Water treatment using stimuli-responsive polymers
Zahra
Abousalman-Rezvani
ab,
Hossein
Roghani-Mamaqani
 *c,
Hossein
Riazi
d and
Ozra
Abousalman-Rezvani
e
*c,
Hossein
Riazi
d and
Ozra
Abousalman-Rezvani
e
aDrug Delivery, Disposition and Dynamics, Monash Institute of Pharmaceutical Sciences, Monash University, 381 Royal Parade, Parkville, Victoria 3052, Australia
bCSIRO, Manufacturing–Biomedical Manufacturing, Ian Wark Laboratory, Research Way, Clayton, VIC 3168, Australia
cFaculty of Polymer Engineering, Sahand University of Technology, P.O. Box: 51335-1996, Tabriz, Iran. E-mail: r.mamaghani@sut.ac.ir
dDepartment of Chemical and Biological Engineering, Drexel University, Philadelphia, PA 19104, USA
eDepartment of Mechanical Engineering, Sharif University of Technology, Tehran, Iran
First published on 11th October 2022
Abstract
Water treatment is a process used to eliminate or reduce chemical and biological contaminants that are potentially harmful to the water supply for human use. Stimuli-responsive polymers are a new category of smart materials used in water treatment via a stimuli-induced purification process and subsequent regeneration of the polymers. Stimuli-responsive polymers dynamically change their physico-chemical properties upon environmental changes. They can undergo shrinkage or expansion, alter their optical properties, and change their electrical characteristics depending on the applied stimuli. In this context, various stimuli-responsive polymer systems such as self-assembled nanostructures, supramolecular polymers, hydrogels, porous nanoparticles, membranes, and responsive coatings can be used in water treatment. As two main water pollution sources, heavy metals and dyes have raised considerable concerns among researchers. Current methods are inefficient at removing these contaminants from water, which may endanger the ecosystem. This review focuses on stimuli-responsive polymers that absorb heavy metal ions, aqueous anions, and dyes through their responsive characteristics. In particular, our objective is to evaluate the effectiveness of different smart polymer systems in water treatment processes. Recent advances in the field of pH-, CO2-, temperature-, and light-responsive polymers in water purification are reported in detail, and other stimuli including magnetic fields, redox systems, and electrical fields are discussed. This review also highlights novel dual- and multi-responsive polymers as these can exhibit favorable synergistic properties. In addition, future directions in water purification through smart polymers, the use of different regeneration processes, and finally the selectivity and efficiency of the removal systems are discussed.
1. Introduction
Water as one of the essential resources of human life is provided by nature, and its quality directly affects the quality of human life. A great part of available freshwater is used in industrial applications and urbanization processes which is no longer suitable for human and animal usage.1 The remaining little amount of freshwater may also contain organic, inorganic, and biological agents, which can exert harmful effects on human life. Biological agents in water may include Vibrio comma, Salmonella typhosa, Yersinia enterocolitica, Escherichia coli, and Shigella dysenteriae.2 In addition, the freshwater in industrial activities may get contaminated by heavy metals, dyes, phenols, detergents, pesticides, polychlorinated biphenyls, and a bunch of other inorganic and organic substances.3,4 Inorganic compounds like calcium, magnesium, potassium, bicarbonate, chloride, nitrate, arsenic, copper, and iron may exist in wastewater.5 Azo-based dyes, capable of converting into aromatic amines, are also the main source of wastewater pollution.6,7Wastewater treatment has remained as a critical global problem despite different technological advancements in this field. To convert wastewater to a potable material, several techniques have been developed to remove the pollutants.8 Coagulation and flocculation are the most effective methods for treating wastewater.9 Addition of some coagulating chemicals, such as Ca(OH)2, NaOH, CaCO3, Al2(SO4)3, organic polymers, and sulfides like pyrite (FeS2), to wastewater can remove organic and inorganic pollutants.10,11 Another commonly used technique for treating wastewater is the adsorption of inorganic and organic contaminants onto activated charcoal.10,12 Membrane filtration, ion exchange, solvent extraction, chemical precipitation, electrochemical treatment, floatation, and oxidation processes are also used to remove inorganic and organic pollutants from wastewater.13–15 Adsorption and membrane filtration are known as the most commonly used water treatment methods since they exhibit highly efficient selective removal of contaminants with an easy procedure for adsorption and regeneration processes.16,17 In these methods, responsive polymers could be extensively used to remove different contaminants from wastewater in the form of solutions, particles, fibers, mats, membranes, etc.18,19
Stimuli-responsive polymers hold great promise for wastewater treatment due to their outstanding properties under different conditions. Temperature-, pH-, light-, CO2-, magneto-, and electro-responsive polymers are being used as absorbents in wastewater treatment. The pH-responsive polymers are based on charged networks, either negatively or positively, and usually exhibit different degrees of swelling at different pH values.20–22 Temperature-responsive polymers respond to the variation of temperature by changing their conformation in the solution state,23 which can be used as a treatment method for wastewater. CO2-responsive polymers contain mostly amine groups, which get protonated and adsorb metal ions and dyes once CO2 is inserted into the system.24,25 Dual and multi-responsive polymers are also sensitive to two or more external stimuli, sometimes endowing them with double power for the removal of dyes and ions from wastewater and eventually in their regeneration process.26Table 1 summarizes the most important studies on water treatment systems using stimuli-responsive polymers, their morphology, the contaminant type, adsorption capacity, selectivity in removal, and the regeneration possibility of the polymer.
| Polymeric system | Morphology | Responsivity | Ion/dye adsorption | Selectivity | Regeneration | Q e (mg g−1) | Ref. |
|---|---|---|---|---|---|---|---|
| a mmol g−1. | |||||||
| Poly(maleic anhydride-alt-styrene) | Particle | pH-Responsive | Methyl blue | No | Yes | 406 | 27 |
| Poly(2-(dimethylamino)ethyl methacrylate)-grafted chitosan | Microsphere | pH-Responsive | Acid green and reactive blue | No | Yes | 991.8, 703.7 | 28 |
| Polylactide/poly[methyl methacrylate-co-2-(2-bromopropionyloxy) ethyl methacrylate] | Cup shaped | pH-Responsive | Rhodamine B | No | No | 7.8 | 29 |
| Acrylamide-2-acrylamido-2-methylpropane sulfonic acid | Hydrogel | pH-Responsive | Cu(II), Cd(II), Pb(II) | Yes | Yes | 0.97, 0.98, and 0.89a | 30 |
| Acrylamide and 2-acrylamido-2-methylpropane sulfonic acid | Hydrogel | pH-Responsive | Cd(II), Cu(II), Fe(III) | Yes | Yes | 510.8, 480.5, 400.2 | 31 |
| Poly(acrylic acid)-4-vinylpyridine | Hydrogel | pH-Responsive | Pb(II) | No | No | 117.9 | 32 |
| Poly(2-hydroxyethyl acrylate-co-2-acrylamido-2-methylpropane sulfonic acid) | Hydrogel | pH-Responsive | Pb2+, Cd2+, Cu2+, and Fe3+ | No | No | 175, 131, 92, 160 | 33 |
| Ferrocene-modified polyacrylic acid | Hydrogel | pH-Responsive | Bisphenol A and methylene blue | No | No | 23.6, 24.64 | 34 |
| Poly(acrylic acid-co-vinylsulfonic acid) | Hydrogel | pH-Responsive | Methylene blue | No | Yes | 1.72 | 35 |
| Gum-graft-poly(2-(dimethylamino)ethyl methacrylate) | Hydrogel | pH-Responsive | Methylene blue and indigo carmine | No | No | 89.28, 101.42 | 36 |
| [(Diallylamino)propyl] phosphonic acid hydrochloride | Resin | pH-Responsive | Cu2+ and Pb2+ | No | No | 3.83 and 10.1a | 37 |
| Acidic tetrapolymer | Resin | pH-Responsive | Cr(III) | No | No | 48.5 | 38 |
| Poly(N,N-dimethylaminoethyl methacrylate) | Resin | pH-Responsive | Cr(VI) | No | Yes | 165 | 39 |
| Carboxylate-rich magnetic chitosan flocculants | Particle | pH-Responsive | Ni(II) and malachite green | No | Yes | 1968 | 40 |
| Zwitterionic aminomethylphosphonate ligands and hydrophobic pendants | Resin | pH-Responsive | Cr(III) | No | Yes | — | 41 |
| Poly(ethylenimine)-grafted carbon nanofiber | Aerogel | pH-Responsive | Cu2+, methyl orange | No | Yes | 103.5, 265.9 | 42 |
| Poly(2-(dimethylamino)ethyl methacrylate)/nanocrystalline cellulose | Hydrogel | pH-Responsive | Methyl orange, acid orange II | No | No | 110, 100 | 43 |
| Poly(amidoamine) | Porous dendrimer gel | pH-Responsive | Methyl orange, tartrazine (TTZ) | Yes | Yes | 550.9, 558.7 | 44 |
| Poly(amidoamine) | Film composite | pH-Responsive | Pb2+, Ni2+, Cd2+, As5+ | No | No | 0.146, 0.094, 0.033, 0.217, 0.511 | 45 |
| Polyaspartamide | Hydrogel | CO2-responsive | Cu2+, Ni2+ | No | No | 88, 172 | 46 |
| Poly(2-dimethylaminoethyl methacrylate) | Octopus-like | CO2-responsive | Cu2+ | No | Yes | 145.1 | 47 |
| Poly(2-dimethylaminoethyl methacrylate) and coumarin | Substrate | CO2-responsive | NO3− | No | No | 3415 | 48 |
| Poly(2-dimethylaminoethyl methacrylate) and coumarin | Substrate | CO2-responsive | NO3− | No | No | 3800 | 25 |
| Poly(4-acryloyloxybenzophenone-co-2-(dimethylamino) ethyl methacrylate) | Cotton coated | CO2-responsive | Methyl orange, methyl blue, naphthol green B | Yes | Yes | 1785.71, 1108.65, 1315.79 | 49 |
| Poly(acrylic acid-2-(dimethylamino)ethyl methacrylate) | Aerogel | CO2-responsive | Cu2+ | No | Yes | 163.7 | 50 |
| Poly(methyl methacrylate-co-spiropyran methacrylate) | Film | Light-responsive | Multiple divalent metal ions | No | No | — | 51 |
| Poly(ethylene oxide) | Nanofiber | Light-responsive | Cu2+, Hg2+, Pb2+, Zn2+, | No | Yes | 0.40, 0.40, 0.35, 0.15a | 52 |
| Poly(vinyl alcohol) | Sponge | Light-responsive | Pb2+ | No | Yes | 5.78 | 53 |
| Poly(methacrylic acid) | BODIPY | Light-responsive | Many ions | Yes | No | — | 54 |
| Poly(N-isopropylacrylamide-co-benzo-18-crown-6-acrylamide) | Hydrogel | Temperature-responsive | Pb2+ | No | No | 140 | 55 |
| Poly(N-isopropylacrylamide-co-acrylic acid) | Hydrogel | Temperature-responsive | Cu2+ | No | No | 67.25 | 56 |
| Poly(N-isopropylacrylamide-co-acrylic acid) | Microgel | Temperature-responsive | Orange II | No | No | — | 57 |
| Poly(N-isopropylacrylamide-co-acryloylamidobenzo-18-crown-6) | Porous membrane | Temperature-responsive | Pb(II) | No | Yes | — | 58 |
| Poly(2-acrylamido-2-methyl-1-propanesulfonic acid-co-vinylimidazole) | Hydrogel | Magnetic-responsive | Cu(II), Cd(II), Fe(II), and Pb(II) | Yes | No | 97.21, 88.43, 117.73, 115.11 | 59 |
| Poly(benzoxazine) | Porous | Magnetic-responsive | Methylene blue and rhodamine B | No | No | — | 60 |
| Polyaniline | Hybrid film | Potential-responsive | Ni2+, Cd2+, Pb2+ | No | Yes | 105, 30, 35 | 61 |
| Polystyrene and poly(N-vinylpyrrolidone) | Porous | Potential-responsive | Cs+ | Yes | Yes | 204 | 62 |
| Poly(N-isopropylacrylamide-co-itaconic acid) | Hydrogel | pH- and temperature-responsive | Safranine T, brilliant green, and brilliant cresyl blue | No | No | 207, 228, 204 | 63 |
| Poly(acrylic acid)-bentonite-FeCo | Hydrogel | pH- and temperature-responsive | Crystal violet | No | No | 31.25 | 64 |
| Poly(2-(dimethylamino)ethyl methacrylate) | Hydrogel | pH- and temperature-responsive | Methyl orange | No | No | 35.3 | 65 |
| P(N-isopropylacrylamide-co-1-vinylimidazole) | Microgel | pH- and temperature-responsive | Orange II | No | Yes | — | 66 |
| Graphene oxide/poly(N-isopropylacrylamide-co-acrylic acid) | Hydrogel | pH- and temperature-responsive | Rhodamine B | No | Yes | 192.932 | 67 |
| Cellulose microfilaments/poly(N-isopropylacrylamide-co-acrylic acid) | Porous sphere | pH- and temperature-responsive | Methylene blue and methyl violet | No | No | 497.5, 840.03 | 68 |
| Carboxymethyl chitosan-graft-poly(N-isopropyl acrylamide-co-diallyl dimethyl ammonium chloride) | Flocculants | pH- and temperature-responsive | Cu2+ | No | No | — | 69 |
| Poly(N-isopropylacrylamide)-grafted chitosan/Fe3O4 | Composite particle | pH-, temperature-, and magnetic-responsive | Nonylphenol | No | No | 123 at pH 9 and 20 °C; 116 at pH 5 and 40 °C | 70 |
| Poly(2-(2-methoxyethoxy)ethyl methacrylate-co-oligo methacrylate-co-acrylic acid) | Hydrogel | pH-, temperature-, and magnetic-responsive | Rhodamine B | No | No | 2.05 | 71 |
| Poly(N-isopropylacrylamide-co-methacrylic acid) | Porous microsphere | pH-, temperature-, and magnetic-responsive | Cu2+ | No | Yes | 2221 | 72 |
| P(N-isopropylacrylamide-N,N’-methylenebisacrylamide)/P(methacrylic acid-ethylene glycol dimethacrylate)/Fe3O4 | IPN hydrogel microsphere | pH-, temperature-, and magnetic-responsive | Congo red, magenta, fluorescein Na | No | No | — | 73 |
| Poly(N-isopropylacrylamide-co-maleic acid-co-1-vinylimidazole) | Hydrogel | pH-, ion-, and temperature responsive | Cu2+ | No | Yes | 21.1 | 74 |
| Poly(ether amine) | Core/shell nanoparticle | Temperature-, pH-, and ionic strength-responsive | Different types of dyes | Yes | No | — | 75 |
| Magnetic chitosan/Carrageenan ampholytic composite | Microsphere | Magnetic-, pH-, and ionic-responsive | Methylene blue, Congo red, Cu(II) and Cr(III) | No | No | 48.35, 60.21, 6.17, 5.04 | 76 |
In this review study, relevant advances in the field of water treatment using stimuli-responsive polymers are briefly presented. The most exciting properties of stimuli-responsive polymers are their easy synthesis routes and compatibilities for treating wastewater. The most recent advances in the removal of dyes and heavy metal ions from water by using stimuli-responsive polymers are discussed. Future directions in wastewater purification using smart polymers, dual- and multi-responsive polymeric systems, and different regeneration processes are also highlighted. Different smart polymers in the form of gels, hybrids, containers, porous particles, fibrous mats, self-assembled structures, membranes, and dendrimers are studied for the removal of ions and dyes from aqueous media, and their beneficial and weak points in the absorption and regeneration processes are also discussed. Adsorption, membrane filtration, and ion-exchange methods seem to best fit with the area of water treatment with stimuli-responsive polymers. The selectivity and efficiency of the treatment and also the limit of detection of the contaminants are important factors in water purification systems. It is expected that this review will lead to further improvement in the field of wastewater treatment in the future.
