Open Access Article
Open Access ArticleSilica gel-induced aryne generation from o-triazenylarylboronic acids as stable solid precursors†
Motoki
Ito
 *,
Yuka
Yamabayashi
,
Mio
Oikawa
,
Emi
Kano
,
Kazuhiro
Higuchi
*,
Yuka
Yamabayashi
,
Mio
Oikawa
,
Emi
Kano
,
Kazuhiro
Higuchi
 and
Shigeo
Sugiyama
*
and
Shigeo
Sugiyama
*
Meiji Pharmaceutical University, 2-522-1 Noshio Kiyose, Tokyo 204-8588, Japan. E-mail: mito@my-pharm.ac.jp; sugiyama@my-pharm.ac.jp
First published on 9th April 2021
Abstract
We report the development of o-triazenylarylboronic acids as new aniline-based aryne precursors. The readily available and shelf-stable solid precursors generate (hetero)arynes under remarkably mild conditions using silica gel as the sole reagent, which subsequently undergo reactions with a range of arynophiles. Furthermore, solid-state aryne reactions under solvent-free conditions were accomplished. Aryne generation proceeded via a dual activation mechanism, as rationalized using Jaffé's plot analysis based on Hammett constants.
Introduction
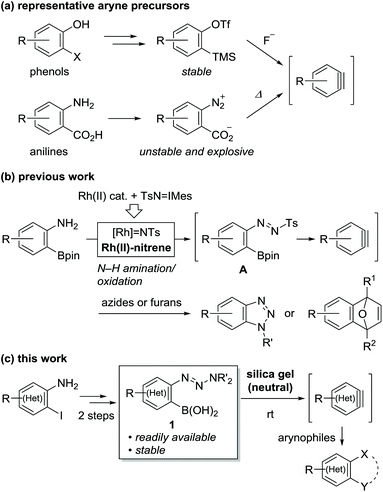
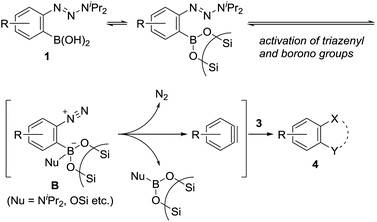
Arynes and heteroarynes are highly reactive synthetically useful reaction intermediates that enable the simultaneous creation of two bonds, including C–C, C–H, and C–X (X = heteroatom) bonds, on adjacent aromatic carbons via reactions with a range of arynophiles.1–3 Because unstable arynes are typically generated in situ, a judicious choice of precursors that generate arynes under conditions compatible with the selected arynophiles is crucial for achieving the desired transformations. Over the last few decades, the use of 2-trimethylsilylphenyl triflates4 in combination with fluoride ions has significantly contributed to the advancement of aryne chemistry, including the expansion of the arynophile scope, elegant reaction design, and syntheses of functional and biologically active compounds (Scheme 1a). These achievements are attributed to the stability and accessibility of the precursors, obtained from ubiquitous phenols, as well as the use of mild reaction conditions. In addition to phenol derivatives, aniline derivatives, represented by benzenediazonium 2-carboxylates, have been used as aryne precursors since the 1960s.5 However, despite their latent synthetic utility associated with the ubiquity of anilines, being comparable to that of phenols, their use is presently limited owing to their explosive character.6 In this context, we envisioned that the development of a methodology for aryne generation from readily available and stable aniline derivatives under mild conditions would contribute to further advancements of aryne chemistry, which would be distinct from that achieved hitherto with phenol-based precursors.7We recently reported the development of a new methodology for aryne generation from o-aminophenylboronates via the in situ preparation of a new aryne precursor, namely, N-tosyldiazene A (Scheme 1b).8 However, under the conditions implemented for the in situ preparation of unstable A, including the generation of reactive Rh(II)-nitrene species from Rh(II) catalyst and iminoiodinane (TsN = IMes), the only applicable arynophiles were found to be azides and furans. These results prompted us to design a more stable precursor based on A, not requiring in situ preparation. After some consideration, we newly designed o-triazenylarylboronic acids 1 by replacing the diazene moiety of A with a triazenyl group, which is well-known as a masked diazonio group.6c,9 Herein, we describe the unexpected discovery of (hetero)aryne generation from o-triazenylarylboronic acids 1 under remarkably mild reaction conditions using neutral silica gel as the sole reagent (Scheme 1c).
