAS PubMed.
- S. Zhao, Z. Tang, S. Guo, M. Han, C. Zhu, Y. Zhou, L. Bai, J. Gao, H. Huang, Y. Li, Y. Liu and Z. Kang, Enhanced activity for CO2 electroreduction on a highly active and stable ternary Au-CDots-C3N4 electrocatalyst, ACS Catal., 2017, 8, 188–197 CrossRef.
- Y. S. Ham, S. Choe, M. J. Kim, T. Lim, S. K. Kim and J. J. Kim, Electrodeposited Ag catalysts for the electrochemical reduction of CO2 to CO, Appl. Catal., B, 2017, 208, 35–43 CrossRef CAS.
- C. Kim, T. Eom, M. S. Jee, H. Jung, H. Kim, B. K. Min and Y. J. Hwang, Insight into electrochemical CO2 reduction on surface-molecule-mediated Ag nanoparticles, ACS Catal., 2016, 7, 779–785 CrossRef.
- C. Jiménez, J. García, R. Camarillo, F. Martínez and J. Rincón, Electrochemical CO2 reduction to fuels using Pt/CNT catalysts synthesized in supercritical medium, Energy Fuels, 2017, 31, 3038–3046 CrossRef.
- M. Ma, H. A. Hansen, M. Valenti, Z. Wang, A. Cao, M. Dong and W. A. Smith, Electrochemical reduction of CO2 on compositionally variant Au-Pt bimetallic thin films, Nano Energy, 2017, 42, 51–57 CrossRef CAS.
- T. Ilyas, F. Raziq, S. Ali, A. Zada, N. Ilyas, R. Shaha, Y. Wang and L. Qiao, Facile synthesis of MoS2/Cu as trifunctional catalyst for electrochemical overall water splitting and photocatalytic CO2 conversion, Mater. Des., 2021, 204, 109674 CrossRef CAS.
- N. Hussain, M. A. Abdelkareem, H. Alawadhi, A. H. Alami and K. Elsaid, Cu2O nanoparticles decorated with MoS2 sheets for electrochemical reduction of CO2 with enhanced efficiency, Appl. Phys. A: Mater. Sci. Process., 2022, 128, 131 CrossRef CAS.
- J. Ye, D. Rao and X. Yan, Regulating the electronic properties of MoSe2 to improve its CO2 electrocatalytic reduction performance via atomic doping, New J. Chem., 2021, 45, 5350–5356 RSC.
- C. Hiragond, H. Kim, J. Lee, S. Sorcar, C. Erkey and S. I. In, Electrochemical CO2 reduction to CO catalyzed by 2D nanostructures, Catalysts, 2020, 10, 98 CrossRef CAS.
- X. Yang, J. Cheng, X. Yang, Y. Xu, W. Sun and J. Zhou, MOF-derived Cu@Cu2O heterogeneous electrocatalyst with moderate intermediates adsorption for highly selective reduction of CO2 to methanol, Chem. Eng. J., 2022, 431, 134171 CrossRef CAS.
- P. Han, X. Yu, D. Yuan, M. Kuang, Y. Wang, A. M. Al-Enizi and G. Zheng, Defective graphene for electrocatalytic CO2 reduction, J. Colloid Interface Sci., 2019, 534, 332–337 CrossRef CAS PubMed.
- R. A. Geioushy, M. M. Khaled, K. Alhooshani, A. S. Hakeem and A. Rinaldi, Graphene/ZnO/Cu2O electrocatalyst for selective conversion of CO2 into n-propanol, Electrochim. Acta, 2017, 245, 456–462 CrossRef CAS.
- C. Zhang, S. Yang, J. Wu, M. Liu, S. Yazdi, M. Ren, J. Sha, J. Zhong, K. Nie, A. S. Jalilov, Z. Li, H. Li, B. I. Yakobson, Q. Wu, E. Ringe, H. Xu, P. M. Ajayan and J. M. Tour, Electrochemical CO2 reduction with atomic iron-dispersed on nitrogen-doped graphene, Adv. Energy Mater., 2018, 8, 1703487 CrossRef.
- M. Li, H. Wang, W. Luo, P. C. Sherrell, J. Chen and J. Yang, Heterogeneous single-atom catalysts for electrochemical CO2 reduction reaction, Adv. Mater., 2020, 32, e2001848 CrossRef PubMed.
- D. Grammatico, A. J. Bagnall, L. Riccardi, M. Fontecave, B. L. Su and L. Billon, Heterogenised molecular catalysts for sustainable electrochemical CO2 reduction, Angew. Chem., Int. Ed., 2022, 61, e202206399 CrossRef CAS PubMed.
