Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Zr-doped BaTaO2N photocatalyst modified with Na–Pt cocatalyst for efficient hydrogen evolution and Z-scheme water splitting†
Huihui
Li
 ab,
Junie Jhon M.
Vequizo
ab,
Junie Jhon M.
Vequizo
 b,
Takashi
Hisatomi
b,
Takashi
Hisatomi
 bc,
Mamiko
Nakabayashi
d,
Jiadong
Xiao
b,
Xiaoping
Tao
b,
Zhenhua
Pan
bc,
Mamiko
Nakabayashi
d,
Jiadong
Xiao
b,
Xiaoping
Tao
b,
Zhenhua
Pan
 b,
Wenpeng
Li
e,
Shanshan
Chen
b,
Wenpeng
Li
e,
Shanshan
Chen
 f,
Zheng
Wang
f,
Zheng
Wang
 g,
Naoya
Shibata
d,
Akira
Yamakata
g,
Naoya
Shibata
d,
Akira
Yamakata
 h,
Tsuyoshi
Takata
b and
Kazunari
Domen
h,
Tsuyoshi
Takata
b and
Kazunari
Domen
 *bi
*bi
aSchool of Materials and Energy, Lanzhou University, 222 South Tianshui Road, Lanzhou 730000, China
bResearch Initiative for Supra-Materials, Interdisciplinary Cluster for Cutting Edge Research, Shinshu University, Nagano-shi, Nagano 380-8553, Japan. E-mail: domen@shinshu-u.ac.jp
cPRESTO, JST, 4-17-1 Wakasato, Nagano-shi, Nagano 380-8553, Japan
dInstitute of Engineering Innovation, The University of Tokyo, Tokyo 113-8656, Japan
eDepartment of Science and Technology, Graduate School of Medicine, Science and Technology, Shinshu University, 4-17-1 Wakasato, Nagano 380-8553, Japan
fSchool of Materials Science and Engineering & National Institute for Advanced Materials, Nankai University, Tianjin 300-350, China
gResearch Center for Eco-Environmental Sciences, Chinese Academy of Sciences, Beijing 100085, China
hFaculty of Natural Science and Technology, Okayama University, 3-1-1, Tsushima-naka, Kita-ku, Okayama, Japan
iOffice of University Professors, The University of Tokyo, 2-11-16 Yayoi, Bunkyo-ku, Tokyo 113-8656, Japan
First published on 15th November 2022
Abstract
BaTaO2N exhibits hydrogen evolution activity under visible light with wavelengths up to 650 nm and is applicable to Z-scheme overall water splitting (ZOWS). However, the insufficient activity and selectivity of BaTaO2N in the presence of redox mediators limit the efficiency of this process. Herein, we report the use of modified BaTaO2N as a hydrogen evolution photocatalyst in combination with BiVO4 and [Fe(CN)6]3−/4− as the oxygen evolution photocatalyst and redox mediator, respectively. Zr-doped BaTaO2N (BaTaO2N:Zr) synthesized by flux-assisted thermal nitridation and decorated with Na and Pt (Na–Pt) as cocatalysts was found to provide higher hydrogen evolution activity than undoped BaTaO2N. Zr doping extended the lifetime of electrons in the BaTaO2N and promoted electron injection into the Na–Pt cocatalysts. Consequently, Na–Pt/BaTaO2N:Zr modified with Cr2O3 to suppress reverse reactions evolved hydrogen from an aqueous K4[Fe(CN)]6 solution. Optimizing the BaTaO2N photocatalyst and the reaction conditions provided a ZOWS system capable of operating under visible light at wavelengths up to 520 nm. This work indicates that ZOWS systems operable under visible light can be constructed based on detailed investigations of photocatalysts, cocatalysts and redox mediators.
Broader contextDirect decomposition of water into hydrogen and oxygen using particulate photocatalysts is a simple and cost-effective approach towards renewable solar hydrogen production on a large scale. Z-scheme overall water splitting (ZOWS) comprising two-step excitation of a hydrogen evolution photocatalyst (HEP) and an oxygen evolution photocatalyst (OEP) has attracted significant interest, because narrow bandgap photocatalysts can be applied to this process if these materials are active during either the hydrogen or oxygen evolution reactions. However, the past ZOWS systems mostly used wide bandgap semiconductors as either HEPs or OEPs, restricting the use of long-wavelength visible light. This work extends the wavelength range of visible light that can be used for ZOWS by improving the performance of a narrow bandgap oxynitride (BaTaO2N) as the HEP and by selecting an appropriate redox couple for electron transfer. This represents an essential advance towards achieving the solar energy conversion efficiency required for practical photocatalytic hydrogen production. The practice of comprehensive refinement of narrow bandgap oxynitride photocatalysts, cocatalysts, and redox mediators effective for ZOWS will guide the research and development of photocatalytic solar hydrogen production. |
Introduction
Z-scheme overall water splitting (ZOWS) comprising two-step excitation of a H2-evolving photocatalyst (HEP) and O2-evolving photocatalyst (OEP) has attracted significant interest as a means of harvesting solar energy. Narrow-bandgap materials can be applied to this process if these compounds are active during either the hydrogen or oxygen evolution reactions, and photocatalyst sheets have exhibited efficient scalable ZOWS.1 The use of p-type semiconductors as HEPs is likely to be essential in the sheet system because the ZOWS mechanism is similar to that for photoelectrochemical cells. Many Cu-based chalcogenides are narrow-bandgap p-type semiconductors, but improving the durability and efficiency of such materials in ZOWS remains challenging.2,3 In contrast, ZOWS systems based on a suspended HEP and OEP together with ionic electron mediators can operate even when both the HEP and OEP are n-type semiconductors. This is possible because charge transfer between the HEP and OEP can occur via photocatalytic reactions of mediator ions instead of at junctions between bulk solid materials.4–8 Many ZOWS systems have been reported to date. As an example, Qi et al. demonstrated a ZrO2/TaON–[Fe(CN)6]3−/4−–BiVO4 system.9 The ZrO2/TaON and BiVO4 photocatalysts can utilize visible light up to 500 and 520 nm, respectively. However, achieving the target solar-to-hydrogen energy conversion efficiency (STH) of 5% or greater required for practical photocatalytic hydrogen production will necessitate the utilization of materials capable of using solar radiation at wavelengths above 530 nm for ZOWS.BaTaO2N has an absorption edge wavelength of 650 nm and so is a promising candidate for photocatalytic water splitting under visible light.10–15 Single-crystalline particulate BaTaO2N can be obtained by RbCl flux-assisted nitridation.16 When loaded with a Pt cocatalyst decorated with Na, this compound exhibits excellent hydrogen evolution activity from aqueous methanol solutions, while unmodified BaTaO2N is inactive.17 The addition of Na ensures wide dispersion of the Pt nanoparticles during the hydrogen reduction treatment and these highly-dispersed nanoparticles effectively catalyze the hydrogen evolution reaction (HER) on the BaTaO2N. Domen et al. reported ZOWS using BaTaO2N as the HEP, WO3 as the OEP and IO3−/I− as the ionic shuttle.16,17 Despite the ability of BaTaO2N to utilize visible light, the use of WO3, which has a wide bandgap, restricts the use of wavelengths above 450 nm by this system. Therefore, it would be an important breakthrough to combine BaTaO2N with OEPs having narrower bandgaps, such as BiVO4.18 Unfortunately, the low activity of BaTaO2N in the presence of reversible redox mediators hampers the realization of such ZOWS systems.