Results and discussion
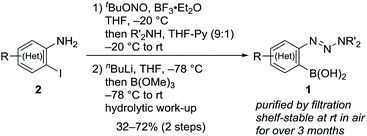
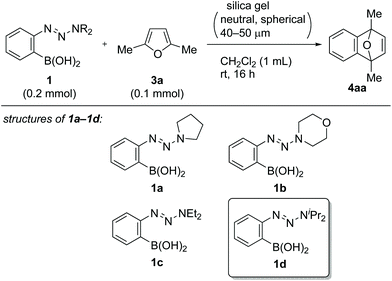
Following a literature procedure,10o-triazenylarylboronic acids 1 were synthesized from o-iodoarylamines 2 in 32–72% yields over two steps, including triazene formation via diazotization followed by borylation via halogen–lithium exchange (Scheme 2). Notably, synthesized 1 were obtained as solids after purification by filtration and were shelf-stable at ambient temperature in air for over three months.Initially, we examined the reaction of 1a with 2,5-dimethylfuran (3a) in CH2Cl2 without the use of additives (Table 1, entry 1). Interestingly, TLC analysis indicated the formation of cycloadduct 4aa, whereas the 1H NMR spectrum of the crude product suggested that the reaction had not occured. Indeed, 4aa was obtained in 36% isolated yield after column chromatography on silica gel (neutral, spherical, 40–50 μm). Inspired by these results, we examined the reaction of 1d (0.2 mmol) in the presence of silica gel.11 The yield of 4aa increased with increasing amounts of silica gel, and a maximum yield of 88% was observed with 200 mg of silica gel (entries 2–4).12 Regarding alkyl substituents on the triazenyl group, isopropyl groups were proven to be optimal, and quantitative 1H NMR yield was obtained with the use of 1d (entries 4–7). Notably, when 1d was used on 5 mmol scale, 4aa was isolated in 92% yield (entry 7). Silica gel displayed virtually no loss of activity when it was used without dryness (entry 8). Although the amount of 1d could be reduced to 1.5 equiv. without significant loss of yield (entry 9), using 1d as the limiting reagent (1d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3a = 1
3a = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) decreased the yield to 59% (entry 10). Solvent screening revealed that polar solvents, such as MeCN and THF, were markedly less effective than CH2Cl2 (entries 11 and 12). In particular, virtually no conversion of 1d was observed in THF. In contrast, the use of toluene provided a yield comparable to that obtained in CH2Cl2 (entry 13). Interestingly, a high product yield was also obtained using hexane, even though 1d was hardly soluble (entry 14).
2) decreased the yield to 59% (entry 10). Solvent screening revealed that polar solvents, such as MeCN and THF, were markedly less effective than CH2Cl2 (entries 11 and 12). In particular, virtually no conversion of 1d was observed in THF. In contrast, the use of toluene provided a yield comparable to that obtained in CH2Cl2 (entry 13). Interestingly, a high product yield was also obtained using hexane, even though 1d was hardly soluble (entry 14).
| Entry | Triazene | Silica gelb (mg) | Variation from standard conditions | Yieldc (%) |
|---|---|---|---|---|
| a Reaction conditions: 1 (0.200 mmol), 3a (0.100 mmol), silica gel in CH2Cl2 (1.0 mL). b Silica gel was used after heating under vacuum to dryness. c Determined by 1H NMR spectroscopy using 1,1,2,2-tetrachloroethane as an internal standard. d Yields in parentheses refer to the yields of the isolated products. e In 5 mmol scale (1d: 10.0 mmol, 3a: 5.00 mmol). f Stabilizer-free. | ||||
| 1 | 1a | 0 | None | ND (36)d |
| 2 | 1a | 40 | None | 33 |
| 3 | 1a | 120 | None | 75 |
| 4 | 1a | 200 | None | 88 |
| 5 | 1b | 200 | None | 71 |
| 6 | 1c | 200 | None | 93 |
| 7 | 1d | 200 | None | Quant. (92)d,e |
