Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Pressure-induced phosphorescence enhancement and piezochromism of a carbazole-based cyclic trinuclear Cu(I) complex†
Mo
Xie
 a,
Xiao-Ru
Chen
b,
Kun
Wu
a,
Xiao-Ru
Chen
b,
Kun
Wu
 a,
Zhou
Lu‡
a,
Zhou
Lu‡
 a,
Kai
Wang
a,
Kai
Wang
 c,
Nan
Li
c,
Rong-Jia
Wei
c,
Nan
Li
c,
Rong-Jia
Wei
 a,
Shun-Ze
Zhan
a,
Shun-Ze
Zhan
 b,
Guo-Hong
Ning
b,
Guo-Hong
Ning
 *a,
Bo
Zou
*a,
Bo
Zou
 *c and
Dan
Li
*c and
Dan
Li
 *a
*a
aCollege of Chemistry and Materials Science, Guangdong Provincial Key Laboratory of Functional Supramolecular Coordination Materials and Applications, Jinan University, Guangzhou, Guangdong 510632, People's Republic of China. E-mail: guohongning@jnu.edu.cn; danli@jnu.edu.cn
bDepartment of Chemistry, Shantou University, Shantou, Guangdong 515063, People's Republic of China
cState Key Laboratory of Superhard Materials, Jilin University, Changchun 130012, People's Republic of China. E-mail: zoubo@jlu.edu.cn
First published on 28th January 2021
Abstract
Interest in piezochromic luminescence has increased in recent decades, even though it is mostly limited to pure organic compounds and fluorescence. In this work, a Cu3Pz3 (Cu3, Pz: pyrazolate) cyclic trinuclear complex (CTC) with two different crystalline polymorphs, namely 1a and 1b, was synthesized. The CTC consists of two functional moieties: carbazole (Cz) chromophore and Cu3 units. In crystals of 1a, discrete Cz–Cu3–Cu3–Cz stacking was found, showing abnormal pressure-induced phosphorescence enhancement (PIPE), which was 12 times stronger at 2.23 GPa compared to under ambient conditions. This novel observation is ascribed to cooperation between heavy-atom effects (i.e., from Cu atoms) and metal–ligand charge-transfer promotion. The infinite π–π stacking of Cz motifs was observed in 1b and it exhibited good piezochromism as the pressure increased. This work demonstrates a new concept in the design of piezochromic materials to achieve PIPE via combining organic chromophores and metal–organic phosphorescence emitters.
Introduction
Piezochromic luminescent compounds are a class of stimuli-responsive materials1,2 which exhibit significant emission colour changes in response to grinding, pressing and stretching, and they show great potential in a wide range of applications, including pressure sensors, optical recording, and memory.3–10 Recent studies revealed that chromic mechanisms of such materials are related to pressure-controllable molecular switches,11–13 phase transition,14,15 conformational transformation,16,17 excited state transformation,18 and aggregation-induced emission (AIE) effect.19,20 To date, the molecular design and material selection for piezochromism are mostly focused on organic molecules.21–25 Because of the weak spin-orbital coupling and a long triplet-state lifetime, the pure organic molecules hardly show phosphorescence at room temperature (rt).26,27 Thus, most of piezochromic luminescent materials display a pressure-induced fluorescence change at rt (e.g., quenched or enhanced intensity and bathochromic/hypsochromic shifts),5,12,20 whereas pressure-induced phosphorescence enhancement (PIPE) materials are highly challenging and still remain unexplored.Cyclic trinuclear complexes (CTCs) with d10 metals are well known for their characteristic M3NxC6−x (M = Au, Ag, or Cu; x = 0, 3, or 6) nine-membered ring and corresponding π-acidity/basicity, and metal–metal interactions (metallophilicity) which play essential roles in photoluminescence (PL) and other properties.28,29 In cooperation with other supramolecular interactions (non-covalent interactions),30 such stimuli-responsive luminescence has often been reported in the CTC family, including piezochromism,31 thermochromism,32–34 solvatochromism, and concentration luminochromism.35 In previous studies, phosphorescence of organic chromophores could be triggered at cryogenic temperatures or even at rt, after forming CTC/organic adducts or if directly serving as ligands.28,36,37 Such luminescence, termed “metal-sensitized phosphorescence”, is attributed to the enhanced intersystem crossing (ISC) rate from singlet excited states to triplet states mediated by an external heavy-atom effect (e.g., Au, Ag, Cu and Hg).38,39 In this way, it is proposed that the external isotropic pressure can enforce the interactions between CTCs and organic chromophores, resulting in alteration of electronic structures of the emission centre and triggering the PIPE. As far as is known, investigations of the luminescence behaviour of such organic chromophore-CTC adducts under high pressure have rarely been reported.