Doping is a simple but effective means of tuning the properties of photocatalysts so as to improve their activity. In the case of BaTaO2N, doping with Zr tends to enhance performance by suppressing the formation of Ta3+ defects during the thermal nitridation process. Abe et al. demonstrated a negative shift in the photoanodic current onset potential when using Zr-doped BaTaO2N.19 Our own group also synthesized BaZrO3–BaTaO2N solid solutions that exhibited improved photocatalytic hydrogen evolution from water under visible light.20 Accordingly, it is expected that the hydrogen evolution activity of BaTaO2N synthesized using nitridation in conjunction with a RbCl flux could be improved by doping with Zr. Another important consideration related to ZOWS systems is the ionic electron mediator, which can induce backward reactions. It is therefore important to improve not only the photoreduction activity but the selectivity for the HER associated with the reduction of the ionic electron mediator. This can be accomplished by surface modification of the BaTaO2N photocatalyst to control the reaction selectivity.
The present work devised an efficient ZOWS system based on Zr-doped BaTaO2N (denoted herein as BaTaO2N:Zr) as the HEP combined with BiVO4 modified with Au and CoOx (denoted as CoOx/Au/BiVO4) as the OEP and [Fe(CN)6]3−/[Fe(CN)6]4− as the redox mediator.9 The BaTaO2N:Zr photocatalyst was additionally loaded with a Pt cocatalyst together with Na, as well as with Cr2O3.21 The resulting ZOWS system (denoted as Cr2O3/Na–Pt/BaTaO2N:Zr–[Fe(CN)6]3−/4−–CoOx/Au/BiVO4) exhibited stable H2 and O2 evolution at the expected stoichiometric molar ratio during a 30 h trial under simulated sunlight with a STH of 0.022%. The effects of the Zr doping, Na–Pt cocatalyst loading and Cr2O3 coating on the ZOWS activity of this material were evaluated.
Results and discussion
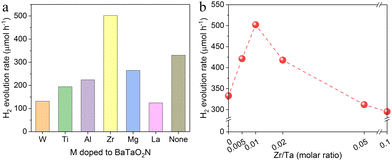
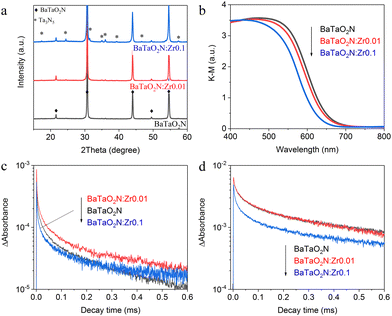
Cation-doped BaTaO2N was prepared via the thermal nitridation of a mixture of the corresponding starting materials with an RbCl flux. The effect of doping was investigated by assessing the hydrogen evolution activity of doped BaTaO2N in an aqueous methanol solution under visible light after loading the Na–Pt cocatalysts. As shown in Fig. 1a, Zr doping enhanced the hydrogen evolution activity of the BaTaO2N in aqueous methanol while the other dopants investigated in this work actually lowered the activity. Zr4+ has the closest valency to Ta5+ and the disturbance of the anion compositions by cationic doping would be minimum, which may account for the lower hydrogen evolution reaction activity. On this basis, the optimal Zr doping amount was also investigated. The incorporation of 1% Zr in the BaTaO2N enhanced the photocatalytic H2 evolution activity to the greatest extent while higher doping levels reduced the activity (Fig. 1b).Fig. 2a presents powder XRD patterns obtained from crystals of BaTaO2N:Zr with Zr/Ta = 0, 0.01 and 0.1. The major product in all cases was evidently a perovskite-type material based on a comparison with the standard JCSD pattern for bulk BaTaO2N.17 However, some small peaks ascribed to Ta3N5 were observed even in the pattern for the undoped BaTaO2N. The formation of Ta3N5 in the undoped BaTaO2N was attributed to a loss of barium through volatilization during the high-temperature nitridation process.22 Upon adding Zr, a larger amount of Ta3N5 was generated as a by-product because Zr4+ ions were substituted into the Ta5+ sites and a corresponding amount of Ta2O5 was segregated and nitrided into Ta3N5. The deficiency of barium species with respect to the B-site elements (tantalum and zirconium) was further confirmed by chemical composition analysis (Table S1, ESI†). The Ba/(Ta + Zr) atomic ratio was found to decrease from 1.01 in BaTaO2N to 0.95 and 0.88 in BaTaO2N:Zr0.01 and BaTaO2N:Zr0.1, respectively. The specific surface areas of undoped BaTaO2N and BaTaO2N:Zr0.01 were 2.3 and 2.9 m2 g−1, respectively, suggesting a decrease in the average particle size of BaTaO2N by Zr doping. This may partly account for the enhancement in the photocatalytic H2 evolution activity at the low doping amount. Notably, the Zr/Ta ratio in the nitrided products was virtually the same as that in the corresponding starting materials. This result indicates that the added Zr species were not lost during the flux-assisted nitridation and subsequent rinsing processes. Despite the substitution of the lower valence element Zr for Ta, the O/(N + O) molar ratio was unchanged, possibly as a consequence of the formation of Ta3N5 (as was indicated by the XRD data). A broad absorption peak in the range of 700–750 nm, characteristic of Ta3N5, was not observed in the DRS spectra (Fig. 2b). However, the addition of Zr shifted the light absorption onset to shorter wavelengths with increasing concentration. Doping with lower valence cations results in exchange of nitride ions with oxide ions, decreasing the nitride ion content of the material to maintain the charge neutrality. As a result, the band gap was widened by doping Zr.
The effect of zirconium doping on the dynamics of photogenerated electrons and holes was studied by TAS. Fig. 2c and d provide transient absorption intensity profiles acquired at 5000 cm−1 (2000 nm) and 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 cm−1 (649 nm), respectively. These data reflect the dynamics of the intraband transitions of free and/or shallowly trapped electrons and photoexcited holes, respectively.23,24 The addition of 1 mol% Zr evidently increased the lifetime of shallowly trapped electrons, in agreement with earlier work by Hojamberdiev et al.,25 but had no appreciable effect on the dynamics of trapped holes. Doping with lower valence cations may suppress the formation of reduced Ta species acting as recombination centers. However, according to the Ta 4f XPS analysis, the chemical states of Ta species were not appreciably changed by Zr-doping, probably due to the low dopant concentration (Fig. S1, ESI†). In-depth analysis of semiconducting properties and defects of particulate oxynitride materials will be required to clarify the physicochemical origin for the prolonged electron lifetime. In contrast, doping with 10 mol% Zr accelerated the decay of both electrons and holes. It is likely that the formation of Ta3N5 impurities, which typically have short carrier lifetimes, promoted charge recombination in the specimens.26 This effect would explain why excessive Zr doping lowered the H2 evolution activity.