2. Water treatment by smart polymers
Different technologies have been developed to remove pollutants from environmental water over the past few decades, which are not usable anymore due to industrial and agricultural polluting materials.77 Heavy metals, inorganic impurities, organic pollutants, and particularly dyes are known as groundwater and surface contaminants.78 Physical adsorption,79 oxidation,80 bioremediation,81 and responsive materials48 are among the technologies being used to address this issue. Stimuli-responsive polymers respond to external and internal stimuli by changing their physical or chemical properties.82 Wastewater treatment by these materials showed impressive results, as shown in Fig. 1. These materials encompass multiple properties, including variable facilities and higher purity after the regeneration step without any solid residue in the sludge. Stimuli-responsive polymers have more efficiency in comparison with other natural or synthetic materials in wastewater treatment.83 | ||
| Fig. 1 Switchable stimuli-responsive polymeric materials in the wastewater treatment process, designed by biorender.com. | ||
Research studies on stimuli-responsive polymer gels for wastewater treatment have rapidly been progressing more than the other products. Polymer gels are classified into three different types: microgels, hydrogels, and interpenetrating networks (IPN).84 The wastewater treatment products mostly rely on stimuli-responsive microgels and hydrogels.85 Smart polymer gels are categorized as chemical (non-destructible, irreversibly destructible, reversibly cleavable, and covalent adaptable gels) and also physical network structures.86–88 Non-destructible polymer gels change their volume sporadically and reversibly in response to external stimuli, such as pH, temperature, light, etc.89,90 As adsorbent materials with a reversible swelling behavior, they undergo adsorption and regeneration cycles effectively.91 Hybrid microgels, gel containers, and nanogels are also used for wastewater treatment.92–95 The hybrid structures with the core of magnetic particles are very effective in the regeneration process due to their easy coagulation and collection by the magnetic field.96–98 Stimuli-responsive porous particles are also used for wastewater treatment. They change their characteristics in different environments originating from a change in their surface area, the capability of taking up solvents with different polarities, and a change in their brittleness. Similar to the stimuli-responsive polymer gels, porous polymer particles show swelling characteristics allowing their application in wastewater treatment. After polymer gels, porous polymer particles are receiving more attention for wastewater treatment. Other families for wastewater treatment are substrates with a grafted responsive polymer, like cellulose, chitosan, cellulose nanocrystals, graphitic layers, carbon nanotubes, etc.99–101 These substrate-grafted stimuli-responsive polymers undergo easy regeneration processes, which is an advantage compared with their counterparts used in wastewater treatment. Dendrimers or hyperbranched polymers, such as poly(amidoamine) and poly(propylene imine), with lots of nitrogen atoms, can also be used in ion- and dye-removal systems.102–104 Fibrous smart polymers and membranes, which could be designed to change their hydrophilicity, surface characteristics, porosity, and charge density, are also known as highly-efficient water treatment methods.105
2.1. pH-Responsive polymers
The pH-responsive polymers change their characteristics including surface activity, chain conformation, and solubility in response to pH variations.106–108 Both negatively- and positively-charged polymers exhibit different degrees of swelling at different pH values depending on their ionic composition.109,110 In general, pH-responsive polymers act as cationic polymers in acidic media and as anionic ones in basic media, showing different self-assembly behaviors depending on their structures. Upon changing from a neutral to a charged state, they can absorb oppositely charged ions and dyes through electrostatic interactions, without leaving any residue, which is highly applicable in wastewater treatment.111 Among the other platforms, hydrogels are a more common type of pH-responsive polymer and they change their size upon a variation in the pH of the media, which can be used in the adsorption and release processes. | ||
| Fig. 2 (A) Schematic illustration for the adsorption and desorption of methyl blue by HPP-NH3+,27 Copyright 2016, Reproduced with permission from American Chemical Society (ACS); (B) working mechanism of chitosan adsorption and desorption of Acid Orange,112 Copyright 2021, Reproduced with permission from Springer; and (C) application of the PAMAM dendrimer for selective and efficient adsorption of anionic MO and tartrazine dyes.44 Copyright 2019, Reproduced with permission from Elsevier Ltd. | ||
Dendritic polymer structures in free or hybrid forms have also been used in the removal of anions from wastewater. Protonation of the amino groups in the structure of PAMAM at low pH values makes it possible to remove negative ions in aqueous systems.113,114 An amine-rich PAMAM dendrimer adsorbent was used for the selective and efficient adsorption of anionic MO and tartrazine dyes by Duan and coworkers,44 as shown in Fig. 2(C). The maximum adsorption capacity towards MO and tartrazine was as high as 680.2 mg g−1 and 689.7 mg g−1, respectively. The dye-adsorbed PAMAM could be readily desorbed in alkaline solution and separated from water by filtration. These dendritic structures could also absorb positive ions in aqueous media at basic pH values, where the regeneration process takes place by applying the pH stimulus and protonation of the amino groups.102,115–117 Interestingly, the positively charged membrane due to the protonation of amino groups shows excellent rejection to Pb2+, Ni2+, Cd2+, As5+, etc.45 Poly(propylene imine) (PPI) dendrimers were also used to remove ions and dyes from aqueous media.23–25 These examples show that the responsive characteristics of amine-containing dendrimers could be used for the removal of anionic dyes and ions and also the regeneration of cationic contaminants.
Poly(acrylic acid) (PAA) is another pH-responsive polymer used for this purpose.32,121,122 A PAA hydrogel was cross-linked via gamma radiation by Barrera-díaz and coworkers123 in order to investigate the ion adsorption behavior. After the grafting of 4-vinyl pyridine to PAA in two different grafting degrees of 26.74 and 48.1%, the product was brought into contact with an aqueous lead solution to determine its Pb2+ removal capacity. To evaluate the effect of pH on the lead removal, the pH range was changed from 2 to 6. The lead ion removal was increased by increasing the pH to 4, and it was slightly decreased by varying the pH from 4 to 6. This behavior originates from the competition between the protons and metal ions to reach the carboxylate active sites. A similar method was used by Wang and coworkers122 for the investigation of Pb2+, Cd2+, Cu2+, and Fe3+ adsorption. The adsorption levels for Pb2+, Cr3+, Cd2+, and Cu2+ were increased from 22 to 175, 51 to 131, 69 to 92, and 102 to 160 mg g−1, respectively, by increasing the pH value. The methylene blue (MB) dye has widely been investigated as a cationic dye in wastewater treatment studies.34,124,125 Environmentally friendly hydrogel adsorbents were prepared by cyclodextrin polymer/ferrocene-modified PAA via host–guest interactions. To remove poisonous organic molecules, including bisphenol A (BPA) and MB, pH changes and hydrophobic interaction were used. The removal mechanism of the organic molecules was considered as electrostatic interaction and hydrogen bonding, and the prepared hydrogel showed excellent potential for water purification. However, the hydrogel showed a low adsorption capacity for BPA in an alkaline solution (pH > 9). The surface charge of the hydrogel plays an essential role in removing positively charged MB, where it has a low adsorption capacity for MB in an acidic solution. Singh and coworkers35 prepared a pH-sensitive poly(acrylic acid-co-vinyl sulfonic acid) hydrogel for the removal of MB from water at pH values of higher than 7, which was reusable in wastewater treatment technology. A pH-responsive gum-grafted PDMAEMA has recently been synthesized to adsorb MB and indigo carmine dyes from wastewater.36 Competition between the high concentration of H+ ions in solution and the positively charged sulfur atoms of MB under acidic conditions led to low adsorption due to the electrostatic attraction forces. Moreover, protonation of the hydroxyl and carbonyl functional groups at low pH values caused a decrease in the amount of the dye adsorbed.
Resins are also used for the removal of ions and dyes from wastewater.37,38,126,127 The adsorption of Cu2+ and Pb2+ ions from wastewater was studied by Ali and coworkers using a resin system.37 Herein, [(diallylamino)propyl] phosphonic acid hydrochloride was prepared through a cycloterpolymerization method, and the effect of pH on the removal of the ions was investigated. The negative charge densities of the prepared polymer/resin were increased by increasing the pH values, which led to an increase in electrostatic force interactions. This process facilitates ion exchange and complexation through the formation of coordination bonds between the functional groups of the resin and metal ions. The same group prepared a cross-linked acidic tetrapolymer via the cyclopolymerization of amino, carboxylate, phosphonate, and sulfonate monomers, and the effect of pH on the removal of Cr(III) ions was investigated in the pH range 3.0–7.0. At pH values of 1–6, Cr(III) exists in the form of Cr(III) and also CrOH2+, Cr3(OH)45+, and Cr2(OH)24+. Therefore, positively charged ions were absorbed on the negatively charged active sites of polymer chains by increasing the pH from 1 to 6.128 In another study, Mazumder and coworkers129 synthesized a pH-responsive resin through the cyclocopolymerization of diallylammonioethanoate and maleic acid. The prepared resin removed MB and Hg(II) from industrial wastewater with high efficacy, and the adsorption capacity was improved by increasing the pH from 2.8 to 5.4. Sánchez and coworkers39 studied the adsorption of Cr(VI) ions from aqueous solution using PDMAEMA synthesized through reversible addition-fragmentation chain transfer (RAFT) polymerization. PDMAEMA chains were protonated at low pH values, because of their amine groups, which resulted in higher removal of Cr(VI) in the pH range of 4 to 6. The ion holding capacity was decreased at pH < 4 and pH > 6, due to the protonation of the PDMAEMA chains, which made the retention of the chromium anions easier in this range of pH values. Desorption of Cr(VI) ions was also investigated in this study. By increasing the pH to the basic values, PDMAEMA chains became neutral and released the anionic ions from the polymer chains. The optimum conditions for the maximum removal of Cr(VI) from water were achieved at pH 4.
Removal of MB has also been investigated by Dragan and coworkers124via IPN composite hydrogels synthesized by the free radical polymerization technique. Polyacrylamide (PAAm) was selected as the hydrogel matrix and native potato starch was selected as the entrapped polymer. The presence of PAAm in the IPN structure resulted in its pH-sensitivity. Repetitive adsorption/desorption analyses showed the capability of the IPN composite hydrogel of maintaining its ion adsorption ability as a re-usable absorbent. A complex copolymer structure to remove cationic heavy metals and dyes was reported by Zheng and coworkers,40 who synthesized multifunctional carboxylate-rich magnetic chitosan flocculants via surface graft copolymerization on magnetite particles. This material showed pH sensitivity due to the presence of amino, hydroxyl, and carboxyl groups on the structure of the flocculating agent. Herein, the removal of Ni(II) and malachite green (MG) was investigated. Polymer grafting on chitosan has significant effects on cationic ion and dye adsorption, because the carboxylic acid groups provide negative charges for attraction of positive ions and dyes. It is shown that the uptake rate of this material is maximum in the pH range of 4.0–8.0. Removal of Cr(III) and organic dyes by a pH-responsive resin containing zwitterionic aminomethyl phosphonate ligands and hydrophobic pendants was carried out by Ali and coworkers.41 They used the quadripolymerization method to prepare the functionalized resin containing hydrophilic motifs of aminomethyl phosphonate and hydrophobic pendants of 6-(biphenyl-4yloxy)hexyl. The effect of pH in the range of 3–7 on the removal of Cr(III) and several dyes, including MO, eriochrome black T, rhodamine B (RhB), methyl red, and MB, was investigated. At pH > 6, dominant species are CrOH2+, Cr3(OH)45+, and Cr2(OH)24+, which were attracted to the negatively charged sites of PO3H− and PO32− on the resin. The mechanism of the removal of ions and dyes is shown in Fig. 3(A). Cellulose nanofibril (CNF)-based aerogels have great potential for commercialization due to their low cost, compressibility, sustainability, high adsorption capacity, rapid adsorption kinetics, and ease of fabrication. By grafting poly(ethyleneimine) (PEI) onto CNFs, aerogel sorbents were prepared to remove Cu2+ and MO from wastewater.42 The aerogel showed a high adsorption capacity over a wide pH range. In addition, its surface charge showed a strong dependency on pH due to the protonation and deprotonation of amine and amino groups upon the change in pH (Fig. 3(B)). At low pH values, PEI endows the aerogel with a positive charge and allows it to adsorb the negatively charged MO via electrostatic attractions.