| 8 | 1d | 200 | Silica gel (undried) | 95 |
| 9 | 1d | 200 |
1d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3a = 1.5 3a = 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
98 |
| 10 | 1d | 200 |
1d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3a = 1 3a = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
59 |
| 11 | 1d | 200 | MeCN instead of CH2Cl2 | 54 |
| 12 | 1d | 200 | THFf instead of CH2Cl2 | NR |
| 13 | 1d | 200 | Toluene instead of CH2Cl2 | 98 |
| 14 | 1d | 200 | Hexane instead of CH2Cl2 | 97 |
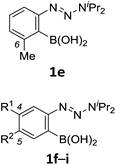
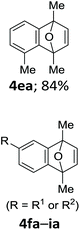
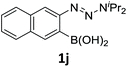
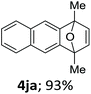
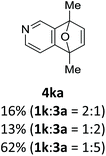
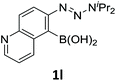
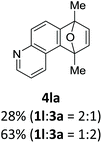
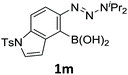
With the optimized conditions in hand, we next examined the performance of functionalized aryne precursors 1e–j and heteroaryne precursors 1k–m (Table 2). Precursors 1e–i bearing electron-donating or electron-withdrawing groups at the 4-, 5-, and 6-position provided 4ea–ia in 68–98% yields (entries 1–7). The results obtained with chlorine-substituted (1f, 1f′) and methoxy-substituted precursors (1g, 1g′) demonstrated that the position of the substituent had little impact on the product yield (entries 2–5). In addition to benzynes, the present protocol was applicable to the reaction of 2,3-naphthalyne (entry 8) and heteroarynes (entries 9–11).3 Under the standard conditions, 3,4-pyridyne precursor 1k resulted in the formation of 4ka in only 16% yield (entry 9). However, we found that the use of an excess amount of arynophile 3a significantly improved the yield to 62%. Similar results were obtained with 5,6-quinolyne precursor 1l, whereby only 2 equiv. of 3a was sufficient to give 4la in 63% yield (entry 10). In contrast to 1k and 1l, 4,5-indolyne precursor 1m afforded 4ma in excellent yields in both cases using 1m and 3a as the limiting reagent (entry 11).
| Entry | Triazene | Product; yielda |
|---|---|---|
| a Isolated yields. | ||
| 1 |
|
|
| 2 | R1 = H, R2 = Cl (1f) | R = Cl (4fa); 98% |
| 3 | R1 = Cl, R2 = H (1f′) | R = Cl (4fa); 95% |
| 4 | R1 = H, R2 = OMe (1g) | R = OMe (4ga); 98% |
| 5 | R1 = OMe, R2 = H (1g′) | R = OMe (4ga); 86% |
| 6 | R1 = H, R2 = CF3 (1h) | R = CF3 (4ha); 68% |
| 7 | R1 = H, R2 = CN (1i) | R = CN (4ia); 96% |
| 8 |
|
|
| 9 |
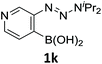
|
|
| 10 |
|
|
| 11 |
|
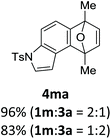
|
Next, we investigated the reaction of 1d with a range of arynophiles (Scheme 3). The precursor was applicable to [4 + 2] and [3 + 2] cycloadditions with various arynophiles, including furan 3b, pyrrole 3c, azides 3d–f, and nitrone 3g. Unfortunately, β-ketoester 3h failed to produce the desired product 4ah. Instead, using enamine 3h′ provided 4ah in 51% yield.13 The reaction with methyl N-methylanthranilate (3i) gave N-phenylated product 4ai in 75% yield along with 22% of N-methylacridone (4ai′).14 Surprisingly, the reactions also proceeded in the absence of solvent by mixing 1d, 3, and silica gel using a magnetic stirrer.15 The formation of 4 under these solvent-free conditions indicated that the aryne had formed via the contact of 1d and silica gel at the solid–solid interface. Furthermore, the generated aryne reacted effectively even with solid arynophiles 3d and 3g. Notably, compared with