- L. Zhang, X. X. Li, Z. L. Lang, Y. Liu, J. Liu, L. Yuan, W. Y. Lu, Y. S. Xia, L. Z. Dong, D. Q. Yuan and Y. Q. Lan, Enhanced cuprophilic interactions in crystalline catalysts facilitate the highly selective electroreduction of CO2 to CH4, J. Am. Chem. Soc., 2021, 143, 3808–3816 CrossRef CAS PubMed.
- A. Gawel, T. Jaster, D. Siegmund, J. Holzmann, H. Lohmann, E. Klemm and U. P. Apfel, Electrochemical CO2 reduction - the macroscopic world of electrode design, reactor concepts & economic aspects, iScience, 2022, 25, 104011 CrossRef CAS PubMed.
- S. Nitopi, E. Bertheussen, S. B. Scott, X. Liu, A. K. Engstfeld, S. Horch, B. Seger, I. E. L. Stephens, K. Chan, C. Hahn, J. K. Norskov, T. F. Jaramillo and I. Chorkendorff, Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte, Chem. Rev., 2019, 119, 7610–7672 CrossRef CAS PubMed.
- M. Konig, J. Vaes, E. Klemm and D. Pant, Solvents and supporting electrolytes in the electrocatalytic reduction of CO2, iScience, 2019, 19, 135–160 CrossRef PubMed.
- W. Xi, R. Ma, H. Wang, Z. Gao, W. Zhang and Y. Zhao, Ultrathin ag nanowires electrode for electrochemical syngas production from carbon dioxide, ACS Sustainable Chem. Eng., 2018, 6, 7687–7694 CrossRef CAS.
- E. P. Delmo, Y. Wang, J. Wang, S. Zhu, T. Li, X. Qin, Y. Tian, Q. Zhao, J. Jang, Y. Wang, M. Gu, L. Zhang and M. Shao, Metal organic framework-ionic liquid hybrid catalysts for the selective electrochemical reduction of CO2 to CH4, Chin. J. Catal., 2022, 43, 1687–1696 CrossRef CAS.
- M. Sun, Z. Bian, W. Cui, X. Zhao, S. Dong, X. Ke, Y. Zhou and J. Wang, Pyrolyzing soft template-containing poly(ionic liquid) into hierarchical n-doped porous carbon for electroreduction of carbon dioxide, Chin. J. Chem. Eng., 2022, 43, 192–201 CrossRef.
- H. Dong, M. Lu, Y. Wang, H. L. Tang, D. Wu, X. Sun and F. M. Zhang, Covalently anchoring covalent organic framework on carbon nanotubes for highly efficient electrocatalytic CO2 reduction, Appl. Catal., B, 2022, 303, 120897 CrossRef CAS.
- D. Faggion Jr., W. D. G. Goncalves and J. Dupont, CO2 electroreduction in ionic liquids, Front. Chem., 2019, 7, 102 CrossRef PubMed.
- J. J. Kaczur, H. Yang, Z. Liu, S. D. Sajjad and R. I. Masel, A review of the use of immobilized ionic liquids in the electrochemical conversion of CO2, Carbon, 2020, 6, 33 CAS.
- S. Mena and G. Guirado, Electrochemical tuning of CO2 reactivity in ionic liquids using different cathodes: From oxalate to carboxylation products, Carbon, 2020, 6, 34 CAS.
- X. Tan, X. Sun and B. Han, Ionic liquid-based electrolytes for CO2 electroreduction and CO2 electroorganic transformation, Natl. Sci. Rev., 2022, 9, nwab022 CrossRef PubMed.
- K. K. Maniam and S. Paul, Ionic liquids and deep eutectic solvents for CO2 conversion technologies-a review, Materials, 2021, 14, 4519 CrossRef CAS PubMed.
- S. A. S. Mohammed, W. Z. N. Yahya, M. A. Bustam and M. G. Kibria, Elucidation of the roles of ionic liquid in CO2 electrochemical reduction to value-added chemicals and fuels, Molecules, 2021, 26, 6962 CrossRef CAS PubMed.
- Y. Huang, G. Cui, Y. Zhao, H. Wang, Z. Li, S. Dai and J. Wang, Preorganization and cooperation for highly efficient and reversible capture of low-concentration CO2 by ionic liquids, Angew. Chem., Int. Ed., 2017, 56, 13293–13297 CrossRef CAS PubMed.
- Y. Huang, G. Cui, H. Wang, Z. Li and J. Wang, Tuning ionic liquids with imide-based anions for highly efficient CO2 capture through enhanced cooperations, J. CO2 Util., 2018, 28, 299–305 CrossRef CAS.