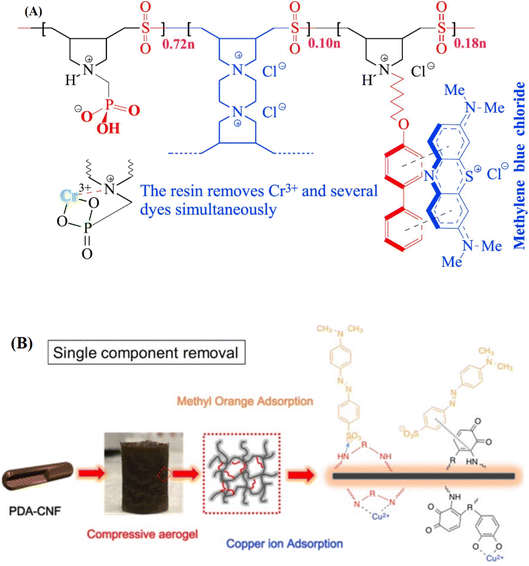 | ||
| Fig. 3 (A) Schematic illustration for the resin containing zwitterionic aminomethyl phosphonate ligands and hydrophobic pendants,41 Copyright 2017, Reproduced with permission from Elsevier Ltd and (B) preparation of PEI onto cellulose nanofibrils in order to remove Cu2+ and MO from wastewater through electrostatic forces,42 Copyright 2019, Reproduced with permission from Elsevier Ltd. | ||
The pH-responsive polymers and copolymers show change in their structure, volume, chain conformation, solubility, and configuration in response to pH variations. The pH-responsive polymers have drawn more attention among the other stimuli-responsive polymers for purification purposes. Reviewing the recent studies on pH-responsive polymers shows that these polymers have high sorbent efficiencies in the form of hydrogels among all the other forms of polymers. Hydrogels, through a change in their functional groups, develop a high binding affinity for various ions and dyes in water. Their significant inherent ability to imbibe a massive volume of water qualifies them to be used as a regenerative smart material in wastewater treatment. Biopolymers such as chitosan are also an important category of pH-responsive polymers in wastewater treatment application.
2.2. CO2-responsive polymers
Although a great number of studies on the adsorption process via responsive polymers is done on the pH-responsive polymers, CO2-responsive polymers have also attracted increasing attention due to their unique advantages, such as being benign, inexpensive, eco-friendly, biocompatible, and less-toxic.90,130 CO2 induces either negative or positive charges on the polymer chains containing special functional groups. Similar to the acids and bases which were triggers of pH-responsive polymers, CO2 works as an external trigger in a polymeric solution while showing distinct advantages like safety and no salt accumulation. CO2-responsive groups are divided into three types of those switch from neutral to cationic, neutral to anionic, and neutral to carbamate salts, which are briefly described in the following.Upon insertion of CO2 in aqueous media, guanidines, amidines, and amines form unstable carbonic acid and switch from neutral to cationic species. The cationic form of polymer chains in the presence of CO2 is suitable for adsorbing negative ions or dyes from the aqueous solution. Furthermore, the easy process of CO2 removal from the system by purging an inert gas such as N2 helps the regeneration of the polymeric adsorbent. The comparison of CO2-responsivity of these functional groups centers on comparing their basicity, which can be calculated from the dissociation constant (pKaH) of the corresponding conjugate acid. Amines with the lowest pKaH are the weakest bases among these functional groups. Although the guanidines and amidines switch to bicarbonate salts more quickly than amines due to their higher pKaH, higher basicity leads to the problematic and slower reverse process.131 Another obstacle for using guanidines and amidines in the CO2-responsive polymers could be attributed to the complex synthesis routes and the risk of decomposition of these materials through the hydrolysis process.132 Consequently, PDMAEMA, poly(dimethylaminoethyl methacrylate), and poly(dimethylaminopropyl methacrylamide as tertiary amine-containing polymers are the most commonly synthesized CO2-responsive polymers. Protonation of polymer chains is required for the adsorption process of anions and dyes from wastewater. Easy deprotonation of the protonated chains is also needed for the regeneration of the adsorbent, which is the main advantage of CO2-responsive tertiary amine-containing polymers. Wide applications of the CO2-responsive polymers originates from their commercial availability and inexpensiveness. CO2-responsive polymers such as PDMAEMA can reversibly be protonated and deprotonated in the presence of water by purging and removal of CO2, which leads to the adsorption and desorption of cationic dyes and pollutants from the water in the wastewater treatment processes.
The guanidines, amidines, and amines which contain an N–H bond without a bulky group switch to carbonate salts instead of bicarbonate salts in the presence of CO2.133 The first report on the application of CO2-responsive polymers for ion removal was reported by Kim and coworkers.46 The synthesized water-soluble polyaspartamide copolymer containing histamine pendants and hydroxyethyl pendant functional groups (PHEA-HIS) was crosslinked by hexamethylene diisocyanate to prepare a hydrogel. The imidazole groups of histamine, which are an example of cyclic amidines with a 5-membered ring, provide the CO2-responsiveness of the prepared hydrogel. The hydrogel undergoes reversible switching of CO2/N2, which affects its solubility and swelling behavior. The swelling ratio and the solubility of the hydrogel decrease to one-third in the presence of CO2, which could be attributed to the protonation of the imidazole groups. The protonated adjacent imidazole units intract together through intra/intermolecular hydrogen bonding,134 increasing the hydrophobicity of the hydrogel and lowering the affinity of the hydrogel for aqueous media and consequently causing shrinkage of the hydrogel. The PHEA-HIS hydrogel showed a reversibile swelling behavior as its solubility changed upon CO2/N2 purging to the solution. The PHEA-HIS hydrogel was used for the adsorption of metal ions, such as Pb2+, Cu2+, and Ni2+, from aqueous media through coordination between ions and the hydrogel. The mechanism of adsorption was similar to the other hydrogels, which are discussed in this study. Not only electrostatic repulsion but also shrinking of the hydrogel in the presence of CO2, which are the results of the protonation of imidazole units, could help desorption and recycling of the prepared adsorbent.
Hu and coworkers135 synthesized a CO2-responsive octopus-like polymeric adsorbent to demonstrate an eco-friendly regeneration concept. Herein, adsorption of various heavy metals relies on the chelating of ions by the adsorbent. The octopus-like polymeric adsorbent was prepared by DMAEMA polymerization from the polyhedral oligomeric silsesquioxane (POSS) core through the electron transfer atom transfer radical polymerization. POSS-PDMAEMA contains eight arms of PDMAEMA with a molecular weight of about 118![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1, which can get protonated in the presence of CO2 in water. Investigation of the pH effect on the adsorption capacity of Cu2+ showed that the optimum pH was recognized to be around 5.5, at which the hydrolysis of copper is prevented. At this pH, the chance of the protonation of the amino pendant groups increases which prevents coordination at lower pH values. The Cu2+ adsorption capacity of POSS-PDMAEMA is found to be about 145.1 mg g−1; it is higher than those of the linear PDMAEMA with a similar arm length. The resulting adsorption capacity was even close to highly porous composite and nanoparticle adsorbents.136 Multiple intra-molecular coordination bonds between ions and the adsorbent led to a higher adsorption capacity than their linear counterparts. The latter ones have higher chain rigidity and show more electrostatic repulsion, compared with the multi-arm structure, which result in inter- and intra-chain coordination hindrance and consequently lower adsorption capacity. The CO2-responsivity of the prepared adsorbent could address the perennial adsorption problems, such as adsorbent recycling, generation of waste sludge, and secondary pollution in wastewater treatment. In this work, heavy metal ions can be adsorbed onto the adsorbent through the chelating mechanism. After the separation of the purified water, the used adsorbent should be recycled for repetitive usage. Thus, CO2 gas purging is carried out to protonate the amine groups of PDMAEMA arms and consequently desorption of heavy metals through the electronic repulsion mechanism. Finally, the N2/air entrance deprotonates the protonated PDMAEMA chains for another treatment process. The close loop shown in Fig. 4(A) demonstrates the CO2-assisted liquid-phase polymer-based retention. Our group also took advantage of the CO2-responsivity of PDMAEMA not only in the recycling process of adsorbents like Hu's work, but also for the removal of negative ions such as nitrate from an aqueous solution.25,48,99 We were the first group who synthesized CO2-switchable adsorbents based on the electrostatic attraction and repulsion for adsorption and desorption of ions, respectively. Dual CO2- and light-responsive CNC-grafted and free block copolymers of DMAEMA and coumarin have been prepared through RAFT polymerization and atom transfer radical polymerization (ATRP). These CNC-grafted and free block copolymers were used for CO2-switchable adsorption/desorption of nitrate ions from aqueous solution.25,122 The induced positive charges on the PDMAEMA chains have potential for the adsorption of negatively charged nitrate ions through electrostatic attraction. The light-responsivity of the coumarin-containing block resulted in bond formation through cycloaddition reactions and consequently crosslinking of polymer chains exposed to UV light (λ > 350 nm). The crosslinking process led to the convenient regeneration of the prepared adsorbent. As shown in Fig. 4(B), after the adsorption process and separation of pure water, two steps are needed for the regeneration of the adsorbent: purging the N2 gas to the adsorbent system for neutralization of the PDMAEMA block and releasing the adsorbed ions and then de-crosslinking of the adsorbent by photo-induced cleavage of the cyclobutane bridges (<250 nm). A CO2-responsive cellulose nanofibril aerogel was successfully prepared from PDMAEMA and carboxylated cellulose nanofibrils via a stepwise cation-induced gelation and freeze drying method (Fig. 4(C)).130 This aerogel exhibited CO2-responsive adsorption of anionic dyes from water with a high adsorption rate and capacity and also outstanding recyclability even after twenty cycles. The maximum adsorption capacities of this aerogel towards MB, naphthol green B (NGB), and MO were 598.8, 621.1 and 892.9 mg g−1, respectively. A CO2-responsive cotton derived from poly(4-acryloyloxybenzophenone-co-2-(dimethylamino) ethyl methacrylate) and cotton was prepared using a dip-coating method (Fig. 4(D)), which showed selective adsorption toward anionic dyes with a high adsorption capacity and rate and also maximum adsorption capacities of 1785.71, 1108.65, and 1315.79 mg g−1 for MO, MB, and NGB, respectively.49 Carboxylic acids and phenols are in anionic form in the absence of CO2 in an aqueous solution. CO2 purging into an aqueous solution decreases the pH value. By decreasing the pH to below the pKa of either carboxylic acids or phenol-containing polymer chains, most functional groups in the form of anionic species become neutral in the aqueous solution. The polymers with these functional groups could be used as CO2-switchable adsorbents of cationic dyes or pollutants from wastewater. Fan et al. synthesized a CO2-responsive chitosan aerogel which is a highly efficient and environmentally friendly adsorbent for the adsorption and recovery of Cu2+ metal ions,50 as shown in Fig. 4(E). The aerogel adsorbent was synthesized from CO2-responsive poly(acrylic acid-2-(dimethylamino)ethyl methacrylate) and chitosan by physicochemical double crosslinking. After the adsorption of Cu2+ by the CO2-responsive chitosan aerogel, Cu2+ could be desorbed by CO2 bubbling.
000 g mol−1, which can get protonated in the presence of CO2 in water. Investigation of the pH effect on the adsorption capacity of Cu2+ showed that the optimum pH was recognized to be around 5.5, at which the hydrolysis of copper is prevented. At this pH, the chance of the protonation of the amino pendant groups increases which prevents coordination at lower pH values. The Cu2+ adsorption capacity of POSS-PDMAEMA is found to be about 145.1 mg g−1; it is higher than those of the linear PDMAEMA with a similar arm length. The resulting adsorption capacity was even close to highly porous composite and nanoparticle adsorbents.136 Multiple intra-molecular coordination bonds between ions and the adsorbent led to a higher adsorption capacity than their linear counterparts. The latter ones have higher chain rigidity and show more electrostatic repulsion, compared with the multi-arm structure, which result in inter- and intra-chain coordination hindrance and consequently lower adsorption capacity. The CO2-responsivity of the prepared adsorbent could address the perennial adsorption problems, such as adsorbent recycling, generation of waste sludge, and secondary pollution in wastewater treatment. In this work, heavy metal ions can be adsorbed onto the adsorbent through the chelating mechanism. After the separation of the purified water, the used adsorbent should be recycled for repetitive usage. Thus, CO2 gas purging is carried out to protonate the amine groups of PDMAEMA arms and consequently desorption of heavy metals through the electronic repulsion mechanism. Finally, the N2/air entrance deprotonates the protonated PDMAEMA chains for another treatment process. The close loop shown in Fig. 4(A) demonstrates the CO2-assisted liquid-phase polymer-based retention. Our group also took advantage of the CO2-responsivity of PDMAEMA not only in the recycling process of adsorbents like Hu's work, but also for the removal of negative ions such as nitrate from an aqueous solution.25,48,99 We were the first group who synthesized CO2-switchable adsorbents based on the electrostatic attraction and repulsion for adsorption and desorption of ions, respectively. Dual CO2- and light-responsive CNC-grafted and free block copolymers of DMAEMA and coumarin have been prepared through RAFT polymerization and atom transfer radical polymerization (ATRP). These CNC-grafted and free block copolymers were used for CO2-switchable adsorption/desorption of nitrate ions from aqueous solution.25,122 The induced positive charges on the PDMAEMA chains have potential for the adsorption of negatively charged nitrate ions through electrostatic attraction. The light-responsivity of the coumarin-containing block resulted in bond formation through cycloaddition reactions and consequently crosslinking of polymer chains exposed to UV light (λ > 350 nm). The crosslinking process led to the convenient regeneration of the prepared adsorbent. As shown in Fig. 4(B), after the adsorption process and separation of pure water, two steps are needed for the regeneration of the adsorbent: purging the N2 gas to the adsorbent system for neutralization of the PDMAEMA block and releasing the adsorbed ions and then de-crosslinking of the adsorbent by photo-induced cleavage of the cyclobutane bridges (<250 nm). A CO2-responsive cellulose nanofibril aerogel was successfully prepared from PDMAEMA and carboxylated cellulose nanofibrils via a stepwise cation-induced gelation and freeze drying method (Fig. 4(C)).130 This aerogel exhibited CO2-responsive adsorption of anionic dyes from water with a high adsorption rate and capacity and also outstanding recyclability even after twenty cycles. The maximum adsorption capacities of this aerogel towards MB, naphthol green B (NGB), and MO were 598.8, 621.1 and 892.9 mg g−1, respectively. A CO2-responsive cotton derived from poly(4-acryloyloxybenzophenone-co-2-(dimethylamino) ethyl methacrylate) and cotton was prepared using a dip-coating method (Fig. 4(D)), which showed selective adsorption toward anionic dyes with a high adsorption capacity and rate and also maximum adsorption capacities of 1785.71, 1108.65, and 1315.79 mg g−1 for MO, MB, and NGB, respectively.49 Carboxylic acids and phenols are in anionic form in the absence of CO2 in an aqueous solution. CO2 purging into an aqueous solution decreases the pH value. By decreasing the pH to below the pKa of either carboxylic acids or phenol-containing polymer chains, most functional groups in the form of anionic species become neutral in the aqueous solution. The polymers with these functional groups could be used as CO2-switchable adsorbents of cationic dyes or pollutants from wastewater. Fan et al. synthesized a CO2-responsive chitosan aerogel which is a highly efficient and environmentally friendly adsorbent for the adsorption and recovery of Cu2+ metal ions,50 as shown in Fig. 4(E). The aerogel adsorbent was synthesized from CO2-responsive poly(acrylic acid-2-(dimethylamino)ethyl methacrylate) and chitosan by physicochemical double crosslinking. After the adsorption of Cu2+ by the CO2-responsive chitosan aerogel, Cu2+ could be desorbed by CO2 bubbling.
 | ||
| Fig. 4 (A) Schematic illustration for the wastewater treatment system mechanism based on CO2-switchable adsorption/desorption,135 Copyright 2017, Reproduced with permission from Elsevier Ltd; (B) CO2-responsive copolymers based on DMAEMA and coumarin monomers and their switchable behavior when exposed to CO2 gas,25 Copyright 2019, Reproduced with permission from Elsevier Ltd; (C) adsorption of MB, NGB, and MO by a CO2-responsive cellulose nanofibril aerogel,130 Copyright 2021, Reproduced with permission from Elsevier Ltd; (D) adsorption of MB, NGB, and MO by CO2-responsive cotton,49 Copyright 2021, Reproduced with permission from American Chemical Society (ACS); and (E) a CO2-responsive chitosan aerogel as an adsorbent for the adsorption and recovery of Cu2+,50 Copyright 2021, Reproduced with permission from Elsevier Ltd. | ||
Reviewing the CO2-responsive polymers, it is understandable that these polymers have extraordinary properties. They are benign, inexpensive, environmentally friendly, and non-toxic triggers, and provide a great opportunity for water treatment. Moreover, CO2-responsive polymers have found many new areas of interest in polymer syntheses like RAFT and ATRP methods. CO2-responsive polymers are rapidly developed as a promising strategy for treating water with high efficiency. The main advantage of CO2-responsive polymers in water treatment is their easy way of regeneration with the insertion of an inert gas into the aqueous media, which could repeatedly be used without any side effects.