the outcome of reactions conducted in solution, no significant loss of yield was observed, except in the cases of 3c and 3h′, despite a lower diffusion rate in the solid-state than in solution. The results suggested that the lifetime of aryne was sufficiently long to encounter the arynophile through diffusion on the silica gel surface. Although a growing number of studies on solid-state reactions have emerged in recent years,11b,d,16,17 our results represent a pioneering example of solid-state intermolecular reactions occuring via short-lived reactive species.16d
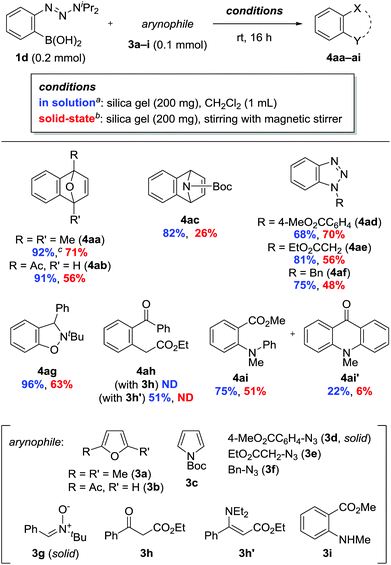 | ||
Scheme 3 Reactions of 1d with arynophiles 3a–i. a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Isolated yields. b Isolated yields. b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1H NMR yields using 1,1,2,2-tetrachloroethane as an internal standard. c 1H NMR yields using 1,1,2,2-tetrachloroethane as an internal standard. c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 mmol scale. 5 mmol scale. | ||
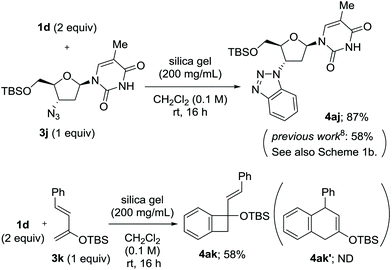
Along with the feasibility of solid-state operation, excellent functional group tolerance is a salient feature of the present method. In particular, the fluoride-free conditions allowed for the use of arynophiles bearing silyl functionalities (Scheme 4). The reaction of 1d and O-tert-butyldimethylsilyl (TBS)-protected zidovudine 3j provided 4aj in a significantly higher yield than that obtained in a previous study, with the added advantage of inexpensive and environmentally benign conditions (Scheme 4a).8 2-Siloxy-1,3-diene 3k, which is sensitive to various conditions, was also applicable as an arynophile to provide [2 + 2] cycloadduct 4ak in 58% yield, while no evidence of [4 + 2] cycloaddition was observed.
To gain insight into the reaction mechanism, we performed a time-course study (Fig. 1). To a series of reaction vessels containing 3a (0.1 mmol), silica gel, and CH2Cl2 was added 1d (0.2 mmol), and each mixture was filtered after stirring for the indicated time to remove silica gel. 1H NMR analysis of the crude mixture after stirring for 5 min indicated that only ∼0.05 mmol of 1d (∼25% of initial amount) was contained in the mixture. The amount of 1d slowly decreased over 4 h. Meanwhile, the formation of 4aa proceeded in >90% yield within 4 h according to the rate of N2 gas evolution.18 This result indicated that 1d was strongly adsorbed on the silica gel surface preceding aryne generation.
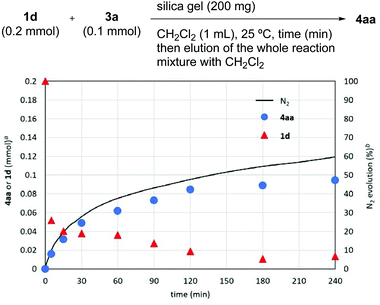 | ||
Fig. 1 Time course studies of the reaction of 1d and 3a. a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Determined by 1H NMR spectroscopy using 1,1,2,2-tetrachloroethane as an internal standard. b Determined by 1H NMR spectroscopy using 1,1,2,2-tetrachloroethane as an internal standard. b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Based on 1d (0.8 mmol). Based on 1d (0.8 mmol). | ||
Next, we performed competition experiments between parent precursor 1d and substituted precursors 1f–h or 1f′, g′, and analyzed the relative rate of the reaction (kR/kH) using Hammett constants based on the triazenyl group (σN) and those based on the borono group (σB) (Fig. 2a).19 As a result, the plots according to Jaffé's eqn (2) and (3),20 derived from the Hammett eqn (1), exhibited linearity with good R2 values, as shown in Fig. 2b and c, respectively (see ESI for details†).