- H. Wang, S. Zhang, J. Wang and Z. Yu, Standard partial molar volumes and viscosity B-coefficients of ionic liquids [Cnmim]Br (n=4, 6, 8) in alcohols at 298.15k, J. Mol. Liq., 2015, 209, 563–568 CrossRef CAS.
- B. A. Rosen, A. Salehi-Khojin, M. R. Thorson, W. Zhu, D. T. Whipple, P. J. A. Kenis and R. I. Masel, Ionic liquid mediated selective conversion of CO2 to CO at low overpotentials, Science, 2011, 334, 643–644 CrossRef CAS PubMed.
- B. Kumar, M. Asadi, D. Pisasale, S. Sinha-Ray, B. A. Rosen, R. Haasch, J. Abiade, A. L. Yarin and A. Salehi-Khojin, Renewable and metal-free carbon nanofibre catalysts for carbon dioxide reduction, Nat. Commun., 2013, 4, 2819 CrossRef.
- J. Choi, T. M. Benedetti, R. Jalili, A. Walker, G. G. Wallace and D. L. Officer, High performance Fe porphyrin/ionic liquid co-catalyst for electrochemical CO2 reduction, Chemistry, 2016, 22, 14158–14161 CrossRef CAS PubMed.
- Z. Min, B. Chang, C. Shao, X. Su, N. Wang, Z. Li, H. Wang, Y. Zhao, M. Fan and J. Wang, Enhancing CO2 electroreduction to syngas by active protons of imidazolium ionic liquids: From performance to mechanism, Appl. Catal., 2022 DOI:10.1016/j.apcatb.2022.122185.
- O. Kuntyi, G. Zozulya, M. Shepida and S. M. Soltani, CO2 electroreduction in organic aprotic solvents: A mini review, J. Chem., 2022, 2022, 1–12 CrossRef.
- S. Jin, Z. Hao, K. Zhang, Z. Yan and J. Chen, Advances and challenges for the electrochemical reduction of CO2 to CO: From fundamentals to industrialization, Angew. Chem., Int. Ed., 2021, 60, 20627–20648 CrossRef CAS PubMed.
- X. Liu, B. Q. Li, B. Ni, L. Wang and H. J. Peng, A perspective on the electrocatalytic conversion of carbon dioxide to methanol with metallomacrocyclic catalysts, J. Energy Chem., 2022, 64, 263–275 CrossRef CAS.
- T. Shi, D. Sridhar, L. Zeng and A. Chen, Recent advances in catalyst design for the electrochemical and photoelectrochemical conversion of methane to value-added products, Electrochem. Commun., 2022, 135, 107220 CrossRef CAS.
- F. Chang, G. Zhan, Z. Wu, Y. Duan, S. Shi, S. Zeng, X. Zhang and S. Zhang, Technoeconomic analysis and process design for CO2 electroreduction to co in ionic liquid electrolyte, ACS Sustainable Chem. Eng., 2021, 9, 9045–9052 CrossRef CAS.
- M. Rumayor, A. Dominguez-Ramos, P. Perez and A. Irabien, A techno-economic evaluation approach to the electrochemical reduction of CO2 for formic acid manufacture, J. CO2 Util., 2019, 34, 490–499 CrossRef CAS.
- M. Jouny, W. Luc and F. Jiao, General techno-economic analysis of CO2 electrolysis systems, Ind. Eng. Chem. Res., 2018, 57, 2165–2177 CrossRef CAS.
- D. Yang, Q. Zhu and B. Han, Electroreduction of CO2 in ionic liquid-based electrolytes, Innovation, 2020, 1, 100016 Search PubMed.
- S. Sharifi Golru and E. J. Biddinger, Effect of additives in aqueous electrolytes on CO2 electroreduction, Chem. Eng. J., 2022, 428, 131303 CrossRef CAS.
- J. M. Vadillo, G. Diaz-Sainz, L. Gomez-Coma, A. Garea and A. Irabien, Chemical and physical ionic liquids in CO2 capture system using membrane vacuum regeneration, Membranes, 2022, 12, 785 CrossRef CAS PubMed.
- C. Ding, A. Li, S. M. Lu, H. Zhang and C. Li, In situ electrodeposited indium nanocrystals for efficient CO2 reduction to CO with low overpotential, ACS Catal., 2016, 6, 6438–6443 CrossRef CAS.
- C. Du, P. Lu and N. Tsubaki, Efficient and new production methods of chemicals and liquid fuels by carbon monoxide hydrogenation, ACS Omega, 2020, 5, 49–56 CrossRef CAS PubMed.
- G. P. Lau, M. Schreier, D. Vasilyev, R. Scopelliti, M. Gratzel and P. J. Dyson, New insights into the role of imidazolium-based promoters for the electroreduction of CO2 on a silver electrode, J. Am. Chem. Soc., 2016, 138, 7820–7823 CrossRef CAS PubMed.