2.3. Temperature-responsive polymers
Temperature-responsive polymers can be introduced to a surface to trap either heavy metal ions or dyes using a lower critical solution temperature (LCST) concept (Fig. 5(A)).137 Temperature-responsive polymers are in the form of chains,138,139 gels,140 hydrogels,55,56,141 and microgels.57,142 Hydrogels can be broken or swollen by applying an external stimulus and are being used in wastewater treatment more than the other systems. Chu and coworkers55 reported a new polymeric lead(II) adsorbent via incorporation of benzo-18-crown-6-acrylamide (BCAm) as a metal ion receptor into the temperature-responsive PNIPAAm hydrogel. Herein, the removal of Pb2+ was investigated using the P(NIPAAm-co-BCAm) hydrogel. Changing the temperature up and down the LCST causes the adsorption and desorption of Pb2+, by a mechanism shown in Fig. 5(B). The hydrogel network is stretched and adsorbs Pb2+ at temperatures lower than the LCST; however, it shrinks and causes a reduction in the adsorption capacity at temperatures above the LCST. The LCST of the P(NIPAM-co-BCAm) hydrogel shifted to higher temperatures in Pb2+ solution due to metal ion complexation.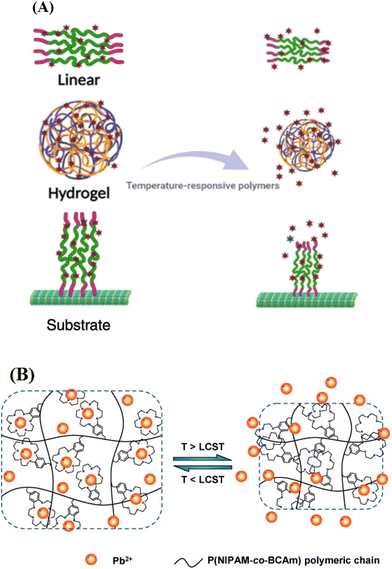 | ||
| Fig. 5 (A) Schematic illustration for temperature-responsive polymers exceeding their LCST and (B) temperature-responsivity of the P(NIPAM-co-BCAm) hydrogel above the LCST which allows the hydrogel to release the adsorbed ions.55 Copyright 2009, Reproduced with permission from Elsevier Ltd. | ||
Hydrogels were synthesized by incorporating acrylic acid as a copper ion absorbent into the temperature-responsive PNIPAAm structure.56 This work showed that the volume phase transition temperature (VPTT) of the hydrogels varied from 32 to 27 °C after the adsorption of Cu2+. The temperature-responsivity of PNIPAAm-co-AA after the adsorption of Cu2+ decreased due to the interference of Cu2+ in the intra-particle hydrogen bonding. Serpe and coworkers57 investigated the removal of organic azo dye molecules (Orange II) by preparation of PNIPAAm and PNIPAAm-co-AAc microgels. They reported an enhanced removal efficiency by increasing the amount of PNIPAAm-co-AAc microgels. Higher amounts of Orange II were adsorbed by rehydration of the microgels, and more Orange II got rid of the solution at high temperatures. In order to detect and remove Pb2+ ions, Chu and coworkers58 reported a gating membrane with poly(N-isopropylacrylamide-co-acryloylamidobenzo-18-crown-6) (poly(NIPAAm-co-AAB18C6)) copolymer chains as functional gates. The PNIPAAm chains show swelling/shrinking behavior and effective removal of Pb2+ ions upon temperature change. After an equal complexation state, increasing the temperature up to the polymer's LCST causes decomplexation of Pb2+ ions from the copolymer. The grafting yield of the poly(NIPAAm-co-AAB18C6)-grafted membrane plays an important role in achieving efficient adsorption of Pb2+ ions. The LCST shifts to a higher temperature during the Pb2+ adsorption analysis, compared to the clean water. This is due to the repulsion between the positively charged complex groups. The more grafting density caused the higher amount of the captured Pb2+. The gating membranes were regenerated by variation of the operating temperature to a high value of 45 °C. Tan and coworkers143 reported the synthesis of temperature-responsive poly(NIPAAm-co-AA) crosslinked with carboxymethyl cellulose through free radical polymerization to remove U(VI) from aqueous solutions. In recent decades, biopolymers are also synthesized with the aim of removing heavy-ion metals and dyes. Tunable biopolymers based on elastin-like polypeptides (ELP) were useful in this field, which represent a “green” technology for environmental issues.138,139 Temperature-responsive polymers always play a crucial role in most wastewater treatment applications. They show remarkable efficiency in wastewater treatment without any by-products during the treatment process.
2.4. Light-responsive polymers
Light-responsive polymers are an important category of smart polymers, which are prepared from incorporation of light-responsive chemicals into a polymer matrix.51,52,144–146 They possess numerous advantages as the triggering stimulus of light includes easy and precise control of on/off switching, utilizes the non-contact application, and have the ability to tune its dosage to change the response strength.147 Therefore, light-responsive polymers can be considered in many studies as efficient adsorbents in wastewater treatment. A light-responsive TiO2/Ag3PO4 nanocomposite immobilized in a spherical polymeric matrix consisting of polysulfone and alginate was recently prepared for the adsorption of different wastewater pollutants like MB, and two emerging pharmaceutical contaminants with significant results.148 Recent studies on heavy metal ion and dye adsorption from wastewater taking advantage of the light-responsivity of polymers are reviewed in the following.Spiropyrans, spirooxazines, oxazines, oxazolidines, azobenzene, boron-dipyrromethene (BODIPY), and other compounds have been widely investigated for the preparation of light-responsive polymers due to their unique features. Spiropyrans are a major group of photochromic materials, which show reversible structural conversion upon the irradiation of ultraviolet light, which results in the formation of an extended conjugated open form of merocyanine (MC).144,149–151 Its adsorption mechanism is based on the negatively charged phenolic oxygen atom in the MC form of a spiropyran which could bind to a metal center. For instance, a methacrylate-based spiropyran-containing copolymer was synthesized by Locklin and coworkers51 to adsorb multiple metal ions. Spin-coated thin methacrylate-based spiropyran-containing copolymers were prepared to investigate the spiropyran's detection behavior. The multiple metal ions’ adsorption processes were evaluated through the MC structure changes due to the hybrid resonance between the two forms of the charged zwitterionic and neutral quinoid. Herein, metal ions are connected to the MC structure via a negatively charged phenolate group of the zwitterionic state (Fig. 6(A)). The results of this study showed that it is possible to design sensors using spiropyran derivatives to identify other metal ions. Photochromic spiropyran-doped silk fibroin poly(ethylene oxide) (PEO) nanofibers have recently been prepared.52 In this work, by irradiating the reversible photoconverting spiropyran alternately with UV and visible lights, the polar MC counterpart was formed. Interactions of the MC molecules with heavy metal ions facilitated the adsorption process. To evaluate the ability of MC phenolate to interact with positively charged species, the response of silk fibroin (SF)/PEO/SP nanofibers to adsorb heavy metal ions (Zn2+, Hg2+, Pb2+, and Cu2+) was investigated. Adsorption of Zn2+ is conducted due to its strong interactions with the SF/PEO matrix. Cu2+, Hg2+, and Pb2+ showed similar adsorption capacities ranging from 0.012 to 0.40 mmol g−1, whereas the adsorption of Zn2+ was 50% lower than the other ions. A photochromic poly(vinyl alcohol) sponge was prepared from spiropyran and poly(vinyl alcohol) sponge using Steglich esterification53 (Fig. 6(C)). The adsorption capacity of the sponge in the light-induced removal of Pb2+ from aqueous solution was 5.78 mg g−1. In addition, regeneration of the sponge to repel Pb2+ was carried out by rinsing it with water under visible light irradiation, which was accompanied by a significant change in the color in the whole process. The adsorption capacity of the sponge can remain over 85% after ten cycles. The selective fluorometric detection of cyanide anions (CN−) in water at room temperature through a poly(N-isopropylacrylamide-co-chitosan) system has also been investigated.152Fig. 6(B) illustrates the structural change of poly(N-isopropylacrylamide-co-chitosan) during the fluorometric CN− sensing, recovery, and regeneration sequence. The CN− determination in total water in the range of 0.5–50 μM has been accomplished in this study. The adsorption mechanism behind the study is the fact that the prepared polymer is simply recovered by heating the solution followed by centrifugation. The CN− removal from the recovered polymer has also been carried out by simple acid treatment. Another study has investigated spiropyran-containing polymer brushes as photoswitchable optical sensors, which show selectivity for various metal ions and drastic changes in surface wettability.153 A spiropyran monomer was copolymerized with MMA from glass substrates using a surface-grafted ATRP initiator. Complexation of the MC form with different metal ions resulted in a metal ion dependent decrease in absorbance and a significant blue shift in the absorbance maxima. The polymer-coated glass substrates showed metal ion selectivity which is observable with the naked eye. With a similar structure to spiropyran, oxazolidine molecules have also been used in ion detection and removal from aqueous solutions.154–156 He and coworkers reported methacrylic acid (MAA) copolymerization with a fluorescent BODIPY monomer via RAFT polymerization, and used the product in the sensitive and selective recognition of Fe3+.54 They reported a detection limit of 1.2 μM even with a BODIPY content of 0.5%, which provides a method to develop water soluble high performance polymer sensors.
 | ||
| Fig. 6 (A) Chemical structure of spiropyran and its corresponding switchability by exposing it to light to adsorb metal ions,51 Copyright 2011, Reproduced with permission from American Chemical Society (ACS); (B) schematic illustration of the light-responsivity behavior of poly(N-isopropylacrylamide-co-chitosan) during the fluorometric CN− sensing, recovery, and regeneration sequence,152 Copyright 2011, Reproduced with permission from American Chemical Society (ACS); and (C) removal of Pb2+ from water using the photochromic poly(vinyl alcohol) sponge prepared from spiropyran and poly(vinyl alcohol),53 Copyright 2021, Reproduced with permission from Elsevier Ltd. | ||
Briefly, light-responsive free polymers, nanoparticles, nanofibers, and membranes are used for the removal of contaminants from water. In these polymeric products, adsorption of ions may cause variations in the fluorescence intensity or wavelength, UV absorbance intensity or wavelength, and states of emission or coloration by ion or dye adsorption. Supramolecular light-responsive polymer structures could also be applicable in ion removal systems. Ion-removal in such polymers could be accompanied by fluorescence or photochromism quenching or intensification or even resonance energy transfer in fluorescence emission or photochromism. Such mechanisms are also applicable in chemosensors, smart inks, and bioimaging in addition to water treatment applications. The most interesting feature of these systems is their coloration upon ion-adsorption, which could be a signal for recognition of a specific ion or dye. Such ion indicators could also work on the basis of fluorometric evaluations in addition to the calorimetric investigations. In most of the cases, visible light irradiation is a common source in the regeneration process. High selectivity, efficiency, and repeatability could be achieved in ion or dye removal from aqueous systems by this category of smart polymers.