| log(kR/kH) = ρNσN + ρBσB | (1) |
| log(kR/kH)/σN = ρN + ρB(σB/σN) | (2) |
| log(kR/kH)/σB = ρN(σN/σB) + ρB | (3) |
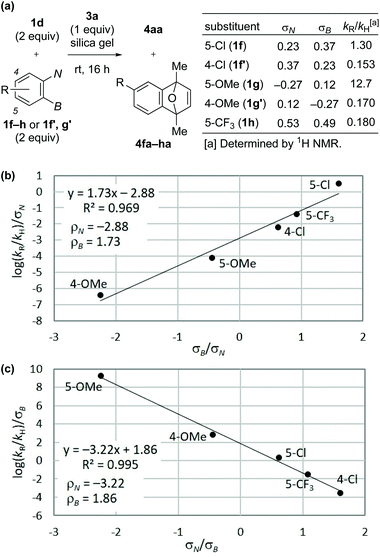 | ||
| Fig. 2 (a) Competition experiments between 1d and 1f–h, or 1f′, g′. (b) Jaffé's plot based on eqn (2). (c) Jaffé's plot based on eqn (3). | ||
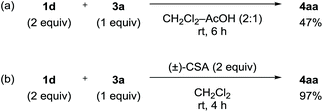
The obtained negative ρN and positive ρB values suggest simultaneous build-up of positive and negative charges on the nitrogen atom and the boron atom, respectively, in the rate-determining step (see ESI for details†). Combining the results in Fig. 1 and 2, we propose a plausible reaction mechanism, as illustrated in Scheme 5. Precursor 1 is adsorbed onto the silica gel surface via the boronate moiety, followed by the formation of zwitterionic intermediate B.21 In other words, triazenyl and borono groups were activated as diazonio and boronate groups, respectively. Upon this dual activation, highly stable precursor 1 is capable of generating an aryne under remarkably mild conditions without heating, photo-irradiation, or the use of acids or bases.9 The role of silica gel remains unclear.22 While the use of excess acetic acid instead of silica gel induced aryne generation from 1d, the yield of 4aa was only 47% (Scheme 6a). Thus, weak acidity of silica gel seems to be insufficient to activate 1. On the other hand, (±)-camphorsulfonic acid [(±)-CSA] led to a formation of 4aa in 97% yield (Scheme 6b). Thus, both heterogeneous conditions using silica gel and homogeneous conditions using Brønsted acid were applicable to aryne generation from 1.
Conclusions
We have developed new aniline-based aryne precursors 1, which generate arynes under remarkably mild conditions through the use of silica gel as the activating agent. The protocol was applicable to a wide range of (hetero)arynes and various arynophiles reacting in solution and in the solid-state. The reaction proceeded via a dual activation mechanism to generate arynes, as rationalized through Jaffé's plot analysis based on Hammett constants. Investigation of further synthetic applications of the protocol as well as mechanistic studies on the role of silica gel are currently in progress.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank T. Koseki of the Analytical Center of Meiji Pharmaceutical University for conducting mass spectrometry measurements. The authors would like to thank Prof. Dr S. Akai for constructive comments on draft of the manuscript.Notes and references
- Books and Reviews on overview of aryne chemistry, see: (a) H. Yoshida, Nucleophilic Coupling with Arynes, in Comprehensive Organic Synthesis, ed. P. Knochel and G. A. Molander, Elsevier, Amsterdam, 2nd edn, 2014, vol. 4, pp. 517–579 Search PubMed; (b) P. M. Tadross and B. M. Stoltz, A Comprehensive History of Arynes in Natural Product Total Synthesis, Chem. Rev., 2012, 112, 3550–3577 CrossRef CAS PubMed; (c) H. Takikawa, A. Nishii, T. Sakai and K. Suzuki, Aryne-based strategy in the total synthesis of naturally occurring polycyclic compounds, Chem. Soc. Rev., 2018, 47, 8030–8056 RSC; (d) J. Shi, L. Li and Yang Li, o-Silylaryl Triflates: A Journey of Kobayashi Aryne Precursors, Chem. Rev., 2021 DOI:10.1021/acs.chemrev.0c01011.
- Selected reviews on development of reactions via arynes, see: (a) T. Ikawa, H. Tokiwa and S. Akai, Experimental and Theoretical Studies on Regiocontrol of Benzyne Reactions Using Silyl and Boryl Directing Groups, J. Synth. Org. Chem., Jpn., 2012, 70, 1123–1133 CrossRef CAS; (b) A. V. Dubrovskiy, N. A. Markina and R. C. Larock, Use of benzynes for the synthesis of heterocycles, Org. Biomol. Chem., 2013, 11, 191–218 RSC; (c) J.-A. Garcia-López and M. F. Greaney, Synthesis of biaryls using aryne intermediates, Chem. Soc. Rev., 2016, 45, 6766–6798 RSC; (d) R. Karmakar and D. Lee, Reactions of arynes promoted by silver ions, Chem. Soc. Rev., 2016, 45, 4459–4470 RSC; (e) J. Shi, Y. Li and Y. Li, Aryne multifunctionalization with benzdiyne and benztriyne equivalents, Chem. Soc. Rev., 2017, 46, 1707–1719 RSC; (f) T. Roy and A. T. Biju, Recent advances in molecular rearrangements involving aryne intermediates, Chem. Commun., 2018, 54, 2580–2594 RSC; (g) S. Ghorai and D. Lee, Aryne-Based Multicomponent Coupling Reactions, Synlett, 2020, 31, 750–771 CrossRef CAS.
