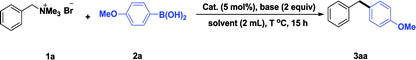Open Access Article
Open Access ArticleCross coupling of benzylammonium salts with boronic acids using a well-defined N-heterocyclic carbene–palladium(II) precatalyst†
Tao
Wang
 *ab,
Jiarui
Guo
ab,
Xiaojuan
Wang
ab,
Han
Guo
ab,
Dingli
Jia
ab,
Hengjin
Wang
ab and
Lantao
Liu
*ab,
Jiarui
Guo
ab,
Xiaojuan
Wang
ab,
Han
Guo
ab,
Dingli
Jia
ab,
Hengjin
Wang
ab and
Lantao
Liu
 *ab
*ab
aHenan Engineering Laboratory of Green Synthesis for Pharmaceuticals, School of Chemistry and Chemical Engineering, Shangqiu Normal University, Shangqiu, Henan 476000, People's Republic of China. E-mail: wt67751726@126.com; liult05@iccas.ac.cn; Fax: +86-0370-2595126; Tel: +86-0370-2595126
bHenan Key Laboratory of Biomolecular Recognition and Sensing, School of Chemistry and Chemical Engineering, Shangqiu Normal University, Shangqiu, Henan 476000, People's Republic of China
First published on 15th February 2019
Abstract
N-heterocyclic carbene–palladium(II)-catalyzed cross-coupling of benzylammonium salts with arylboronic acids for the synthesis of diarylmethane derivatives via C–N bond activation has been developed. Notably, in the presence of the easily prepared and bench-stable Pd-PEPPSI precatalyst, the Csp3–N bond activation of the benzylammonium salt even proceeded smoothly in isopropanol at room temperature.
Studies on synthetic methods of diarylmethane derivatives have attracted considerable attention because the compounds are important structural units in organic synthesis, materials science and pharmaceutical development.1 Among the synthetic approaches explored, transition metal catalyzed Suzuki coupling is one of the most important and frequently used methods. Over the past decade, the most popular strategies for the cross coupling of benzyl halides2 and benzyl sulfonates3 with aryl boric acid using Pd-catalysis have been reported. Disappointingly, some obvious drawbacks are involved with the use of benzyl halides and benzyl sulfonates as the electrophiles. These reagents are sometimes difficult in terms of substrate tolerance and storage. During recent years, the transition metal catalyzed Suzuki coupling reactions by the C–N bonds cleavage have been developed.4 Among them, the cross coupling of quaternary ammonium salts has been quite well explored because they are more easily available from amine precursors or benzyl halides and they are also stable to long-term storage. Since the pioneering work of MacMillan and co-workers in 2003,5 with aryltrimethylammonium salts as the electrophiles in various catalytic reactions such as cross-coupling,6 C–H arylation,7 borylation8 and reductive carboxylation reactions9 have been carried out. Although excellent results have been obtained, the optimization and development of cross-coupling reactions involving Csp3–N bond cleavage of benzylammonium salts under mild conditions, such as in aqueous media or at room temperature, are still worthwhile projects.10 In this present contribution, we have developed efficient catalytic systems11–13 for the Suzuki–Miyaura coupling of benzyl chlorides with arylboronic acids, producing the corresponding diarylmethane derivatives in high yields. In a recent communication, the N-heterocyclic carbene–palladium(II) complexes were also found to be active catalysts for the Suzuki–Miyaura cross-coupling of N-acylsuccinimides with arylboronic acids via C–N bond activation.14 Considering our successful experience with the applications of this complexes in the cross-coupling reaction, we then turned our recent interest to the coupling reaction between benzylammonium salt and arylboronic acids for the formation of diarylmethane derivatives. In such context, we herein report the first example of NHC–Pd-PEPPSI catalyzed coupling reactions of benzylammonium salts with arylboronic acids via Csp3–N bond activation under mild conditions (Scheme 1).