400 cm−1 (649 nm), respectively. These data reflect the dynamics of the intraband transitions of free and/or shallowly trapped electrons and photoexcited holes, respectively.23,24 The addition of 1 mol% Zr evidently increased the lifetime of shallowly trapped electrons, in agreement with earlier work by Hojamberdiev et al.,25 but had no appreciable effect on the dynamics of trapped holes. Doping with lower valence cations may suppress the formation of reduced Ta species acting as recombination centers. However, according to the Ta 4f XPS analysis, the chemical states of Ta species were not appreciably changed by Zr-doping, probably due to the low dopant concentration (Fig. S1, ESI†). In-depth analysis of semiconducting properties and defects of particulate oxynitride materials will be required to clarify the physicochemical origin for the prolonged electron lifetime. In contrast, doping with 10 mol% Zr accelerated the decay of both electrons and holes. It is likely that the formation of Ta3N5 impurities, which typically have short carrier lifetimes, promoted charge recombination in the specimens.26 This effect would explain why excessive Zr doping lowered the H2 evolution activity.
Fig. 3 shows a TEM image of a crystal of the cocatalyst-loaded BaTaO2N:Zr0.01, which exhibited the best H2 evolution activity. A nearly cubic particle with 100–300 nm in size with clear facets can be seen. Note that rod-like particles typically formed by Ta3N5 crystals were not observable due to the low amount. The well-defined SAED pattern with no additional diffraction spots (inset to Fig. 3) and the clear lattice fringes observed by HRTEM (inset to Fig. 3) indicate that these BaTaO2N:Zr0.01 particles were single crystals. Elemental mapping of the cross section of a BaTaO2N:Zr0.01 sample with STEM-EDS (Fig. 4) confirmed that the Zr dopant was not segregated from the BaTaO2N particles. Fig. 4 also shows that the other four elements (Ba, Ta, O and N) were uniformly distributed throughout the BaTaO2N:Zr crystals. These results indicate the homogeneous composition and single-crystalline nature of the BaTaO2N:Zr0.01 crystals.
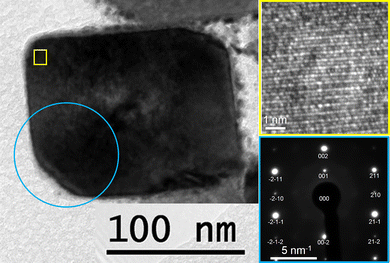 | ||
| Fig. 3 TEM, HRTEM (inset) and SAED (inset) images of the cross-section of a Cr2O3/Na–Pt loaded BaTaO2N:Zr0.01 sample. | ||
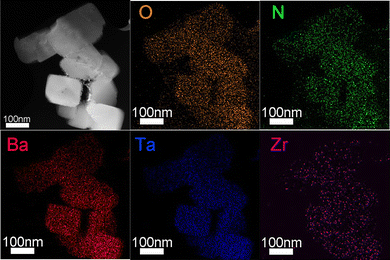 | ||
| Fig. 4 ADF-STEM image and corresponding STEM-EDS elemental maps of the cross-section of a Cr2O3/Na–Pt loaded BaTaO2N:Zr0.01 sample. | ||
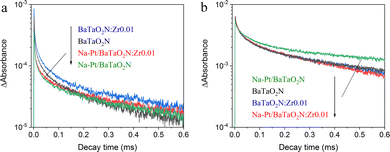
TAS was also used to examine the effect of zirconium doping on the interaction of the photocatalyst and the Na–Pt cocatalyst (Fig. 5a and b). The addition of the Na–Pt cocatalyst to the BaTaO2N was found to accelerate the decay of electrons while inhibiting that of holes, similar to the findings of our previous work.17 This effect occurred because the Na–Pt cocatalyst captured electrons in the BaTaO2N. Interestingly, the acceleration of the electron decay following cocatalyst loading was stronger in the case of the BaTaO2N:Zr0.01, suggesting that electron injection into the Na–Pt cocatalyst was promoted by Zr doping. However, the decay of holes was not retarded appreciably. Zr doping may induce deep-trapping and quenching of photoexcited holes by modifying the anion composition that, in turn, affects the valence band structure of the BaTaO2N. Additional in-depth spectroscopic investigations will be required to better explain the TAS observations in the present work.
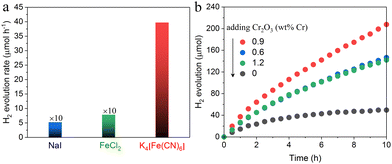
The hydrogen evolution activity of a HEP in an aqueous solution containing an ionic redox mediator depends on the characteristics of the mediator, such as the redox potential, number of electrons involved in the redox reaction, and adsorption properties.9 Thus, an appropriate redox mediator for the present ZOWS system was determined by experimental screening. The hydrogen evolution activities of BaTaO2N:Zr0.01 samples loaded with the Na–Pt cocatalyst followed by the photodeposition of Cr2O3 from aqueous K4[Fe(CN)6], NaI or FeCl2 solutions were evaluated. Here, Cr2O3 was employed to suppress backward reactions (i.e., the reduction of oxidized forms of the redox shuttle).27 As seen in Fig. 6a, the Cr2O3/Na–Pt/BaTaO2N:Zr0.01 was almost inactive in aqueous solutions containing I− or Fe2+ shuttle ions, while the same photocatalyst exhibited decent activity when [Fe(CN)6]4− was employed as the electron donor. The [Fe(CN)6]3−/[Fe(CN)6]4− redox couple has a more negative redox potential than the IO3−/I− or Fe3+/Fe2+ redox mediators.9 As a result, photogenerated holes could be transferred from the valence band of the BaTaO2N:Zr0.01 to the [Fe(CN)6]4− ions more efficiently. In addition, the [Fe(CN)6]3−/[Fe(CN)6]4− redox couple works in less extreme pH environments (pH 6–7) than the Fe3+/Fe2+ mediator (pH 2–3, at which Cr2O3 is unstable). The former couple is also cycled by accepting/donating one electron instead of multiple electrons such as the six electrons required by the IO3−/I− shuttle. These features allowed the Cr2O3/Na–Pt/BaTaO2N:Zr0.01 to evolve hydrogen efficiently and steadily, and so the [Fe(CN)6]3−/[Fe(CN)6]4− redox shuttle shows promise as a component of ZOWS systems.
