Recent development of a magneto-optical nanoplatform for multimodality imaging of pancreatic ductal adenocarcinoma
Xuan
Zhang
ab,
Zhiming
Zeng
ab,
Huiyi
Liu
a,
Li
Xu
a,
Xin
Sun
c,
Jing
Xu
*b and
Guosheng
Song
 *a
*a
aState Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha 410082, P. R. China. E-mail: songgs@hnu.edu.cn
bDepartment of Ophthalmology and Otolaryngology, National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha 410008, P. R. China. E-mail: xujing1981@csu.edu.cn
cCollege of Mechanical and Electrical Engineering, Central South University, Changsha 410083, P. R. China
First published on 26th January 2022
Abstract
Pancreatic ductal adenocarcinoma (PDAC) is the most common type of pancreatic cancer. Given its inconspicuous and atypical early symptoms and hidden location, most patients have already reached the terminal stage before diagnosis. At present, the diagnosis of PDAC mainly depends on serological and imaging examinations. However, serum tests cannot identify specific tumor locations and each imaging technology has its own defects, bringing great challenges to the early diagnosis of PDAC. Therefore, it is of great significance to find new strategies for the early and accurate diagnosis of PDAC. In recent years, a magneto-optical nanoplatform integrating near infrared fluorescence, photoacoustic, magnetic resonance imaging, etc. has attracted widespread attention, giving full play to the complementary advantages of each imaging modality. Herein, we summarize the recent advances of imaging modalities in the diagnosis of pancreatic cancer, and then discuss in detail the construction and modification of magneto or/and optical probes for multimodal imaging, and advances in early diagnosis using the combination of various imaging modalities, which can provide potential tools for the early diagnosis or even intraoperative navigation and post-treatment follow-up of PDAC patients.
1. Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the most prevalent and aggressive primary malignancy of the pancreas, with an overall 5-year survival rate of less than 10%, making it one of the deadliest types of cancers in the world.1,2 In the United States, pancreatic carcinoma is estimated to cause over 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 deaths in both genders in 2021, ranked third after pulmonary cancer, breast or prostate cancer and colorectal cancer.3 The most important factor determining the prognosis of tumor patients is their clinical stage.1 Unfortunately, the majority of PDAC patients have already reached the terminal-stage before diagnosis, as a result of the inconspicuous and atypical early symptoms and hidden location of this type of malignant tumor.4,5 The 5-year survival rate of early-stage patients is approximately 60 times higher than that of terminal-stage patients.6 Therefore, improving the early diagnosis rate of PDAC patients is the key to increasing their five-year survival rate.7,8
000 deaths in both genders in 2021, ranked third after pulmonary cancer, breast or prostate cancer and colorectal cancer.3 The most important factor determining the prognosis of tumor patients is their clinical stage.1 Unfortunately, the majority of PDAC patients have already reached the terminal-stage before diagnosis, as a result of the inconspicuous and atypical early symptoms and hidden location of this type of malignant tumor.4,5 The 5-year survival rate of early-stage patients is approximately 60 times higher than that of terminal-stage patients.6 Therefore, improving the early diagnosis rate of PDAC patients is the key to increasing their five-year survival rate.7,8
At present, PDAC continues to exhibit a poor prognosis due to the difficulty in early diagnosis.9,10 The pancreas is a retroperitoneal organ located posterior to the stomach, between the spleen and the duodenum. Due to the interference of gases in the gastrointestinal tract, it is difficult to obtain clear and high-quality images of the early primary tumor and its distant metastasis accurately using the current clinical examination methods.11 Moreover, the present commonly used clinical diagnostic methods (serological and imaging examinations) have their own limitations. Specifically, CEA and CA19-9 are unable to determine the tumor location; in addition, the sensitivity and speciality of these two cancer antigens are not ideal.8,12,13 Computed tomography (CT) imaging has difficulties in detecting microtumors less than 1 cm in diameter, differentiating benign and malignant lesions, and displays the disadvantage of ionizing radiation.14,15 Magnetic resonance imaging (MRI) has the shortcomings of long scanning time, high cost and low sensitivity in the detection of PDAC.16 Endoscopic ultrasound imaging (EUS) has successfully detected PDAC microtumors in some areas, but is heavily dependent on the dexterity of the operator and the condition and cooperation of the patient, and has an average detection rate lower than 50% when microtumors are smaller than 1 cm in diameter.17,18 Moreover, contrast media used in CT and MRI scanning are nephrotoxic and can hardly enter PDAC tissue due to the high interstitial fluid pressure and low microvessel density.19–21 Therefore, it is of great significance to develop new strategies for the early and accurate diagnosis of PDAC.22,23
In order to overcome the above difficulties, various magneto-optical nanoplatforms integrating fluorescence imaging, photoacoustic imaging, magnetic resonance imaging, etc. have been designed.24 These magneto-optical nanoplatforms can specifically target PDAC cells via recognition of specific molecular receptors or the urokinase-type plasminogen activator receptor (uPAR) and cell-surface associated mucin 1 (MUC1), which are highly expressed specifically in PDAC cells.25 A magneto-optical nanoplatform can also be designed to respond to specific biomarkers, including pH, oxidative stress, metal ions, anoxia and specific enzymes such as matrix metalloproteinases (MMPs).19,26 Furthermore, the magneto-optical nanoplatform have a longer circulation time in blood compared to conventional contrast medium, along with the advantage of strong signal expression in lesions in multi-imaging modalities such as MRI, fluorescence imaging (FI) and photoacoustic imaging (PAI), thus achieving long term and high contrast multi-modality imaging of PDAC. This technology overcomes the limitations of traditional imaging examinations, showing higher sensitivity and specificity, therefore bringing hope to achieve a higher early and accurate diagnosis rate of PDAC, and offers a great value in intraoperative navigation and post-treatment follow-up of PDAC patients.
In this review, we summarized the advances in recent years regarding imaging modalities in the diagnosis of pancreatic cancer, and discussed in detail the construction and modification of probes for the magneto-optical nanoplatform, the advances and potential application of the magneto-optical nanoplatform in the early diagnosis of PDAC, as well as the future development and challenges of this novel imaging method (Fig. 1).
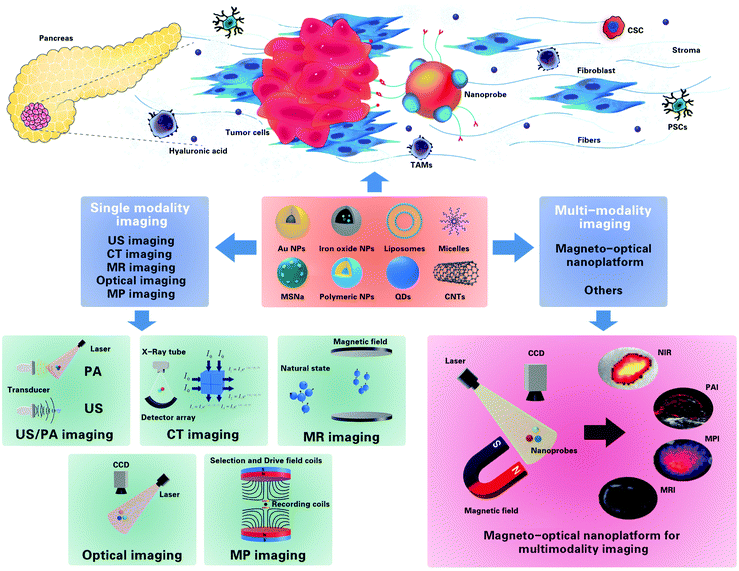 | ||
| Fig. 1 Illustration of various nanoparticles that can be easily modified or loaded with imaging agents for imaging of PDAC, and their use in various imaging modalities for the diagnosis of DPAC. | ||
2. Imaging modalities in the diagnosis of PDAC
Currently, there are no reliable blood markers that can diagnose early-stage PDAC, which is also often asymptomatic. Therefore, medical imaging plays an important role in the early diagnosis of PDAC. Common clinical imaging methods for the diagnosis of PDAC include ultrasound (US), CT, MRI, and EUS. In this part, we summarize the characteristics and limitations of the current imaging methods applied in the diagnosis of PDAC, and potential imaging modalities for the early diagnosis of PDAC, along with recent advances in nanomaterials modified for the single-modality imaging of PDAC (Fig. 2).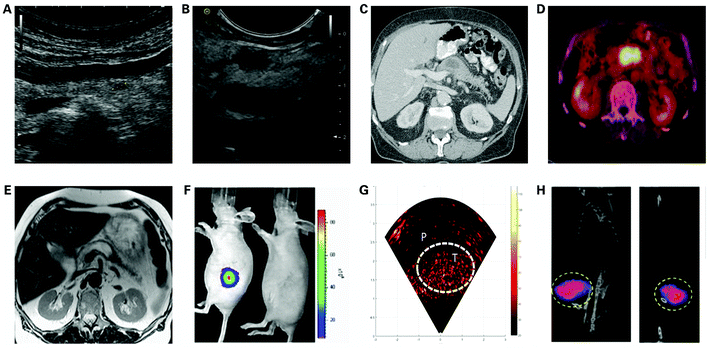 | ||
| Fig. 2 Single imaging modalities for the diagnosis of PDAC, including (A) transabdominal ultrasound imaging; (B) endoscopic ultrasound imaging. Reproduced with permission from ref. 120. Copyright 2020. KSUM; (C) computed tomography. Reproduced with permission from ref. 141. Copyright 2008. Elsevier; (D) positron-emission tomography/CT. Reproduced with permission from ref. 142. Copyright 2018. The Authors. Licensee IntechOpen; (E) magnetic resonance imaging. Reproduced with permission from ref. 141. Copyright 2008. Elsevier; (F) optical imaging-NIR. Reproduced with permission from ref. 143. Copyright 2015. Elsevier; (G) optical imaging-PAI. Reproduced with permission from ref. 144. Copyright 2019. Elsevier; (H) magnetic particle imaging. Reproduced with permission. Copyright 2019. ACS. | ||
2.1. Overview of imaging modalities in PDAC diagnosis
CT is often seen as the initial imaging method for evaluating patients suspected with PDAC, with an overall sensitivity of about 89% and specificity of around 90%.30 CT uses ionizing radiation or X-ray beams that rotate around and pass through the patient, in combination with an electronic detector array that records the detected density patterns and generates images of a “slice” or “cut” of tissues through complex reconstruction methods.31 CT is the best modality for the assessment of locoregional and nodal pancreatic tumors and has been validated as the reference standard. Moreover, triphasic cross-sectional imaging and the use of thin slices can be used to assess respectability and visualize important vessels and anatomic relationships. However, CT has the disadvantage of ionizing radiation, and PDAC microtumors are often overlooked on CT, with a reported sensitivity of only 58%–77% when masses are less than 2 cm in diameter.27,32 Positron-emission tomography (PET)/CT has been gradually applied to early diagnosis of PDAC, but remains a controversial modality, with the reported high detection rates of PDAC tumors being smaller than 2 cm diameter of 81–100%, yet also low sensitivity to 50% in other reported studies.33,34
Magnetic resonance imaging (MRI) is one of the most widely used imaging methods in hospitals and clinics for medical diagnosis, staging and follow-up of disease, with a reported PDAC diagnosis specificity equivalent to CT of about 89%.35 MRI is based on the excitement and detection of the change in the direction of the rotational axis of protons found in the water forming living tissues, with the advantages of high spatial resolution, unlimited tissue penetration and no involvement of X-rays or use of ionizing radiation, and therefore serves as a great alternative when imaging methods such as US, X-ray and CT cannot accurately detect lesions and assess disease progression, especially of soft tissues of the brain or abdomen.36 MRI can comprehensively obtain sectional images in any direction, three-dimensional volume images, and even four-dimensional images of spatial spectral distribution, including qualitative information such as the characteristics of anatomical structures of tissues and organs, tumor blood supply and tumor vascular distribution, and therefore can be used to accurately detect and locate tumors as well as monitor tumor growth. However, MRI has a limited value in distinguishing PDAC from mass-forming chronic pancreatitis, and as with CT imaging, MRI is weak in detecting microtumors.16