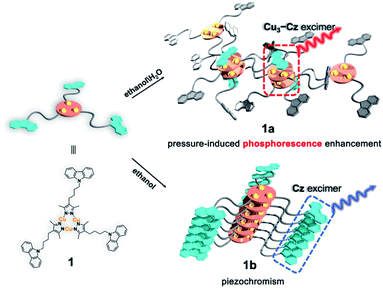
In this work, a carbazole-based Cu(I) CTC (1) was designed by linking classic organic chromophore carbazole40 (Cz) and Cu3Pz3 CTCs (Cu3 Pz: pyrazolate) with a non-conjugated flexible n-butyl chain. Two crystalline polymorphs, denoted as 1a and 1b, are obtained and exhibit two different stacking models leading to the formation of [Cz–Cu3–Cu3–Cz] excimers and Cz excimer/aggregator, respectively (Scheme 1). Therefore, metal-sensitized phosphorescence and the ligand-dominated luminescence are observed for 1a and 1b, respectively. Furthermore, 1a shows an unprecedented PIPE phenomenon, which has never been reported in piezochromic materials, and 1b exhibits conventional carbazole characteristic piezochromic luminescence with a good linear relationship of intensity versus external pressure.
Results and discussion
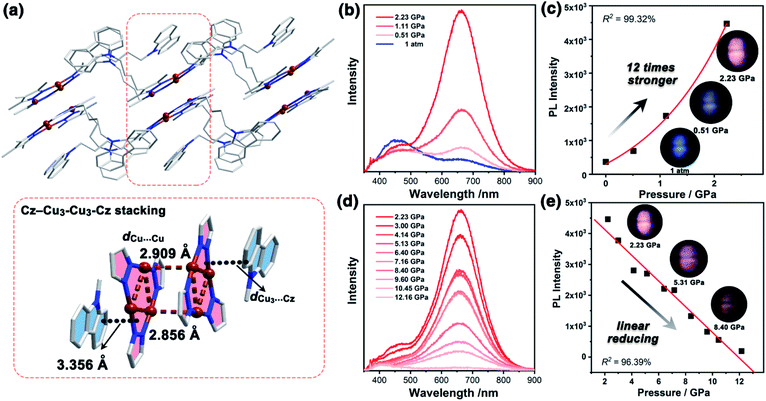
The yellow block-shaped crystals of 1a, or colourless fibrous crystals of 1b were obtained by mixing 9-(4-(3,5-dimethyl-1H-pyrazol-4-yl)butyl)-carbazole ligand (HL) and Cu2O in anhydrous ethanol or ethanol/water under solvothermal conditions (see ESI† for further details). The freshly synthesized single-crystal samples of 1a and 1b were characterized by powder X-ray diffraction (PXRD) and infrared (IR) spectroscopy, confirming their phase purity (Fig. S7 and S8, ESI†). The single X-ray crystallographic analysis of 1a and 1b revealed that both featured the nine-membered Cu3N6 CTC composed of Cu3 units and Cz motifs (Fig. 1a and 2a).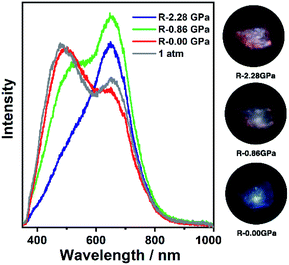 | ||
| Fig. 2 The PL spectra and photographs of 1a in a pressure relief process from 2.28 GPa to 0.00 GPa. The gray line (marked as 1 atm) is the PL spectrum before compression. | ||
Unlike the conventional sandwich-like ternary adducts (i.e., CTC–organic aromatic–CTC),41,42 unprecedented discrete adducts with a Cz–Cu3–Cu3–Cz stacking model were observed in 1a (Fig. 1a). Specifically, two adjacent Cu3 units adopted a dimer-of-trimer configuration with short intertrimer dCu⋯Cu of 2.854 and 2.909 Å, implying that there were strong Cu⋯Cu interactions between the Cu3 units. Moreover, one set of Cz groups adducted with the Cu3–Cu3 dimer and the intermolecular plane-to-plane distance between the Cu3 unit and the Cz group (dCu3⋯Cz) was 3.360 Å, which indicated weak π-acid⋯base interactions (Fig. 1a). It is worth noting that the other Cz groups were randomly arranged and no noticeable interactions between Cu3 or Cz units were observed (Fig. S10 and S11, ESI†).
1a exhibits a UV-vis absorption profile similar to that of the proligand HL in either CH2Cl2 solution or solid state (Fig. S14 and S15, ESI†). The absorption bands of 1a at about 290 and 340 nm can be attributed to ligand-centred (LC) transition. The variable-excitation wavelength PL spectra of 1a at rt were remarkably different from those of the proligand (Fig. S16 and S17, ESI†). As shown in Fig. S18 (ESI†), 1a has low-energy (LE) dominated dual emission bands at about 400 nm (high-energy, HE band) and 680 nm (LE band) with a large LE/HE intensity ratio of 100 (λex = 280 nm). The HE band was consistent with the solid emission of the proligand in both emission energy and band profile, which can be assigned as a LC fluorescence. The LE band intensity was significantly larger compared with that of the proligand, and it remained at a certain strength even under LE excitation (i.e., λex = 360 nm), indicating that the LE band was not limited to the emission of the proligand. Upon increasing the excitation energy, the emission colour changed from blue-white to red (Fig. S18b, ESI†) in the CIE-1931 chromaticity diagram.