- Reviews on chemistry of heteroarynes, see: (a) A. E. Goetz, T. K. Shah and N. K. Garg, Pyridynes and indolynes as building blocks for functionalized heterocycles and natural products, Chem. Commun., 2015, 51, 34–45 RSC; (b) Y. Nakamura, S. Yoshida and T. Hosoya, Recent Advances in Synthetic Hetaryne Chemistry, Heterocycles, 2019, 98, 1623–1677 CrossRef.
- Y. Himeshima, T. Sonoda and H. Kobayashi, Fluoride-Induced 1,2-Elimination of o-Trimethylsilylphenyl Triflate to Benzyne under Mild Conditions, Chem. Lett., 1983, 1211–1214 CrossRef CAS.
- (a) L. Friedman and F. M. Logullo, Benzynes via Aprotic Diazotization of Anthranilic Acids: A Convenient Synthesis of Triptycene and Derivatives, J. Am. Chem. Soc., 1963, 85, 1549 CrossRef CAS; (b) G. Wittig and R. W. Hoffmann, 1,2,3-Benzothiadiazole 1,1-Dioxide, Org. Synth., 1967, 47, 4 CrossRef CAS; (c) C. D. Campbell and C. W. Rees, Reactive intermediates. Part I. Synthesis and oxidation of 1- and 2-aminobenzotriazole, J. Chem. Soc. C, 1969, 742–747 RSC; (d) K. Okuma, Y. Qu, N. Fujiie and N. Nagahora, Old and New Aryne Precursor, Anthranilic Acid: Multicomponent Reaction of Benzyne with Quinolines or Imines and Pronucleophiles, Chem. Lett., 2020, 49, 446–449 CrossRef CAS; (e) L. Li, Y. Li, N. Fu, L. Zhang and S. Luo, Catalytic Asymmetric Electrochemical α-Arylation of Cyclic β-Ketocarbonyls with Anodic Benzyne Intermediates, Angew. Chem., Int. Ed., 2020, 59, 14347–14351 CrossRef CAS PubMed.
- (a) F. M. Logullo, A. H. Seitz and L. Friedman, Benzenediazonium-2-carboxylate and Biphenylene, Org. Synth., 1968, 48, 12 CrossRef CAS; (b) A. V. Kelleghan, C. A. Busacca, M. Sarvestani, I. Volchkov, J. M. Medina and N. K. Garg, Safety Assessment of Benzyne Generation from a Silyl Triflate Precursor, Org. Lett., 2020, 22, 1665–1669 CrossRef CAS PubMed; (c) C. Schotten, S. K. Leprevost, L. M. Yong, C. E. Hughes, K. D. M. Harris and D. L. Browne, Comparison of the Thermal Stabilities of Diazonium Salts and Their Corresponding Triazenes, Org. Process Res. Dev., 2020, 24, 2336–2341 CrossRef CAS.
- For recent examples of development of new strategies for arynes generation, see: (a) T. R. Hoye, B. Baire, D. Niu, P. H. Willoughby and B. P. Woods, The hexadehydro-Diels–Alder reaction, Nature, 2012, 490, 208–212 CrossRef CAS PubMed; (b) J. Shi, H. Xu, D. Qiu, J. He and Y. Li, Selective Aryne Formation via Grob Fragmentation from the [2+2] Cycloadducts of 3-Triflyloxyarynes, J. Am. Chem. Soc., 2017, 139, 623–626 CrossRef CAS PubMed; (c) M. Minoshima, K. Uchida, Y. Nakamura, T. Hosoya and S. Yoshida, Acylalkylation of Arynes Generated from o-Iodoaryl Triflates with Hydrosilanes and Cesium Fluoride, Org. Lett., 2021, 23, 1868–1873 CrossRef CAS PubMed.
- M. Ito, A. Tanaka, K. Hatakeyama, E. Kano, K. Higuchi and S. Sugiyama, One-pot generation of benzynes from 2-aminophenylboronates via a Rh(II)-catalyzed N–H amination/oxidation/elimination cascade process, Org. Chem. Front., 2020, 7, 64–68 RSC.