At present, PL is the most commonly used method in optical imaging. PL probes include quantum dots, lanthanide NPs, fluorescence dye, etc., amongst which fluorescence imaging is the most widely used in the clinic.42 The basic principle of fluorescence imaging is to inject a combination of optical nanoparticles and fluorescent dyes into cells or animals; the combination absorbs light at a certain wavelength range, and the electrons in optical nanoparticles are then excited to a higher and more unstable energy state, relaxes to their ground state and emits a photon of light of a specific wavelength range which can be detected using a fluorescence imaging system, so as to achieve tracking, and qualitative and quantitative analysis of optical nanoparticles.43
Chemiluminescence is a kind of optical radiation phenomenon where light is generated through chemiexcitation during the process of the chemical reaction, in which substances are activated by oxidation and form an unstable oxidized highly energetic intermediate, which decomposes or transfers energy to the neighboring fluorophores, and relaxes to its ground state, during which it emits luminescence.44,45 Unlike traditional fluorescence imaging methods, which suffer from photobleaching and autofluorescence, CL eliminates excitation light, thereby permitting deep-tissue imaging with ultrahigh sensitivity and no background noise from biological tissues.46–49
Raman spectroscopy (RS) has gained enormous interest as a physicochemical technique, due to its outstanding chemical specificity during identification and quantification of specific substances, providing vibration fingerprint-like spectra of chemical and biological materials without interference from water.50 However, non-resonant RS is a weak scattering technique, limiting its development in clinical imaging. Surface-enhanced Raman spectroscopy (SERS) can provide up to 108 enhancement of the Raman signal, allowing the detection of low concentration analytes, showing great potential as an excellent tool for clinical diagnosis.51
Photoacoustic imaging (PAI), also called optoacoustic imaging, is a novel biomedical imaging method based on the use of laser-generated ultrasound that has emerged over the last decade, which combines the advantages of optical imaging and ultrasound imaging, showing both high contrast and high spatial resolution in imaging, bringing great potential and expectation for the early diagnosis of PDAC.52–54 In PAI, based on the photoacoustic effect, modulated electromagnetic radiation is pulsed to biological tissue. The irradiated tissue then expands rapidly after absorption of laser energy due to thermoelasticity and excites broadband ultrasound waves, which provide high contrast, high temporal and spatial resolution, and non-invasive images.55–57 In contrast to fluorescence imaging, the acoustic signal of PAI is positively correlated with the optical absorption intensity of imaging tissues, instead of relying on the detection of emitted light from imaging tissues, providing PAI with a greater penetration depth than pure optical imaging modalities like FI.58–60
As a magnetic imaging modality, unlike MRI, magnetic particle imaging (MPI) directly maps the spatial distribution of magnetic nanoparticle contrast agents in vivo using a time-varying magnetic field, instead of indirectly mapping through the MRI signal.61 MPI exploits the magnetization characteristic of nanoparticle contrast agents to localize the spatial distribution of the nanoparticles, and therefore the sensitivity and resolution of MPI are determined by nanoparticle contrast agent characteristics, showing great potential of development with the advances of materials science. MPI also shows great potential in clinical imaging with a near-perfect contrast, and no obscuring background tissue, due to zero manifesting of the MPI signal from human tissue which is diamagnetic. Moreover, MPI is quantitative at any depth with low-frequency magnetic fields where there is zero depth attenuation.62–64 The above advantages make MPI an excellent alternative in PDAC diagnosis over standard imaging techniques CT and MRI.
2.2. Advances in nanomaterials for single-modality imaging of PDAC
Imaging examination plays an important role in the clinical diagnosis of PDAC, popular imaging techniques include CT, MRI, US and EUS. However, each imaging technology has its own defects, particularly in the detection of microtumors less than 1 cm in diameter or depending too much on the operator experience and patient condition, bringing great challenges to the early diagnosis of PDAC. With the use of contrast agents, healthy tissue and lesions can be better distinguished. However, traditional contrast agents suffer from several drawbacks and limitations, such as lack of specificity and organ toxicity, leading to a focus of attention from researchers on novel nanomaterials.65 Nanoprobes have the advantages of small size, strong targeting ability, great degradability, and little stimulation to biological tissues. They can also be synthesized with different coatings that can be modified to include various desirable functionalities, showing great potential as an auxiliary means for the early diagnosis of PDAC.66,67 Recent advances in nanoprobes for single-modality imaging of PDAC will be introduced in the following contents.Hyaluronic acid (HA) is a substance naturally produced by the human body, serves as a component of the extracellular matrix, is mainly produced to retain water and lubricate tissues, and participates in cell proliferation, healing of wounds, and cancer metastasis.78–80 Self-assembled HA nanoparticles have been extensively investigated by researchers as cancer diagnosis and treatment agents due to their biocompatibility, biodegradability and targeting characteristics.81,82 Qi et al. designed a PDAC targeted ICG nanoprobe termed NanoICG, by physiochemically entrapping indocyanine Green into hyaluronic acid derived nanoparticles, intended for the detection of pancreatic cancer83 (Fig. 3A–C). Contrast-enhancement of pancreatic cancer by NanoICG was verified in vivo, and unlike traditional contrast agents that can hardly enter the PDAC tissue, NanoICG can accumulate significantly in PDAC tissue through the EPR effect and demonstrate contrast-enhancement for pancreatic lesions relative to disease-free portions of the pancreas, with negligible cytotoxicity to healthy pancreatic epithelial cells and nearly no signs of chemotaxis or phagocytosis, suggesting that NanoICG is a promising potential contrast agent for the early detection of PDAC and intraoperative guidance of tumor removal.
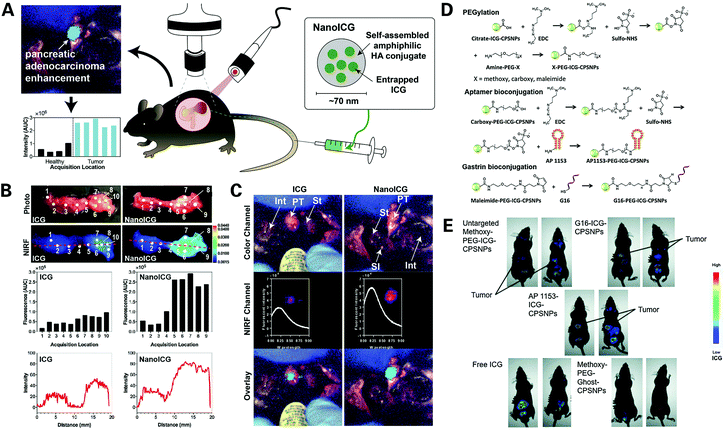 | ||
| Fig. 3 Novel ICG dye synthesized nanoprobes for the optical imaging of PDAC: (A) illustration of the structure and characterization of the NanoICG and general content of the experiment; (B) surgical navigation images of pancreatic tumor contrast-enhanced with ICG (left) or NanoICG (right) 24 h post intravascular injection, with NanoICG displaying a stronger signal; (C) ex vivo assessment of ICG and NanoICG accumulation in pancreatic tumor via photo images, NIRF images, analysis of the fluorescence intensity excited with medium laser power, and plots of NIRF image intensity values along the red dashed line. Reproduced with permission from ref. 83. Copyright 2018. Elsevier; (D) synthesis of targeted AP1153–ICG–CPSNPs; (E) assessment of CPSNP uptake by PANC-1 orthotopic tumors in vivo via whole-body NIR imaging 15 h post intravascular injection, regarding AP1153–ICG–CPSNPs, unloaded empty particles (methoxy–PEG–GHOST–CPSNPs), or free ICG. Reproduced with permission from ref. 86. Copyright 2017. Mary Ann Liebert. | ||
Calcium phosphosilicate nanoparticles (CPSNPs) were developed to deliver imaging agents and drugs for the diagnosis and treatment of human cancer.84,85 This material is an amorphous calcium phosphate, designed to encapsulate particles of various shapes and sizes, including chemotherapeutics and imaging agents such as indocyanine Green. This particle has the advantages of small size, being biocompatible and biodegradable, are able to remain intact in blood but dissolve intracellularly, making it a fine vehicle for carrying imaging probes and target ligands. Clawson et al. designed a novel nanoprobe termed AP1153-ICG-CPSNPs using CPSNPs doped with ICG and coupled with AP1153, a type of DNA aptamer (AP) that binds to and describes the characterization and targeting efficacy of the G-protein-coupled cholecystokinin B receptor (CCKBR), which is constitutively expressed in PDAC86 (Fig. 3D). The novel AP1153-ICG-CPSNPs were assessed in vivo showing enhanced cellular uptake of this nanoparticle in tumor cells compared to non-targeted ICGs and no uptake in the brain. The particles demonstrated high PDAC-selectivity, and therefore hold promise for the achievement of identifying precursor lesions and early pancreatic lesions before they progress to full-blown PDAC, as well as improving the chemotherapeutic treatments for PDAC patients, so as to improve the patient prognosis.
Albumin has attracted much attention as a carrier for nanoprobe integration.87,88 Human serum albumin (HSA) is a protein synthesized by the liver, consists of 585 amino acids, and is the most abundant protein in human blood plasma. HSA is widely used in the biotechnology industry, due to its characteristics of non-toxicity, non-immunogenicity and long blood circulation time.89 HSA contains several hydrophobic binding pockets, making it an ideal transporter for conjugating imaging probes and targeting ligands. Han et al. developed a novel enzyme-sensitive albumin-based gemcitabine (GEM) delivery nanoplatform termed HAS-GEM/IR780, by conjugating GEM to HSA, a safe natural carrier, and then complexing it with NIR dye IR780![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 (Fig. 4). The performance of the HAS-GEM/IR780 complex was tested in vitro and in vivo, and showed an enhanced accumulation and long-term retention over 72 hours compared to free IR780, along with superior tumor inhibition activity compared to free GEM, indicating that HAS-GEM/IR780 serves as a promising agent for the early detection and practical treatment of pancreatic tumors.
90 (Fig. 4). The performance of the HAS-GEM/IR780 complex was tested in vitro and in vivo, and showed an enhanced accumulation and long-term retention over 72 hours compared to free IR780, along with superior tumor inhibition activity compared to free GEM, indicating that HAS-GEM/IR780 serves as a promising agent for the early detection and practical treatment of pancreatic tumors.