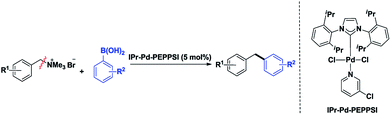 | ||
| Scheme 1 NHC–Pd(II) catalyzed coupling reactions of benzylammonium salts with arylboronic acids via Csp3–N bond activation. | ||
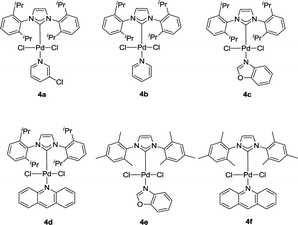
It is initiated by investigating the crossing coupling of 4-methoxyphenyl boronic acid with benzyltrimethylammonium bromide 1a, which is readily prepared quantitatively via the reaction of trimethylamine and benzyl bromide. The details were shown in Table 1. The choice of base is crucial to the yield of the reaction (Table 1, entries 1–9).15 In the presence of the IPr–Pd-PEPPSI complex 4a as the catalyst, K3PO4·3H2O as the base in isopropanol at 70 °C, we were delighted to observe that the reaction gave the corresponding diarylmethanes quantitatively. Several other solvents including THF, EtOH, 1,4-dioxane, toluene, CH3CN and H2O were tested, and the yield was not enhanced further (Table 1, entries 10–15). When 2.0 mol% of complex 4a was tested, the yield of cross coupling product was obtained in 82% yield (Table 1, entry 16). It is worth mentioning that the IPr–Pd-PEPPSI complex 4a still gave good yield at room temperature (Table 1, entry 18). Further screening of NHC–Pd(II) catalysts demonstrates that the coordination environment of the NHC–Pd(II) complexes had an obvious effect on the yield (Table 1, entries 18–23). When a solvent mixture of isopropanol and water was tested, the yield of the product reduced to 76% (Table 1, entry 24). In this case, heating to 50 °C was found to be necessary (Table 1, entry 25). Then the performance of the other five NHC–Pd(II) complexes 4b–f in this reaction condition was examined, and IPr–Pd-PEPPSI complex 4a was found to be optimal (Table 1, entry 25 vs. entries 26–30).
| Entry | Cat. | Base | Solvent | Temp (°C) | Yieldc (%) |
|---|---|---|---|---|---|
| a All reactions were carried out using 1a (0.20 mmol), 2a (0.40 mmol), base (2.0 equiv.), cat. (5.0 mol%) in solvent (0.1 M) for 15 h. b Cat. (2.0 mol%). c Isolated yields. | |||||
| 1 | 4a | KOtBu | iPrOH | 70 | 89 |
| 2 | 4a | Na2CO3 | iPrOH | 70 | Trace |
| 3 | 4a | K2CO3 | iPrOH | 70 | 91 |
| 4 | 4a | NaHCO3 | iPrOH | 70 | Trace |
| 5 | 4a | K3PO4 | iPrOH | 70 | 70 |
| 6 | 4a | K3PO4·3H2O | iPrOH | 70 | >99 |
| 7 | 4a | NaOAc | iPrOH | 70 | Trace |
| 8 | 4a | NaOH | iPrOH | 70 | 90 |
| 9 | 4a | KOH | iPrOH | 70 | 98 |
| 10 | 4a | K3PO4·3H2O | THF | 70 | 47 |
| 11 | 4a | K3PO4·3H2O | 1,4-Dioxane | 70 | 98 |
| 12 | 4a | K3PO4·3H2O | EtOH | 70 | 88 |
| 13 | 4a | K3PO4·3H2O | Toluene | 70 | 30 |
| 14 | 4a | K3PO4·3H2O | CH3CN | 70 | 59 |
| 15 | 4a | K3PO4·3H2O | H2O | 70 | 54 |
| b16 | 4a | K3PO4·3H2O | iPrOH | 70 | 82 |
| 17 | 4a | K3PO4·3H2O | iPrOH | 50 | 99 |
| 18 | 4a | K 3 PO 4 ·3H 2 O | i PrOH | rt | 96 |
| 19 | 4b | K3PO4·3H2O | iPrOH | rt | 92 |
| 20 | 4c | K3PO4·3H2O | iPrOH | rt | 90 |
| 21 | 4d | K3PO4·3H2O | iPrOH | rt | 22 |
| 22 | 4e | K3PO4·3H2O | iPrOH | rt | 42 |
| 23 | 4f | K3PO4·3H2O | iPrOH | rt | 16 |
| 24 | 4a | K3PO4·3H2O |
iPrOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1 H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
rt | 75 |
| 25 | 4a | K 3 PO 4 ·3H 2 O |
i
PrOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) H