Upon cooling to 200 K or a lower temperature, 1a showed a new broad emission band at 560 nm, whose emission energy was temperature-independent (Fig. S20a, ESI†). To better understand the steady state PL, density functional theory (DFT) and time-dependent density functional theory (TDDFT) calculations were conducted and the calculations based on singlet monomeric 1a geometry revealed that the low-lying singlet excited states were mainly localized on the Cz moieties, until the metal-to-ligand charge transfer (MLCT) character became dominant in the S14 state (Table S4, ESI†). These results suggested that 1a will produce 1LC fluorescence upon LE excitation to the S1–S13 states. By exciting 1a to the S14 or higher energy singlet states by increasing excitation energy, the ISC process might be generated by metal copper participation. The Cz–Cu3–Cu3–Cz stacking model of 1a is divided into two theoretical models for clarity, denoted as dimer (a) or (b), which contained Cu3–Cu3 dimers or Cu3–Cz adducts, respectively. The lowest single–triplet excited states of both monomer and dimer (a) were localized on the Cz moieties (Table S5, ESI†), which can be assigned as metal-sensitized ligand centred (MSLC) phosphorescence. The charge transfer (3LC/3MLCT) excited state based on the Cu3–Cz interaction contributed a little (9%) to the triplet emission of dimer (b), suggesting that the copper metal directly participated in the triplet electronic transition rather than just sensitizing the LC emission. Therefore, the LE dual emissions of 1a can be assigned as the 3LC of the monomer (λem = 560 nm) and the domination of the MSLC mixed with the 3MLCT assisted phosphorescence of the Cz–Cu3–Cu3–Cz excimer (λem = 680 nm). Taking advantage of the Cu3–Cz interactions, the heavy atom effects of copper atoms promoted the ISC rate and the generation of low-energy phosphorescence, leading to MSLC phosphorescence dominated emission of 1a. The proposed complete photophysical processes are summarized in Fig. S23 (ESI†).
It is known that pure carbazole possesses excellent piezoluminochromism.40 The unique mixed MSLC/MLCT phosphorescence of Cz-based CTC 1a promoted further study of the PL under high pressure. The isotropic hydrostatic pressure was directly applied via a diamond anvil cell (DAC)43 on crystal samples of 1a (see ESI† for experimental details). At ambient conditions, 1a exhibited LC dominated blue emission, under LE excitation (355 nm). As the external pressure increased, 1a presented constant fluorescence reduction and phosphorescence enhancement. The intensity of the LE phosphorescence showed exponential growth and was greatly increased: 12 times stronger at 2.23 GPa than that at 1 atm (10−4 GPa), whereas the HE emission intensity was slightly weakened, resulting in a remarkable colour change from weak blue to bright pink (Fig. 1b and c). Such PIPE is rare in the piezochromic luminescent materials. When the pressure was greater than 2.23 GPa, the emission intensity decreased linearly (Fig. 1d, e and S24, ESI†). The reduced LE emission and enhanced HE emission recorded during a pressure relief process from 2.28 GPa to 0 GPa (Fig. 2) indicated that the PIPE phenomenon of 1a was reversible. To further explain these results, TDDFT calculations were performed based on the T1 geometry of 1a at 1 atm and 2.5 GPa (Fig. S28–S30, ESI†). Under ambient conditions, the T1 state was mainly composed of 3LC transition of Cz moieties (79%) and LLCT (ligand-to-ligand charge transfer) transition of Cu3–Cz (9%). Pressurizing 1a to 2.5 GPa, the 3MLCT/3LLCT transition became dominant in the T1 state (65%) while the percentage of 3LC transition of Cz moieties was reduced to 14%. In addition, when the pressure increased from 1 atm to 2.5 GPa, the Cu contribution in the MLCT increased from 6.46% to 11.75%. These computational results indicated the external pressure strength of the Cu3–Cz interactions, resulting in the increase of the 3MLCT/3LLCT contribution in the excited state. Combining the crystal structure analysis and the computational results, it can be postulated that the weak LE emission band is attributed to MSLC phosphorescence due to the rather weak Cu3–Cz interactions and the negligible Cu contribution to the MLCT state at ambient conditions. When the pressure was increased to 2.23 GPa, the intensity of the LE phosphorescence was greatly enhanced, which could reasonably be attributed to a cooperative effect between heavy-atom effects and MLCT promotion because of the shortening of dCu3⋯Cz as well as the significant increase of Cu3–Cz interactions and Cu contribution in the MLCT (Fig. 4a).