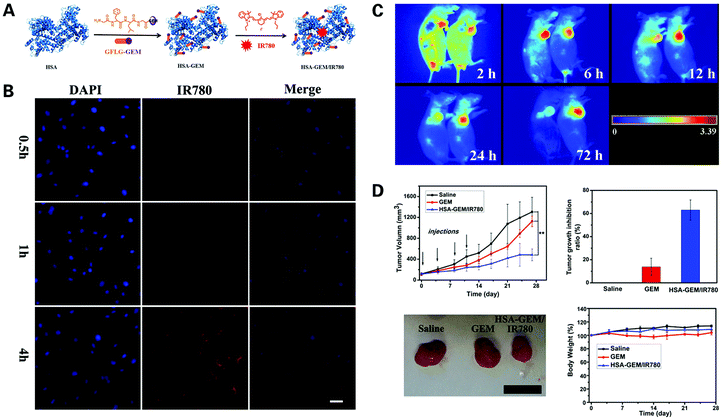 | ||
| Fig. 4 Novel IR780 dye synthesized nanoprobe for the optical imaging of PDAC: (A) synthesis of HAS–GEM/IR780 complex; (B) assessment of HAS–GEM/IR780 in vitro via confocal imaging for 0.5 h, 1 h and 4 h post incubation; (C) assessment of HAS–GEM/IR780 in vivo via NIR imaging post injection of free IR780 (left) and HAS–GEM/780 (right); (D) analysis of tumor growth in mice treated with saline, GEM, and HAS–GEM/IR780, respectively, with a scale bar of 2 cm. Reproduced with permission from ref. 90. Copyright 2017. Elsevier. | ||
Mesoporous nanomaterial with its high specific surface, unique pores volume and size is another novel material drawing great interest in diverse application fields including catalysis, drug delivery and imaging.91,92 Mesoporous silica is a recent development in nanotechnology, and as a mesoporous form of silica, it exhibits greater loading capacity, is easy to prepare and shows controllable particle and pore size.93,94 Mesoporous silica nanoparticles (MSN NPs) can be modified to attach imaging probes and targeting ligands, making it a great vehicle for imaging agent synthesis. Li et al. designed a novel biodegradable mesoporous silica nanoparticle under 100 nm in diameter termed bMSN@Cy7.5-FA NP as an imaging agent for the detection and quantification of tumor metastasis, by conjugating cyanine 7.5 NHS ester (Cy7.5) and folic acid (FA), a common target ligand that can selectively bind to folate receptors (FRs) overexpressed on the surface of tumor cells95 (Fig. 5). These new bMSN@Cy7.5-FA NPs were tested in vitro showing no toxicity, good biocompatibility and great targeted cellular uptake, accumulating in the cytoplasm of tumor cells. Then, the in vivo experiments showed significantly higher fluorescence intensity compared to non-targeted Cy7.5, with a strong signal intensity even after 12 h, indicating that bMSN@Cy7.5-FA NPs had good stability and excellent potential as agents for carrying out the early detection of PDAC.
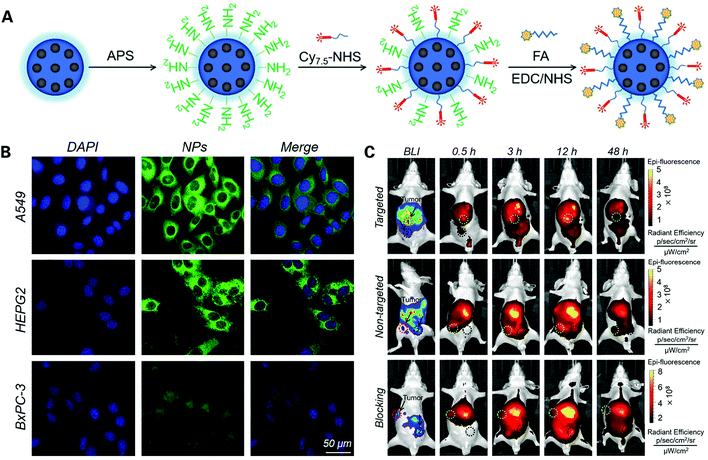 | ||
| Fig. 5 Novel Cy7.5 dye synthesized nanoprobe for optical imaging of PDAC: (A) construction of bMSN@Cy7.5–FA NPs; (B) assessment of bMSN@Cy7.5–FA NPs in vitro via confocal FI in different pancreatic cancer cell lines; (C) assessment of bMSN@Cy7.5–FA NPs in vivo via bioluminescence imaging and fluorescence imaging after 48 hours. Reproduced with permission from ref. 95. Copyright 2018. Elsevier. | ||
Wang et al. designed a dendrimer-based gene-free loading vector with a mean diameter of 110.9 nm, using third-generation dendrigraft poly-L-lysine (DGL) as a nanocarrier scaffold and modified it with gadolinium (Gd), a common type of T1-weighted contrast agent, and cell-penetrating peptides (CPPs), which are short 30-residue synthetic peptides that can enhance the diffusion of agents through cells, thereby facilitating the cellular uptake of nanoparticles administered regionally in the tumor microenvironment105 (Fig. 6). Cellular uptake and loaded gene expression of the novel vector were assessed in vitro, and the permeability of the vector in tumor stroma and the distribution of transfected gene expression were evaluated in vivo. The novel nanoparticle displayed luciferases strictly expressed in the pancreatic tumor region, with higher luminescence intensity and permeability compared with unmodified Gd-based contrast agents, verifying the ability of the novel vector to successfully target and deliver loaded particles to the selected tumor region, while limiting its expression in the targeted tumor tissue, showing great potential in the application of the early and accurate diagnosis of PDAC, as well as guidance in PDAC treatment and tumor change monitoring in patient follow up.
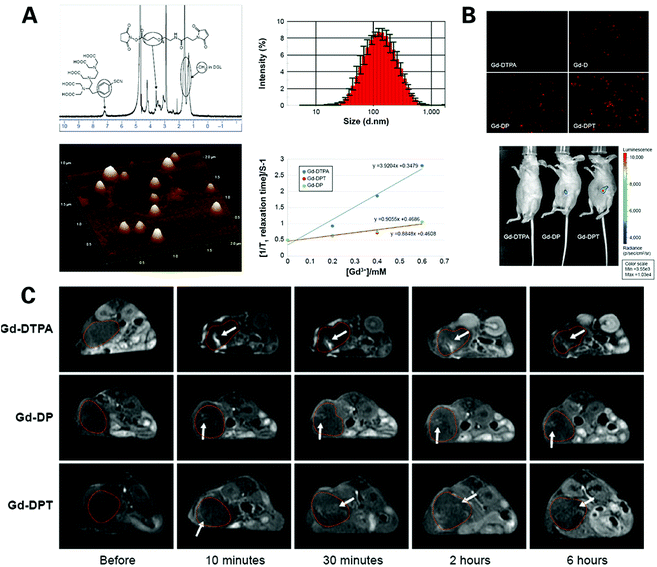 | ||
| Fig. 6 Gadolinium based nanoprobes for MR imaging of PDAC: (A) characteristics of Gd–DPT/pRFP; (B) assessment of gene transfection in vitro and in vivo via FI of Gd–DTPA/pRFP (Gd–DTPA), Gd–DGL/pRFP (Gd–D), Gd–DP/pRFP (Gd–DP), and Gd–DPT/pRFP (Gd–DPT); (C) real-time T1 weighted MR imaging of Gd–DPT/pRFP (Gd–DTPA), Gd–DP/pRFP (Gd–DPT/pDPT) diffusion in vivo. Reproduced with permission from ref. 105. Copyright 2015. Dove Press. | ||
However, Gd-based contrast agents have the disadvantages of potential toxicity, especially the risk of causing fatal nephrogenic systematic fibrosis (NSF) and metabolism difficulty.103 Iron oxide nanoparticle based T2 contrast agents have the advantages of non-nephrotoxicity and avoiding the risk of NSF101 (Fig. 7). He et al. designed a biomarker-targeted nanoparticle-based contrast agent termed CXCR4–USPIO for pancreatic cancer cell specific magnetic resonance imaging.106 The complex was produced by bioconjugating ultrasmall superparamagnetic iron oxide (USPIO) nanoparticles used in magnetic resonance imaging of pancreatic tissue for its ability to offer a significant contrast-enhancement effect, with chemokine receptor 4 (CXCR4) monoclonal antibody, a receptor which has been found to be highly expressed in pancreatic cancer cell lines and primary pancreatic tumors but not in normal pancreatic tissues. The complex was assessed compared to BSA (bovine serum albumin)–USPIO and USPIO. They found a strong correlation between the T2 enhancement ratio in the CXCR4–USPIO group and the CXCR4 protein expression levels (peptide relative Frey values and mean fluorescence signal intensity) in four different pancreatic cancer cell lines, indicating that the T2 enhancement ratio of CXCR4–USPIO nanoparticles could be used to semi-quantitatively assess CXCR4 expression levels in cells, showing great potential to achieve PDAC diagnosis at the cellular level.
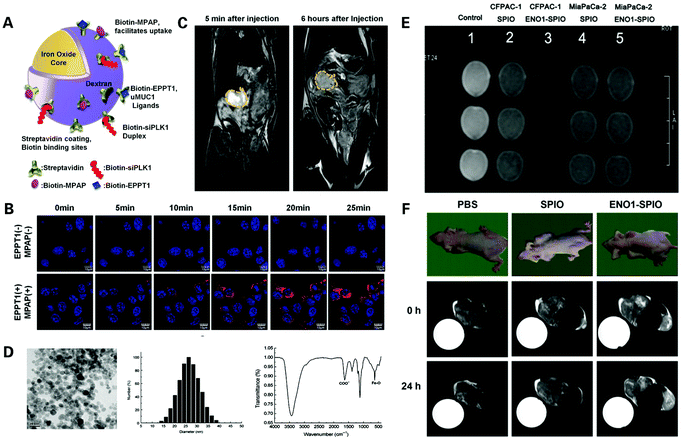 | ||
| Fig. 7 Iron oxide nanoparticle based nanoprobes for the MR imaging of PDAC: (A) schematic structure of siPLK1–StAv–SPION conjugated to MPAP, EPPT1 and siPLK1; (B) assessment of accumulation of siPLK1–StAv–SPION coupled with or without EPPT1 and MPAP, respectively, in vitro via confocal FI over 25 min; (C) assessment of siPLK1–StAv–SPION in vivo via MRI pre and 6 h post injection. Reproduced with permission from ref. 107. Copyright 2016. BMJ; (D) characteristics of ENO1-Dex-g-PCL/SPIO nanoparticles; (E) assessment of ENO1-Dex-g-PCL/SPIO nanoparticles in vitro via MRI; (F) assessment of ENO1-Dex-g-PCL/SPIO nanoparticles in vivo via MRI. Reproduced with permission from ref. 108. Copyright 2020. Wiley. | ||
Later, Mahajan et al. designed a complex termed siPLK1–StAv–SPION, by coupling superparamagnetic iron oxide nanoparticles (SPIONs) with polo-like kinase-1 (siOLK1), a siRNA directed against the cell cycle-specific serine–threonine–kinase107 (Fig. 7A–C). The complex was designed not only to assess targeted delivery efficiency in vivo by tumor imaging, but can also serve the purpose of PDAC therapy by delivery of siPLK1, and therefore can be applied to the early diagnosis, treatment and therapeutic effect observation of PDAC. The complex was assessed in vivo and showed significant accumulation of siPLK1–StAv–SPIONs in PDAC and inhibition of tumor growth due to silencing of polo-like kinase 1 (PLK1), a proto-oncogene overexpressed in tumor cells, verifying the PDAC targeting function and therapeutic effectiveness of siPLK1–StAv–SPIONs.