Pt nanoparticles were well used as a cocatalyst for hydrogen evolution.28,29 Note that it was important to apply a Cr2O3 layer to the Na–Pt nanoparticles to suppress backward reactions during the HER. As shown in Fig. 6b, without Cr2O3 deposition, hydrogen evolution ceased after a few hours due to the progression of backward reactions. With the optimal amount of Cr2O3, the H2 evolution performance of the Na–Pt/BaTaO2N:Zr0.01 was promoted because the access of [Fe(CN)6]3− to the Na–Pt cocatalyst was suppressed.30 The optimum amount of Cr based on photodeposition was found to be 0.9 wt% with respect to the photocatalyst mass. Greater amounts of Cr actually lowered the hydrogen evolution activity because of the excessive photodeposition of Cr2O3 in the presence of methanol. The action spectrum of a Cr2O3/Na–Pt/BaTaO2N:Zr0.01 photocatalyst for the hydrogen evolution reaction from aqueous K4[Fe(CN)6] solution is provided in Fig. S2 (ESI†). The photocatalyst exhibited the hydrogen evolution activity up to 640 nm, which was consistent with the absorption edge of the material. The AQY at 420 nm was measured to be 0.4%.
Fig. 7 provides ADF-STEM, TEM, and STEM-EDS elemental maps of BaTaO2N:Zr0.01 loaded with 0.23 wt% Na 0.3 wt% Pt and Cr2O3 (0.9 wt% Cr). The loading of Pt nanoparticles along with Na was studied in our recent work.17 According to our previous work, the Pt nanoparticles in this sample had sizes in the range of 2–8 nm. The addition of Na improved the dispersion and structural stability of the Pt cocatalyst, although some cocatalyst particles are seen to have aggregated to form larger secondary particles on the edges of the BaTaO2N crystal. Na species were not observed because of the low loading amount and the poor sensitivity of EDS for this element. The Cr species appeared to be present in the same positions as the Pt, suggesting the formation of a layer of Cr2O3 covering the Pt nanoparticles as in earlier reports,21 but were distributed over a somewhat wider area.
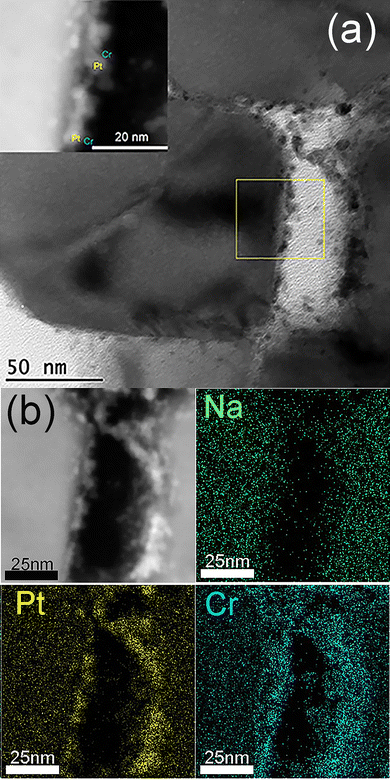 | ||
| Fig. 7 (a) TEM and ADF-STEM (inset) images and (b) ADF-STEM image along with STEM-EDS elemental maps of the cross-section of a 0.9 wt% Cr2O3/0.23 wt% Na 0.3 wt% Pt/BaTaO2N:Zr0.01 sample. | ||
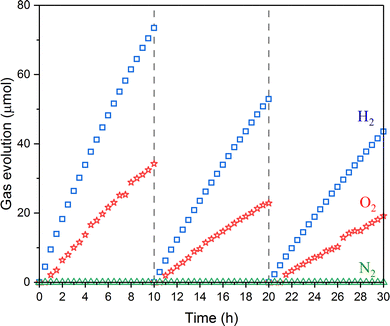
The BaTaO2N:Zr0.01 photocatalyst loaded with Cr2O3/Na–Pt cocatalysts was found to function as a HEP in a ZOWS system in combination with CoOx/Au/BiVO4 as the OEP and [Fe(CN)6]3−/[Fe(CN)6]4− as a redox mediator (Fig. 8), whereas the amounts of gaseous products were below the detection limits in the absence of the redox mediator. The same HEP and OEP showed H2 and O2 evolution activities from aqueous solutions containing the corresponding redox mediators, respectively (Fig. S3, ESI†). These observations indicate the occurrence of Z-scheme via the electron transfer mediated by the [Fe(CN)6]3−/[Fe(CN)6]4− redox couple. The ZOWS performance of this system was examined under simulated sunlight with evacuation of the test apparatus followed by the reintroduction of Ar to 10 kPa at 10 h intervals. Both H2 and O2 were evolved at a molar ratio close to the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric ratio, and the STH of this redox-mediated ZOWS system was determined to be 0.022% in the initial run. The AQY values at 420 nm and 520 nm under monochromatic light were 1.5% and 0.2% respectively (Fig. S4, ESI†). Compared with the previously reported BaTaO2N-WO3 ZOWS system, this system using BiVO4 instead of WO3 was able to harvest longer-wavelength visible light up to approximately 520 nm. This performance was enabled by doping with 1 mol% Zr, which promoted H2 evolution on the BaTaO2N, as well as by the modification of the Na–Pt cocatalyst with Cr2O3 to inhibit reverse reactions and the use of [Fe(CN)6]3−/[Fe(CN)6]4− as a suitable redox mediator.
1 stoichiometric ratio, and the STH of this redox-mediated ZOWS system was determined to be 0.022% in the initial run. The AQY values at 420 nm and 520 nm under monochromatic light were 1.5% and 0.2% respectively (Fig. S4, ESI†). Compared with the previously reported BaTaO2N-WO3 ZOWS system, this system using BiVO4 instead of WO3 was able to harvest longer-wavelength visible light up to approximately 520 nm. This performance was enabled by doping with 1 mol% Zr, which promoted H2 evolution on the BaTaO2N, as well as by the modification of the Na–Pt cocatalyst with Cr2O3 to inhibit reverse reactions and the use of [Fe(CN)6]3−/[Fe(CN)6]4− as a suitable redox mediator.