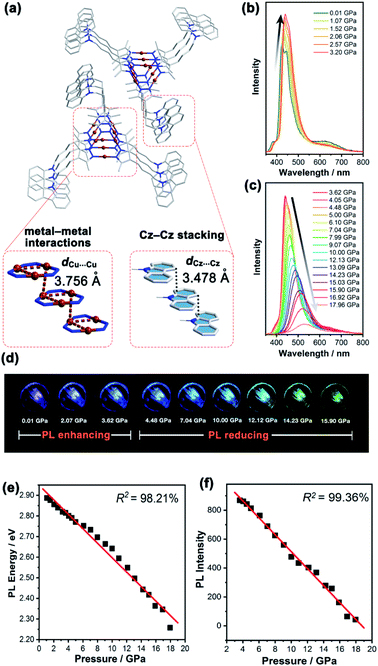
In contrast, the Cu3 units in 1b packed as a stair-step infinite chain with one pair of weak intertrimer Cu⋯Cu interactions with a relatively long intermolecular Cu⋯Cu distance (dCu⋯Cu) of 3.756(3) Å at 298 K (Fig. 3a). In addition, the Cz motifs adopted a J-type aggregation along the a-axis, whereby the two adjacent Cz groups tightly stacked through π–π interactions with an intermolecular Cz⋯Cz plane-to-plane distance (dCz⋯Cz) of about 3.480 Å (Fig. 3a). 1b presented different intensity ratios of higher and lower absorption bands in UV-vis absorption compared with that of 1a, which explained the different crystal colours of 1a and 1b (Fig. S12 and S13, ESI†). In sharp contrast, 1b shows HE dominated dual emission with a much smaller LE/HE intensity ratio of 1.25 (λex = 280 nm) than that of 1a (Fig. S19a, ESI†). The HE structured bands in the range of 350–500 nm were similar to those for the proligand (Fig. S16 and S17, ESI†) which can be attributed to the LC emission upon LE excitation (i.e., λex = 360 nm). The excitation wavelength-dependent emission colour changes were also found for 1b (Fig. S19b, ESI†). The DFT and TDDFT calculations revealed that the low-lying singlet excited states were Cz localized, confirming the 1LC feature of the HE emission of 1b. Both the lowest triplet states of monomeric and dimeric 1b were a 3LC feature (Table S6, ESI†), therefore the LE emission band of 1b can be attributed to the MSLC phosphorescence which experiences the ISC from the 1MLCT state to the 3MLCT state and the internal conversion (IC) of the triplet state. Such a proposed photophysical mechanism has been commonly observed in other CTC systems.44 Because of the well-ordered J-type aggregation of the Cz motifs, 1b exhibited aggregate/excimer emission with a lower energy in the solid state, which further supported the lower energy broad emission of 1b compared to that of the proligand. Combining the experimental and computational studies, 1a showed phosphorescence dominated emission, but 1b featured similar emission behaviours to the proligand, revealing that the stacking mode of the organic chromophore in the crystals would be an essential factor for determining the PL properties of the metal–organic complex.
Upon external pressure, the PL of 1b showed a continuous red shift, accompanied by two-step changes including emission enhancement (0.01–3.62 GPa) and emission reduction (3.62–17.96 GPa) under 355 nm excitation (Fig. 3b–d). These observations were similar to the piezoluminochromism of pure Cz (ref. 40) and agreed well with the previously mentioned suggestion for the Cz-centred characteristic luminescence of 1b. Interestingly, both PL energy and intensity (or the integrated area in Fig. S25, ESI†) showed a linear relationship versus the external pressure as shown in Fig. 3e and f, respectively. The emission spectra and photographs taken during the decompression process indicate that the piezoluminochromic phenomena of 1b (Fig. S26, ESI†) was reversible. The pressure-dependent structural simulations and frontier molecular orbital (FMO) analysis (Fig. S30–S34, ESI†) of 1b revealed that the dCz⋯Cz was the only structural parameter to change consistently with the cell volume change. It is a key factor that influences the overlap of molecular orbitals participating in the PL transition, leading to a continuous bathochromic shift in the PL energy. As the Cz groups were well separated from the Cu3 moieties with non-conjugated n-butyl chains and well packed to form an infinite and column-like Cz aggregator via π–π interactions in the crystals, 1b mainly exhibited the Cz/Cz excimer-based luminescence and the contribution of MLCT to the PL were negligible (Fig. 4b), and which are not favoured for d10-metal CTCs.39
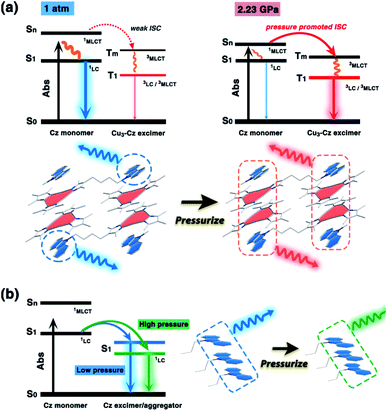 | ||
| Fig. 4 A schematic diagram of the (a) pressure-induced phosphorescence enhancement of 1a, and (b) the piezochromism of 1b. The dominant luminescent components are highlighted. | ||
Conclusions