2.5. Other responsive polymers
Purification of wastewater includes a series of chemical processes that can be conducted via other responsive polymers to eliminate pollutants.157,158 Population explosion and the expansion of urban areas increased adverse impacts on water resources, which necessitates other responsive polymers to be involved in the treatment of wastewater streams. Other responsive polymeric systems for decontamination of wastewater include magnetic-responsive composites,59,60,157,159–165 salt-responsive polymers,162 and potential-responsive polymers.61,62 In this regard, Sahiner and coworkers160 prepared a 2-acrylamide-2-methyl-1-AMPS hydrogel containing magnetic iron particles as a magnetic-responsive composite, and used it for the removal of toxic metal ions, such as Cd(II), Co(II), Fe(II), Pb(II), Ni(II), Cu(II), and Cr(III) from wastewater. Fig. 7 schematically illustrates the magnetic-responsive particle and its application for metal ion removal. Herein, ionization and polar interactions are due to –SO3H and –CONH2 groups, which are also responsible for the swelling behavior of the hydrogel. Interactions between the hydrogel and metal ions originate from ion–ion and dipole–ion pairs. The adsorption capacity of the system depends on the extent of the magnetic responsiveness of metal nanoparticles which interact with the hydrogel. The sequence for the adsorption of magnetic particles by this composite is Cd(II) > Pb(II) > Co(II) > Cu(II) > Ni(II) > Fe(II) > Cr(III). | ||
| Fig. 7 Schematic illustration for the magnetic-responsive AMPS hydrogel structure and its utilization for metal ion removal. This composite was used for toxic metal ion removal from wastewater through ion–ion and dipole–ion interactions.160 Copyright 2009, Reproduced with permission from Elsevier Ltd. | ||
Poly(2-acrylamide-2-methyl-1-propanesulfonic acid-co-vinyl imidazole) (P(AMPS-co-VI)) hydrogels are the other magnetic-responsive polymers used for wastewater treatment.59 By incorporating Fe(II) and Fe(III) ions into the P(AMPS-co-VI) hydrogel's network, magnetic-responsive hydrogel nanocomposites were prepared and used for the selective removal of toxic metal ions, like Cu(II), Cd(II), Fe(II), and Pb(II). Desorption studies were executed to evaluate the ability of these nanocomposite hydrogels as a reusable tool for toxic metal ion removal. Comparing PAMPS and PVI adsorption behavior showed that the hydrogels have significant selectivity for metal ion adsorption. The PAMPS hydrogels have a higher binding ability to Fe(II) than Cu(II) and more selectivity for Fe(II). The reusability investigation of the absorbents showed that hydrogels can be tailored for specific uptake of one component. For example, while the PAMPS hydrogel has more selectivity for Fe(II), the PVI hydrogel has higher selectivity for Cu(II). The results showed that the desorption step can be repeated up to 5 cycles allowing these reusable materials to be used in environmental applications. Magnetic Fe3O4@carbon nanofibers (Fe3O4@CNFs) based on polybenzoxazine precursors were synthesized to adsorb the MB and RhBdyes from aqueous solutions.60 These Fe3O4 nanocrystal-impregnated carbon fibers exhibited excellent magnetic separation performance. The results showed excellent removal of both MB and RhB dyes within 15 and 25 min by Fe3O4@CNFs, which can be attributed to the porous structure of the nanofibers. Moreover, the aromatic structure of dyes allows the formation of π–π interaction between the dyes and π-conjugation of the CNFs, which is the driving force behind the adsorption process. Shen and coworkers166 synthesized Fe3O4 magnetic nanoparticles with different average particle sizes to treat wastewater streams contaminated with heavy metal ions, such as Ni(II), Cu(II), Cd(II), and Cr(VI). The adsorption percentage of Cu2+, Cr6+, Cd2+, and Ni2+ at pH 4.0 was 99.8, 97.6, 84.7, and 88.5, respectively. Recently, poly(maleic anhydride)-graft-poly(vinyl alcohol)-modified magnetic nanoparticles were obtained as an efficient adsorbent of Ag+, Ni2+, Cd2+, Pb2+, and Co2+ ions from water.167 High sustainability, easy reusability, and facile regeneration were reported for this system.
The other technology used for wastewater treatment is based on electropotential-responsive polymers. Hao and coworkers61 reported an electropotential-responsive hybrid film system, which includes a layered α-zirconium phosphate (α-ZrP) nanosheet and a polyaniline (PANI)-intercalated chain. The nitrogen atoms in the PANI chains showed a high electroactivity due to the protons on the electro-negative α-ZrP nanosheets and their ion interchange. The mechanism of ion capture is based on the change of operating potential to the electrode to catch heavy metals onto the film from solutions. The negatively-charged inorganic nanosheets were shaped via electrophoretic deposition. In addition, a conducting polymer was formed through electro-initiated polymerization on the anode. The PANI chains are reduced at an electrode with a potential of −0.2 V. Therefore, they carry away the protons from the α-ZrP sheets, resulting in hydrated Ni2+ ions imbedded into the electronegative layered α-ZrP gallery. On the other hand, when the film potential achieved the maximum value of 0.8 V, it caused weight loss in the electrode. Consequently, the PANI chains were oxidized, resulting in the hydrated Ni2+ ions which were released from the layered α-ZrP gallery to the solution. The same mechanism is valid for capturing Cd2+ and Pb2+ ions by α-ZrP/PANI hybrid films. Catching the Cd2+ and Ni2+ onto ZrP is facilitated by outer-sphere complex formation, whereas the Pb2+ uptake happens due to the inner-sphere complex formation with ZrP. It is demonstrated that the α-ZrP/PANI hybrid films can remove heavy metals through the potential-responsive behavior in the presence of other cations, which is vital for industrial applications. Wang and coworkers62 also reported an electropotential-responsive material for wastewater treatment. Electropotential-responsive ion-exchange nanomaterials (MPiX) and γ-Fe2O3@nickel hexacyanoferrate (NiHCF) were synthesized to remove Cs+ from water. This three-dimensionally ordered macroporous (3DOM) MPiX exhibited rapid ion insertion because of the open and interconnected porous channels and high affinity of NiHCF to Cs+. This work has shown that the Cs+ adsorbed 3DOM MPiX could be regenerated by changing the potential of a magnetic electrode in an electromagnetic coupling regeneration system. The capacity of the 3DOM MPiX toward Cs+ is nearly over 98%, which remained constant in each cycle. The maximum removal capacity for Cs+ was reported as 204 mg g−1. The 3DOM MPiX illustrates a higher efficiency for Cs+ removal compared to the other metal ions. The Cs+ removal efficiency was not improved by the addition of Li+, Na+, and K+, showing that 3DOM MPiX can selectively capture Cs+ from the mixed ion solution. In another excellent and interesting work, reactive dyes were adsorbed from wastewater through hydrogen bonding and van der Waals interactions.61 Due to the covalent bonding between the –OH groups of poly(vinyl alcohol) (PVA) and the vinyl sulfone groups of reactive dyes (RB5), the crosslinking process occurs. All the dyes’ adsorption processes are represented in Fig. 8. Due to covalent bonding between the functional groups of electropotential-responsive polymers and RB5, polymers were crosslinked by dyes because of the nucleophilic addition reaction. Moreover, there is another possible reaction for dye adsorption through the van der Waals interaction.
 | ||
| Fig. 8 Adsorption mechanism of the crosslinking-induced precipitation of PVA from wastewater through the hydrogen bonding and van der Waals interactions,61 Copyright 2014, Reproduced with permission from the Royal Society of Chemistry (RSC). | ||
2.6. Dual- and multi-stimuli-responsive polymers
Dual and multi-responsive polymers respond to more than one stimuli, and have an important role in the stimuli-purification of wastewater and stimuli-regeneration processes.168 The recently published studies in this subject are reviewed in the following. To obtain polymers with sensitivity towards both temperature and pH, monomers of temperature-sensitive polymers and pH-sensitive monomers are combined in a polymer structure.156,169,170 These dual pH- and temperature-responsive polymers have found large applications in the removal of different ions and dyes. For example, Özkahraman and coworkers63 synthesized poly(NIPAAm-co-itaconic acid) hydrogels by free radical polymerization to remove cationic dyes from aqueous solutions. The synthesized hydrogels were temperature- and pH-responsive due to the nature of the used monomers. To determine the effect of temperature on swelling, the LCST of the hydrogel was calculated in the range of 33.7–36.5 °C. It was observed that increasing the content of the hydrophilic comonomer caused the LCST to shift up to 40–50 °C. A sharp decrease in the adsorption capacity occurred at such high temperatures. At high pH values, ionization of the COOH groups caused higher adsorption capacities. In a highly alkaline medium, the sodium ions cannot be dissociated from the carbonyl groups, which largely decreased the adsorption capacity value. The higher number of COO− groups in the solution caused higher interaction between the dyes and the hydrogel. Strong hydrophilic COO− groups facilitate the adsorption of cationic dyes. In another study, the synthesis of a poly(acrylic acid)-bentonite-FeCo (PAA-B-FeCo) hydrogel nanocomposite was carried out by Sonawane and coworkers.64 The strength and stability of this hydrogel allows its application in the removal of harmful ions in wastewater treatment. The response of the nanocomposite hydrogel was investigated using the cationic dye crystal violet (CV) at different temperatures and pH values. The optimum temperature and pH were 35 °C and 11, respectively, as the hydrogel was prone to high adsorption of dyes due to the easy dissociation of COO− ions at higher pH values. The adsorption of the dye was increased by increasing the temperature. The hydrogel network was relaxed at higher temperatures, which resulted in easy diffusion of the CV molecules into the hydrogel and increased the adsorption rate. The maximum removal efficiency of about 87% was achieved at 35 °C. Dissociation of the COOH groups of acrylic acid to COO− ions at high pH values resulted in higher adsorption of the CV dye from wastewater. Therefore, electrostatic attraction between the positively charged dyes and the negatively charged COO− ions led to an increase in the dye removal.There are many research studies on the simultaneous adjustment of pH and temperature to remove ions and dyes from water.171–173 Vishalakshi and coworkers synthesized a gellan gum-graft poly(DMAEMA) hydrogel (GG-g-PDMAEMA) using a microwave irradiation technique.65 The presence of PDMAEMA endows the hydrogel with pH- and temperature-responsive characters. In this study, GG-g-PDMAEMA was studied to remove MO dyes from wastewater. The suitability of GG-g-PDMAEMA as a potential adsorbent for the removal of anionic dyes from dye-polluted water samples was appreciable. The maximum adsorption capacity was achieved at pH 3, which may be attributed to the electrostatic interaction between the protonated tertiary amino groups of the polymer chains and the negative charge of the MO dyes. Thermodynamic calculations demonstrate that the adsorption is exothermic and non-spontaneous at high temperatures. Śliwa and coworkers66 reported microgels of the NIPAAm copolymer with 1-vinyl imidazole (P(NIPAAm-co-Vim)) for the uptake and release of a dye (Orange II) using its pH and temperature responsive behavior. The zeta potential measurements demonstrated that P(NIPAAm-co-Vim) shows a positive charge at pH 4 and a negative charge at pH 9. The efficiency of dye loading by the microgel was 87% at pH 4 at room temperature. Variation of pH from 4 to 9 caused the complete release of the loaded dye from the microgel. In addition, the copolymer particles become more hydrophobic upon increasing the temperature above the LCST. The pH value was highly increased at temperatures of around the LCST. The high potency of P(NIPAAm-co-Vim) to catch Orange II originates from electrostatic attractions at low pH values. By swelling the polymer network at temperatures below the LCST, leaking of the Orange II dye from the particles was observed. The other significant result in this study is that the dye bonded to P(NIPAAm-co-Vim) particles at pH 4 was entirely removable by switching the pH value to 9. Liu and coworkers67 also synthesized a dual-responsive graphene oxide/poly(NIPAAm-co-AA) hydrogel as an adsorbent for RhB and Imidacloprid. Xiao and coworkers68 reported the removal of MB and methyl violet dyes from wastewater using cellulose microfilaments/poly(NIPAAm-co-AA) spheres. Similar to the other reports, the negatively-charged groups adsorbed the cationic dyes through electrostatic interactions. The regeneration process was also conducted based on their thermo-responsive properties. Dyes were released by increasing the temperature up to 60 °C. Yang and coworkers reported the flocculation of heavy metal ions and antibiotics from livestock wastewater by a dual pH- and temperature-responsive flocculant, carboxymethyl chitosan-graft-poly(N-isopropyl acrylamide-co-diallyl dimethyl ammonium chloride).69 The flocculation performance of this polymer was evaluated for copper(II) and tetracycline at different temperatures and pH values.
Dual light- and temperature-responsive and also light- and pH-responsive polymers could also be applicable in wastewater treatment applications. Dual light- and temperature-responsive polymers are prepared by incorporation of light-responsive moieties into the temperature-responsive polymer structures.174,175 In addition, dual light- and pH-responsive polymers are prepared by insertion of light-responsive compounds into the pH-responsive polymer structure.176 The synergistic effects of light and temperature, light-controlling of temperature responsivity or temperature-controlling of light-responsivity, may be useful in the removal of ions and dyes and subsequent regeneration of the absorbent systems. The light-responsivity of dual light- and temperature-responsive polymers could control the LCST of the polymer and consequently its hydrophilicity, which plays a key role in the removal of contaminants and regeneration of the polymers. Such a behavior could be more complex in dual light- and pH-responsive polymers, which requires a more detailed investigation.
Multi-responsive polymers shed light on the wastewater treatment methods. PNIPAAm-grafted chitosan/Fe3O4 composite particles as a pH- and temperature-responsive magnetic adsorbent were designed for the removal of nonylphenol from water,70 as shown in Fig. 9(A). The responses to pH and temperature were used in surface-charge variation and also for adjusting hydrophilic/hydrophobic characteristics, respectively. The composite particles presented relatively high adsorption capacities for nonylphenol from water (123 mg g−1 at pH 9 and 20 °C; 116 mg g−1 at pH 5 and 40 °C). Such a high adsorption capacity, high selectivity, and reusability of the composite particles showed their high application potential in the removal of nonylphenol. Removal of the RhB dye was reported by multiple stimuli-responsive organic/inorganic hybrid hydrogels of poly(2-(2-methoxy ethoxy)ethyl methacrylate-co-oligo (ethylene glycol) methacrylate-co-AA) (PMOA) loaded with magnetic attapulgite/Fe3O4 (AT-Fe3O4) nanoparticles.71 The capability of this complex pH-, temperature-, and magnetic field-responsive hydrogel in the dye adsorption process was investigated. The PMOA/AT-Fe3O4 hydrogel with an LCST of about 33 °C was used to study the effect of temperature on dye adsorption at 25, 30, and 35 °C. The adsorption of the dye was significantly increased by increasing the temperature from 25 to 30 °C, and then was slowly decreased. By increasing the temperature to 30 °C, removal of RhB reached 95%, which is attributed to the polymer chain extension in the hydrogel network at temperatures below the LCST. On the other hand, a lower adsorption efficiency was observed due to shrinking of the hydrogel at temperatures above the LCST. The hydrogels containing the RhB dyes could be separated under the application of magnetic fields. The magnetic-responsive characteristics of the hydrogel allow its directional movement. Investigation of the effect of pH on dye adsorption in the pH range of 3.29 to 9.15 showed that the adsorption capacity increased due to the dissociation of the COOH group's by increasing the pH. At low pH values, a very low dissociation of the –COOH groups of the hydrogel was observed.71 These external stimulants were also used in different polymers by Tian and coworkers.177 The pH, temperature, and magnetic triple-responsive poly(NIPAAm-co-methacrylic acid) porous microspheres (PMS) were synthesized and used for the adsorption of Cu2+. The pH-responsive characteristics of the Fe3O4/PMS were investigated by determination of the hydrodynamic diameter (Dh) in the aqueous solution. The Dh value of the microspheres increased with increasing pH, because of the protonation of the PMAA's carboxyl groups. The effect of temperature on these microspheres was also studied by UV–vis spectroscopy, and the results showed that the transmittance of the microspheres was constantly decreased by increasing the temperature at pH 1.4. The transmittance was rapidly decreased by changing the temperature within the range of 32 to 33 °C, which indicates that the microspheres showed a temperature-responsive feature controlled by the pH value of the solution. Fig. 9(B) shows the effect of pH on Cu2+ adsorption by the Fe3O4/PMS complex system. The adsorption capacity was gradually increased from pH 3 to 4, which shows that such triple-responsive porous polymeric microspheres can be used as smart adsorbents in water treatment applications.