Furthermore, Wang et al. designed a novel SPIO nanoparticle (30 nm in diameter) targeting Enolase 1 (ENO1) that is a glycolytic enzyme located on the PDAC cell membrane involved in the development, invasion, metastasis and chemoresistance of PDAC, by coupling poly(epsilon-caprolactone)-grafted dextran (Dex-g-PCL)/SPIO nanoparticles with the ENO1 antibody108 (Fig. 7D–F). The tumor detection efficacy of the nanoparticle was tested, showing a stronger signal enhancement of the MRI signal after treatment of ENO1–SPIO compared to SPIO in vitro and in vivo. A signal enhancement was still overserved 24 h after ENO1–SPIO injection, while the MRI signal intensity was fully recovered to the pre-injection level at 24 h after SPIO injection. These findings indicated that the nanoparticles had satisfactory superparamagnetism and the ability to significantly enhance the detection of PDAC by MRI in vitro and in vivo, greatly increasing the efficiency of PDAC detection and bringing hope to achieving a higher early diagnosis rate of PDAC.
Moreover, Zou et al. generated a novel microminiature nanocomposite of only 23.6 nm diameter termed IONPs–PEG–MCC triple scAbs, by conjugating triple single chain antibodies (scAbs) including scAbMUC4, scAbCEACAM6, scFvCD44v6 and MCC triple scAbs to the surface of polyethylene glycol modified IONPs (IONPs–PEG).109 IONPs–PEG–MCC triple scAbs as a bi-functional nanocomposite could be used as a negative MR contrast agent for the early and precise diagnosis of PDAC, as well as play a helpful role in PDAC treatment. Magnetic iron oxide nanoparticles (IONPs) are ultra-small superior contrast agents used in magnetic resonance imaging that can be passively retained in pancreatic tumors due to the EPR effect, as well as be modified to specifically target tumors by artificially attaching tumor-associated biomarkers to its surface. Moreover, instead of attaching a single biomarker, a combination of three biomarkers MUC4, CEACAM6 and CD44v6, all proven in research studies to be potential in the imaging and treatment of pancreatic cancer, was used to promote the sensitivity of PDAC detection and diagnosis. The imaging performance and anti-pancreatic cancer effect of the nanocomposite were evaluated, displaying a decreased T2-weighted signal intensity of the tumor region of interest (tROI) after injection of modified IONPs compared to non-modified IONPs, and with the increase of scAbs modified to IONPs, the T2 signal intensity of tROI was decreased to a greater level. Notably, while non-modified IONPs were found to be obviously gathered in the spleen, the modified IONPs gathered specifically in tumor regions. The IONPs–PEG–MCC triple scAbs also exhibited the ability to significantly inhibit tumor growth. These findings confirmed IONPs–PEG–MCC triple scAbs as an excellent dual-functional nanocomposite that can be used in both the diagnosis and treatment of PDAC.
Earlier reports of nanomaterials for X-ray contrast agents were mainly based on emulsions or liposomes, and nowadays, more extensive nanoparticle imaging agents based on X-ray contrasting elements including gold, silver, tantalum, bismuth, lanthanides, etc. have emerged.113,114 Nanomaterials also give us synthetic control over size, shape, and composition, which can be designed for various biomedical applications. They also have the advantage of higher payloads per entity, resulting in fewer amounts of contrast agents needed in patients, lowering the renal toxicity and providing more compatibility for patients with impaired kidney function. X-ray nanoparticle agents are generally composed of a contrast generating core coated by compounds that can provide desired pharmacological or physicochemical properties including silica, proteins, polymers, lipids, etc.113 For metal core-based nanoparticle agents, gold has received extensive attention for its high K-edge energy, low density and high biocompatibility.115,116 Moreover, gold core-based nanoparticles have exhibited a high uptake rate in PDAC cells, showing potential as a promising candidate for CT imaging of PDAC. Currently, CT is still commonly used in PDAC detection, and therefore the development of nanoparticle contrast agents targeting PDAC for CT imaging can greatly enhance the specificity and sensitivity for the early diagnosis of PDAC.
Contrast agents are commonly used in other imaging modalities, but contrast agents for US imaging have long been lacking.119,120 Many novel microbubble ultrasound contrast agents (UCAs) have emerged in the past decade filling out this vacancy and have been approved recently for abdominal mass characterization. Microbubble US contrast agents are mainly composed of an outer shell of a lipid, albumin, or other desirable material, containing a gas core, greatly increasing the blood circulation time and reducing the risk of coalescence, and unlike CT and MR contrast agents, microbubble contrast agents are not filtered by the kidneys with no renal toxicity and do not extravasate into interstitial space. The shell material is ultimately metabolized by the liver and the gas contained is exhaled after bubble dissolution.121 This novel contrast agent can be modified to target PDAC cells specifically or be used as drug-encapsulating vehicles, to provide sensitive real-time imaging or targeted therapy for PDAC, leading to a precise and early detection of PDAC and a better treatment effect for PDAC patients.
3. Multimodality imaging in the diagnosis of PDAC
Although there are various imaging techniques for the detection of PDAC, all of them have their own advantages as well as limitations. Therefore, at present, single use of any one imaging method alone cannot yet achieve an early and accurate diagnosis of PDAC. Therefore, it is of great significance to develop a new strategy of integrating 2–3 imaging methods into one imaging system, termed multimodal imaging, which can combine the complementary advantages of different imaging modalities.122,123 Herein, we mainly summarize and discuss the recent advances of multimodality imaging in the early diagnosis of PDAC.3.1. Magneto-optical nanoplatform for the multimodality imaging of PDAC
Both optical imaging and magnetic resonance imaging have their own advantages and limitations. Optical imaging offers a wide spectrum and deep tissue penetration, but has low spatial resolution and cannot obtain comprehensive three-dimensional anatomical structure images.38,68 Also, PAI displays the disadvantage of not being able to image through bones or air-filled structures, and there is no mature commercial PDAC PAI system available yet.124 Thus, the clinical application of PAI in the early diagnosis of PDAC is still under exploration. As for MRI, although it is non-invasive and has no use of ionizing radiation, MRI is expensive, has low sensitivity, cannot obtain real-time imaging and may be uncomfortable for patients with claustrophobia due to its long examination time and narrow exam space.36 In addition, though the development and application of signal enhancing contrast agents have provided improvement of the specificity and sensitivity of MRI to a certain extent, the contrast agents themselves also have many problems yet to be solved. Gd3+ based T1 contrast agents are nephrotoxic, SPIONPs based T2 contrast agents have great imaging performance and low nephrotoxicity, but has low stability in vivo and may cause serious DNA and protein damage, even systemic inflammatory due to the production of reactive oxygen species (ROS).98,103,107 The use of magneto-optical nanoplatform is a novel multimodality imaging method combining optical imaging and MRI, so as to produce the complementary effect. Multimodal imaging nanoparticles are generally a complex of optical luminescent dyes and MRI contrast agents, which can be applied to optical imaging and MRI imaging at the same time, and when the nanoparticles can generate the photothermal effect, they can also be used in photoacoustic imaging.125,126 In this case, not only can these nanoparticles specifically provide PDAC targeted comprehensive three-dimensional anatomical images of pancreatic tumors, but can also obtain real-time visual information of the tumors.127,128 Recent advances in the magneto-optical nanoplatform for the multimodality imaging of PDAC will be elaborated in the following contents (Fig. 8).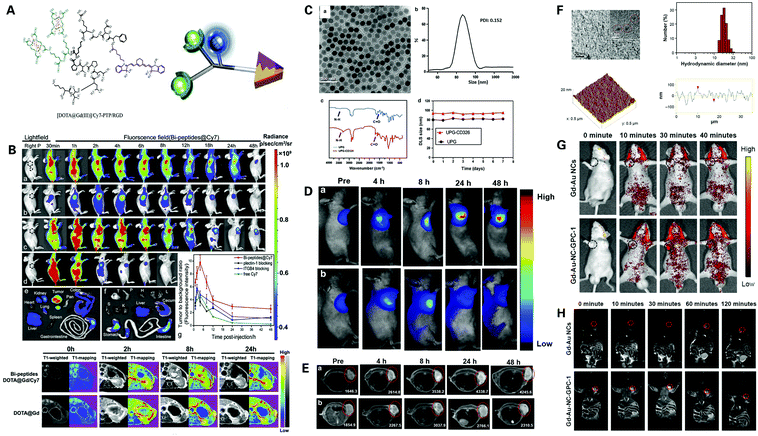 | ||
| Fig. 8 Magneto-optical nanoplatform for the multimodality imaging of PDAC: (A) schematic structure of Gd–Cy7–PTP/RGD; (B) bio-distribution and assessment of Gd–Cy7–PTP/RGD (a) in vivo via dual-modality imaging, compared with plectin-1 McAb (b), ITGB4 McAb blocking (c) and free Cy7 (d). Reproduced with permission from ref. 129. Copyright 2018. Elsevier; (C) characteristics of UPG–CD326 micelles; assessment of UPG–CD326 micelles (a) in vivo via real-time upconversion luminescence imaging (D) and real-time T1-weighted MRI (E) compared with non-targeted UPG (b). Reproduced with permission from ref. 131. Copyright 2018. BMC; (F) characteristics of Gd–Au–NC–GPC-1; assessment of Gd–Au–NC–GPC-1 in vivo via real-time FI (G) and real-time MRI (H) compared with Gd–Au NCs. Reproduced with permission from ref. 132. Copyright 2018. Dove Press. | ||
MR contrast agents can be conjugated with fluorescence imaging agents to provide complementary imaging of PDAC. Gd-based materials are commonly used in modifying MR-fluorescent agents, by conjugating them with fluorescent dyes and targeting ligands. Wang et al. designed a novel bispecific molecular probe termed Gd–Cy7–PTP/RGD aimed to be used for both MRI and NIRF of pancreatic cancer129 (Fig. 8A and B). The novel bispecific molecular probe was synthesized by conjugating Gd, a common T1-weighted contrast agent, cyanine 7 (Cy7), a common NIRF dye, the peptide PTP that binds to plectin-1 which is specifically overexpressed on the surface of PDAC cells, and the peptide RGD that targets integrin widely expressed on pancreatic duct epithelial cells and angiogenesis in malignant tumors. The bispecific probes were evaluated in vitro and in vivo and showed that the combination of PTP and RGD modified probes provided a great increase in the targeting efficiency of PDAC in vitro and in vivo compared to non-modified agents and agents modified with a single peptide. The bispecific probes not only target pancreatic neoplasms but also target angiogenesis in tumors at the same time, thereby producing a multi-level targeting effect. NIRF-guided intraoperative delineation of surgical margins and tumor excision was achieved in vivo under the navigation of MR/NIRF bimodality imaging, which provided high spatial resolution, high sensitivity, high specificity and real-time visualization simultaneously, promoting further exploration and development of multi-specific probes for the early diagnosis and treatment guidance of PDAC.