In summary, a new piezochromic material, carbazole-based Cu(I) CTCs, consisting of a carbazole chromophore and Cu3Pz3 functional moieties linked by a non-conjugated alkyl chain, was designed. Significant stacking conformations were observed in the two crystalline polymorphs of 1a and 1b: discrete Cu3–Cz adducts via π-acid⋯base interactions were found in 1a, whereas the infinite π–π stacking of Cz groups and a stair-step infinite chain of Cu3 motifs were obtained in 1b. Like pure carbazole molecules, upon increasing the external pressure, the emission maximum of 1b gradually red-shifted and the intensity of the fluorescence band slightly increased before gradually decreasing. However, the unprecedented phenomenon of the pressure-induced phosphorescence enhancement (PIPE) of 1a was discovered, which was a rare case in the field of piezochromic luminescence. Theoretical and experimental investigations illustrated that the increase of the LE phosphorescence band intensity originated from the Cu3–Cz excimer in 1a. External heavy atom effects induced MSLC phosphorescence and then promoted MLCT events from the Cu3 unit to the Cz groups cooperatively contributed to the enhancement of the LE phosphorescence band from 1 atm to 2.5 GPa. These studies demonstrated a novel strategy for designing a new class of piezochromic materials with PIPE properties via the combination of external heavy atom effects and MLCT promotion. The excellent responses to pressure of 1a and 1b make them promising materials for use as pressure sensors or in pressure detection.Experimental section
Materials
All starting materials were purchased from commercial sources and used as received without further purification. Detailed characterization methods are included in the ESI.†The eluent selected was dichloromethane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) petroleum ether (1
petroleum ether (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8). Next K2CO3 (80 mol, 11.04 g), acetylacetone (60 mmol, 6 mL), and a small amount of 18-crown-6 was added to a three-necked round bottom flask containing 100 mL of acetone. Then 1-bromobutyl 4-oxazole (20 mmol, 6.038 g) was dissolved in 60 mL of acetone and placed in a dropping funnel and attached to a three-necked flask. After installing the device, nitrogen gas was added and the mixture was stirred at rt for 30 min. After 30 min, the temperature was raised to 50 °C, and the 1-bromobutyl 4-oxazole solution in the dropping funnel was gradually added dropwise to the three-necked flask, and the mixture was heated to reflux, and the nitrogen was left flowing for 17 h. After the reaction was completed, distilled water was added, and the mixture was extracted with a dichloromethane solution, and then steamed, and purified by column chromatography. The eluent selected was dichloromethane
8). Next K2CO3 (80 mol, 11.04 g), acetylacetone (60 mmol, 6 mL), and a small amount of 18-crown-6 was added to a three-necked round bottom flask containing 100 mL of acetone. Then 1-bromobutyl 4-oxazole (20 mmol, 6.038 g) was dissolved in 60 mL of acetone and placed in a dropping funnel and attached to a three-necked flask. After installing the device, nitrogen gas was added and the mixture was stirred at rt for 30 min. After 30 min, the temperature was raised to 50 °C, and the 1-bromobutyl 4-oxazole solution in the dropping funnel was gradually added dropwise to the three-necked flask, and the mixture was heated to reflux, and the nitrogen was left flowing for 17 h. After the reaction was completed, distilled water was added, and the mixture was extracted with a dichloromethane solution, and then steamed, and purified by column chromatography. The eluent selected was dichloromethane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) petroleum ether (1
petroleum ether (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) and colorless needle-like crystals were obtained, which was the intermediate HL-2: 3-(4-(9H-carbazol-9-yl)butyl)pentane-2,4-dione with a yield of about 51%. 1H-NMR (400 MHz, CD2Cl2) δ 8.13 (d, J = 7.8 Hz, 1H), 7.48 (m, 2H), 7.26 (dd, J = 7.4, 6.9 Hz, 1H), 4.35 (t, J = 7.2 Hz, 1H), 3.58 (t, J = 7.1 Hz, 1H), 2.12 (m, 3H), 1.92 (m, 1H), 1.84 (m, 1H), 1.35 (m, 1H) ppm. 13C-NMR (400 MHz, CD2Cl2) δ 204.0, 140.4, 125.6, 122.7, 120.2, 118.8, 108.7, 68.1, 42.7, 29.2, 28.8, 27.8, 25.3 ppm. Elemental analysis for C21H23NO2, calcd (%): C 78.47, H 7.21, N 4.36; found (%): C 78.67, H 7.40, N 4.37.
10) and colorless needle-like crystals were obtained, which was the intermediate HL-2: 3-(4-(9H-carbazol-9-yl)butyl)pentane-2,4-dione with a yield of about 51%. 1H-NMR (400 MHz, CD2Cl2) δ 8.13 (d, J = 7.8 Hz, 1H), 7.48 (m, 2H), 7.26 (dd, J = 7.4, 6.9 Hz, 1H), 4.35 (t, J = 7.2 Hz, 1H), 3.58 (t, J = 7.1 Hz, 1H), 2.12 (m, 3H), 1.92 (m, 1H), 1.84 (m, 1H), 1.35 (m, 1H) ppm. 13C-NMR (400 MHz, CD2Cl2) δ 204.0, 140.4, 125.6, 122.7, 120.2, 118.8, 108.7, 68.1, 42.7, 29.2, 28.8, 27.8, 25.3 ppm. Elemental analysis for C21H23NO2, calcd (%): C 78.47, H 7.21, N 4.36; found (%): C 78.67, H 7.40, N 4.37.