- It is reported that o-triazenylbenzoic acids generate arynes upon heating, and photo-irradiation. (a) J. Nakayama, M. Yoshida and O. Simamura, N-Alkyldiarylamines from 3-Alkyl-3-aryl-1-(2-carboxyphenyl)triazenes, Bull. Chem. Soc. Jpn., 1975, 48, 2397–2398 CrossRef CAS; (b) M. Hori, T. Kataoka, H. Shimizu and N. Ueda, Reaction of benzothiazoline with benzyne-generation of novel heterocyclic sulfur ylide, benzothiazolinium s-ylide, Tetrahedron Lett., 1981, 22, 3071–3074 CrossRef CAS; (c) C. May and C. J. Moody, A concise synthesis of the antitumour alkaloid ellipticine, J. Chem. Soc., Chem. Commun., 1984, 926–927 RSC; (d) P. C. Buxton and H. Heaney, Aryne formation from 1-(3′-carboxyaryl)-3,3-dimethyl triazenes, Tetrahedron, 1995, 51, 3929–3938 CrossRef CAS; (e) A. W. Gann, J. W. Amoroso, V. J. Einck, W. P. Rice, J. J. Chambers and N. A. Schnarr, A Photoinduced, Benzyne Click Reaction, Org. Lett., 2014, 16, 2003–2005 CrossRef CAS PubMed.
- (a) D. Horhant, J.-J. Liang, M. Virboul, C. Poriel, G. Alcarasz and J. Rault-Berthelot, Dispirofluorene-indenofluorene (DSFIF): Synthesis, Electrochemical, and Optical Properties of a Promising New Family of Luminescent Materials, Org. Lett., 2006, 8, 257–260 CrossRef CAS PubMed; (b) C.-Y. Liu, A. Gavryushin and P. Knochel, Synthesis of Functionalized o-, m-, and p-Terphenyl Derivatives by Consecutive Cross-Coupling Reactions of Triazene-Substituted Arylboronic Esters, Chem. – Asian J., 2007, 2, 1020–1030 CrossRef CAS PubMed; (c) L. Xu, W. Yang, L. Zhang, M. Miao, Z. Yang, X. Xu and H. Ren, Brönsted Acid-Mediated Intramolecular Cyclization of Biaryl Triazenes for the Synthesis of Fluorenes and 9,10-Dihydro-Phenanthrenes, J. Org. Chem., 2014, 79, 9206–9221 CrossRef CAS PubMed.
- (a) A. K. Banerjee, M. S. Laya and W. J. Vera, Silica gel in organic synthesis, Russ. Chem. Rev., 2001, 70, 971–990 CrossRef CAS; (b) S. Onitsuka, Y. Z. Jin, A. C. Shaikh, H. Furuno and J. Inanaga, Silica Gel-Mediated Organic Reactions under Organic Solvent-Free Conditions, Molecules, 2012, 17, 11469–11483 CrossRef CAS PubMed; (c) Y. Jin, J. Li, L. Peng and C. Gao, Discovery of neat silica gel as a catalyst: an example of S → O acetyl migration reaction, Chem. Commun., 2015, 51, 15390–15393 RSC; (d) C. Bolm, R. Mocci, C. Schumacher, M. Turberg, F. Puccetti and J. G. Hernándeɀ, Mechanochemical Activation of Iron Cyano Complexes: A Prebiotic Impact Scenario for the Synthesis of α-Amino Acid Derivatives, Angew. Chem., Int. Ed., 2018, 57, 2423–2426 CrossRef CAS PubMed.
- Reaction time was set to 16 h because of the difficulty in accurately monitoring the reaction by TLC analysis. In Table 2 and Schemes 3 and 4, dried silica gel was used to ensure reproducibility.
- R. Li, H. Tang, H. Fu, H. Ren, X. Wang, C. Wu, C. Wu and F. Shi, Arynes Double Bond Insertion/Nucleophilic Addition with Vinylogous Amides and Carbodiimides, J. Org. Chem., 2014, 79, 1344–1355 CrossRef CAS PubMed.
- J. Zhao and R. C. Larock, Synthesis of Xanthones, Thioxanthones, and Acridones by the Coupling of Arynes and Substituted Benzoates, J. Org. Chem., 2007, 72, 583–588 CrossRef CAS PubMed.
- The reactions were performed by adding arynophile 3 and 1d sequentially to silica gel while stirring. After stirring 16 h, the solid mixtures were eluted with THF to avoid reaction during elution (see Table 1, entry 12), and the product yields were determined by 1H NMR. No reaction of 1d with 3a was observed without stirring.