Later, Li et al. designed a dual-modal imaging nanoprobe termed dendron-grafted polylysine (DGL–U11), by using third generation dendron G3 of DGL (DGL–G3) as the platform, due to its desirable properties of good biocompatibility, biodegradability, distinct sizes, etc.130 to load U11, a peptide targeting receptor uPAR overexpressed in pancreatic tumors, Gd3+-diethylenetriamine pentaacetic acid, a T1-weighted contrast agent, and Cy5.5, a NIRF imaging dye. The nanoprobe was assessed in vitro and in vivo showing a significantly higher fluorescence intensity in DGL–U11 nanoparticle incubated tumor cells compared to non-targeted agents, and an increasingly enhanced MR signal and fluorescence signal in different tumor stages compared to non-targeted control. The above findings confirm that this novel uPAR-targeted dual-modal nanoprobe serves as an excellent contrast agent in the targeted imaging of precancerous PanINs and PDAC lesions in both MR and NIRF imaging simultaneously, providing both high sensitivity and high spatial resolution, bringing great hope to the early diagnosis of PDAC.
Furthermore, Han et al. designed a novel micelle probe termed UPG–CD326, by synthesizing gadolinium ion-doped upconversion nanoparticles (UCNPs) that can be used in magnetic resonance imaging and fluorescence imaging simultaneously, with anti-human CD326 monoclonal antibody targeting CD326, a transmembrane glycoprotein overexpressed on pancreatic cancer stem cells131 (Fig. 8C–E). The micelles were assessed in vitro and in vivo and exhibited superior imaging properties, long-time stability and good biocompatibility, showing a higher fluorescence and MR signal intensity compared to non-targeted agents, indicating a significant enhancement of the cellular uptake of micelles through a CD326 antigen–antibody mediated endocytosis process, exhibiting both passive and active CD326 targeting abilities of the targeted micelles, even after 48 h, while non-targeted micelles only showed passive tumor targeting ability via the EPR effect, verifying the excellent targeting function of UPG–CD326 micelles. These findings demonstrated great potential for achieving an early and accurate diagnosis of PDAC in the future.
Moreover, Huang et al. designed a dual-modal imaging probe termed NCs; Gd–Au–NC–GPC-1 by conjugating Gd–Au nanoclusters with Glypica-1 (GPC-1) antibody targeting GPC-1, a type of cell surface heparan sulfate proteoglycan highly expressed in pancreatic cancer cells132 (Fig. 8F–H). The probe was assessed in vitro and in vivo, displaying an enhanced MR and fluorescent signal compared to non-targeted probes, suggesting that Gd–Au–NC–GPC-1 can target pancreatic cancer cells selectively in both fluorescence imaging and MR imaging. FI displayed high sensitivity but could only provide whether Gd–Au–NC–GPC-1 was targeted to the tumor and give an approximate position in vivo, however, this limitation was remedied via MR imaging which could clearly demonstrate the exact location of the tumor. Therefore, this novel dual-modal FI/MRI probe showed great potential in the application of early diagnosis of PDAC.
Iron oxide nanoparticles can be easily synthesized with a fluorescent dye and targeting ligand, making it a popular choice for the MR-fluorescencet imaging of PDAC. Medina et al. designed a novel nanoparticle by encapsulating iron nanoparticles and ICG into cationic sphingomyelin (SM) consisting of liposomes that had an RA-96 Fab fragment conjugated on its surface in order to increase the tumor homing ability.133 The targeting ability and MRI photoacoustic visibility of RA-96-targeted liposomes encapsulating iron nanoparticles and ICG were tested in vitro and in vivo exhibiting increased association of ICG-encapsulated liposomes coated with RA-96 Fab fragments in vitro compared to non-targeted agents in FI, and increased accumulation of RA-96-targeted nanoparticles in tumor sites compared to non-targeted controls in vivo via FI and MRI, indicating that RA-96-targeted iron nanoparticles and ICG-encapsulated liposomes can be applied to imaging pancreatic tumors using a variety of optical and magnetic imaging techniques or even be suitable for drug delivery in PDAC treatment.
Furthermore, Wang et al. designed a novel dual-modality molecular imaging contrast agent termed MN–EPPT, by conjugating dextran-coated iron oxide nanoparticles to NIRF dye Cy5.5 and a peptide EPPT targeting uMUCI, an overexpressed and underglycosylated biomarker on over half of human cancers.134 The novel contrast agent was tested in vitro and in vivo, showing a significantly reduced accumulation of MN–EPPT, as to a lower level of uMUCI expression in both MRI and optical imaging in the treatment group using gemcitabine as chemotherapy compared to the saline control. Histopathological results confirmed the findings above showing a normal prevalent glandular structure in the gemcitabine group and high-grade PanIN lesions and CIS in the saline control group. This novel contrast agent showed great potential in the early diagnosis and evaluation of treatment assessment of PDAC.
3.2. Other multi-modality imaging of PDAC
At present, multimodal imaging nanoparticles are generally a combination of optical luminescent dyes and MRI contrast agents, but agents of other imaging modalities commonly used in the diagnosis of PDAC can also be integrated to synergistically provide more accurate imaging of PDAC, contributing to earlier and more accurate diagnosis, guidance in surgery and treatment, as well as the assessment of therapeutic outcomes. US and optical imaging can be combined to obtain anatomic, target specific and real-time imaging of PDAC. Barrefelt et al. designed a novel nanoprobe termed VivoTag 680 MBs, by labelling air-filled polyvinyl alcohol microbubbles (PVA–MBs), a newly introduced contrast agent for US imaging, with a NIR fluorophore, VivoTag 680.135 The novel nanoparticle was tested in vivo via US imaging showing highly fluorescent signals only in PDAC tumor surrounding tissues but not inside the tumor, which successfully demonstrated the poor vascularization of PDAC, indicating the potential application of PVA–MBs as a multimodal imaging contrast agent for early PDAC diagnosis. PET/CT and EUS may also be combined to complement the high sensitivity of PET/CT and high specificity of EUS.136MR contrast agents can also be conjugated with both optical imaging agents and X-ray contrast agents forming a triple-modality platform to obtain comprehensive imaging of PDAC. Zhao et al. designed a core–shell structured gold nanorod (AuNR) to be used as a contrast agent for multimodal imaging applied to the early diagnosis of PDAC137 (Fig. 9). The nanoparticles are composed of a AuNR core with a mesoporous silica outside layer, which was coated with a gadolinium oxide carbonate shell, and the resulting AuNR–SiO2–Gd can then be used in MRI, CT and optical imaging. The AuNR–SiO2–Gd NPs were assessed in vitro showing higher MRI contrast compared to Gadovist, higher X-ray attenuation compared to Visipaque, and strong PA contrast enhancement within the examined range of 680–970 nm with a peak absorbance at around 800 nm. The nanoparticles were then tested in vivo, showing high accumulation of AuNR–SiO2–Gd NPs in surrounding tissues but little distribution throughout the tumor caused by dense stoma infiltration and hypovascularity, leading to a negative contrast within the tumor portion in CT/PAI and a positive contrast in MRI. These findings suggest that AuNR–SiO2–Gd NPs have great potential as a multimodal contrast medium for the early detection of PDAC, hoping to improve early diagnosis and benefit patient outcomes.
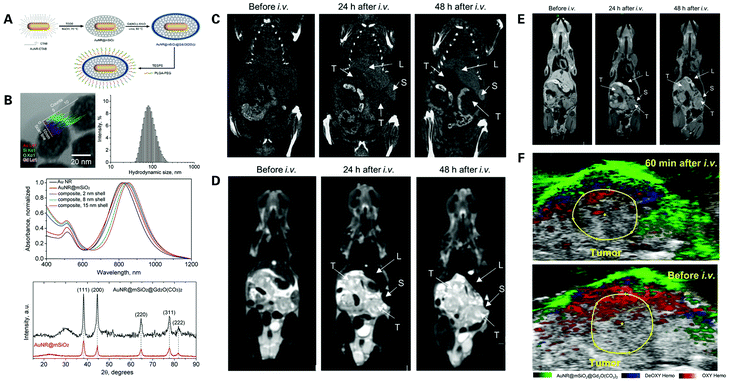 | ||
| Fig. 9 Nanoprobes for the triple-modality imaging of PDAC: (A) schematic synthesis procedure of AuNR@mSiO2@Gd2O(CO3)2 NPs; (B) characteristics of AuNR@mSiO2@Gd2O(CO3)2 NPs; assessment of AuNR@mSiO2@Gd2O(CO3)2 NPs in vivo via whole-body CT imaging (C), T2-weighted MR imaging (D), T1-weighted MR imaging (E), and PA imaging (F) at different time periods. Reproduced with permission from ref. 137. Copyright 2020. ACS. | ||
Contrast agents of potential imaging modalities for PDAC diagnosis can also be integrated to provide complementary and more accurate imaging of PDAC. MPI, as an emerging imaging modality with great potential in the application of PDAC diagnosis, traces the spatial distribution of magnetic nanoparticle agents and detects the change in iron electronic magnetization, unlike MRI which measures the change in water proton nuclear magnetization, and therefore provides much higher sensitivity than MRI, with nearly zero background signal and zero signal attenuation for analysis of in-depth tissues.62 Therefore, nanomaterials that can be used in both MPI and optical imaging may show greater advantages in the early detection of cancer compared to current imaging examinations. Though also a magnetic imaging modality, there is a great difference in physics between MPI and MRI, and therefore common MRI contrast materials like iron oxide nanoparticles are not ideal for MPI. Song et al. discovered that FeCo nanoparticles that bear a poly(ethylene glycol) decorated graphic carbon shell provide an MPI signal intensity much higher than the signals from SPIONs at the same molar concentration of iron138 (Fig. 10F–K). Notably, the novel nanoparticle was tested and it showed both photothermal and magnetothermal properties, with high optical absorbance in the NIR region (700–1200 nm wavelength), indicating that this nanoparticle is suitable as a tracer in both MPI and optical imaging, showing great potential in the early detection of PDAC after modification adding targeting functions.