The diketone intermediate was added to a flask containing hydrazine hydrate (80%, 14 mL), ethanol (100 mL), and kept at 70 °C for 15 h, and the ethanol evaporated to give a yellow oil. After freezing in a refrigerator for one week, the yellow oil was taken out, and a white solid was observed. After washing with a diethyl ether solution, it was filtered and dried to give a product HL [9-(4-(3,5-dimethyl-1H-pyrazol-4-yl)butyl)-9H-carbazole] with a yield of about 39%. 1H-NMR (400 MHz, CD2Cl2) δ 8.13 (d, J = 7.8 Hz, 1H), 7.50 (m, 2H), 7.25 (dd, J = 7.9, 6.7, 1H), 4.35 (t, J = 7.2 Hz, 1H), 2.38 (t, J = 7.6 Hz, 1H), 2.13 (s, 3H), 1.90 (m, 1H), 1.56 (m, 1H) ppm. 13C-NMR (400 MHz, CD2Cl2) δ 141.8, 140.4, 125.6, 122.7, 120.2, 118.7, 115.0, 108.7, 43.0, 28.7, 28.2, 22.8, 10.6 ppm. IR spectrum (KBr, cm−1): 3203 (w), 3147 (w), 3089 (w), 3042 (w), 3007 (w), 2928 (m), 2856 (w), 1626 (m), 1592 (m), 1482 (m), 1451 (m), 1413 (m), 1383 (w), 1325 (m), 1298 (w), 1238 (m), 1206 (m), 1177 (w), 1153 (m), 1104 (w), 1066 (w), 1020 (w), 1000 (w), 924 (w), 901 (w), 850 (w), 771 (w), 749 (s), 721 (s), 628 (w), 615 (w), 556 (w), 527 (w), 445 (w), 421 (m). Elemental analysis for C21H23N3, calcd (%): C 79.46, H 7.30, N 13.24; found (%): C 79.61, H 7.41, N 13.41. Detailed characterization of HL and the intermediates are included in the ESI.†
Synthesis of 1a
A solvo thermal method45 was used to synthesize the target complex 1a and 1b. The Cu2O (4.32 mg, 0.03 mmol), HL (9.51 mg, 0.03 mmol), 1.5 mL of ethanol and 1.5 mL of water were added to a hard glass tube with an inner diameter of 8 mm, and the tube was sealed and heated to 140 °C for 72 h. After cooling the tube and its contents to rt at a rate of 3 °C per h, the contents were filtered and washed with ethanol to give a light yellow block crystal with a yield of about 52%. Elemental analysis for C63H66N9Cu3, calcd (%): C 66.15, H 6.06, N 10.74; found (%): C 66.38, H 5.84, N 11.06.Synthesis of 1b
The Cu2O (4.22 mg, 0.03 mmol), HL (9.51 mg, 0.03 mmol) and 3 mL of ethanol were added to a hard glass tube with an inner diameter of 8 mm, and the tube was sealed and heated to 140 °C for 72 h. After cooling the tube and its contents to rt at a rate of 3 °C per h, the contents were then filtered and washed with ethanol to give a colorless filamentous crystal with a yield of about 70%. Elemental analysis for C63H66N9Cu3, calcd (%): C 66.38, H 5.84, N 11.06; found (%): C 66.12, H 5.65, N 10.96.Crystallographic studies
Single-crystal X-ray diffraction (SCXRD) data for 1a and 1b were collected by an Cryostream system (Oxford Cryosystems) on a XtaLAB PRO MM007 DW diffractometer system equipped with a RA-Micro7HF-MR-DW(Cu/Mo) X-ray generator and a Pilatus3R-200K-A detector (Rigaku, Japan), Cu Kα, λ = 1.54178 Å. The numerical absorption corrections were applied using the ABSCOR program. The data set temperatures were 296 K and 100 K for 1a and 298 K for 1b. For 1a, the structures were solved using direct methods, which yielded the positions of all the non-hydrogen atoms. These were first refined isotropically and then anisotropically. All the hydrogen atoms of the ligands were placed in calculated positions with fixed isotropic thermal parameters and included in the structure factor calculations in the final stage of the full-matrix least-squares refinement.For 1b, the low quality of the crystal structure of the sample was due to poor data and a disorder problem of the flexible n-butyl chain and the carbazole ring, respectively. The cell parameters and atomic position of the CTC rings and heavy atom were generated from the SCXRD data by the Patterson method.
All the calculations were performed using the system of computer programs.46,47 The crystal data and structure refinement parameters are summarized in Table S1 (ESI†). Selected bond lengths and angles are given in Table S2 (ESI†).
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 21731002, 21725304, 21975104, and 21901085), the Guangdong Major Project of Basic and Applied Research (2019B030302009), and the high-performance public computing service platform of Jinan University. G.-H. Ning thanks the Guangdong Basic and Applied Basic Research Foundation (2019B151502024), the Guangdong Province Pearl River Scholar Funded Scheme (2019), and the Fundamental Research Funds for the Central Universities (21619315) for their financial support.Notes and references
- A. J. McConnell, C. S. Wood, P. P. Neelakandan and J. R. Nitschke, Chem. Rev., 2015, 115, 7729–7793 CrossRef CAS.