- F. Li, F. Mocci, X. Zhang, X. Ji and A. Laaksonen, Ionic liquids for CO2 electrochemical reduction, Chin. J. Chem. Eng., 2021, 31, 75–93 CrossRef CAS.
- F. Liang, J. Zhang, Z. Hu, C. Ma, W. Ni, Y. Zhang and S. Zhang, Intrinsic defect-rich graphene coupled cobalt phthalocyanine for robust electrochemical reduction of carbon dioxide, ACS Appl. Mater. Interfaces, 2021, 13, 25523–25532 CrossRef CAS PubMed.
- T. Oguma and K. Azumi, Improvement of electrochemical reduction of CO2 using the potential-pulse polarization method, Electrochemistry, 2020, 88, 451–456 CrossRef CAS.
- W. Guo, J. Bi, Q. Zhu, J. Ma, G. Yang, H. Wu, X. Sun and B. Han, Highly selective CO2 electroreduction to CO on Cu–Co bimetallic catalysts, ACS Sustainable Chem. Eng., 2020, 8, 12561–12567 CrossRef CAS.
- R. Zhang, J. Yang, X. Zhao, H. Yang, H. Li, B. Ji, G. Zhou, X. Ma and D. Yang, Electrochemical deposited zeolitic imidazolate frameworks as an efficient electrocatalyst for CO2 electrocatalytic reduction, ChemCatChem, 2022, 14, e202101653 CAS.
- T. Kunene, A. Atifi and J. Rosenthal, Selective CO2 reduction over rose's metal in the presence of an imidazolium ionic liquid electrolyte, ACS Appl. Energy Mater., 2019, 3, 4193–4200 CrossRef.
- I. Ganesh, BMIM-BF4 RTIL: Synthesis, characterization and performance evaluation for electrochemical CO2 reduction to CO over Sn and MoSi2 cathodes, Carbon, 2020, 6, 47 CAS.
- S. Yu, A. J. Wilson, G. Kumari, X. Zhang and P. K. Jain, Opportunities and challenges of solar-energy-driven carbon dioxide to fuel conversion with plasmonic catalysts, ACS Energy Lett., 2017, 2, 2058–2070 CrossRef CAS.
- F. Ju, J. Zhang and W. Lu, Efficient electrochemical reduction of CO2 to CO in ionic liquid/propylene carbonate electrolyte on ag electrode, Catalysts, 2020, 10, 1102 CrossRef CAS.
- Y. Hu, J. Feng, X. Zhang, H. Gao, S. Jin, L. Liu and W. Shen, Efficient electrochemical reduction of CO2 to CO in ionic liquids, ChemistrySelect, 2021, 6, 9873–9879 CrossRef CAS.
- B. Ratschmeier and B. Braunschweig, Cations of ionic liquid electrolytes can act as a promoter for CO2 electrocatalysis through reactive intermediates and electrostatic stabilization, J. Phys. Chem. C, 2021, 125, 16498–16507 CrossRef CAS.
- H. Wang, D. Yang, J. Yang, X. Ma, H. Li, W. Dong, R. Zhang and C. Feng, Efficient electroreduction of CO2 to CO on porous ZnO nanosheets with hydroxyl groups in ionic liquid-based electrolytes, ChemCatChem, 2021, 13, 2570–2576 CrossRef.
- A. V. Rudnev, K. Kiran and P. Broekmann, Specific cation adsorption: Exploring synergistic effects on CO2 electroreduction in ionic liquids, ChemElectroChem, 2020, 7, 1897–1903 CrossRef CAS.
- M. Zeng, Y. Liu, Y. Hu and X. Zhang, High-efficient CO2 electrocatalysis over nanoporous Au film enabled by a combined pore engineering and ionic liquid-mediated approach, Chem. Eng. J., 2021, 425, 131663 CrossRef CAS.
- W. Guo, X. Tan, J. Bi, L. Xu, D. Yang, C. Chen, Q. Zhu, J. Ma, A. Tayal, J. Ma, Y. Huang, X. Sun, S. Liu and B. Han, Atomic indium catalysts for switching CO2 electroreduction products from formate to CO, J. Am. Chem. Soc., 2021, 143, 6877–6885 CrossRef CAS PubMed.
- Y. Wu, C. Chen, X. Yan, X. Sun, Q. Zhu, P. Li, Y. Li, S. Liu, J. Ma, Y. Huang and B. Han, Boosting CO2 electroreduction over a cadmium single-atom catalyst by tuning of the axial coordination structure, Angew. Chem., Int. Ed., 2021, 60, 20803–20810 CrossRef CAS PubMed.