The H2 evolution rate obtained for the Cr2O3/Na–Pt modified BaTaO2N:Zr0.01 in an aqueous [Fe(CN)6]4− solution (37 μmol h−1; Fig. 6) was slightly lower than the O2 evolution rate for CoOx/Au/BiVO4 in a [Fe(CN)6]3− solution (51 μmol h−1; Fig. S3, ESI†). These data indicate that the rate-determining component of this ZOWS system was the Cr2O3/Na–Pt/BaTaO2N:Zr0.01 photocatalyst. In fact, the present system using Cr2O3/Na–Pt/BaTaO2N:Zr as the HEP showed inferior AQY and STH values compared with an earlier system using RhCrOx/ZrO2/TaON.9 Thus, it is still necessary to improve the preparation of the BaTaO2N:Zr so that more photoexcited charge carriers become available at the surface and to enhance the injection of electrons into the cocatalysts. The present ZOWS system was also found to lose 27% of its original activity when reused, unlike a previously reported system using ZrO2/TaON loaded with a Rh2−yCryO3 cocatalyst as the HEP.9 The evolution of N2 was not detected and thus deterioration of the BaTaO2N:Zr0.01 photocatalyst during the 30 h trial was likely negligible. The XRD pattern of the Cr2O3/Na–Pt/BaTaO2N:Zr0.01 photocatalyst was also not changed appreciably during the hydrogen evolution reaction (Fig. S5A, ESI†). Therefore, the activity loss was presumably a result of the degradation of the Cr2O3/Na–Pt cocatalyst. Degradation of the hydrogen evolution activity of Na–Pt/BaTaO2N was also observed in our previous work.17 XPS analysis of the Cr2O3/Na–Pt/BaTaO2N:Zr0.01 photocatalyst before and after a ZOWS reaction suggests that the BaTaO2N:Zr0.01 photocatalyst was apparently intact while the Pt cocatalyst was slightly oxidized during the reaction (Fig. S5, ESI†). This may be associated with the degradation of the HER and ZOWS activities. Our previous work showed that coloading of an appropriate water oxidation cocatalyst can improve the durability of the cocatalyst by reducing the probability of hole injection into the hydrogen evolution cocatalyst.31,32 At present, it is difficult to coload BaTaO2N with hydrogen evolution cocatalysts and oxygen evolution cocatalysts without sacrificing photocatalytic activity because of the insufficient reactivity and stability of the materials. The development of new technologies for the preparation and modification of narrow-bandgap particulate oxynitride photocatalysts will be vital to the future design of efficient solar-to-chemical energy conversion processes.
Experimental
Synthesis of Zr-doped BaTaO2N
BaTaO2N:Zr samples were synthesized by RbCl (95.0%; FUJIFILM Wako Pure Chemical Corporation) flux-assisted nitridation of a mixture of ZrO(NO3)2·2H2O (99.5%; Wako Pure Chemical Industries, Ltd.), BaCO3 (99.9%; Kanto Chemical Co., Inc.) and Ta2O5 (99.9%; High Purity Chemicals). Note that all chemicals employed in this study were used directly as received. In a typical synthesis, a certain amount of the mixture (in which the Ba/Ta molar ratio was 1.1 regardless of the Zr/Ta molar ratio) and RbCl were blended thoroughly so that the solute concentration in RbCl flux was 10 mol% and the total mass of solute and RbCl flux was 2 g. The mixture was subsequently heated under an ammonia flow (200 mL min−1) at 1223 K for 8 h, based on a procedure previously reported by our group.17 The resulting BaTaO2N:Zr was washed with water and dried to give the final product. The BaTaO2N:Zr specimens having varying Zr doping levels are referred to herein as BaTaO2N:Zrx, where x represents the Zr/Ta molar ratio and ranges from 0 to 0.1. BaTaO2N specimens doped with other cations at the cation/Ta molar ratio of 0.01 were also prepared for comparison purposes.Synthesis of BiVO4
BiVO4 was prepared by a hydrothermal procedure.9 In this process, 5 mmol of NH4VO3 (99.0%; FUJIFILM Wako Pure Chemical Corporation) and 5 mmol of Bi(NO3)3·5H2O (99.5%; FUJIFILM Wako Pure Chemical Corporation) were dissolved in 50 mL of a 2.0 M nitric acid solution (65%; FUJIFILM Wako Pure Chemical Corporation). The pH of this solution was then adjusted to 1.0 with an ammonia solution (28 wt%; FUJIFILM Wako Pure Chemical Corporation) while stirring the mixture, which generated a light-yellow precipitate. The solution was allowed to age for 2 h with stirring, after which the precipitate was removed and transferred to a Teflon-lined stainless-steel autoclave with a capacity of 100 mL and was hydrothermally treated at 453 K for 11 h. The single phase of diffraction structure of as-prepared BiVO4 (PDF No. 14-0688) was confirmed as shown in Fig. S6 (ESI†).9Deposition of cocatalysts and characterization
The cocatalysts were deposited on the BaTaO2N:Zr by impregnation followed by hydrogen reduction.17 In a typical process, a 110 mg quantity of BaTaO2N:Zr was immersed in an aqueous solution containing NaOH (0.1 mol L−1; FUJIFILM Wako Pure Chemical Corporation) and H2PtCl6·6H2O (98.5%; FUJIFILM Wako Pure Chemical Corporation) as the precursors. The amounts of Pt and Na in the solution were 0.3 and 0.23 wt%, respectively, with respect to the photocatalyst mass. The solution was subsequently heated on a boiling water bath, after which the product was completely dried and then heated at 523 K for 30 min in a flow of 10% H2 in N2. The resulting Na–Pt/BaTaO2N:Zr was additionally modified with Cr2O3 by photodeposition to suppress backward reactions.21 This was accomplished by dispersing a quantity of the Na–Pt/BaTaO2N:Zr powder in 100 mL of an aqueous solution of methanol (15 vol%), after which K2CrO4 (99.0%; FUJIFILM Wako Pure Chemical Corporation) was added as a Cr6+ precursor without pH adjustment. The amount of Cr2O3 was equivalent to a loading of 0.9 wt% Cr with respect to the photocatalyst mass. After complete degassing, the suspension was irradiated with visible light (420 nm < λ < 800 nm) for 1 h. The photocatalyst was then removed by filtering, washed with ultrapure water and dried at 313 K under vacuum.The deposition of Au and CoOx as reduction and oxidation cocatalysts, respectively, on the BiVO4 was carried out using a stepwise photodeposition process previously reported in the literature.9 The prepared BiVO4 (150 mg) was dispersed in deionized water to which a specific amount of HAuCl4·4H2O (99.0%; FUJIFILM Wako Pure Chemical Corporation) was added, equivalent to 0.2 wt% Au relative to the oxide mass. Thereafter, the suspension was irradiated under visible light (420 nm < λ < 800 nm) for 1 h, after which the powder was collected by filtration, washed and dried. The as-obtained powder was subsequently loaded with CoOx (0.5 wt%) by photodeposition. This was accomplished by dispersing the powder in 100 mL of a 50 mM sodium potassium buffer solution (KH2PO4, 99.5%; FUJIFILM Wako Pure Chemical Corporation; 44 mM; Na2HPO4, 99.0%; FUJIFILM Wako Pure Chemical Corporation; 6 mM) at pH 6.0. A quantity of K3[Fe(CN)6] (99.0%; FUJIFILM Wako Pure Chemical Corporation) sufficient to give a concentration of 5 mM was added along with a specific amount of Co(NO3)2·6H2O (98.0%; FUJIFILM Wako Pure Chemical Corporation), and the mixture was illuminated for 1 h. The resulting CoOx/Au/BiVO4 has been shown to work as an excellent OEP in ZOWS systems involving [Fe(CN)6]3−/[Fe(CN)6]4− as the redox mediator.9
X-Ray diffraction (XRD) patterns were acquired using a Rigaku MiniFlex 300 powder diffractometer with a Cu Kα radiation source (λ = 1.5418 Å). The data were analyzed after mathematically removing the contribution from the Kα2 line. (Scanning) transmission electron microscopy ((S)TEM) images, energy dispersive X-ray spectroscopy (EDS) mapping images, selected area electron diffraction (SAED) patterns, high-resolution transmission electron microscopy (HRTEM) and annular dark-field (ADF) STEM images were obtained with a JEOL JEM-2800 system equipped with an Oxford Instruments X-MAX 100TLE SDD detector. Diffuse reflectance spectroscopy (DRS) data were acquired with an ultraviolet-visible-near-infrared spectrometer (V-670, JASCO) and converted from reflectance to the Kubelka–Munk function. The Ba, Ta and Zr concentrations in the BaTaO2N:Zr were determined by inductively coupled plasma-atomic emission spectroscopy (ICP-AES; ICPS-8100, Shimadzu). The oxygen and nitrogen contents of the BaTaO2N were obtained using an oxygen–nitrogen combustion analyzer (Horiba, EMGA-620W). X-Ray photoelectron spectroscopy (XPS) was performed using a PHI Quantera II spectrometer with an Al Kα radiation source. All binding energies were referenced to the C 1s peak (284.8 eV) arising from adventitious carbon. The specific surface area of the samples was calculated from nitrogen adsorption isotherms measured at 77 K with a BELSORP Mini II apparatus (MicrotracBEL) using the Brunauer–Emmett–Teller method.