- P. Theato, B. S. Sumerlin, R. K. O'Reilly and T. H. Epps III, Chem. Soc. Rev., 2013, 42, 7055–7056 RSC.
- Z. Fu, K. Wang and B. Zou, Chin. Chem. Lett., 2019, 30, 1883–1894 CrossRef CAS.
- L. Bai, P. Bose, Q. Gao, Y. Li, R. Ganguly and Y. Zhao, J. Am. Chem. Soc., 2016, 139, 436–441 CrossRef.
- T. Ono, Y. Tsukiyama, A. Taema, H. Sato, H. Kiyooka, Y. Yamaguchi, A. Nagahashi, M. Nishiyama, Y. Akahama, Y. Ozawa, M. Abe and Y. Hisaeda, ChemPhotoChem, 2018, 2, 416–420 CrossRef CAS.
- H. Yan, F. Yang, D. Pan, Y. Lin, J. N. Hohman, D. Solis-Ibarra, F. H. Li, J. E. P. Dahl, R. M. K. Carlson, B. A. Tkachenko, A. A. Fokin, P. R. Schreiner, G. Galli, W. L. Mao, Z. X. Shen and N. A. Melosh, Nature, 2018, 554, 505–510 CrossRef CAS.
- Y. Gu, H. Liu, R. Qiu, Z. Liu, C. Wang, T. Katsura, H. Zhang, M. Wu, M. Yao, H. Zheng, K. Li, Y. Wang, K. Wang, B. Yang, Y. Ma and B. Zou, J. Phys. Chem. Lett., 2019, 10, 5557–5562 CrossRef CAS.
- Q. Li, Z. Chen, B. Yang, L. Tan, B. Xu, J. Han, Y. Zhao, J. Tang and Z. Quan, J. Am. Chem. Soc., 2020, 142, 1786–1791 CrossRef CAS.
- M. S. Kwon, J. Gierschner, J. Seo and S. Y. Park, J. Mater. Chem. C, 2014, 2, 2552 RSC.
- A. Pucci, Sensors, 2019, 19, 4969 CrossRef CAS.
- D. A. Davis, A. Hamilton, J. Yang, L. D. Cremar, D. Van Gough, S. L. Potisek, M. T. Ong, P. V. Braun, T. J. Martínez, S. R. White, J. S. Moore and N. R. Sottos, Nature, 2009, 459, 68–72 CrossRef CAS.
- J. W. Chung, Y. You, H. S. Huh, B.-K. An, S.-J. Yoon, S. H. Kim, S. W. Lee and S. Y. Park, J. Am. Chem. Soc., 2009, 131, 8163–8172 CrossRef CAS.
- Y. Wang, X. Tan, Y.-M. Zhang, S. Zhu, I. Zhang, B. Yu, K. Wang, B. Yang, M. Li, B. Zou and S. X.-A. Zhang, J. Am. Chem. Soc., 2015, 137, 931–939 CrossRef CAS.
- J. Kunzelman, M. Kinami, B. R. Crenshaw, J. D. Protasiewicz and C. Weder, Adv. Mater., 2008, 20, 119–122 CrossRef CAS.
- B. S. Li, X. D. Wen, R. P. Li, Z. W. Wang, P. G. Clem and H. Y. Fan, Nat. Commun., 2014, 5, 1–7 Search PubMed.
- W. Z. Yuan, Y. Tan, Y. Gong, P. Lu, J. W. Y. Lam, X. Y. Shen, C. Feng, H. H.-Y. Sung, Y. Lu, I. D. Williams, J. Z. Sun, Y. Zhang and B. Z. Tang, Adv. Mater., 2013, 25, 2837–2843 CrossRef CAS.
- S. Bergantin, M. Moret, G. Buth and F. P. A. Fabbiani, J. Phys. Chem. C, 2014, 118, 13476–13483 CrossRef CAS.
- Q. Qi, J. Qian, X. Tan, J. Zhang, L. Wang, B. Xu, B. Zou and W. Tian, Adv. Funct. Mater., 2015, 25, 4005–4010 CrossRef CAS.
- N. Li, Y. Gu, Y. Chen, L. Zhang, Q. Zeng, T. Geng, L. Wu, L. Jiang, G. Xiao, K. Wang and B. Zou, J. Phys. Chem. C, 2019, 123, 6763–6767 CrossRef CAS.
- H. Liu, Y. Gu, Y. Dai, K. Wang, S. Zhang, G. Chen, B. Zou and B. Yang, J. Am. Chem. Soc., 2020, 142, 1153–1158 CrossRef CAS.
- Y. Liu, Q. Zeng, B. Zou, Y. Liu, B. Xu and W. Tian, Angew. Chem., Int. Ed., 2018, 57, 15670–15674 CrossRef CAS.
- X. Sun, X. Wang, W. Liang, C. Gao, Z. Sui, M. Liu, R. Dai, Z. Wang, X. Zheng and Z. Zhang, J. Phys. Chem. C, 2018, 122, 15861–15867 CrossRef CAS.