- G. A. Olah, Beyond oil and gas: The methanol economy, Angew. Chem., Int. Ed., 2005, 44, 2636–2639 CrossRef CAS PubMed.
- X. Sun, Q. Zhu, X. Kang, H. Liu, Q. Qian, Z. Zhang and B. Han, Molybdenum-bismuth bimetallic chalcogenide nanosheets for highly efficient electrocatalytic reduction of carbon dioxide to methanol, Angew. Chem., Int. Ed., 2016, 55, 6771–6775 CrossRef CAS PubMed.
- D. X. Yang, Q. G. Zhu, C. J. Chen, H. Z. Liu, Z. M. Liu, Z. J. Zhao, X. Y. Zhang, S. J. Liu and B. X. Han, Selective electroreduction of carbon dioxide to methanol on copper selenide nanocatalysts, Nat. Commun., 2019, 10, 677 CrossRef CAS PubMed.
- L. Lu, X. Sun, J. Ma, D. Yang, H. Wu, B. Zhang, J. Zhang and B. Han, Highly efficient electroreduction of CO2 to methanol on palladium-copper bimetallic aerogels, Angew. Chem., Int. Ed., 2018, 57, 14149–14153 CrossRef CAS PubMed.
- W. Guo, S. Liu, X. Tan, R. Wu, X. Yan, C. Chen, Q. Zhu, L. Zheng, J. Ma, J. Zhang, Y. Huang, X. Sun and B. Han, Highly efficient CO2 electroreduction to methanol through atomically dispersed Sn coupled with defective CuO catalysts, Angew. Chem., Int. Ed., 2021, 60, 21979–21987 CrossRef CAS PubMed.
- P. Li, J. Bi, J. Liu, Q. Zhu, C. Chen, X. Sun, J. Zhang and B. Han, In situ dual doping for constructing efficient CO2-to-methanol electrocatalysts, Nat. Commun., 2022, 13, 1965 CrossRef CAS PubMed.
- T. Sheng and S. G. Sun, Identifying the significance of proton-electron transfer in CH4 production on Cu (100) in CO2 electro-reduction, J. Electroanal. Chem., 2017, 793, 184–187 CrossRef CAS.
- H. Pan and C. J. Barile, Electrochemical CO2 reduction to methane with remarkably high faradaic efficiency in the presence of a proton permeable membrane, Energy Environ. Sci., 2020, 13, 3567–3578 RSC.
- S. Chen, B. Wang, J. Zhu, L. Wang, H. Ou, Z. Zhang, X. Liang, L. Zheng, L. Zhou, Y. Q. Su, D. Wang and Y. Li, Lewis acid site-promoted single-atomic Cu catalyzes electrochemical CO2 methanation, Nano Lett., 2021, 21, 7325–7331 CrossRef CAS PubMed.
- Y. Wu, C. Chen, X. Yan, R. Wu, S. Liu, J. Ma, J. Zhang, Z. Liu, X. Xing, Z. Wu and B. Han, Enhancing CO2 electroreduction to CH4 over Cu nanoparticles supported on n-doped carbon, Chem. Sci., 2022, 13, 8388–8394 RSC.
- Y. Wang, Z. Chen, P. Han, Y. Du, Z. Gu, X. Xu and G. Zheng, Single-atomic Cu with multiple oxygen vacancies on ceria for electrocatalytic CO2 reduction to CH4, ACS Catal., 2018, 8, 7113–7119 CrossRef CAS.
- X. Kang, Q. Zhu, X. Sun, J. Hu, J. Zhang, Z. Liu and B. Han, Highly efficient electrochemical reduction of CO2 to CH4 in an ionic liquid using a metal-organic framework cathode, Chem. Sci., 2016, 7, 266–273 RSC.
- X. Sun, X. Kang, Q. Zhu, J. Ma, G. Yang, Z. Liu and B. Han, Very highly efficient reduction of CO2 to CH4 using metal-free n-doped carbon electrodes, Chem. Sci., 2016, 7, 2883–2887 RSC.
- X. Liu, H. Yang, J. He, H. Liu, L. Song, L. Li and J. Luo, Highly active, durable ultrathin MoTe2 layers for the electroreduction of CO2 to CH4, Small, 2018, 14, e1704049 CrossRef PubMed.
- L. Wang, Z. Luo, S. Feng, J. Ou, S. Luo, K. Yan and C. Wu, Synthesis of MOF-derived hybrids for efficient electrocatalytic reduction of CO2 to syngas, Catal. Lett., 2022 DOI:10.1007/s10562-022-04089-x.
- R. Yun, B. Zhang, F. Zhan, Z. Xin, T. Sheng and Z. Shi, Electrocatalysis CO2 to tunable syngas upon Fe clusters catalyst dispersed on bamboo-like NCT, Inorg. Chem., 2022, 61, 9375–9380 CrossRef CAS PubMed.