2
O (1 H
2
O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) 1)
|
50 | 97 |
| 26 | 4b | K3PO4·3H2O |
iPrOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1 H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
50 | 91 |
| 27 | 4c | K3PO4·3H2O |
iPrOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1 H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
50 | 90 |
| 28 | 4d | K3PO4·3H2O |
iPrOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1 H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
50 | 86 |
| 29 | 4e | K3PO4·3H2O |
iPrOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1 H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
50 | 6 |
| 30 | 4f | K3PO4·3H2O |
iPrOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1 H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
50 | Trace |
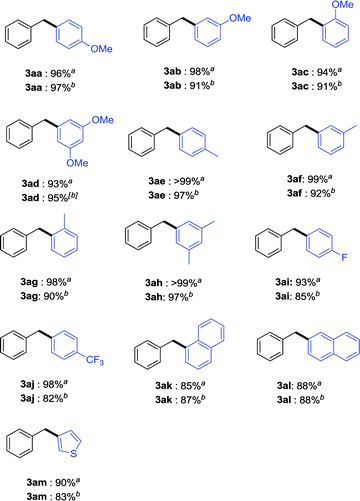
Since the reaction was performed in isopropanol at room temperature as well as in a solvent mixture consisting of isopropanol (1.0 mL) and water (1.0 mL) at 50 °C rather well, they were both applied as the reaction conditions in the following experiments to explore the scope of the cross coupling. As shown in Table 2, a series of aromatic boronic acids with benzyltrimethylammonium bromide were investigated in iPrOH in the presence of 5.0 mol% complex 4a and 2.0 equiv. K3PO4·3H2O at room temperature for 15 h. Gratifyingly, most of the coupling reaction proceeded rapidly and efficiently to provide the corresponding diarylmethane derivatives in excellent yields. It seems that the electronic effect and the steric effect of the substituents on the aromatic boronic acids have little effect on the reaction efficiency. No matter electron-donating (3aa–3ah) or -withdrawing (3ai–3aj) groups on the phenyl ring of boronic acids, good to excellent yields were obtained. The reaction was quite feasible with benzyltrimethylammonium bromide when the ortho-substituted aryl boronic acid was used (3ac and 3ag). In addition, in the case of 1-naphthylboronic acid or 2-naphthylboronic acid afforded in high reaction efficiency under the present reaction conditions (3ak–3al). Particularly, when heteroaromatic boronic acid, such as thienyl, was used as the substrate, high yield of the corresponding product was always observed (3am). Subsequently, a series of aromatic boronic acids with benzyltrimethylammonium bromide were investigated in iPrOH–H2O in the presence of 5.0 mol% complex 4a and 2.0 equiv. K3PO4·3H2O at 50 °C for 15 h. All of the above substrates still worked well to afford the desired products in good to almost quantitative yields.
a All reactions were carried out using 1a (0.20 mmol), 2 (0.40 mmol), K3PO4·3H2O (2.0 equiv.), cat. 4a (5.0 mol%) in iPrOH (0.1 M) at room temperature for 15 h.
b All reactions were carried out using 1a (0.20 mmol), 2 (0.40 mmol), K3PO4·3H2O (2.0 equiv), cat. 4a (5.0 mol%) in mixture solvent (iPrOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O [v H2O [v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) / /![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v] = 1 v] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 0.1 M) at 50 °C for 15 h. 1, 0.1 M) at 50 °C for 15 h.