- (a) D. Tan and F. García, Main group mechanochemistry: from curiosity to established protocols, Chem. Soc. Rev., 2019, 48, 2274–2292 RSC; (b) A. Porcheddu, E. Colacino, L. De Luca and F. Delogu, Metal-Mediated and Metal-Catalyzed Reactions Under Mechanochemical Conditions, ACS Catal., 2020, 10, 8344–8394 CrossRef CAS; (c) Y. Monguchi, Y. Fujita, S. Hashimoto, M. Ina, T. Takahashi, R. Ito, K. Nozaki, T. Maegawa and H. Sajiki, Palladium on carbon-catalyzed solvent-free and solid-phase hydrogenation and Suzuki–Miyaura reaction, Tetrahedron, 2011, 67, 8628–8634 CrossRef CAS; (d) T. Seo, T. Ishiyama, K. Kubota and H. Ito, Solid-state Suzuki–Miyaura cross-coupling reactions: olefin-accelerated C–C coupling using mechanochemistry, Chem. Sci., 2019, 10, 8202–8210 RSC.
- For generation and reaction of arynes under solvent-free conditions, see: (a) N. Pavliček, B. Schuler, S. Collazos, N. Moll, D. Pérez, E. Guitián, G. Meyer, D. Peña and L. Gross, On-surface generation and imaging of arynes by atomic force microscopy, Nat. Chem., 2015, 7, 623–628 CrossRef PubMed; (b) M. V. Sulleiro, S. Quiroga, D. Peña, D. Pérez, E. Guitián, A. Criado and M. Prato, Microwave-induced covalent functionalization of few-layer graphene with arynes under solvent-free conditions, Chem. Commun., 2018, 54, 2086–2089 RSC.
- The reaction was performed at 25 °C using 1d (0.8 mmol), 3a (0.4 mmol), silica gel (800 mg), and CH2Cl2 (4 mL). Rate of N2 evolution was measured using an eudiometer apparatus. W.-B. Liu, D. P. Schuman, Y.-F. Yang, A. A. Toutov, Y. Liang, H. F. T. Klare, N. Nesnas, M. Oestreich, D. G. Blackmond, S. C. Virgil, S. Banerjee, R. N. Zare, R. H. Grubbs, K. N. Houk and B. M. Stoltz, Potassium tert-Butoxide-Catalyzed Dehydrogenative C–H Silylation of Heteroaromatics: A Combined Experimental and Computational Mechanistic Study, J. Am. Chem. Soc., 2017, 139, 6867–6879 CrossRef CAS PubMed.
- For Hammett plot analysis of o-(trimethylsilylphenyl) triflates, see: S. Liu, Y. Li and Y. Lan, Mechanistic Study of the Fluoride-Induced Activation of a Kobayashi Precursor: Pseudo-SN2 Pathway via a Pentacoordinated Silicon Ate Complex, Eur. J. Org. Chem., 2017, 6349–6353 CrossRef CAS.
- (a) H. H. Jaffé, Application of the Hammett Equation to Fused Ring Systems, J. Am. Chem. Soc., 1954, 76, 4261–4264 CrossRef; (b) K. H. Jensen, J. D. Webb and M. S. Sigman, Advancing the Mechanistic Understanding of an Enantioselective Palladium-Catalyzed Alkene Difunctionalization Reaction, J. Am. Chem. Soc., 2010, 132, 17471–17482 CrossRef CAS PubMed; (c) M. Simonetti, R. Kuniyil, S. A. Macgregor and I. Larrosa, Benzoate Cyclometalation Enables Oxidative Addition of Haloarenes at a Ru(II) Center, J. Am. Chem. Soc., 2018, 140, 11836–11847 CrossRef CAS PubMed.
- No reaction was observed with
2-(trimethylsilyl)phenyltriazene under the conditions using silica gel.
. - No reaction was observed with the use of other adsorbents, such as alumina (neutral or basic) and MS 4A. Compared to that of spherical silica gel (40–50 μm, specific surface area: 630–730 m2 g−1), crushed-shape silica gel (40–63 μm, specific surface area: 480–540 m2 g−1) displayed lower activity. In addition, recycling of spherical silica gel slightly diminished the activity. Thus, the activity of silica gel depends on the properties of their surface. Additionaly, we consider that the silica gel did not play the role of a photo-catalyst, because quantitative conversion of 1d was observed in the dark. See ESI for details.†.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures, and spectral data. See DOI: 10.1039/d1qo00385b |
| This journal is © the Partner Organisations 2021 |