- J. Xiong, Syngas production via carbon dioxide electroreduction over Cds nanorods, Int. J. Electrochem. Sci., 2021, 16, 210369 CrossRef CAS.
- F. Marques Mota, D. L. T. Nguyen, J. E. Lee, H. Piao, J. H. Choy, Y. J. Hwang and D. H. Kim, Toward an effective control of the H2 to CO ratio of syngas through CO2 electroreduction over immobilized gold nanoparticles on layered titanate nanosheets, ACS Catal., 2018, 8, 4364–4374 CrossRef CAS.
- H. Xie, S. Chen, F. Ma, J. Liang, Z. Miao, T. Wang, H. L. Wang, Y. Huang and Q. Li, Boosting tunable syngas formation via electrochemical CO2 reduction on Cu/In2O3 core/shell nanoparticles, ACS Appl. Mater. Interfaces, 2018, 10, 36996–37004 CrossRef CAS PubMed.
- I. Hjorth, M. Nord, M. Rønning, J. Yang and D. Chen, Electrochemical reduction of CO2 to synthesis gas on CNT supported CuxZn1-x O catalysts, Catal. Today, 2020, 357, 311–321 CrossRef CAS.
- A. S. Varela, N. Ranjbar Sahraie, J. Steinberg, W. Ju, H. S. Oh and P. Strasser, Metal-doped nitrogenated carbon as an efficient catalyst for direct CO2 electroreduction to CO and hydrocarbons, Angew. Chem., Int. Ed., 2015, 54, 10758–10762 CrossRef CAS PubMed.
- S. Y. Zhang, Y. Y. Yang, Y. Q. Zheng and H. L. Zhu, Ag-doped Co3O4 catalyst derived from heterometallic MOF for syngas production by electrocatalytic reduction of CO2 in water, J. Solid State Chem., 2018, 263, 44–51 CrossRef CAS.
- N. Meng, C. Liu, Y. Liu, Y. Yu and B. Zhang, Efficient electrosynthesis of syngas with tunable CO/H2 ratios over Znx Cd1-xS-amine inorganic-organic hybrids, Angew. Chem., Int. Ed., 2019, 58, 18908–18912 CrossRef CAS PubMed.
- M. Beheshti, S. Kakooei, M. C. Ismail and S. Shahrestani, Investigation of CO2 electrochemical reduction to syngas on Zn/Ni-based electrocatalysts using the cyclic voltammetry method, Electrochim. Acta, 2020, 341, 135976 CrossRef CAS.
- A. Goeppert, M. Czaun, J. P. Jones, G. K. Surya Prakash and G. A. Olah, Recycling of carbon dioxide to methanol and derived products - closing the loop, Chem. Soc. Rev., 2014, 43, 7995–8048 RSC.
- M. E. Dry, The fischer–tropsch process: 1950–2000, Catal. Today, 2002, 71, 227–241 CrossRef CAS.
- P. C. Munasinghe and S. K. Khanal, Biomass-derived syngas fermentation into biofuels: Opportunities and challenges, Bioresour. Technol., 2010, 101, 5013–5022 CrossRef CAS PubMed.
- J. R. Rostrup-Nielsen, Syngas in perspective, Catal. Today, 2002, 71, 243–247 CrossRef CAS.
- J. R. Rostrup-Nielsen, New aspects of syngas production and use, Catal. Today, 2000, 63, 159–164 CrossRef CAS.
- J. Xu, X. Li, W. Liu, Y. Sun, Z. Ju, T. Yao, C. Wang, H. Ju, J. Zhu, S. Wei and Y. Xie, Carbon dioxide electroreduction into syngas boosted by a partially delocalized charge in molybdenum sulfide selenide alloy monolayers, Angew. Chem., Int. Ed., 2017, 56, 9121–9125 CrossRef CAS PubMed.
- C. Guan, J. Liu, C. Cheng, H. Li, X. Li, W. Zhou, H. Zhang and H. J. Fan, Hybrid structure of cobalt monoxide nanowire @ nickel hydroxidenitrate nanoflake aligned on nickel foam for high-rate supercapacitor, Energy Environ. Sci., 2011, 4, 4496–4499 RSC.
- W. Zhu, Y. J. Zhang, H. Zhang, H. Lv, Q. Li, R. Michalsky, A. A. Peterson and S. Sun, Active and selective conversion of CO2 to CO on ultrathin au nanowires, J. Am. Chem. Soc., 2014, 136, 16132–16135 CrossRef CAS PubMed.