- K. Nagura, S. Saito, H. Yusa, H. Yamawaki, H. Fujihisa, H. Sato, Y. Shimoikeda and S. Yamaguchi, J. Am. Chem. Soc., 2013, 135, 10322–10325 CrossRef CAS.
- B. Xu, Y. Mu, Z. Mao, Z. Xie, H. Wu, Y. Zhang, C. Jin, Z. Chi, S. Liu, J. Xu, Y.-C. Wu, P.-Y. Lu, A. Lien and M. R. Bryce, Chem. Sci., 2016, 7, 2201–2206 RSC.
- Y. Gong, S. He, Y. Li, Z. Li, Q. Liao, Y. Gu, J. Wang, B. Zou, Q. Li and Z. Li, Adv. Opt. Mater., 2020, 8, 1902036 CrossRef CAS.
- L. Huang, C. Qian and Z. Ma, Chem.–Eur. J., 2020, 26, 11914–11930 CrossRef CAS.
- J. Yang, M. Fang and Z. Li, InfoMat, 2020, 2, 791–806 CrossRef CAS.
- J. Zheng, Z. Lu, K. Wu, G.-H. Ning and D. Li, Chem. Rev., 2020, 120, 9675–9742 CrossRef CAS.
- J. Zheng, H. Yang, M. Xie and D. Li, Chem. Commun., 2019, 55, 7134–7146 RSC.
- Q. Li and Z. Li, Acc. Chem. Res., 2020, 53, 962–973 CrossRef CAS.
- C. H. Woodall, S. Fuertes, C. M. Beavers, L. E. Hatcher, A. Parlett, H. J. Shepherd, J. Christensen, S. J. Teat, M. Intissar, A. Rodrigue-Witchel, Y. Suffren, C. Reber, C. H. Hendon, D. Tiana, A. Walsh and P. R. Raithby, Chem.–Eur. J., 2014, 20, 16933–16942 CrossRef CAS.
- X.-C. Shan, F.-L. Jiang, D.-Q. Yuan, H.-B. Zhang, M.-Y. Wu, L. Chen, J. Wei, S.-Q. Zhang, J. Pan and M.-C. Hong, Chem. Sci., 2013, 4, 1484–1489 RSC.
- S.-Z. Zhan, M. Li, X.-P. Zhou, J.-H. Wang, J.-R. Yang and D. Li, Chem. Commun., 2011, 47, 12441–12443 RSC.
- J.-H. Wang, M. Li, J. Zheng, X.-C. Huang and D. Li, Chem. Commun., 2014, 50, 9115–9118 RSC.
- W.-X. Ni, M. Li, J. Zheng, S.-Z. Zhan, Y.-M. Qiu, S. W. Ng and D. Li, Angew. Chem., Int. Ed., 2013, 52, 13472–13476 CrossRef CAS.
- R. Galassi, M. A. Rawashdeh-Omary, H. V. R. Dias and M. A. Omary, Comments Inorg. Chem., 2019, 39, 287–348 CrossRef CAS.
- O. Elbjeirami, M. D. Rashdan, V. Nesterov and M. A. Rawashdeh-Omary, Dalton Trans., 2010, 39, 9465 RSC.
- S.-Z. Zhan, F. Ding, X.-W. Liu, G.-H. Zhang, J. Zheng and D. Li, Inorg. Chem., 2019, 58, 12516–12520 CrossRef CAS.
- L.-R. Xing, Z. Lu, M. Li, J. Zheng and D. Li, J. Phys. Chem. Lett., 2020, 11, 2067–2073 CrossRef CAS.
- Y. Gu, K. Wang, Y. Dai, G. Xiao, Y. Ma, Y. Qiao and B. Zou, J. Phys. Chem. Lett., 2017, 8, 4191–4196 CrossRef CAS.
- M. M. Olmstead, F. Jiang, S. Attar and A. L. Balch, J. Am. Chem. Soc., 2001, 123, 3260–3267 CrossRef CAS.
- H. V. Rasika Dias and C. S. Palehepitiya Gamage, Angew. Chem., Int. Ed., 2007, 46, 2192–2194 CrossRef.
- P. F. McMillan, Chem. Commun., 2003, 8, 919–923 RSC.
- L. D. Earl, J. K. Nagle and M. O. Wolf, Inorg. Chem., 2014, 53, 7106–7117 CrossRef CAS.
- R.-J. Wei, H.-G. Zhou, Z.-Y. Zhang, G.-H. Ning and D. Li, CCS Chem., 2020, 2, 2045–2053 CrossRef.
- C. B. Hübschle, G. M. Sheldrick and B. Dittrich, J. Appl. Crystallogr., 2011, 44, 1281–1284 CrossRef.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112–122 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental and calculation details; general characterization data, including IR, PXRD, and TGA; crystal data and structure refinement parameters; UV-vis spectra; varied-temperature solid-state emission spectra; detailed photophysical data and proposed Jablonski diagrams; linear relationships; detailed TDDFT results; and pressure-dependent simulation results. CCDC 2043666–2043668. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0sc07058k |
| ‡ Present address: Department of Chemistry, University of North Texas, Denton, Texas 76203, USA. |
| This journal is © The Royal Society of Chemistry 2021 |