 | ||
| Fig. 9 (A) Nonylphenol adsorption from water using PNIPAAm-grafted chitosan/Fe3O4 composite particles as a pH- and temperature-responsive magnetic adsorbent,70 Copyright 2015, Reproduced with permission from American Chemical Society (ACS); (B) schematic illustration for the effect of pH on Cu2+ adsorption by the multi-responsive Fe3O4/PMS system,177 Copyright 2016, Reproduced with permission from John Wiley & Sons Ltd; (C) schematic illustration of the pH-, ion, and temperature-responsive polymer adsorbent through the ionization of carboxyl groups and protonation of imidazole groups,74 Copyright 2017, Reproduced with permission from American Chemical Society (ACS); and (D) structure illustration of (a) the core-crosslinked hybrid nanoparticles prepared based on hyperbranched poly(ether amine) and (b) the core-crosslinked hybrid particles obtained by hydrolyzing the amine groups in the molecules,75 Copyright 2012, Reproduced with permission from John Wiley & Sons Ltd. | ||
IPN hydrogel microspheres were synthesized using temperature-sensitive crosslinked PNIPAAm and pH-sensitive crosslinked PMAA.73 Herein, the hydrogel's temperature- and pH-responsive behavior was evaluated by measuring the variation of the hydrodynamic diameter. The Fe3O4 nanoparticles were also incorporated into the IPN hydrogel microspheres to endow them with magnetic properties. The adsorption/desorption behavior of different dyes and then the separation of the dye-loaded magnetic hydrogel microspheres from the aqueous media were investigated under a magnetic field. The volume phase transition in this pH-responsive hydrogel resulted from the protonation and deprotonation of carboxylic acid groups at lower and higher pH values. Moreover, investigating the temperature-responsive volume phase transition of this hydrogel showed that the formation of inter/intramolecular hydrogen bonding among the carboxyl and amide groups at low pH values resulted in its UCST character. On the other hand, the breakdown of inter/intramolecular hydrogen bonding at higher pH values (pH 10) cancelled out the LCST behavior. The magnetic IPN hydrogel microspheres were utilized to remove organic dyes from aqueous solutions at pH 10. The hydrophobic nature of the dye favored both adsorption and recovery of the dyes from dye aqueous solution as the operation temperature was altered above and below the LCST. An electrostatic repulsion between the anionic dye and hydrogel microspheres was reported while the cationic dye achieved maximum adsorption from the dye solution above the LCST. On the other hand, the recovery was insignificant at temperatures below the LCST due to the electrostatic attraction with the negatively charged microspheres. Shan and coworkers74 have recently synthesized pH-, ion-, and thermo-responsive poly(NIPAAm-co-maleic acid-co-VI) (P(NIPAM-MA-VI)) adsorbents by free radical polymerization to remove Cu2+ ions from wastewater, as shown in Fig. 9(C). The ion-adsorbed P(NIPAM-MA-VI) can be regenerated using hydrochloric acid and be reused in the adsorption process. Ionization of the carboxyl groups was increased by increasing the pH, which resulted in a stronger electrostatic repulsion. These changes in the polymer structure caused the polymer chains to stretch hard and form a cluster. This behavior is responsible for the shift of the LCST to a higher temperature. In consequence, more hydrogen bonding was established between the polymer chains and water molecules. In this situation, imidazole groups were protonated and some of the carboxyl groups got ionized. Therefore, interactions between the imidazole and carboxylate ions in the solution make it difficult for the polymer chains to aggregate. The imidazole groups got ionized with few carboxylate ions at pH 1, and the repulsive interactions among the imidazole cations caused collapse of the polymer gel. The carboxyl and imidazole groups can form complexes with heavy metal ions. Each Cu2+ ion attracts four imidazole groups or two negatively-charged carboxyl groups by a chelate connection. A higher pH value is favorable for Cu2+ adsorption due to the deprotonation of imidazole and ionization of the carboxyl groups, enhancing the adsorption ability of polymer chains. The maximum adsorption capacity was obtained as 21.1 mg g−1 at 60 °C and a pH of 5.
Core/shell polymer nanoparticles are also nominated for application in water treatment. In this regard, inverse micelles with a hydrophilic core and a hydrophobic shell could encapsulate dyes and then remove them from wastewater streams generated in the textile and paper industries.75 Jiang and coworkers75 synthesized various core-crosslinked hybrid nanoparticles based on hyperbranched poly(ether amine), which exhibited sharp multi-response to temperature, pH, and ionic strength in an aqueous solution. As shown in Fig. 9(D), the core-crosslinked hybrid particles were obtained after the hydrolysis of the amine groups. These nanoparticles are responsive to pH because of the tertiary amine groups that can get protonated or deprotonated at different pH values. Moreover, the responsivity of the nanoparticles to ionic strength was also evaluated via UV–vis spectroscopy. These nanoparticles showed a strong response to ionic strength in an aqueous solution due to the typical salting-out effect. They also studied the adsorption capability of five synthesized core-crosslinked hybrid nanoparticles onto twelve hydrophilic dyes in water. Dyes can be divided into three types: anionic, cationic, and neutral. The nanoparticles established a host–guest interaction with the guest dyes with varying strength. The results showed that by increasing the hydrophobic chains of the nanoparticles, their interaction with the guest dyes increases, regardless of the charge states of the dyes and the nanoparticles. The electrostatic interaction has a minimal effect on the host–guest interaction.
Other types of multi-responsive polymers also show highly efficient adsorption capacity for both cationic and anionic dyes and heavy metal ions in wastewater.76 Ampholytic polyelectrolyte microspheres were used to examine the adsorption capacity of the cationic dye, MB, and the anionic dye, Congo Red (CR), as well as the metal ions of Cu(II) and Cr(III) from aqueous solutions. The adsorption capacity was gradually increased by increasing the contact time for both the dyes and metal ions. The adsorption capacity of microspheres for MB and CR was reduced by strengthening the NaCl concentration. This result showed that the adsorption process takes place even in high ionic strength media. The effect of pH was also investigated, which shows that the adsorption capacity for CR decreases due to the electrostatic repulsion between the free sulfate groups and CR at low pH values. Enhancement of electrostatic interactions between chitosan and Carrageenan (CRG) due to the protonation of amino groups on chitosan increased the adsorption capacity of MB. By increasing the pH value, electrostatic interactions between chitosan and CRG were observed. Experimental results showed that the ampholytic microspheres were changed to positively charged chitosan and negatively charged metal ions in wastewater because of their strong electrostatic and chelating affinities. Moreover, the microspheres can be recycled, reused, and biodegraded in soil, showing great potential in water purification.
Multi-responsive polymers in the form of gel nanoparticles, core–shell structures, and grafted polymers to substrates are highly applicable for water treatment applications. Controlling adsorption and regeneration processes by different stimuli makes it easy to remove any contaminant from water by molecular interaction mechanisms (e.g. by pH, light, temperature, etc.) and then collect the contaminant-loaded small size polymers by their aggregation (e.g. by magnetic field, temperature, etc.). Therefore, although the design and synthesis of such multi-responsive polymers are complicated, their controlling parameters may result in the easy removal of dyes, ions, etc. from the water and then the regeneration of the absorbent. In the case of membranes and fibrous adsorbents, there would not be any requirement for aggregation of the adsorbent, but instead the need for physical desorption in the regeneration process makes it necessary to use multi-responsive polymers. Finally, selectivity, repeatability, and high efficiency for contaminant removal are still the highly important parameters which should be considered.
3. Conclusion, future, and challenges
Water is one of the most important natural resources and its quality is crucial for human life. During the last few decades, environmental issues related to the chemical and biological contaminations of water have become an important concern. Therefore, numerous systems have been introduced for the purification of water. Stimuli-responsive polymers, with high selectivity and strong affinity for targeted chemicals, have particularly high potential for water treatment applications. This review focuses on smart polymers which are able to treat water and provide clean water from low-quality sources. The development of highly-efficient response mechanisms and stimuli-responsive polymers for water purification applications is the current challenge in this field. The important challenge of water treatment is its highly-expensive process and operation. While developments in polymer chemistry may solve numerous important challenges in industrial applications, the process of combining, producing, and deploying polymer products is very complex, expensive, and time-consuming. The easily synthesized smart hydrogels, aerogels, porous nanoparticles, substrates, membranes, and fibers containing responsive polymers have been considered to widely address this problem in water treatment. Most of the reviewed papers take advantage of smart polymers to be combined or modified to produce advanced materials with relevant features for water treatment with increased pollutant removal efficiencies. The other issue with the water treatment processes is the regeneration of the removal systems. Stimuli-responsive polymers could solve such problems by their reversibility to different stimuli. Stimuli-adsorption and desorption of ions and dyes from water could be the main beneficial features of the smart polymers in water treatment in the future. Among different stimuli, light as a physical stimulus will largely be considered since it is non-destructive, easily available, and low in cost, and can be applied locally and externally. It is shown that aggregation is needed for the polymer particles, nano and microgels, hybrid nanoparticles, and core–shell particles. However, fibrous polymers or polymer membranes could solve this issue in the future. Taking these factors into consideration, we remain positive about the future potential of stimuli-responsive polymers as selective adsorbents for water treatment and a wide variety of other applications. In the future, biopolymers including chitosan, lignin, modified cellulose and its derivatives, and also their combination with other smart polymers would be able to play a crucial role in wastewater treatment applications. A process that is selective, efficient, repeatable, and has a low limit of detection for all harmful contaminants should be chosen for water purification.Abbreviations
| AA | Acrylic acid |
| AAB18C6 | Acryloylamidobenzo-18-crown-6 |
| AAm | Acrylamide |
| AAM/MPS | 2-Acrylamide-2-methyl propane sulfonic acid |
| AMPS | 2-Acrylamido-2-methyl-1-propanesulfonic acid |
| AMPs | Methylpropane sulfonic acid |
| ATRP | Atom transfer radical polymerization |
| α-ZrP | α-Zirconium phosphate |
| BCAm | Benzo-18-crown-6-acrylamide |
| BPA | Bisphenol A |
| CNC | Crystalline nanocellulose |
| CNF | Cellulose nanofibril |
| CR | Congo red |
| CRG | Carrageenan |
| CV | Crystal violet |
| ELP | Elastin-like polypeptides |
| HPP-NH3+ | Poly(maleic anhydride-alt-styrene) |
| IPN | Interpenetrated networks |
| LCST | Lower critical solution temperature |
| MB | Methylene blue |
| MC | Merocyanine |
| MG | Malachite green |
| MO | Methyl orange |
| MPiX | Ion-exchange nanomaterials |
| PAA | Poly(acrylic acid) |
| PAAm | Polyacrylamide |
| PANI | Polyaniline |
| PDMAEMA | Poly(2-(dimethylamino)ethyl methacrylate) |
| PEI | Polyethyleneimine |
| PEO | Poly(ethylene oxide) |
| PHEA-HIS | Histamine pendant copolymer |
| PMAA | Poly(methacrylic acid) |
| PMOA | Poly(2-(2-methoxy ethoxy)ethyl methacrylate-co-oligo (ethylene glycol) methacrylate-co-acrylic acid) |
| PMS | Porous microspheres |
| PNIPAAm | Poly(N-isopropyl acrylamide) |
| POSS | Polyhedral oligomeric silsesquioxane |
| PS | Potato starch |
| PVA | Poly(vinyl alcohol) |
| PVI | Poly(vinylimidazole) |
| Qe | Adsorption capacity |
| RAFT | Reversible addition-fragmentation chain transfer |
| RhB | Rhodamine B |
| SF | Silk fibroin |
| Vim | 1-Vinylimidazole |
| VPTT | Volume phase transition temperature |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the Iran National Science Foundation (Project No. 99021685).References
- J. F. J. R. Pesqueira, M. F. R. Pereira and A. M. T. Silva, J. Cleaner Prod., 2020, 261, 121078 CrossRef CAS.
- Z. Song, C. Williams and R. G. Edyvean, Desalination, 2004, 164, 249–259 CrossRef CAS.
- G. Lettinga, Antonie van Leeuwenhoek, 1995, 67, 3–28 CrossRef CAS PubMed.
- M. Zhang, Z. Zhang, Y. Peng, L. Feng, X. Li, C. Zhao and K. Sarfaraz, Int. J. Biol. Macromol., 2020, 156, 289–301 CrossRef CAS PubMed.
- Y. Li, M. Li, K. Xiao and X. Huang, J. Cleaner Prod., 2020, 246, 118964 CrossRef CAS.
- E. Forgacs, T. Cserháti and G. Oros, Environ. Int., 2004, 30, 953–971 CrossRef CAS PubMed.
- H. Chen, H. Xu, T. M. Heinze and C. E. Cerniglia, J. Ind. Microbiol. Biotechnol., 2009, 36, 1459–1466 CrossRef CAS PubMed.
- A. H. Khan, N. A. Khan, S. Ahmed, A. Dhingra, C. P. Singh, S. U. Khan, A. A. Mohammadi, F. Changani, M. Yousefi, S. Alam, S. Vambol, V. Vambol, A. Khursheed and I. Ali, J. Cleaner Prod., 2020, 269, 122411 CrossRef CAS.
- O. J. Hao, H. Kim and P.-C. Chiang, Crit. Rev. Environ. Sci. Technol., 2000, 30, 449–505 CrossRef CAS.
- F. Fu and Q. Wang, J. Environ. Manage., 2011, 92, 407–418 CrossRef CAS PubMed.
- M. Lee, I. S. Paik, I. Kim, H. Kang and S. Lee, J. Hazard. Mater., 2007, 144, 208–214 CrossRef CAS PubMed.
- A. Jusoh, L. Su Shiung, N. Ali and M. J. M. M. Noor, Desalination, 2007, 206, 9–16 CrossRef CAS.
- J. Landaburu-Aguirre, V. García, E. Pongrácz and R. L. Keiski, Desalination, 2009, 240, 262–269 CrossRef CAS.