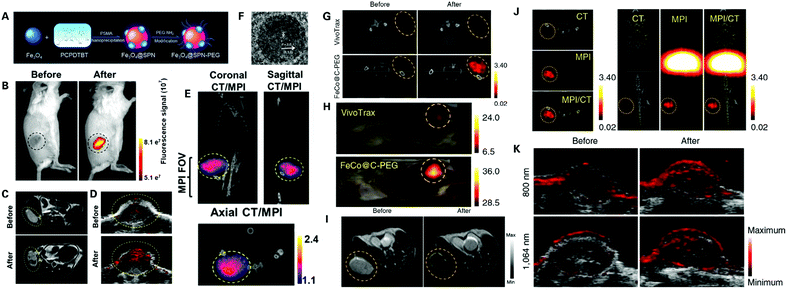 | ||
| Fig. 10 Potential nanoprobes for the multimodality imaging of PDAC: (A) schematic synthesis of MMPF NPs; assessment of MMPF NPs in vivo via FI (B), T2-MRI (C), PAI (D), and MPI/CT (E). Reproduced with permission from ref. 139. Copyright 2019. ACS; (F) high-resolution TEM image of FeCo@C–PEG; (G and H) assessment of FeCo@CPEG in mice bearing breast tumors via MPI/CT compared with VivoTrax; assessment of FeCo@CPEG in mice bearing breast tumors via MRI (I), CT, MPI and MPI/CT (J), and PAI (K). Reproduced with permission from ref. 138. Copyright 2020. Nature. | ||
MPI contrast nanoparticles can even be modified to be used in MPI, MRI, PAI and FI, combining all complementary advantages of all four imaging modalities, providing a precise and comprehensive assessment of tumor lesions. Song et al. designed a novel multimodality nanoparticle termed MMPF NPs for imaging tumor in vivo via MPI, MRI, PAI and FI139 (Fig. 10A–E). The novel nanoparticles were assessed and showed long blood circulation time with a half-life of 49 h, and high tumor uptake. Notably, MMPF NPs offered ultrasensitive real-time imaging of tumors via MPI. MMPF NPs have been tested in breast and brain tumors via simultaneous MPI, MRI, PAI and FI showing outstanding contrast between tumor lesions and normal tissues, demonstrating great potential in the application of cancer diagnosis. The novel nanoparticle also showed great potential in the early and accurate detection of PDAC after modification with PDAC specific targeting ligands, or even be used to monitor PDAC treatment via a desired modification.
PAI is a novel biomedical imaging method and there is no mature commercial PDAC PAI system available yet, and the application of PAI in early diagnosis of PDAC is limited to pre-clinical studies. However, compared with luminescence imaging, PAI can provide deeper tissue penetration and relatively higher sensitivity, showing great potential in the clinical application of early PDAC diagnosis.60 By modifying agents that can be used in both magnetic imaging and PAI, the combination of both imaging modalities endow better accuracy and higher penetration depth to lesion visualization. Chen et al. designed a nanoprobe based on Prussian blue (PB) which can be used to combine MRI and PAI for deep tissue imaging.140 The PB nanoparticle was designed to function as an indicator for sensing peroxynitrite (ONOO−), a reactive oxygen species (ROS) in pathological and physiological prosses, to detect drug-induced liver injury. Such a magnetic/photoacoustic nanoplatform for multi-modality imaging can be referred to in the development of novel nanomaterials for multi-modality imaging in the early diagnosis of solid tumors.
4. Conclusion and prospects
This review comprehensively introduced imaging methods for the diagnosis of PDAC and highlighted the current magneto-optical nanoplatform in multimodality imaging for the enhanced early diagnosis of PDAC. We believe that with an in-depth understanding of biological interactions that occur in the unique PDAC microenvironment, it is increasingly important for materials scientists, clinicians, and other researchers to integrate ideas into the development of a novel magneto-optical nanoplatform both preclinically and clinically, especially in the areas of multimodality imaging for achieving an early diagnosis of PDAC. We hope this review can inspire the design and fabrication of nanoprobes in multimodality imaging for achieving an early diagnosis of pancreatic cancer, so as to raise the clinical benefits in PDAC patients.Although many researchers made a lot of effort to improve the early diagnosis rate of pancreatic cancer via the development of multimodality imaging particularly the magneto-optical nanoplatform. Many challenges remain, and we need to explore new strategies to solve the below problems. The specificity of the nanoplatform for distinguishing PDCA cells from normal pancreatic cells is not enough, resulting in excessive interference by background signals. To address this challenge, it is expected to develop an “off–on” switchable nanoplatform with a specific function assisted by a responsive polymer, i-motif DNA, or even a chemical bond, which can selectively turn on the imaging signals triggered by the stimuli in tumor tissues while keeping the imaging signals powered off in normal tissues, so as to further amplify the imaging signal in PDAC tissue and reduce the interference signal of the background, thereby improving the early diagnosis rate of PDCA. Furthermore, patient derived xenotransplantation models or genetically engineered mouse models (GEMMs) are recommended to verify the function of the magneto-optical nanoplatform, so as to promote these nanomedicines from bench to bedside. Moreover, many newly emerged imaging modalities such as MPI and PAI show great potential in the early detection of PDAC, however, the development of novel nanomaterials for these imaging methods in the diagnosis of PDAC is still lacking. Meanwhile, nanoparticle contrast agents for the current commonly used imaging methods such as CT and US are also lacking, appealing more exploration in these fields, to help achieve a higher early and accurate diagnosis rate of PDAC.
Author contributions
X. Z., J. X., and G. S. designed the review, X. Z. wrote the manuscript, Z. Z., L. X., H. L., S. X., J. X. and G. S. edited the paper. J. X. and G. S. revised the paper. All authors gave final approval to the article.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (grants U21A20287, 51872088) and the Science and Technology Project of Hunan Province (2020RC3022; 2020SK2014).References
- A. Vincent, J. Herman, R. Schulick, R. H. Hruban and M. Goggins, Lancet, 2011, 378, 607–620 CrossRef.
- E. Riquelme, Y. Zhang, L. Zhang, M. Montiel, M. Zoltan, W. Dong, P. Quesada, I. Sahin, V. Chandra, A. San Lucas, P. Scheet, H. Xu, S. Hanash, L. Feng, J. Burks, K. Do, C. Peterson, D. Nejman, C. Tzeng, M. Kim, C. Sears, N. Ajami, J. Petrosino, L. Wood, A. Maitra, R. Straussman, M. Katz, J. White, R. Jenq, J. Wargo and F. McAllister, Cell, 2019, 178, 795–806 CrossRef CAS PubMed.
- R. Siegel, K. Miller, H. Fuchs and A. Jemal, Ca-Cancer J. Clin., 2021, 71, 7–33 CrossRef PubMed.
- H. Sung, J. Ferlay, R. Siegel, M. Laversanne, I. Soerjomataram, A. Jemal and F. Bray, Ca-Cancer J. Clin., 2021, 71, 209–249 CrossRef PubMed.
- M. Schmidt-Hansen, S. Berendse and W. Hamilton, Pancreas, 2016, 45, 814–818 CrossRef PubMed.
- A. Bengtsson, R. Andersson and D. Ansari, Sci. Rep., 2020, 10, 16425 CrossRef PubMed.
- T. Schnelldorfer, A. Ware, M. Sarr, T. Smyrk, L. Zhang, R. Qin, R. Gullerud, J. Donohue, D. Nagorney and M. Farnell, Ann. Surg., 2008, 247, 456–462 CrossRef PubMed.
- L. Zhang, S. Sanagapalli and A. Stoita, World J. Gastroenterol., 2018, 24, 2047–2060 CrossRef PubMed.
- A. Singhi, E. Koay, S. Chari and A. Maitra, Gastroenterology, 2019, 156, 2024–2040 CrossRef PubMed.
- L. Zhang, S. Sanagapalli and A. Stoita, World J. Gastroenterol., 2018, 24, 2047–2060 CrossRef PubMed.
- V. Goral, Asian Pac. J. Cancer Prev., 2015, 16, 5619–5624 CrossRef PubMed.
- D. Marrelli, S. Caruso, C. Pedrazzani, A. Neri, E. Fernandes, M. Marini, E. Pinto and F. Roviello, Am. J. Surg., 2009, 198, 333–339 CrossRef CAS PubMed.
- S. Su, S. Qin, W. Chen, W. Luo and H. Jiang, World J. Gastroenterol., 2015, 21, 4323–4333 CrossRef CAS PubMed.
- R. Prokesch, L. Chow, C. Beaulieu, R. Bammer and R. Jeffrey, Radiology, 2002, 224, 764–768 CrossRef PubMed.
- N. Balci and R. Semelka, Eur. J. Radiol., 2001, 38, 105–112 CrossRef CAS PubMed.
- M. Barral, B. Taouli, B. Guiu, D. M. Koh, A. Luciani, R. Manfredi, V. Vilgrain, C. Hoeffel, M. Kanematsu and P. Soyer, Radiology, 2015, 274, 45–63 CrossRef PubMed.
- S. van Asselt, A. Brouwers, H. van Dullemen, E. van der Jagt, A. Bongaerts, I. Kema, K. Koopmans, G. Valk, H. Timmers, W. de Herder, R. Feelders, P. Fockens, W. Sluiter, E. de Vries and T. Links, Gastrointest. Endosc., 2015, 81, 159–167 CrossRef PubMed.
- W. Wang, A. Shpaner, S. Krishna, W. Ross, M. Bhutani, E. Tamm, G. Raju, L. Xiao, R. Wolff, J. Fleming and J. Lee, Gastrointest. Endosc., 2013, 78, 73–80 CrossRef PubMed.
- S. Dougan, Cancer J., 2017, 23, 321–325 CrossRef PubMed.
- A. Rucki and L. Zheng, World J. Gastroenterol., 2014, 20, 2237–2246 CrossRef PubMed.
- J. McCarroll, J. Teo, C. Boyer, D. Goldstein, M. Kavallaris and P. Phillips, Front. Physiol., 2014, 5, 2 Search PubMed.
- W. Tummers, J. Willmann, B. Bonsing, A. Vahrmeijer, S. Gambhir and R. Swijnenburg, Pancreas, 2018, 47, 675–689 CrossRef PubMed.
- D. Shahbazi-Gahrouei, P. Moradi Khaniabadi, B. Moradi Khaniabadi and S. Shahbazi-Gahrouei, J. Res. Med. Sci., 2019, 24, 38 CrossRef CAS PubMed.
- N. Lamichhane, S. Sharma, Parul, A. K. Verma, I. Roy and T. Sen, Biomedicines, 2021, 9, 288 CrossRef CAS PubMed.
- B. Ren, M. Cui, G. Yang, H. Wang, M. Feng, L. You and Y. Zhao, Mol. Cancer, 2018, 17, 108 CrossRef PubMed.
- D. W. Townsend, J. Nucl. Med., 2008, 49, 938–955 CrossRef PubMed.
- T. Tamada, K. Ito, N. Kanomata, T. Sone, A. Kanki, A. Higaki, M. Hayashida and A. Yamamoto, Eur. Radiol., 2016, 26, 646–655 CrossRef PubMed.
- T. Iiboshi, K. Hanada, T. Fukuda, S. Yonehara, T. Sasaki and K. Chayama, Pancreas, 2012, 41, 523–529 CrossRef PubMed.
- W. Wang, A. Shpaner, S. G. Krishna, W. A. Ross, M. S. Bhutani, E. P. Tamm, G. S. Raju, L. Xiao, R. A. Wolff, J. B. Fleming and J. H. Lee, Gastrointest. Endosc., 2013, 78, 73–80 CrossRef PubMed.
- J. R. Treadwell, H. M. Zafar, M. D. Mitchell, K. Tipton, U. Teitelbaum and J. Jue, Pancreas, 2016, 45, 789–795 CrossRef CAS PubMed.
- L. W. Goldman, J. Nucl. Med. Technol., 2007, 35, 115–128 CrossRef PubMed.
- K. M. Horton and E. K. Fishman, Am. J. Roentgenol., 2002, 178, 827–831 CrossRef PubMed.
- I. Matsumoto, S. Shirakawa, M. Shinzeki, S. Asari, T. Goto, T. Ajiki, T. Fukumoto, K. Kitajima and Y. Ku, Clin. Gastroenterol. Hepatol., 2013, 11, 712–718 CrossRef PubMed.