|
|---|
|
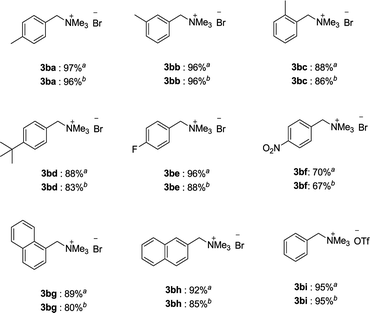
Inspired by these results and our attention was next turned to the cross coupling reaction of benzylammonium salts with 4-methoxyphenyl boronic acid. As shown in Table 3, the reactions proceed smoothly to afford diarylmethanes in excellent yields. Roughly, the electron-donating group in the phenyl ring of benzylammonium salts showed some beneficial effect on the yields of the catalysis products. Benzylammonium salts bearing fluorine substituent showed good reactivity in this transformation (3be). Substrate 1 having naphthalene ring substituent was also suitable for such transformation to afford products 3bg and 3bh in good yields under appropriate conditions. In addition, when benzyltrimethylammonium triflate was used as the substrate, high yield of the corresponding product was always observed (3bi).
a All reactions were carried out using 1 (0.20 mmol), 2a (0.40 mmol), K3PO4·3H2O (2.0 equiv.), cat. 4a (5.0 mol%) in iPrOH (0.1 M) at room temperature for 15 h.
b All reactions were carried out using 1 (0.20 mmol), 2a (0.40 mmol), K3PO4·3H2O (2.0 equiv.), cat. 4a (5.0 mol%) in mixture solvent (iPrOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O [v H2O [v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) / /![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v] = 1 v] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, (0.1 M)) at 50 °C for 15 h. 1, (0.1 M)) at 50 °C for 15 h.
|
|---|
|
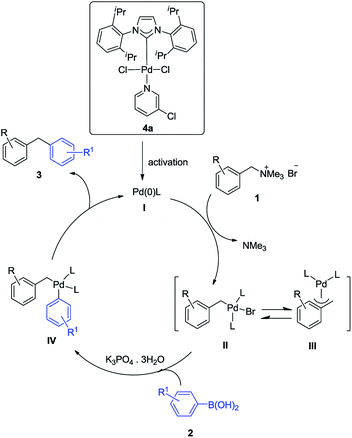
On the basis of the mechanism of previous reports10b and our results, a putative reaction mechanism was then proposed in Scheme 2. First, oxidative addition of Pd(0) I produced in situ, with benzyltrimethylammonium salt 1 formed intermediate II with the release of trimethylamine. Then a transmetalation reaction of the intermediate II with aryl boronic acid converted to intermediate IV, which followed by reductive elimination to the product 3 with simultaneous regeneration of the Pd(0) catalyst.
Conclusions
In summary, we have developed the first example of NHC–Pd(II) catalyzed cross-coupling of benzylammonium salts with arylboronic acids to form diarylmethane derivatives, a very important skeleton in synthetic chemistry. The current process tolerates broad scope with respect to both the boronic acid and benzylammonium salts under mild conditions. Further exploration of these N-heterocyclic carbene–palladium(II) complexes and their catalytic applications in other reactions is in progress.Experimental
General remarks
The catalytic reactions were carried out under a nitrogen atmosphere. Benzylammonium salts were prepared according to the literature method.16 The N-heterocyclic carbene–palladium(II) complexes were synthesized according to our previous report.11b Solvents were dried by standard methods and freshly distilled prior to use. All other chemicals were used as purchased. 1H and 13C NMR spectra were recorded on a Bruker DPX 400 instrument using TMS as an internal standard.General procedure for the cross-coupling of benzylammonium salts with arylboronic acids
A Schlenk flask was charged with the required benzylammonium salts 1a (0.20 mmol, 46.0 mg), (4-methoxyphenyl)boronic acid (0.40 mmol, 60.8 mg), N-heterocyclic carbene–palladium(II) complex 4a (5 mol%, 6.8 mg), K3PO4·3H2O (2.0 equiv., 106.5 mg), and iPrOH (0.1 M) [or iPrOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O [v
H2O [v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) /
/![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v] = 1
v] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 0.1 M]. The mixture was stirred at room temperature [or 50 °C] for 15 h under N2. After cooling, the mixture was evaporated and the product was isolated by by preparative TLC on silica gel plates eluting with CH2Cl2/petroleum ether to afford the diarylmethane. The purified products were identified by NMR spectra and their analytical data are given in the ESI.†
1, 0.1 M]. The mixture was stirred at room temperature [or 50 °C] for 15 h under N2. After cooling, the mixture was evaporated and the product was isolated by by preparative TLC on silica gel plates eluting with CH2Cl2/petroleum ether to afford the diarylmethane. The purified products were identified by NMR spectra and their analytical data are given in the ESI.†
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge financial support from the National Natural Science Foundation of China (No. U1504207, 21572126), the Key Science Research of Education Committee in Henan Province (19A150035) and the Program of Science and Technology Innovation Talents of Henan Province (2018JQ0011).Notes and references
- (a) J. D. Houwer and B. U. W. Maes, Synthesis, 2014, 2533 Search PubMed; (b) S. Messaoudi, A. Hamze, O. Provot, B. Tréguier, J. Rodrigo De Losada, J. Bignon, J.-M. Liu, J. Wdzieczak Bakala, S. Thoret, J. Dubois, J.-D. Brion and M. Alami, ChemMedChem, 2011, 6, 488 CrossRef CAS; (c) A. V. Cheltsov, M. Aoyagi, A. Aleshin, E. C.-W. Yu, T. Gilliland, D. Zhai, A. A. Bobkov, J. C. Reed, R. C. Liddington and R. Abagyan, J. Med. Chem., 2010, 53, 3899 CrossRef CAS; (d) B. Liegault, J.-L. Renaud and C. Bruneau, Chem. Soc. Rev., 2008, 37, 290 RSC.
- (a) V. Ramakrishna, M. J. Rani and N. D. Reddy, Eur. J. Org. Chem., 2017, 48, 7238 CrossRef; (b) G. Zhao, K. Zhang, L. Wang, J. Li, D. Zou, Y. Wu and Y. Wu, Tetrahedron Lett., 2015, 56, 6700 CrossRef CAS; (c) Z. Guan, B. Li, G. Hai, X. Yang, T. Li and B. Tan, RSC Adv., 2014, 4, 36437 RSC; (d) M. Micksch, M. Tenne and T. Strassner, Organometallics, 2014, 33, 3966 CrossRef CAS; (e) F. Chahdoura, C. Pradel and M. Gomez, Adv. Synth. Catal., 2013, 355, 3648 CrossRef CAS; (f) K. Karami, C. Rizzoli and M. M. Salah, J. Organomet. Chem., 2011, 696, 940 CrossRef CAS; (g) A. John, M. M. Shaikh, R. J. Butcher and P. Ghosh, Dalton Trans., 2010, 39, 7353 RSC; (h) O. Diebolt, V. Jurcik, R. Correada Costa, P. Braunstein, L. Cavallo, S. P. Nolan, A. M. Z. Slawin and C. S. Cazin, J. Organometallics, 2010, 29, 1443 CrossRef CAS; (i) R. Singh, M. S. Viciu, N. Kramareva, O. Navarro and S. P. Nolan, Org. Lett., 2005, 7, 1829 CrossRef CAS; (j) S. M. Nobre and A. L. Monteiro, Tetrahedron Lett., 2004, 45, 8225 CrossRef CAS.
- X.-X. Wang, B.-B. Xu, W.-T. Song, K.-X. Sun and J.-M. Lu, Org. Biomol. Chem., 2015, 13, 4925 RSC.
- (a) C. Liu, G. Li, S. Shi, G. Meng, R. Lalancette, R. Szostak and M. Szostak, ACS Catal., 2018, 8, 9131 CrossRef CAS; (b) C. Wang, L. Huang, F. Wang and G. Zou, Tetrahedron Lett., 2018, 59, 2299 CrossRef CAS; (c) P. Lei, G. Meng and M. Szostak, ACS Catal., 2017, 7, 1960 CrossRef CAS; (d) P. Lei, G. Meng, Y. Ling, J. An, S. P. Nolan and M. Szostak, Org. Lett., 2017, 19, 6510 CrossRef CAS; (e) G. Meng, R. Szostak and M. Szostak, Org. Lett., 2017, 19, 3596 CrossRef CAS; (f) P. Lei, G. Meng, Y. Ling, J. An and M. Szostak, J. Org. Chem., 2017, 82, 6638 CrossRef CAS; (g) P. Lei, G. Meng, S. Shi, Y. Ling, J. An, R. Szostak and M. Szostak, Chem. Sci., 2017, 8, 6525 RSC; (h) G. Meng, R. Lalancette, R. Szostak and M. Szostak, Org. Lett., 2017, 19, 4656 CrossRef CAS; (i) G. Meng and M. Szostak, Org. Lett., 2015, 17, 4364 CrossRef CAS.