- E. Samper, M. Rodríguez, M. A. De la Rubia and D. Prats, Sep. Purif. Technol., 2009, 65, 337–342 CrossRef CAS.
- X. Li, G.-M. Zeng, J.-H. Huang, C. Zhang, Y.-Y. Fang, Y.-H. Qu, F. Luo, D. Lin and H.-L. Liu, J. Membr. Sci., 2009, 337, 92–97 CrossRef CAS.
- K. Dutta and S. De, J. Mater. Chem. A, 2017, 5, 22095–22112 RSC.
- H. Namdar, A. Akbari, R. Yegani and H. Roghani-Mamaqani, J. Environ. Chem. Eng., 2021, 9, 104685 CrossRef CAS.
- H. F. M. Austria, T. M. Subrahmanya, O. Setiawan, J. Widakdo, Y.-H. Chiao, W.-S. Hung, C.-F. Wang, C.-C. Hu, K.-R. Lee and J.-Y. Lai, J. Mater. Chem. A, 2021, 9, 21510–21531 RSC.
- A. Hai, K. Rambabu, B. Govindan, F. Banat and M. Naushad, in Smart Polymer Nanocomposites, Elsevier, 2021, pp. 313–350 Search PubMed.
- G. Kocak, C. Tuncer and V. Bütün, Polym. Chem., 2017, 8, 144–176 RSC.
- H. Hemmatpour, V. Haddadi-Asl and H. Roghani-Mamaqani, Polymer, 2015, 65, 143–153 CrossRef CAS.
- H. Hemmatpour, V. Haddadi-Asl, F. Khanipour, M. C. A. Stuart, L. Lu, Y. Pei, H. Roghani-Mamaqani and P. Rudolf, Eur. Polym. J., 2022, 180, 111583 CrossRef CAS.
- R. Hoogenboom, in Smart Polymers and their Applications, Elsevier, 2019, pp. 13–44 Search PubMed.
- M. F. Cunningham and P. G. Jessop, Macromolecules, 2019, 52, 6801–6816 CrossRef CAS.
- Z. Abousalman-Rezvani, P. Eskandari, H. Roghani-Mamaqani, H. Mardani and M. Salami-Kalajahi, Polymer, 2019, 182, 121830 CrossRef.
- Y. Liu, H. Zhong, X. Li, Z. Bao, Z. Cheng, Y. Zhang and C. Li, Environ. Technol., 2020, 1–13 Search PubMed.
- Y. Qin, L. Wang, C. Zhao, D. Chen, Y. Ma and W. Yang, ACS Appl. Mater. Interfaces, 2016, 8, 16690–16698 CrossRef CAS PubMed.
- B. Xu, H. Zheng, H. Zhou, Y. Wang, K. Luo, C. Zhao, Y. Peng and X. Zheng, J. Mol. Liq., 2018, 256, 424–432 CrossRef CAS.
- I. S. Saha, Mater. Sci. Eng., C, 2019, 104, 109894 CrossRef PubMed.
- H. Kaşgöz, A. Durmuş and A. Kaşgöz, Polym. Adv. Technol., 2008, 19, 213–220 CrossRef.
- A. M. Atta, H. S. Ismail and A. M. Elsaaed, J. Appl. Polym. Sci., 2012, 123, 2500–2510 CrossRef CAS.
- E. Ramírez, S. G. Burillo, C. Barrera-Díaz, G. Roa and B. Bilyeu, J. Hazard. Mater., 2011, 192, 432–439 CrossRef PubMed.
- Z. Li, Y. Wang, N. Wu, Q. Chen and K. Wu, Environ. Sci. Pollut. Res., 2013, 20, 1511–1525 CrossRef CAS PubMed.
- N. Hou, R. Wang, F. Wang, J. Bai, T. Jiao, Z. Bai, L. Zhang, J. Zhou and Q. Peng, Colloids Surf., A, 2019, 579, 123670 CrossRef CAS.
- R. Singh, D. Pal, A. Mathur, A. Singh, M. A. Krishnan and S. Chattopadhyay, React. Funct. Polym., 2019, 144, 104346 CrossRef.
- P. Bidarakatte Krishnappa and V. Badalamoole, Int. J. Biol. Macromol., 2019, 122, 997–1007 CrossRef CAS PubMed.
- S. A. Ali, I. W. Kazi and N. Ullah, Ind. Eng. Chem. Res., 2015, 54, 9689–9698 CrossRef CAS.
- T. A. Saleh, S. A. Haladu and S. A. Ali, Chem. Eng. J., 2015, 269, 9–19 CrossRef CAS.
- J. Sánchez, C. Espinosa, F. Pooch, H. Tenhu, G. d. C. Pizarro and D. P. Oyarzún, React. Funct. Polym., 2018, 127, 67–73 CrossRef.
- B. Liu, X. Chen, H. Zheng, Y. Wang, Y. Sun, C. Zhao and S. Zhang, Carbohydr. Polym., 2018, 181, 327–336 CrossRef CAS PubMed.
- S. A. Ali, I. B. Rachman and T. A. Saleh, Chem. Eng. J., 2017, 330, 663–674 CrossRef CAS.
- J. Tang, Y. Song, F. Zhao, S. Spinney, J. da Silva Bernardes and K. C. Tam, Carbohydr. Polym., 2019, 208, 404–412 CrossRef CAS PubMed.
- S.-A. Safavi-Mirmahalleh, M. Salami-Kalajahi and H. Roghani-Mamaqani, Environ. Sci. Pollut. Res., 2020, 27, 28091–28103 CrossRef CAS PubMed.
- Y. Duan, Y. Song and L. Zhou, J. Colloid Interface Sci., 2019, 546, 351–360 CrossRef CAS PubMed.
- W.-P. Zhu, J. Gao, S.-P. Sun, S. Zhang and T.-S. Chung, J. Membr. Sci., 2015, 487, 117–126 CrossRef CAS.
- N. B. Tran, J. Y. Kim, Y.-C. Kim, Y. J. Kim and J.-H. Kim, J. Appl. Polym. Sci., 2016, 133, 43305 Search PubMed.
- Y. Bai, Y. N. Liang and X. Hu, Chemosphere, 2017, 185, 1157–1163 CrossRef CAS PubMed.
- Z. Abousalman-Rezvani, P. Eskandari, H. Roghani-Mamaqani and M. Salami-Kalajahi, Carbohydr. Polym., 2019, 225, 115247 CrossRef PubMed.
- L. Yang, Y. Zhan, R. Yu, J. Lan, J. Shang, B. Dou, H. Liu, R. Zou and S. Lin, ACS Appl. Mater. Interfaces, 2021, 13, 2694–2709 CrossRef CAS PubMed.
- S. Fan, J. Chen, C. Fan, G. Chen, S. Liu, H. Zhou, R. Liu, Y. Zhang, H. Hu, Z. Huang, Y. Qin and J. Liang, J. Hazard. Mater., 2021, 416, 126225 CrossRef CAS PubMed.
- K. H. Fries, J. D. Driskell, G. R. Sheppard and J. Locklin, Langmuir, 2011, 27, 12253–12260 CrossRef CAS PubMed.
- M. E. Genovese, G. Caputo, G. Nanni, C. Setti, M. Bustreo, G. Perotto, A. Athanassiou and D. Fragouli, ACS Appl. Mater. Interfaces, 2017, 9, 40707–40715 CrossRef CAS PubMed.
- G. Liu, M. Wang, H. Gao, C. Cui and J. Gao, Eur. Polym. J., 2021, 161, 110828 CrossRef CAS.
- S. He, L. Xiao, L. Marin, Y. Bai and X. Cheng, Eur. Polym. J., 2021, 150, 110428 CrossRef CAS.
- X.-J. Ju, S.-B. Zhang, M.-Y. Zhou, R. Xie, L. Yang and L.-Y. Chu, J. Hazard. Mater., 2009, 167, 114–118 CrossRef CAS PubMed.
- J. J. Chen, A. L. Ahmad and B. S. Ooi, J. Environ. Chem. Eng., 2013, 1, 339–348 CrossRef CAS.
- D. Parasuraman and M. J. Serpe, ACS Appl. Mater. Interfaces, 2011, 3, 2732–2737 CrossRef CAS PubMed.
- Z. Liu, F. Luo, X.-J. Ju, R. Xie, Y.-M. Sun, W. Wang and L.-Y. Chu, J. Mater. Chem. A, 2013, 1, 9659 RSC.
- O. Ozay, S. Ekici, Y. Baran, S. Kubilay, N. Aktas and N. Sahiner, Desalination, 2010, 260, 57–64 CrossRef CAS.
- Y. Si, T. Ren, Y. Li, B. Ding and J. Yu, Carbon, 2012, 50, 5176–5185 CrossRef CAS.
- Z. Wang, Y. Feng, X. Hao, W. Huang and X. Feng, J. Mater. Chem. A, 2014, 2, 10263–10272 RSC.
- Z. Wang, S. Guo, Z. Wu, H. Fan, G. Guan and X. Hao, Sep. Purif. Technol., 2017, 187, 199–206 CrossRef CAS.
- B. Özkahraman, I. Acar and S. Emik, Polym. Bull., 2011, 66, 551–570 CrossRef.
- S. R. Shirsath, A. P. Hage, M. Zhou, S. H. Sonawane and M. Ashokkumar, Desalination, 2011, 281, 429–437 CrossRef CAS.
- J. S. Karthika and B. Vishalakshi, Int. J. Biol. Macromol., 2015, 81, 648–655 CrossRef CAS PubMed.
- T. Śliwa, M. Jarzębski, E. Andrzejewska, M. Szafran and J. Gapiński, React. Funct. Polym., 2017, 115, 102–108 CrossRef.
- G. Yao, S. Li, J. Xu and H. Liu, J. Chem. Eng. Data, 2019, 64, 4054–4065 CrossRef CAS.
- Y. Li, H. Xiao, Y. Pan, M. Zhang and Y. Jin, J. Hazard. Mater., 2019, 377, 88–97 CrossRef CAS PubMed.
- Z. Yang, S. Jia, N. Zhuo, W. Yang and Y. Wang, Chemosphere, 2015, 141, 112–119 CrossRef CAS PubMed.
- Y. Zhen, Z. Ning, Z. Shaopeng, D. Yayi, Z. Xuntong, S. Jiachun, Y. Weiben, W. Yuping and C. Jianqiang, ACS Appl. Mater. Interfaces, 2015, 7, 24446–24457 CrossRef CAS PubMed.
- Z. Yuan, Y. Wang, X. Han and D. Chen, J. Appl. Polym. Sci., 2015, 132, 42244 CrossRef.
- J. Wang, W. Zhang, Y. Qian, B. Deng and W. Tian, Macromol. Mater. Eng., 2016, 301, 1132–1141 CrossRef CAS.
- H. Ahmad, M. Nurunnabi, M. M. Rahman, K. Kumar, K. Tauer, H. Minami and M. A. Gafur, Colloids Surf., A, 2014, 459, 39–47 CrossRef CAS.
- J. Cheng, G. Shan and P. Pan, Ind. Eng. Chem. Res., 2017, 56, 1223–1232 CrossRef CAS.
- R. Wang, B. Yu, X. Jiang and J. Yin, Adv. Funct. Mater., 2012, 22, 2606–2616 CrossRef CAS.
- X. Liang, J. Duan, Q. Xu, X. Wei, A. Lu and L. Zhang, Chem. Eng. J., 2017, 317, 766–776 CrossRef CAS.
- M. N. Chong, B. Jin, C. W. K. Chow and C. Saint, Water Res., 2010, 44, 2997–3027 CrossRef CAS PubMed.
- I. Oller, S. Malato and J. A. Sánchez-Pérez, Sci. Total Environ., 2011, 409, 4141–4166 CrossRef CAS PubMed.
- S. A. Snyder, S. Adham, A. M. Redding, F. S. Cannon, J. DeCarolis, J. Oppenheimer, E. C. Wert and Y. Yoon, Desalination, 2007, 202, 156–181 CrossRef CAS.
- S. A. Snyder, E. C. Wert, D. J. Rexing, R. E. Zegers and D. D. Drury, Ozone: Sci. Eng., 2006, 28, 445–460 CrossRef CAS.
- H. Guo, S. Luo, L. Chen, X. Xiao, Q. Xi, W. Wei, G. Zeng, C. Liu, Y. Wan, J. Chen and Y. He, Bioresour. Technol., 2010, 101, 8599–8605 CrossRef CAS PubMed.
- P. Theato, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 6677–6687 CrossRef CAS.
- A. Nath and P. P. Pande, Adv. Sci., Eng. Med., 2020, 12, 105–107 CrossRef CAS.
- P. Mohammadzadeh Pakdel and S. J. Peighambardoust, Carbohydr. Polym., 2018, 201, 264–279 CrossRef CAS PubMed.
- P. Mohammadzadeh Pakdel and S. J. Peighambardoust, J. Environ. Manage., 2018, 217, 123–143 CrossRef CAS PubMed.
- S. Shahi, H. Roghani-Mamaqani, S. Talebi and H. Mardani, Polym. Chem., 2022, 13, 161–192 RSC.
- S. Shahi, H. Roghani-Mamaqani, S. Talebi and H. Mardani, Coord. Chem. Rev., 2022, 455, 214368 CrossRef CAS.
- S. Shahi, H. Roghani-Mamaqani, R. Hoogenboom, S. Talebi and H. Mardani, Chem. Mater., 2022, 34, 468–498 CrossRef CAS.
- J. R. Koduru, R. R. Karri and N. M. Mubarak, in Sustainable Polymer Composites and Nanocomposites, Springer International Publishing, Cham, 2019, pp. 759–781 Search PubMed.
- P. Eskandari, H. Roghani-Mamaqani, M. Salami-Kalajahi and Z. Abousalman-Rezvani, J. Mol. Liq., 2020, 310, 113234 CrossRef CAS.
- W. A. Laftah, S. Hashim and A. N. Ibrahim, Polym.-Plast. Technol. Eng., 2011, 50, 1475–1486 CrossRef CAS.
- X. Zhou, Y. Guo, F. Zhao, W. Shi and G. Yu, Adv. Mater., 2020, 32, 2007012 CrossRef PubMed.
- J. Cai, D. Zhang, W. Xu, W.-P. Ding, Z.-Z. Zhu, J.-R. He and S.-Y. Cheng, J. Agric. Food Chem., 2020, 68, 9725–9732 CrossRef CAS PubMed.
- M. Akter, M. Bhattacharjee, A. K. Dhar, F. B. A. Rahman, S. Haque, T. U. Rashid and S. M. F. Kabir, Gels, 2021, 7, 30 CrossRef CAS PubMed.