- W. Metzger, R. Westfall, A. Hermann and P. Lyman, Nickel foam substrate for nickel metal hydride electrodes and lightweight honeycomb structures, Int. J. Hydrogen Energy, 1998, 23, 1025–1029 CrossRef CAS.
- G. Yang, W. Xu, K. Tian, D. Su, J. Xu, H. Chen and Y. Zhang, Controllable syngas production on gold nanowires/nickel foam electrode in non-aqueous system, J. Colloid Interface Sci., 2020, 579, 290–296 CrossRef CAS PubMed.
- D. Yang, Q. Zhu, X. Sun, C. Chen, W. Guo, G. Yang and B. Han, Electrosynthesis of a defective indium selenide with 3D structure on a substrate for tunable CO2 electroreduction to syngas, Angew. Chem., Int. Ed., 2020, 59, 2354–2359 CrossRef CAS PubMed.
- P. Qin, S. Yang, P. Zhan, M. Chu, G. Li, Z. Si, J. Shi, L. Lu, B. Han and T. Tan, Two-dimensional PdMo curved nanosheets for tunable CO2 electrocatalytic reduction to syngas, Cell Rep. Phys. Sci., 2021, 2, 100619 CrossRef CAS.
- C. Q. Xi, J. K. Sang, A. Q. Wu, J. Yang, X. P. Qi, W. B. Guan, J. X. Wang and S. C. Singhal, Electrochemical performance and durability of flat-tube solid oxide electrolysis cells for H2O/CO2 co-electrolysis, Int. J. Hydrogen Energy, 2022, 47, 10166–10174 CrossRef CAS.
- H. Y. Jeong, M. Balamurugan, V. S. K. Choutipalli, E. S. Jeong, V. Subramanian, U. K. Sim and K. T. Nam, Achieving highly efficient CO2 to CO electroreduction exceeding 300 mA cm−2 with single-atom nickel electrocatalysts, J. Mater. Chem. A, 2019, 7, 10651–10661 RSC.
- Y. Huang, X. Zhang, X. Zhang, H. Dong and S. Zhang, Thermodynamic modeling and assessment of ionic liquid-based CO2 capture processes, Ind. Eng. Chem. Res., 2014, 53, 11805–11817 CrossRef CAS.
- J. M. Spurgeon and B. Kumar, A comparative technoeconomic analysis of pathways for commercial electrochemical CO2 reduction to liquid products, Energy Environ. Sci., 2018, 11, 1536–1551 RSC.
- A. Paturska, M. Repele and G. Bazbauers, Economic assessment of biomethane supply system based on natural gas infrastructure, Energy Procedia, 2015, 72, 71–78 CrossRef.
- M. A. Adnan and M. G. Kibria, Comparative techno-economic and life-cycle assessment of power-to-methanol synthesis pathways, Appl. Energy, 2020, 278, 115614 CrossRef CAS.
- Y. J. Guo, G. D. Li, J. B. Zhou and Y. Liu, Comparison between hydrogen production by alkaline water electrolysis and hydrogen production by pem electrolysis, IOP Conf. Ser.: Earth Environ. Sci., 2019, 371, 042022 CrossRef.
- G. C. B. 2021, https://www.globalcarbonproject.org/, 2021, vol. 2022 Search PubMed.
- D. Steward, T. Ramsden and J. Zuboy, H2A Central Hydrogen Production Model, Version 3 User Guide, National Renewable Energy Laboratory, 2012 Search PubMed.
- J. Andersson, E. Furusjö, E. Wetterlund, J. Lundgren and I. Landälv, Co-gasification of black liquor and pyrolysis oil: Evaluation of blend ratios and methanol production capacities, Energy Convers. Manage., 2016, 110, 240–248 CrossRef CAS.
- S. Redl, S. Sukumara, T. Ploeger, L. Wu, T. Olshoj Jensen, A. T. Nielsen and H. Noorman, Thermodynamics and economic feasibility of acetone production from syngas using the thermophilic production host moorella thermoacetica, Biotechnol. Biofuels, 2017, 10, 150 CrossRef PubMed.
- V. Baghdjian, S. A. European methanol market mulls February contract price.
- Methane price, https://www.globalpetrolprices.com/methane_prices/, 2022, [accessed 12 Sep. 2022].
- Natural gas price, https://markets.businessinsider.com/commodities/natural-gas-price, 2022, [accessed 10 Oct. 2022].
- Methanol price trend and forecast, https://www.chemanalyst.com/Pricing-data/methanol-1, 2022, [accessed 18 Oct. 2022].
- S. Kibria Nabil, S. McCoy and M. G. Kibria, Comparative life cycle assessment of electrochemical upgrading of CO2 to fuels and feedstocks, Green Chem., 2021, 23, 867–880 RSC.