- S. B. Blakey and D. W. C. MacMillan, J. Am. Chem. Soc., 2003, 125, 6046 CrossRef CAS.
- (a) G. Li, Y. Chen and J. Xia, Chin. J. Org. Chem., 2018, 38, 1949 CrossRef; (b) X.-Y. Liu, H.-B. Zhu, Y.-J. Shen, J. Jiang and T. Tu, Chin. J. Chem., 2017, 28, 350 CAS; (c) D. M. Shacklady-McAtee, K. M. Roberts, C. H. Basch, Y.-G. Song and M. P. Watson, Tetrahedron, 2014, 70, 4257 CrossRef CAS; (d) J. T. Reeves, D. R. Fandrick, Z. Tan, J. J. Song, H. Lee, N. K. Yee and C. H. Senanayake, Org. Lett., 2010, 12, 4388 CrossRef CAS; (e) P. Maity, D. M. Shacklady-McAtee, G. P. A. Yap, E. R. Sirianni and M. P. Watson, J. Am. Chem. Soc., 2013, 135, 280 CrossRef CAS.
- (a) H. J. Davis, M. T. Mihai and R. J. Phipps, J. Am. Chem. Soc., 2016, 138, 12759 CrossRef CAS; (b) F. Zhu, J.-L. Tao and Z.-X. Wang, Org. Lett., 2015, 17, 4926 CrossRef CAS.
- (a) J. Hu, H. Sun, W. Cai, X. Pu, Y. Zhang and Z. Shi, J. Org. Chem., 2016, 81, 14 CrossRef CAS; (b) C. H. Basch, K. M. Cobb and M. P. Watson, Org. Lett., 2016, 18, 136 CrossRef CAS; (c) H. Zhang, S. Hagihara and K. Itami, Chem. Eur. J., 2015, 21, 16796 CrossRef CAS PubMed.
- T. Moragas, M. Gaydou and R. Martin, Angew. Chem., Int. Ed., 2016, 55, 5053 CrossRef CAS PubMed.
- (a) P. L. Türtscher, H. J. Davis and R. J. Phipps, Synthesis, 2018, 50, 793 CrossRef; (b) T. Wang, S. Yang, S. Xu, C. Han, G. Guo and J. Zhao, RSC Adv., 2017, 7, 15805 RSC.
- (a) T. Wang, K. Xu, A. Zhang, W. Wang and L. Liu, Chin. J. Org. Chem., 2018, 38, 259 CrossRef; (b) T. Wang, H. Xie, L. Liu and W.-X. Zhao, J. Organomet. Chem., 2016, 804, 73 CrossRef CAS.
- T. Wang, K. Xu, W. Wang, A. Zhang and L. Liu, Transition Met. Chem., 2018, 43, 347 CrossRef CAS.
- T. Wang, L. Liu, K. Xu, H. Xie, H. Shen and W.-X. Zhao, RSC Adv., 2016, 6, 100690 RSC.
- T. Wang, J. Guo, H. Wang, H. Guo, D. Jia, W. Zhang and L. Liu, J. Organomet. Chem., 2018, 877, 80 CrossRef CAS.
- K. Ouyang and Z. Xi, Acta Chim. Sinica., 2013, 71, 13 CrossRef CAS.
- (a) S. Yu, S. Liu, Y. Lan, B. Wan and X. Li, J. Am. Chem. Soc., 2015, 137, 1623 CrossRef CAS PubMed; (b) K. H. Jensen and J. E. Hanson, Chem. Mater., 2002, 14, 918 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Characterization data and NMR spectra of the catalysis products. See DOI: 10.1039/c8ra10439e |
| This journal is © The Royal Society of Chemistry 2019 |