- K. Okano, K. Kakinoki, S. Akamoto, M. Hagiike, H. Usuki, Y. Yamamoto, Y. Nishiyama and Y. Suzuki, World J. Gastroenterol., 2011, 17, 231–235 CrossRef PubMed.
- Y. Wang, F. H. Miller, Z. E. Chen, L. Merrick, K. J. Mortele, F. L. Hoff, N. A. Hammond, V. Yaghmai and P. Nikolaidis, RadioGraphics, 2011, 31, E47–E64 CrossRef PubMed.
- V. P. Grover, J. M. Tognarelli, M. M. Crossey, I. J. Cox, S. D. Taylor-Robinson and M. J. McPhail, J. Clin. Exp. Neuropsychol., 2015, 5, 246–255 Search PubMed.
- C. Wang, Z. Wang, T. Zhao, Y. Li, G. Huang, B. Sumer and J. Gao, Biomaterials, 2018, 157, 62–75 CrossRef CAS PubMed.
- G. Pirovano, S. Roberts, S. Kossatz and T. Reiner, J. Nucl. Med., 2020, 61, 1419–1427 CrossRef CAS PubMed.
- M. L. James and S. S. Gambhir, Physiol. Rev., 2012, 92, 897–965 CrossRef CAS PubMed.
- C. Hoogstins, L. Boogerd, B. Sibinga Mulder, J. Mieog, R. Swijnenburg, C. van de Velde, A. Farina Sarasqueta, B. Bonsing, B. Framery, A. Pèlegrin, M. Gutowski, F. Cailler, J. Burggraaf and A. Vahrmeijer, Ann. Surg. Oncol., 2018, 25, 3350–3357 CrossRef PubMed.
- C. Wang, Z. Wang, T. Zhao, Y. Li, G. Huang, B. D. Sumer and J. Gao, Biomaterials, 2018, 157, 62–75 CrossRef CAS PubMed.
- Y. Peng, B. Xiong, L. Peng, H. Li, Y. He and E. S. Yeung, Anal. Chem., 2015, 87, 200–215 CrossRef CAS PubMed.
- F. Stuker, J. Ripoll and M. Rudin, Pharmaceutics, 2011, 3, 229–274 CrossRef CAS PubMed.
- M. E. Roth-Konforti, C. R. Bauer and D. Shabat, Angew. Chem., Int. Ed., 2017, 56, 15633–15638 CrossRef CAS PubMed.
- C. Lu, C. Zhang, P. Wang, Y. Zhao, Y. Yang, Y. Wang, H. Yuan, S. Qu, X. Zhang and G. Song, Chem, 2020, 6, 2314–2334 CAS.
- N. Hananya, A. Eldar Boock, C. R. Bauer, R. Satchi-Fainaro and D. Shabat, J. Am. Chem. Soc., 2016, 138, 13438–13446 CrossRef CAS PubMed.
- M. Yang, J. Huang, J. Fan, J. Du, K. Pu and X. Peng, Chem. Soc. Rev., 2020, 49, 6800–6815 RSC.
- B. Wang, Y. Wang, Y. Wang, Y. Zhao and X. B. Zhang, Anal. Chem., 2020, 92, 4154–4163 CrossRef CAS PubMed.
- Y. Wang, L. Shi, Z. Ye, K. Guan, L. Teng, J. Wu, X. Yin, G. Song and X. Zhang, Nano Lett., 2020, 20, 176–183 CrossRef CAS PubMed.
- M. Blanco-Formoso and R. Alvarez-Puebla, Int. J. Mol. Sci., 2020, 21, 2253 CrossRef CAS PubMed.
- T. Moore, A. Moody, T. Payne, G. Sarabia, A. Daniel and B. Sharma, Biosensors, 2018, 8, 46 CrossRef PubMed.
- W. Li and X. Chen, Nanomedicine, 2015, 10, 299–320 CrossRef CAS PubMed.
- J. Glatz, N. Deliolanis, A. Buehler, D. Razansky and V. Ntziachristos, Opt. Express, 2011, 19, 3175–3184 CrossRef CAS PubMed.
- S. Tzoumas and V. Ntziachristos, Philos. Trans. R. Soc., A, 2017, 375, 20170262 CrossRef PubMed.
- W. Zhu, M. Chen, Y. Liu, Y. Tian, Z. Song, G. Song and X. Zhang, Nanoscale, 2019, 11, 20630–20637 RSC.
- B. Yin, Y. Wang, C. Zhang, Y. Zhao, Y. Wang, L. Teng, Y. Yang, Z. Zeng, S. Huan, G. Song and X. Zhang, Anal. Chem., 2019, 91, 15275–15283 CrossRef CAS PubMed.
- Y. Ma, L. Xu, B. Yin, J. Shang, F. Chen, J. Xu, Z. Song, B. Nan, G. Song and X. Zhang, Nano Lett., 2021, 21, 4484–4493 CrossRef CAS PubMed.
- Y. Zhou, J. Yao and L. V. Wang, J. Biomed. Opt., 2016, 21, 61007 CrossRef PubMed.
- Z. Yuan and H. Jiang, Opt. Lett., 2009, 34, 1714–1716 CrossRef CAS PubMed.
- L. Zeng, G. Ma, J. Lin and P. Huang, Small, 2018, 14, e1800782 CrossRef PubMed.
- J. Haegele, T. Sattel, M. Erbe, K. Luedtke-Buzug, M. Taupitz, J. Borgert, T. Buzug, J. Barkhausen and F. Vogt, RoeFo, Fortschr. Geb. Roentgenstr. Nuklearmed., 2012, 184, 420–426 CrossRef CAS PubMed.
- N. Talebloo, M. Gudi, N. Robertson and P. Wang, Magn. Reson. Imaging, 2020, 51, 1659–1668 CrossRef PubMed.
- P. Goodwill, E. Saritas, L. Croft, T. Kim, K. Krishnan, D. Schaffer and S. Conolly, Adv. Mater., 2012, 24, 3870–3877 CrossRef CAS PubMed.
- G. Song, M. Chen, Y. Zhang, L. Cui, H. Qu, X. Zheng, M. Wintermark, Z. Liu and J. Rao, Nano Lett., 2018, 18, 182–189 CrossRef CAS PubMed.
- B. R. Smith and S. S. Gambhir, Chem. Rev., 2017, 117, 901–986 CrossRef CAS PubMed.
- Z. Zhou and Z. Lu, Adv. Drug Delivery Rev., 2017, 113, 24–48 CrossRef CAS PubMed.
- J. Wolfram, M. Zhu, Y. Yang, J. Shen, E. Gentile, D. Paolino, M. Fresta, G. Nie, C. Chen, H. Shen, M. Ferrari and Y. Zhao, Curr. Drug Targets, 2015, 16, 1671–1681 CrossRef CAS PubMed.
- V. Pansare, S. Hejazi, W. Faenza and R. Prud'homme, Chem. Mater., 2012, 24, 812–827 CrossRef CAS PubMed.
- G. Song, C. Liang, X. Yi, Q. Zhao, L. Cheng, K. Yang and Z. Liu, Adv. Mater., 2016, 28, 2716–2723 CrossRef CAS PubMed.
- Kenry, Y. Duan and B. Liu, Adv. Mater., 2018, 30, 1802394 CrossRef PubMed.
- S. Zhu, R. Tian, A. Antaris, X. Chen and H. Dai, Adv. Mater., 2019, 31, e1900321 CrossRef PubMed.
- H. Dai, X. Wang, J. Shao, W. Wang, X. Mou and X. Dong, Small, 2021, 17, e2102646 CrossRef PubMed.
- H. Dai, Z. Cheng, T. Zhang, W. Wang, J. Shao, W. Wang, Y. Zhao, X. Dong and L. Zhong, Chin. Chem. Lett., 2021 DOI:10.1016/j.cclet.2021.11.079.
- D. Sheng, T. Liu, L. Deng, L. Zhang, X. Li, J. Xu, L. Hao, P. Li, H. Ran, H. Chen and Z. Wang, Biomaterials, 2018, 165, 1–13 CrossRef CAS PubMed.
- C. Yue, P. Liu, M. Zheng, P. Zhao, Y. Wang, Y. Ma and L. Cai, Biomaterials, 2013, 34, 6853–6861 CrossRef CAS PubMed.
- H. Han, H. Wang, Y. Chen, Z. Li, Y. Wang, Q. Jin and J. Ji, Nanoscale, 2016, 8, 283–291 RSC.
- N. Kosaka, M. Ogawa, P. L. Choyke, N. Karassina, C. Corona, M. McDougall, D. T. Lynch, C. C. Hoyt, R. M. Levenson, G. V. Los and H. Kobayashi, Bioconjugate Chem., 2009, 20, 1367–1374 CrossRef CAS PubMed.
- K. Choi, H. Chung, K. Min, H. Yoon, K. Kim, J. Park, I. Kwon and S. Jeong, Biomaterials, 2010, 31, 106–114 CrossRef CAS PubMed.
- G. Abatangelo, V. Vindigni, G. Avruscio, L. Pandis and P. Brun, Cells, 2020, 9, 1743 CrossRef CAS PubMed.
- M. Litwiniuk, A. Krejner, M. S. Speyrer, A. R. Gauto and T. Grzela, Wounds, 2016, 28, 78–88 Search PubMed.
- J. M. Wickens, H. O. Alsaab, P. Kesharwani, K. Bhise, M. Amin, R. K. Tekade, U. Gupta and A. K. Iyer, Drug Discovery Today, 2017, 22, 665–680 CrossRef CAS PubMed.
- S. Arpicco, P. Milla, B. Stella and F. Dosio, Molecules, 2014, 19, 3193–3230 CrossRef PubMed.
- B. Qi, A. Crawford, N. Wojtynek, M. Holmes, J. Souchek, G. Almeida-Porada, Q. Ly, S. Cohen, M. Hollingsworth and A. Mohs, Nanomedicine, 2018, 14, 769–780 CrossRef CAS PubMed.
- D. M. Tacelosky, A. E. Creecy, S. S. Shanmugavelandy, J. P. Smith, D. F. Claxton, J. H. Adair, M. Kester and B. M. Barth, Discovery Med., 2012, 13, 275–285 Search PubMed.
- A. K. Burwell, L. J. Litkowski and D. C. Greenspan, J. Dent. Res., 2009, 21, 35–39 CAS.
- G. Clawson, T. Abraham, W. Pan, X. Tang, S. Linton, C. McGovern, W. Loc, J. Smith, P. Butler, M. Kester, J. Adair and G. Matters, Nucleic Acid Ther., 2017, 27, 23–35 CrossRef CAS PubMed.
- G. Giordano, M. Pancione, N. Olivieri, P. Parcesepe, M. Velocci, T. Di Raimo, L. Coppola, G. Toffoli and M. R. D'Andrea, World J. Gastroenterol., 2017, 23, 5875–5886 CrossRef CAS PubMed.
- E. Kianfar, J. Nanobiotechnol., 2021, 19, 159 CrossRef CAS PubMed.
- Y. Ishima and T. Maruyama, Yakugaku Zasshi, 2016, 136, 39–47 CrossRef CAS PubMed.