Transient absorption (TA) spectroscopic measurements were carried out using a Nd:YAG laser system (Continuum, Surelite I; pulse duration: 6 nm) equipped with custom-built spectrometers.33 The probe beams coming from the halogen lamp (producing visible light) and MoSi2 coil (producing IR light) were used in this measurement for monitoring the absorption signals at 649 and 2000 nm, respectively. In the case of 2000 nm probing, the beam was focused on the sample and was transmitted from the sample and then detected by an mercury cadmium telluride detector. For the 649 nm probing, we used reflection mode, of which the incident probe beam was focused on the sample and then the reflected light from the sample was detected by a Si photodetector. The output electric signal was amplified with an AC-coupled amplifier (Stanford Research Systems, SR560, 1 MHz). The time resolution of the spectrometer was limited to 1 μs by the response of the photodetectors. One thousand responses were accumulated to obtain the transient profiles (that is, the decay curves) at 649 and 2000 nm. The various BaTaO2N:Zr photocatalysts loaded with Na and/or Pt were photoexcited using 470 nm pump pulses with a fluence of 3 mJ pulse−1. Each powder sample was fixed on a circular CaF2 substrate by drop-casting at a density of 1.24 mg cm−2. These TAS analyses were carried out in a vacuum at room temperature.
Photocatalytic H2 evolution reaction and OWS reaction
The HER over each BaTaO2N photocatalyst was performed at room temperature in a Pyrex top-irradiation reaction vessel connected to a glass closed gas circulation system. In each trial, a quantity of Na–Pt/BaTaO2N:Zr (100 mg) was dispersed in an aqueous methanol solution (15 vol%, 150 mL) using a magnetic stirrer. The vessel was subsequently evacuated several times to ensure complete air removal. The activity of a photocatalyst can depend on the pressure in the reaction system.16 Thus, to minimize the effects of changes in pressure resulting from gas evolution and heating of the reaction solution during irradiation, Ar was introduced into the reaction system prior to the reaction to a pressure of approximately 10 kPa. The reaction was initiated by irradiation with a 300 W Xe lamp fitted with a cutoff filter and a dichroic mirror, emitting at 420 nm < λ < 800 nm. A flow of cooling water was used to keep the suspension at approximately 283 K throughout the trial. The evolved gases were analyzed by gas chromatography (GC; Shimadzu, GC-8A with a thermal conductivity detector, MS-5 A columns and Ar as the carrier gas). The H2 evolution rates obtained from aqueous solutions of NaI, FeCl2 and K4[Fe(CN)6] and the O2 evolution rates from an aqueous K3[Fe(CN)6] solution were also examined. In each case, an aqueous solution (150 mL) containing the photocatalyst sample (100 mg) was prepared and the pH was adjusted to a specific value for NaI (pH 6–7, 2 mM), FeCl2 (pH 2.3, 2 mM), K4[Fe(CN)6] (pH 6, 2 mM) and K3[Fe(CN)6] (pH 6, 5 mM). An aqueous HNO3 solution was used to adjust the pH of the FeCl2 solution while the pH values of the K4[Fe(CN)6] and K3[Fe(CN)6] solutions were maintained at 6 using a 50 mM sodium potassium buffer solution.ZOWS reactions were carried out using the same reactor and system described above. In each experiment, Cr2O3/Na–Pt/BaTaO2N:Zr (70 mg) and CoOx/Au/BiVO4 (100 mg), acting as the HEP and OEP, respectively, were suspended in 150 mL of an aqueous sodium potassium buffer solution (pH 6.0, 25 mM) containing K4[Fe(CN)6]·3H2O (99.5%; FUJIFILM Wako Pure Chemical Corporation; 6 mM). After complete degassing, Ar gas was introduced to the reaction system to an initial pressure of 10 kPa. Following this, the suspension was irradiated with either a Xe lamp or solar simulator (AM 1.5G, 92 mW cm−2, 5.5 × 5.5 cm), and a cooling water system was employed to maintain the solution at 283 K, as described above. The evolved gases were also analyzed by the same GC instrumentation.
Apparent quantum yield (AQY) and STH
The AQY values for the ZOWS reactions were determined using a procedure similar to that described in our previous paper.34 The same light source was employed but it was equipped with various band-pass filters. The number of incident photons illuminating the reaction cell was measured using a grating spectroradiometer (LS-100, EKO Instruments). The AQY was then calculated as| AQY (%) = [A × R]/I × 100, | (1) |
The STH values for ZOWS reactions under simulated solar radiation were calculated as
| STH (%) = (R(H2) × ΔGr)/(P × S) × 100, | (2) |
Conclusions
This work demonstrated the effective modification of a BaTaO2N photocatalyst by Zr doping and by incorporating a Na–Pt cocatalyst together with a Cr2O3 coating to produce a HEP for ZOWS. Doping with 1 mol% Zr extended the lifetime of electrons and promoted electron injection into the Na–Pt cocatalyst, leading to improved H2 evolution activity for an aqueous methanol solution under visible light. The Cr2O3 coating also allowed the Na–Pt/BaTaO2N:Zr0.01 photocatalyst to evolve H2 from an aqueous [Fe(CN)6]3−/4− solution.A ZOWS system based on Cr2O3/Na–Pt/BaTaO2N:Zr0.01 as the HEP, CoOx/Au/BiVO4 as the OEP and [Fe(CN)6]3−/4− as redox ions evolved H2 and O2 under visible light up to 520 nm. The STH efficiency of this process was 0.022%. This newly developed ZOWS system was able to harvest visible light with longer wavelengths than earlier systems utilizing TaON as the HEP or WO3 as the OEP. This achievement was made possible by improving the performance of the BaTaO2N as the HEP and also by the selection of [Fe(CN)6]3−/4− as an appropriate redox couple. It is therefore important to optimize the photocatalyst, cocatalysts and reaction conditions to enhance the activity of ZOWS systems operating under long-wavelength visible light.