- B. Endrődi, G. Bencsik, F. Darvas, R. Jones, K. Rajeshwar and C. Janáky, Continuous-flow electroreduction of carbon dioxide, Prog. Energy Combust. Sci., 2017, 62, 133–154 CrossRef.
- S. Verma, B. Kim, H. R. Jhong, S. Ma and P. J. Kenis, A gross-margin model for defining technoeconomic benchmarks in the electroreduction of CO2, ChemSusChem, 2016, 9, 1972–1979 CrossRef CAS PubMed.
- A. Sternberg, C. M. Jens and A. Bardow, Life cycle assessment of CO2-based C1-chemicals, Green Chem., 2017, 19, 2244–2259 RSC.
- T. T. H. Nguyen, T. Yamaki, S. Taniguchi, A. Endo and S. Kataoka, Integrating life cycle assessment for design and optimization of methanol production from combining methane dry reforming and partial oxidation, J. Cleaner Prod., 2021, 292, 125970 CrossRef CAS.
- A. Sternberg and A. Bardow, Life cycle assessment of power-to-gas: Syngas vs methane, ACS Sustainable Chem. Eng., 2016, 4, 4156–4165 CrossRef CAS.
- R. Farahipour and A. T. Karunanithi, Life cycle environmental implications of CO2 capture and sequestration with ionic liquid 1-butyl-3-methylimidazolium acetate, ACS Sustainable Chem. Eng., 2014, 2, 2495–2500 CrossRef CAS.
- J. Artz, T. E. Müller, K. Thenert, J. Kleinekorte, R. Meys, A. Sternberg, A. Bardow and W. Leitner, Sustainable conversion of carbon dioxide: An integrated review of catalysis and life cycle assessment, Chem. Rev., 2017, 118, 434–504 CrossRef PubMed.
- A. S. Miriam Werder, Life cycle assessment of the conventional and solar thermal production of zinc and synthesis gas, Energy, 2000, 25, 395–409 CrossRef.
- D. Xu, K. Li, B. Jia, W. Sun, W. Zhang, X. Liu and T. Ma, Electrocatalytic CO2 reduction towards industrial applications, Carbon Energy, 2023, 5(1) DOI:10.1002/cey2.230.
- Z. Duan, R. Sun, C. Zhu and I. M. Chou, An improved model for the calculation of CO2 solubility in aqueous solutions containing Na+, K+, Ca2+, Mg2+, Cl−, and SO42−, Mar. Chem., 2006, 98, 131–139 CrossRef CAS.
- S. Kaneco, K. Iiba, H. Katsumata, T. Suzuki and K. Ohta, Effect of sodium cation on the electrochemical reduction of CO2 at a copper electrode in methanol, J. Solid State Electrochem., 2006, 11, 490–495 CrossRef.
- F. Zhang, H. Zhang and Z. Liu, Recent advances in electrochemical reduction of CO2, Curr. Opin. Green Sustainable Chem., 2019, 16, 77–84 CrossRef.
- Acetonitrile Price Trend and Forecast, https://www.chemanalyst.com/Pricing-data/acetonitrile-1105, 2022, [accessed 15 Oct. 2022].
- Dimethylformamide Price Trend and Forecast, https://www.chemanalyst.com/Pricing-data/dimethylformamide-dmf-1167, 2022, [accessed 15 Oct. 2022].
- Eu sodium carbonate market report: Consumption, suppliers, buyers and forecast – indexbox, https://www.Globenewswire.Com/en/news-release/2022/06/08/2458413/0/en/eu-sodium-carbonate-market-report-consumption-suppliers-buyers-and-forecast-indexbox.Html, 2021, [accessed 14 jan. 2023].
- L. Yuan, L. Zhang, J. Feng, C. Jiang, J. Feng, C. Li, S. Zeng and X. Zhang, Upscaling studies for efficiently electric-driven CO2 reduction to CO in ionic liquid-based electrolytes, Chem. Eng. J., 2022, 450, 138378 CrossRef CAS.
- S. P. F. Costa, A. M. O. Azevedo, P. Pinto and M. Saraiva, Environmental impact of ionic liquids: Recent advances in (eco)toxicology and (bio)degradability, ChemSusChem, 2017, 10, 2321–2347 CrossRef CAS PubMed.
- M. Bystrzanowska, F. Pena-Pereira, L. Marcinkowski and M. Tobiszewski, How green are ionic liquids? - a multicriteria decision analysis approach, Ecotoxicol. Environ. Saf., 2019, 174, 455–458 CrossRef CAS PubMed.
- N. Adhikari and D. R. Joshi, An overview on common organic solvents and their toxicity, Int. J. Pharm., 2019, 28, 1–18 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2im00055e |
| This journal is © Institute of Process Engineering of CAS 2023 |