- H. Han, J. Wang, T. Chen, L. Yin, Q. Jin and J. Ji, J. Colloid Interface Sci., 2017, 507, 217–224 CrossRef CAS PubMed.
- M. Gisbert-Garzarán, D. Lozano and M. Vallet-Regí, Int. J. Mol. Med., 2020, 21, 9696 Search PubMed.
- S. Jafari, H. Derakhshankhah, L. Alaei, A. Fattahi, B. S. Varnamkhasti and A. A. Saboury, Biomed. Pharmacother., 2019, 109, 1100–1111 CrossRef CAS PubMed.
- M. Manzano and M. Vallet-Regí, J. Mater. Sci. Mater. Med., 2018, 29, 65 CrossRef PubMed.
- A. Ravindran Girija and S. Balasubramanian, Nanotheranostics, 2019, 3, 1–40 CrossRef PubMed.
- H. Li, K. Li, Y. Dai, X. Xu, X. Cao, Q. Zeng, H. He, L. Pang, J. Liang, X. Chen and Y. Zhan, Nanomedicine, 2018, 14, 1867–1877 CrossRef CAS PubMed.
- G. H. Glover, Crit. Care Nurs. Clin. North Am., 2011, 22, 133–139 Search PubMed.
- H. Yuan, Y. Zhao, C. Yang, C. Zhang and X. Zhang, Sci. China: Chem., 2020, 63, 924–935 CrossRef CAS.
- D. Zhu, F. Liu, L. Ma, D. Liu and Z. Wang, Int. J. Mol. Sci., 2013, 14, 10591–10607 CrossRef PubMed.
- A. Heshmatzadeh Behzadi, Z. Farooq, J. H. Newhouse and M. R. Prince, Medicine, 2018, 97, e0055 CrossRef PubMed.
- J. Pellico, C. M. Ellis and J. J. Davis, Contrast Media Mol. Imaging, 2019, 2019, 1845637 Search PubMed.
- S. M. Dadfar, K. Roemhild, N. I. Drude, S. von Stillfried, R. Knüchel, F. Kiessling and T. Lammers, Adv. Drug Delivery Rev., 2019, 138, 302–325 CrossRef CAS PubMed.
- S. Sammet, Abdom. Radiol., 2016, 41, 444–451 CrossRef PubMed.
- H. Li and T. J. Meade, J. Am. Chem. Soc., 2019, 141, 17025–17041 CrossRef CAS PubMed.
- J. Lux and A. D. Sherry, Curr. Opin. Chem. Biol., 2018, 45, 121–130 CrossRef CAS PubMed.
- Q. Wang, J. Li, S. An, Y. Chen, C. Jiang and X. Wang, Int. J. Nanomed., 2015, 10, 4479–4490 CrossRef CAS PubMed.
- Y. He, W. Song, J. Lei, Z. Li, J. Cao, S. Huang, J. Meng, H. Xu, Z. Jin and H. Xue, Acta Radiol., 2012, 53, 1049–1058 CrossRef PubMed.
- U. Mahajan, S. Teller, M. Sendler, R. Palankar, C. van den Brandt, T. Schwaiger, J. Kühn, S. Ribback, G. Glöckl, M. Evert, W. Weitschies, N. Hosten, F. Dombrowski, M. Delcea, F. Weiss, M. Lerch and J. Mayerle, Gut, 2016, 65, 1838–1849 CrossRef CAS PubMed.
- L. Wang, H. Yin, R. Bi, G. Gao, K. Li and H. Liu, J. Cell. Mol. Med., 2020, 24, 5751–5757 CrossRef CAS PubMed.
- J. Zou, S. Chen, Y. Li, L. Zeng, G. Lian, J. Li, S. Chen, K. Huang and Y. Chen, Nanoscale, 2020, 12, 4473–4490 RSC.
- C. Cronin, P. Prakash and M. Blake, Am. J. Roentgenol., 2010, 195, W5–W13 CrossRef PubMed.
- B. Yeh, P. FitzGerald, P. Edic, J. Lambert, R. Colborn, M. Marino, P. Evans, J. Roberts, Z. Wang, M. Wong and P. Bonitatibus, Adv. Drug Delivery Rev., 2017, 113, 201–222 CrossRef CAS PubMed.
- N. Lee, S. Choi and T. Hyeon, Adv. Mater., 2013, 25, 2641–2660 CrossRef CAS PubMed.
- J. Hsu, L. Nieves, O. Betzer, T. Sadan, P. Noël, R. Popovtzer and D. Cormode, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2020, 12, e1642 Search PubMed.
- A. L. Brown, P. C. Naha, V. Benavides-Montes, H. I. Litt, A. M. Goforth and D. P. Cormode, Chem. Mater., 2014, 26, 2266–2274 CrossRef CAS PubMed.
- Y. C. Dong, M. Hajfathalian, P. S. N. Maidment, J. C. Hsu, P. C. Naha, S. Si-Mohamed, M. Breuilly, J. Kim, P. Chhour, P. Douek, H. I. Litt and D. P. Cormode, Sci. Rep., 2019, 9, 14912 CrossRef PubMed.
- R. D. Ross, L. E. Cole, J. M. Tilley and R. K. Roeder, Chem. Mater., 2014, 26, 1187–1194 CrossRef CAS.
- A. Sofuni, T. Tsuchiya and T. Itoi, J. Med. Ultrason., 2020, 47, 359–376 CrossRef PubMed.
- D. Maresca, A. Lakshmanan, M. Abedi, A. Bar-Zion, A. Farhadi, G. J. Lu, J. O. Szablowski, D. Wu, S. Yoo and M. G. Shapiro, Annu. Rev. Chem. Biomol. Eng., 2018, 9, 229–252 CrossRef PubMed.
- W. Chong, V. Papadopoulou and P. Dayton, Abdom. Radiol., 2018, 43, 762–772 CrossRef PubMed.
- C. Dietrich and C. Jenssen, Ultrasonography, 2020, 39, 105–113 CrossRef PubMed.
- V. Paefgen, D. Doleschel and F. Kiessling, Front. Pharmacol., 2015, 6, 197 Search PubMed.
- G. Song, Y. Chao, Y. Chen, C. Liang, X. Yi, G. Yang, K. Yang, L. Cheng, Q. Zhang and Z. Liu, Adv. Funct. Mater., 2016, 26, 8243–8254 CrossRef CAS.
- P. Wang, F. Zhou, K. Guan, Y. Wang, X. Fu, Y. Yang, X. Yin, G. Song, X. Zhang and W. Tan, Chem. Sci., 2019, 11, 1299–1306 RSC.
- G. Pirovano, S. Roberts, S. Kossatz and T. Reiner, J. Nucl. Med., 2020, 61, 1419–1427 CrossRef CAS PubMed.
- M. Wu and J. Shu, Contrast Media Mol. Imaging, 2018, 2018, 1382183 Search PubMed.
- K. M. Bennett, J. Jo, H. Cabral, R. Bakalova and I. Aoki, Adv. Drug Delivery Rev., 2014, 74, 75–94 CrossRef CAS PubMed.
- X. Cai, Q. Zhu, Y. Zeng, Q. Zeng, X. Chen and Y. Zhan, Int. J. Nanomed., 2019, 14, 8321–8344 CrossRef CAS PubMed.
- J. R. Fink, M. Muzi, M. Peck and K. A. Krohn, J. Nucl. Med., 2015, 56, 1554–1561 CrossRef CAS PubMed.
- Q. Wang, H. Yan, Y. Jin, Z. Wang, W. Huang, J. Qiu, F. Kang, K. Wang, X. Zhao and J. Tian, Biomaterials, 2018, 183, 173–184 CrossRef CAS PubMed.
- H. Li, P. Wang, W. Gong, Q. Wang, J. Zhou, W. Zhu and Y. Cheng, Adv. Healthcare Mater., 2018, 7, 1700912 CrossRef PubMed.
- Y. Han, Y. An, G. Jia, X. Wang, C. He, Y. Ding and Q. Tang, J. Nanobiotechnol., 2018, 16, 7 CrossRef PubMed.
- X. Huang, C. Fan, H. Zhu, W. Le, S. Cui, X. Chen, W. Li, F. Zhang, Y. Huang, D. Sh, Z. Cui, C. Shao and B. Chen, Int. J. Nanomed., 2018, 13, 2585–2599 CrossRef CAS PubMed.
- O. P. Medina, R. J. Tower, T. P. Medina, F. Ashkenani, L. Appold, M. Bötcher, L. Huber, O. Will, Q. Ling and C. Hauser, Curr. Pharm. Des., 2022, 28, 313–323 CrossRef PubMed.
- P. Wang, B. Yoo, S. Sherman, P. Mukherjee, A. Ross, P. Pantazopoulos, V. Petkova, C. Farrar, Z. Medarova and A. Moore, Int. J. Cancer, 2016, 139, 712–718 CrossRef CAS PubMed.
- Å. Barrefelt, Y. Zhao, M. K. Larsson, G. Egri, R. V. Kuiper, J. Hamm, M. Saghafian, K. Caidahl, T. B. Brismar, P. Aspelin, R. Heuchel, M. Muhammed, L. Dähne and M. Hassan, Biochem. Biophys. Res. Commun., 2015, 464, 737–742 CrossRef PubMed.
- S. Tang, G. Huang, J. Liu, T. Liu, L. Treven, S. Song, C. Zhang, L. Pan and T. Zhang, Eur. J. Radiol., 2011, 78, 142–150 CrossRef PubMed.
- Y. Zhao, F. Ye, T. Brismar, X. Li, R. He, R. Heuchel, R. El-Sayed, N. Feliu, W. Zheng, S. Oerther, J. Dutta, W. Parak, M. Muhammed and M. Hassan, ACS Appl. Mater. Interfaces, 2020, 12, 53665–53681 CrossRef CAS.
- G. Song, M. Kenney, Y. S. Chen, X. Zheng, Y. Deng, Z. Chen, S. X. Wang, S. S. Gambhir, H. Dai and J. Rao, Nat. Biomed. Eng., 2020, 4, 325–334 CrossRef CAS.
- G. Song, X. Zheng, Y. Wang, X. Xia, S. Chu and J. Rao, ACS Nano, 2019, 13, 7750–7758 CrossRef CAS PubMed.
- F. Chen, L. Teng, C. Lu, C. Zhang, Q. Rong, Y. Zhao, Y. Yang, Y. Wang, G. Song and X. Zhang, Anal. Chem., 2020, 92, 13452–13461 CrossRef CAS PubMed.
- P. Peddu, A. Quaglia, P. Kane and J. Karani, Crit. Rev. Oncol. Hematol., 2009, 70, 12–23 CrossRef CAS PubMed.
- C. J. Mclaren, D. Day, D. Croagh, A. Strickland and E. Segelov, in Advances in Pancreatic Cancer, ed. L. Rodrigo, 2019, ch. 4, pp. 73–94 Search PubMed.
- V. Blanco, T. Latif, Z. Chu and X. Qi, Transl. Oncol., 2015, 8, 196–203 CrossRef PubMed.
- I. Steinberg, D. Huland, O. Vermesh, H. Frostig, W. Tummers and S. Gambhir, Photoacoustics, 2019, 14, 77–98 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2022 |