Author contributions
The manuscript was written through contributions of all authors. H. L. designed and performed the experiments, wrote and edited the manuscript. J. J. M. V. acquired the TAS spectra and analyzed these data together with A. Y. T. H. edited the manuscript and discussed the scientific results as a supervisor. M. N. characterized the morphology and composition of each specimen using STEM-EDS and HRTEM together with N. S. J. X., Z. P. W. L. and W. Z. discussed the scientific results. X. T. and S. C. discussed the synthesis of BiVO4. T. T. provided resources as a supervisor. K. D. edited the manuscript and provided resources as a supervisor. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the National Natural Science Foundation of China (grant no. 51402139), the China Scholarship Council (grant no. 201906185004 and 202008440289), the Artificial Photo-synthesis Project (ARPChem) of the New Energy and Industrial Technology Development Organization (NEDO), and by JST, PRESTO, Japan (grant no. JPMJPR20T9). Part of this study was supported by the University of Tokyo Advanced Characterization Nanotechnology Platform as part of the Nanotechnology Platform Project sponsored By the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan (grant no. JPMXP09A-21-UT-0046). W. L. thanks 2021 MEXT Scholarship with Embassy Recommendation for financial support. The authors thank Ms Michiko Obata of Shinshu University for her assistance during the XPS analyses. The authors thank Dr Taro Yamada, Ms Keiko Kato and Ms Yasuko Kuromiya of the University of Tokyo for their assistance in the ICP-OES and oxygen–nitrogen combustion analyses.Notes and references
- Q. Wang, T. Hisatomi, Q. Jia, H. Tokudome, M. Zhong, C. Wang, Z. Pan, T. Takata, M. Nakabayashi, N. Shibata, Y. Li, I. Sharp, A. Kudo, T. Yamada and K. Domen, Scalable water splitting on particulate photocatalyst sheets with a solar-to-hydrogen energy conversion efficiency exceeding 1%, Nat. Mater., 2016, 15, 611–615 CrossRef CAS PubMed.
- K. Iwashina, A. Iwase, Y. Ng, R. Amal and A. Kudo, Z-Schematic Water Splitting into H2 and O2 Using Metal Sulfide as a Hydrogen-Evolving Photocatalyst and Reduced Graphene Oxide as a Solid-State Electron Mediator, J. Am. Chem. Soc., 2015, 137, 604–607 CrossRef CAS.
- S. Chen, J. Vequizo, Z. Pan, T. Hisatomi, M. Nakabayashi, L. Lin, Z. Wang, K. Kato, A. Yamakata, N. Shibata, T. Takata, T. Yamada and K. Domen, Surface Modifications of (ZnSe)0.5(CuGa2.5Se4.25)0.5 to Promote Photocatalytic Z-Scheme Overall Water Splitting, J. Am. Chem. Soc., 2021, 143, 10633–10641 CrossRef CAS PubMed.
- J. Nasir, A. Munir, N. Ahmad, T. Haq, Z. Khan and Z. Rehman, Photocatalytic Z-Scheme Overall Water Splitting: Recent Advances in Theory and Experiments, Adv. Mater., 2021, 33, 2105195 CrossRef PubMed.
- B. Ng, L. Putri, X. Kong, Y. The, P. Pasbakhsh and S. Chai, Z-Scheme Photocatalytic Systems for Solar Water Splitting, Adv. Sci., 2020, 7, 1903171 CrossRef CAS PubMed.
- Y. Miseki and K. Sayama, Photocatalytic Water Splitting for Solar Hydrogen Production Using the Carbonate Effect and the Z-Scheme Reaction, Adv. Energy Mater., 2019, 9, 1801294 CrossRef.
- K. Maeda, Z-Scheme Water Splitting Using Two Different Semiconductor Photocatalyst, ACS Catal., 2013, 3, 1486–1503 CrossRef CAS.
- R. Zhang, L. Zhang, Q. Zheng, P. Gao, J. Zhao and J. Yang, Direct Z-Scheme Water Splitting Photocatalyst Based on Two-Dimensional van der Waals Heterostructures, J. Phys. Chem. Lett., 2018, 9, 5419–5424 CrossRef CAS PubMed.
- Y. Qi, Y. Zhao, Y. Gao, D. Li, Z. Li, F. Zhang and C. Li, Redox-Based Visible-Light-Driven Z-Scheme Overall Water Splitting with Apparent Quantum Efficiency Exceeding 10%, Joule, 2018, 2, 2393–2402 CrossRef CAS.
- M. Hojamberdiev, K. Yubuta, J. Vequizo, A. Yamakata, S. Oishi, K. Domen and K. Teshima, NH3-Assisted Flux Growth of Cube-like BaTaO2N Submicron Crystals in a Completely Ionized Nonaqueous High-Temperature Solution and Their Water Splitting Activity, Cryst. Growth Des., 2015, 15, 4663–4671 CrossRef CAS.
- S. Wei, S. Chang, F. Yang, Z. Fu, G. Liu and X. Xu, Stable and efficient solar-driven photoelectrochemical water splitting into H2 and O2 based on a BaTaO2N photoanode decorated with CoO microflowers, Chem. Commun., 2021, 57, 4412–4415 RSC.
- H. Zhang, S. Wei and X. Xu, Mg modified BaTaO2N as an efficient visible-light-active photocatalyst for water oxidation, J. Catal., 2020, 383, 135–143 CrossRef CAS.
- S. Wei, G. Zhang and X. Xu, Activating BaTaO2N by Ca modifications and cobalt oxide for visible light photocatalytic water oxidation reactions, Appl. Catal., B, 2018, 237, 373–381 CrossRef CAS.
- B. Dong, Y. Qi, J. Cui, B. Liu, F. Xiong, X. Jiang, Z. Li, Y. Xiao, F. Zhang and C. Li, Synthesis of BaTaO2N oxynitride from Ba-rich oxide precursor for construction of visible-light-driven Z-scheme overall water splitting, Dalton Trans., 2017, 46, 10707–10713 RSC.
- K. Hibino, M. Yashima, T. Oshima, K. Fujii and K. Maeda, Structures, electron density and characterization of novel photocatalysts, (BaTaO2N)1-x(SrWO2N)x solid solutions, Dalton Trans., 2017, 46, 14947–14956 RSC.