- S. Fallah, H. R. Mamaghani, R. Yegani, N. Hajinajaf and B. Pourabbas, Adv. Compos. Hybrid Mater., 2020, 3, 187–193 CrossRef CAS.
- F. S. A. Khan, N. M. Mubarak, Y. H. Tan, R. R. Karri, M. Khalid, R. Walvekar, E. C. Abdullah, S. A. Mazari and S. Nizamuddin, Environ. Sci. Pollut. Res., 2020, 27, 43526–43541 CrossRef CAS PubMed.
- P. Bober, I. M. Minisy, U. Acharya, J. Pfleger, V. Babayan, N. Kazantseva, J. Hodan and J. Stejskal, Synth. Met., 2020, 260, 116266 CrossRef CAS.
- A. M. Atta, Y. M. Moustafa, A. O. Ezzat and A. I. Hashem, Nanomaterials, 2019, 10, 71 CrossRef PubMed.
- P. Eskandari, Z. Abousalman-Rezvani, H. Roghani-Mamaqani and M. Salami-Kalajahi, J. Mol. Liq., 2020, 318, 114301 CrossRef CAS.
- V. Adavan Kiliyankil, B. Fugetsu, I. Sakata, Z. Wang and M. Endo, J. Colloid Interface Sci., 2021, 582, 950–960 CrossRef CAS PubMed.
- M. Hadid, H. Noukrati, H. Ben youcef, A. Barroug and H. Sehaqui, Cellulose, 2021, 28, 7893–7908 CrossRef CAS.
- I. Sohail, I. A. Bhatti, A. Ashar, F. M. Sarim, M. Mohsin, R. Naveed, M. Yasir, M. Iqbal and A. Nazir, J. Mater. Res. Technol., 2020, 9, 498–506 CrossRef CAS.
- C. Wei, L. Lin, Y. Zhao, X. Zhang, N. Yang, L. Chen and X. Huang, ACS Appl. Mater. Interfaces, 2020, 12, 19130–19139 CrossRef CAS PubMed.
- M. B. Wazir, M. Daud, F. Ali and M. A. Al-Harthi, J. Mol. Liq., 2020, 315, 113775 CrossRef CAS.
- N. Puri, A. Gupta and A. Mishra, J. Cleaner Prod., 2021, 322, 129051 CrossRef CAS.
- J. Hu, G. Zhang, Z. Ge and S. Liu, Prog. Polym. Sci., 2014, 39, 1096–1143 CrossRef CAS.
- A. S. Hoffman, Adv. Drug Delivery Rev., 2012, 64, 18–23 CrossRef.
- G. Yao, W. Bi and H. Liu, Colloids Surf., A, 2020, 588, 124393 CrossRef CAS.
- G. Sharma, B. Thakur, A. Kumar, S. Sharma, M. Naushad and F. J. Stadler, Carbohydr. Polym., 2020, 241, 116258 CrossRef CAS PubMed.
- S. Bhattacharya, F. Eckert, V. Boyko and A. Pich, Small, 2007, 3, 650–657 CrossRef CAS PubMed.
- S. Dai, P. Ravi and K. C. Tam, Soft Matter, 2008, 4, 435 RSC.
- W. Wang, J. Hu, R. Zhang, C. Yan, L. Cui and J. Zhu, Cellulose, 2021, 28, 897–909 CrossRef CAS.
- W. Xiao, B. Yan, H. Zeng and Q. Liu, Carbon, 2016, 105, 655–664 CrossRef CAS.
- F. Banisheykholeslami, M. Hosseini and G. Najafpour Darzi, Int. J. Biol. Macromol., 2021, 177, 306–316 CrossRef CAS PubMed.
- Y. Wang and Q. Lu, Cellulose, 2020, 27, 2173–2187 CrossRef CAS.
- C. Chen, J. Kang, J. Shen, S. Zhao, B. Wang, Z. Chen and Q. Chen, Chemosphere, 2021, 262, 127836 CrossRef CAS PubMed.
- L. Luan, B. Tang, S. Ma, L. Sun, W. Xu, A. Wang and Y. Niu, J. Mol. Liq., 2021, 330, 115634 CrossRef CAS.
- B. K. Preetha and B. Vishalakshi, J. Environ. Chem. Eng., 2020, 8, 103608 CrossRef CAS.
- E. M. Ahmed, J. Adv. Res., 2015, 6, 105–121 CrossRef CAS PubMed.
- U. Yildiz, Ö.F Kemik and B. Hazer, J. Hazard. Mater., 2010, 183, 521–532 CrossRef CAS PubMed.
- Y. Tian, M. Wu, R. Liu, Y. Li, D. Wang, J. Tan, R. Wu and Y. Huang, Carbohydr. Polym., 2011, 83, 743–748 CrossRef CAS.
- J. Wang, F. Liu and J. Wei, Polym. Bull., 2011, 67, 1709–1720 CrossRef CAS.
- E. Ramírez, S. G. Burillo, C. Barrera-díaz, G. Roa and B. Bilyeu, J. Hazard. Mater., 2011, 192, 432–439 CrossRef PubMed.
- E. S. Dragan and D. F. Apopei, Chem. Eng. J., 2011, 178, 252–263 CrossRef CAS.
- M. Sakthivel, D. S. Franklin and S. Guhanathan, Ecotoxicol. Environ. Saf., 2016, 134, 427–432 CrossRef CAS PubMed.
- Z. A. Jamiu, T. A. Saleh and S. A. Ali, J. Hazard. Mater., 2017, 327, 44–54 CrossRef CAS PubMed.
- S. A. Ali, O. C. S. Al Hamouz and N. M. Hassan, J. Hazard. Mater., 2013, 248–249, 47–58 CrossRef CAS PubMed.
- T. A. Saleh, S. A. Haladu and S. A. Ali, Chem. Eng. J., 2015, 269, 9–19 CrossRef CAS.
- S. A. Ali, I. Y. Yaagoob, M. A. J. Mazumder and H. A. Al-Muallem, J. Hazard. Mater., 2019, 369, 642–654 CrossRef CAS PubMed.
- L. Yang, Y. Zhan, Y. Gong, E. Ren, J. Lan, R. Guo, B. Yan, S. Chen and S. Lin, J. Hazard. Mater., 2021, 405, 124194 CrossRef CAS PubMed.
- L. M. Scott, T. Robert, J. R. Harjani and P. G. Jessop, RSC Adv., 2012, 2, 4925 RSC.
- J. Y. Quek, P. J. Roth, R. A. Evans, T. P. Davis and A. B. Lowe, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 394–404 CrossRef CAS.
- A. Darabi, P. G. Jessop and M. F. Cunningham, Chem. Soc. Rev., 2016, 45, 4391–4436 RSC.
- J. R. Moon, B. S. Kim and J. H. Kim, Bull. Korean Chem. Soc., 2006, 27, 981–985 CrossRef CAS.
- Y. Bai, Y. N. Liang and X. Hu, Chemosphere, 2017, 185, 1157–1163 CrossRef CAS PubMed.
- Y.-H. Chen and F.-A. Li, J. Colloid Interface Sci., 2010, 347, 277–281 CrossRef CAS PubMed.
- M. Cao, Y. Shen, Z. Yan, Q. Wei, T. Jiao, Y. Shen, Y. Han, Y. Wang, S. Wang, Y. Xia and T. Yue, Chem. Eng. J., 2021, 405, 126647 CrossRef CAS.
- J. Kostal, A. Mulchandani and W. Chen, Macromolecules, 2001, 34, 2257–2261 CrossRef CAS.
- J. Kostal, A. Mulchandani, K. E. Gropp and W. Chen, Environ. Sci. Technol., 2003, 37, 4457–4462 CrossRef CAS PubMed.
- K. Yamashita, T. Nishimura and M. Nango, Polym. Adv. Technol., 2003, 14, 189–194 CrossRef CAS.
- K. Yamashita, T. Nishimura, K. Ohashi, H. Ohkouchi and M. Nango, Polym. J., 2003, 35, 545–550 CrossRef CAS.
- D. Parasuraman and M. J. Serpe, ACS Appl. Mater. Interfaces, 2011, 3, 4714–4721 CrossRef CAS PubMed.
- J. Tan, S. Xie, G. Wang, C. W. Yu, T. Zeng, P. Cai and H. Huang, Polymer, 2020, 12, 151 CAS.
- N. Shao, Y. Zhang, S. Cheung, R. Yang, W. Chan, T. Mo, K. Li and F. Liu, Anal. Chem., 2005, 77, 7294–7303 CrossRef CAS PubMed.
- S. Venkata Mohan, N. Chandrasekhar Rao, K. Krishna Prasad and J. Karthikeyan, Waste Manag., 2002, 22, 575–582 CrossRef CAS.
- H. Roghani-Mamaqani and Z. Tajmoradi, Photoresponsive Polymers, in Smart Stimuli-Responsive Polymers, Films, and Gels, Wiley-VCH GmbH, 2022, ch. 2 Search PubMed.
- H. Wang, Y. Wu, M. Feng, W. Tu, T. Xiao, T. Xiong, H. Ang, X. Yuan and J. W. Chew, Water Res., 2018, 144, 215–225 CrossRef CAS PubMed.
- C. T. Mehmood, Z. Zhong, H. Zhou, C. Zhang and Y. Xiao, RSC Adv., 2020, 10, 36349–36362 RSC.
- A. Abdollahi, H. Roghani-Mamaqani and B. Razavi, Prog. Polym. Sci., 2019, 98, 101149 CrossRef CAS.
- A. Abdollahi, K. Sahandi-Zangabad and H. Roghani-Mamaqani, Langmuir, 2018, 34, 13910–13923 CrossRef CAS PubMed.
- A. Abdollahi, H. Alidaei-Sharif, H. Roghani-Mamaqani and A. Herizchi, J. Mater. Chem. C, 2020, 8, 5476–5493 RSC.
- Y. Shiraishi, S. Sumiya, K. Manabe and T. Hirai, ACS Appl. Mater. Interfaces, 2011, 3, 4649–4656 CrossRef CAS PubMed.
- K. Fries, S. Samanta, S. Orski and J. Locklin, Chem. Commun., 2008, 6288 RSC.
- B. Razavi, H. Roghani-Mamaqani and M. Salami-Kalajahi, ACS Appl. Mater. Interfaces, 2022, 14, 41433–41446 CrossRef CAS PubMed.
- B. Razavi, H. Roghani-Mamaqani and M. Salami-Kalajahi, J. Mol. Struct., 2023, 1271, 134021 CrossRef.
- J. Zhang, L.-Y. Chu, Y.-K. Li and Y. M. Lee, Polymer, 2007, 48, 1718–1728 CrossRef CAS.
- X. Hu, Y. Hu, G. Xu, M. Li, Y. Zhu, L. Jiang, Y. Tu, X. Zhu, X. Xie and A. Li, Environ. Res., 2020, 180, 108796 CrossRef CAS PubMed.
- S. Yadav, A. Asthana, R. Chakraborty, B. Jain, A. K. Singh, S. A. C. Carabineiro and M. A. B. H. Susan, Nanomaterials, 2020, 10, 170 CrossRef CAS PubMed.
- G. Bayramoğlu and M. Y. Arica, J. Hazard. Mater., 2007, 144, 449–457 CrossRef PubMed.
- O. Ozay, S. Ekici, Y. Baran, N. Aktas and N. Sahiner, Water Res., 2009, 43, 4403–4411 CrossRef CAS PubMed.
- L. Uzun, A. Kara, B. Osman, E. Yılmaz, N. Beşirli and A. Denizli, J. Appl. Polym. Sci., 2009, 114, 2246–2253 CrossRef CAS.
- H. Chen, J. Yang, S. Xiao, R. Hu, S. M. Bhaway, B. D. Vogt, M. Zhang, Q. Chen, J. Ma, Y. Chang, L. Li and J. Zheng, Acta Biomater., 2016, 40, 62–69 CrossRef CAS PubMed.
- I. Safarik, L. F. T. Rego, M. Borovska, E. Mosiniewicz-Szablewska, F. Weyda and M. Safarikova, Enzyme Microb. Technol., 2007, 40, 1551–1556 CrossRef CAS.
- I. Safarik and M. Safarikova, Phys. Procedia, 2010, 9, 274–278 CrossRef CAS.
- Y. Zhang, S. Xu, Y. Luo, S. Pan, H. Ding and G. Li, J. Mater. Chem., 2011, 21, 3664 RSC.
- Y. F. Shen, J. Tang, Z. H. Nie, Y. D. Wang, Y. Ren and L. Zuo, Sep. Purif. Technol., 2009, 68, 312–319 CrossRef CAS.
- X. Liu, J. Guan, G. Lai, Q. Xu, X. Bai, Z. Wang and S. Cui, J. Cleaner Prod., 2020, 253, 119915 CrossRef CAS.
- Y. Li, D. Xie, J. Xiao, W. Wu, L. Zhang, H. Xiao and J. Chen, J. Cleaner Prod., 2020, 258, 120867 CrossRef CAS.
- E. Zeinali, V. Haddadi-Asl and H. Roghani-Mamaqani, RSC Adv., 2014, 4, 31428–31442 RSC.
- M. Haqani, H. Roghani-Mamaqani and M. Salami-Kalajahi, Cellulose, 2017, 24, 2241–2254 CrossRef CAS.
- H.-Y. Yu, W. Li, J. Zhou, J.-S. Gu, L. Huang, Z.-Q. Tang and X.-W. Wei, J. Membr. Sci., 2009, 343, 82–89 CrossRef CAS.
- K. Pan, X. Zhang, R. Ren and B. Cao, J. Membr. Sci., 2010, 356, 133–137 CrossRef CAS.
- B. Özkahraman, I. Acar and S. Emik, Clean: Soil, Air, Water, 2011, 39, 658–664 Search PubMed.
- A. Abdollahi, H. Roghani-Mamaqani, B. Razavi and M. Salami-Kalajahi, Polym. Chem., 2019, 10, 5686–5720 RSC.
- F. D. Jochum and P. Theato, Chem. Soc. Rev., 2013, 42, 7468–7483 RSC.
- D. Schmaljohann, Adv. Drug Delivery Rev., 2006, 58, 1655–1670 CrossRef CAS PubMed.
- J. Wang, W. Zhang, Y. Qian, B. Deng and W. Tian, Macromol. Mater. Eng., 2016, 301, 1132–1141 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2022 |