- Z. Wang, Y. Luo, T. Hisatomi, J. Vequizo, S. Suzuki, S. Chem, M. Nakabayashi, L. Lin, Z. Pan, N. Kariya, A. Yamakata, N. Shibata, T. Takata, K. Teshima and K. Domen, Sequential cocatalyst decoration on BaTaO2N towards highly-active Z-scheme water splitting, Nat. Commun., 2021, 12, 1005 CrossRef CAS PubMed.
- H. Li, D. Lu, S. Chem, T. Hisatomi, J. Vequizo, J. Xiao, Z. Wang, L. Lin, Q. Xiao, Y. Sun, Y. Miseki, K. Sayama, A. Yamakata, T. Takata and K. Domen, A Na-containing Pt cocatalyst for efficient visible-light-induced hydrogen evolution on BaTaO2N, J. Mater. Chem. A, 2021, 9, 13851–13854 RSC.
- A. Iwase, Y. Udagawa, S. Yoshino, Y. Ng, R. Amal and A. Kudo, Solar Water Splitting under Neutral Conditions Using Z-Scheme Systems with Mo-Doped BiVO4 as an O2-Evolving Photocatalyst, Energy Technol., 2019, 7, 1900358 CrossRef.
- M. Higashi, Y. Yamanaka, O. Tomita and R. Abe, Fabrication of cation-doped BaTaO2N photoanodes for efficient photoelectrochemical water splitting under visible light irradiation, APL Mater., 2015, 3, 104418 CrossRef.
- K. Maeda, D. Lu and K. Domen, Solar-Driven Z-scheme Water Splitting Using Modified BaZrO3-BaTaO2N Solid Solutions as Photocatalysts, ACS Catal., 2013, 3, 1026–1033 CrossRef CAS.
- K. Maeda, K. Teramura, D. Lu, N. Saito, Y. Inoue and K. Domen, Noble-Metal/Cr2O3 Core/Shell Nanoparticles as a Cocatalyst for Photocatalytic Overall Water Splitting, Angew. Chem., 2006, 118, 7970–7973 CrossRef.
- Y. Luo, Z. Wang, S. Suzuki, K. Yubuta, N. Kariya, T. Hisatomi, K. Domen and K. Teshima, Fabrication of Single-Crystalline BaTaO2N from Chloride Fluxes for Photocatalytic H2 Evolution under Visible Light, Cryst. Growth Des., 2020, 20, 255–261 CrossRef CAS.
- A. Yamakata and J. Vequizo, Curious Behaviors of Photogenerated Electrons and Holes at the Defects on Anatase, Rutile, and Brookite TiO2 Powders: A Review, J. Photochem. Photobiol., C, 2019, 40, 234–243 CrossRef CAS.
- A. Yamakata, J. Vequizo, T. Ogawa, K. Kato, S. Tsuboi, N. Furutani, M. Ohtsuka, S. Muto, A. Kuwabara and Y. Sakata, Core-Shell Double Doping of Zn and Ca on β–Ga2O3 photocatalysts for Remarkable Water Splitting, ACS Catal., 2021, 11, 1911–1919 CrossRef CAS.
- M. Hojamberdiev, R. Vargas, Z. Kadirova, K. Kato, H. Sena, A. Krasnov, A. Yamakata, K. Teshima and M. Lerch, Unfolding the Role of B Site-Selective Doping of Aliovalent Cations on Enhancing Sacrificial Visible Light-Induced Photocatalytic H2 and O2 Evolution over BaTaO2N, ACS Catal., 2022, 12, 1403–1414 CrossRef CAS.
- J. Vequizo, M. Hojamberdiev, K. Teshima and A. Yamakata, Role of CoOx cocatalyst on Ta3N5 photocatalysts studied by transient visible to mid-infrared absorption spectroscopy, J. Photochem. Photobiol., A, 2018, 358, 315–319 CrossRef CAS.
- M. Yoshida, K. Takanabe, K. Maeda, A. Ishikawa, J. Kubota, Y. Sakata, Y. Ikezawa and K. Domen, Role and Function of Noble-Metal/Cr-Layer Core/Shell Structure Cocatalysts for Photocatalytic Overall Water Splitting Studied by Model Electrodes, J. Phys. Chem. C, 2009, 113, 10151–10157 CrossRef CAS.
- L. Lin, Z. Lin, J. Zhang, X. Cai, W. Lin, Z. Yu and X. Wang, Molecular-level insights on the reactive facet of carbon nitride single crystals photocatalysing overall water splitting, Nat. Catal., 2020, 3, 649–655 CrossRef CAS.
- M. Liu, C. Wei, H. Zhuzhang, J. Zhou, Z. Pan, W. Lin, Z. Yu, G. Zhang and X. Wang, Fully Condensed Poly (Triazine Imide) Crystals: Extended π-Conjugation and Structural Defects for Overall Water Splitting, Angew. Chem., Int. Ed., 2022, 61, e202113389 CAS.
- M. Qureshi, T. Shinagawa, N. Tsiapis and K. Takanabe, Exclusive Hydrogen Generation by Electrocatalysts Coated with an Amorphous Chromium-Based Layer Achieving Efficient Overall Water Splitting, ACS Sustainable Chem. Eng., 2017, 5, 8079–8088 CrossRef CAS.
- H. Lyu, T. Hisatomi, Y. Goto, M. Yoshida, T. Higashi, M. Katayama, T. Takata, T. Minegishi, H. Nishiyama, T. Yamada, Y. Sakata, K. Asakura and K. Domen, An Al-doped SrTiO3 Photocatalyst maintaining sunlight-driven overall water splitting activity for over 1000 h of constant illumination, Chem. Sci., 2019, 10, 3196–3201 RSC.
- T. Ohno, L. Bai, T. Hisatomi, K. Maeda and K. Domen, Photocatalytic Water Splitting Using Modified GaN:ZnO Solid Solution under Visible Light: Long-Time Operation and Regeneration of Activity, J. Am. Chem. Soc., 2012, 134, 8254–8259 CrossRef CAS PubMed.
- A. Yamakata, M. Kawaguchi, T. Nishimura, T. Minegishi, J. Kubota and K. Domen, Behavior and Energy States of Photogenerated Charge Carriers on Pt- or CoOx-Loaded LaTiO2N photocatalysts: Time-Resolved Visible to Mid-Infrared Absorption Study, J. Phys. Chem. C, 2014, 118, 23897–23906 CrossRef CAS.
- Z. Wang, T. Hisatomi, R. Li, K. Sayama, G. Liu, K. Domen, C. Li and L. Wang, Efficiency Accreditation and Testing Protocols for Particulate Photocatalysts toward Solar Fuel Production, Joule, 2021, 5, 344–359 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Characterization results including those obtained using ICP-OES and N–O analysis; photocatalytic HER data. See DOI: https://doi.org/10.1039/D2EY00031H |
| This journal is © The Royal Society of Chemistry 2023 |