A ‘sulfonyl-azide-free’ (SAFE) aqueous-phase diazo transfer reaction for parallel and diversity-oriented synthesis†
Dmitry
Dar’in
,
Grigory
Kantin
and
Mikhail
Krasavin
 *
*
Institute of Chemistry, Saint Petersburg State University, Saint Petersburg 199034, Russian Federation. E-mail: m.krasavin@spbu.ru
First published on 8th April 2019
Abstract
Diazo transfer reactions are notoriously associated with the use of potentially explosive sulfonyl azides. The first ‘sulfonyl-azide-free’ (SAFE) protocol for producing diazo compounds from their active-methylene precursors via the Regitz diazo transfer reaction was developed and has displayed a remarkable substrate scope. It can be applied to generating arrays of diazo compounds for further evolution via combinatorial chemistry and a range of scaffold-generating transformations.
Diazo compounds are distinctly versatile reactive building blocks which can be activated, with the loss of a nitrogen molecule, to give rise to carbene or metal carbenoid intermediates.1 Besides their appeal in synthetic organic chemistry, diazo compounds display a growing significance as tools for chemical biology.2 Perhaps the most popular method of preparing diazo compounds is currently the Regitz diazo transfer from a sulfonyl azide to an active methylene substrate.3 Although this process is generally clean and high-yielding, potential explosion hazards4 associated with diazo transfer reagents preclude their use on an industrial scale and may even be hindering active exploration of the diazo chemical space. In particular, there have been no examples of diazo compound synthesis in an array format, which would ultimately enable combinatorial library generation based on the vast diazo chemistry.
The continued search for safer alternatives to tosyl azide (the most commonly used diazo transfer reagent5) has delivered such promising reagents as p-acetamidobenzenesulfonyl azide,6p-dodecylbenzenesulfonyl azide,7 and imidazole-1-sulfonyl azide hydrogen sulfate8 as well as polymer-supported9 and ionic liquid sulfonyl azides.10 However, all these reagents are nonetheless prone to exothermal decomposition and, therefore, should be handled with care.
In principle, the risks associated with diazo transfer reagents can be avoided by preparing them in situ. Such an approach has not been realized for diazo transfer reactions to-date except for one isolated example reported by Hoveyda11 and the recently reported noteworthy examples of generating diazo compounds in a flow reactor described by Maguire and Collins.12 Considering this methodology void, we became interested in exploring diazo transfer reactions without a need to prepare, isolate and handle the hazardous reagent which could be instead generated in situ.
Any in situ protocol is potentially associated with increased by-product formation. It is especially the case with diazo transfer reagents where a large molecular entity (the sulfonyl portion) is already destined to become a by-product and needs to be effectively removed upon completion of the reaction. Our attention was drawn by m-carboxybenzenesulfonyl azide which has been successfully employed as a diazo transfer reagent in organic solvents13a–f and in water.13g Although its preparation has never been attempted in an aqueous medium, we saw it as a suitable lead for the development of a ‘sulfonyl-azide-free’ (SAFE) protocol for the preparation of diazo compounds in water (Fig. 1).
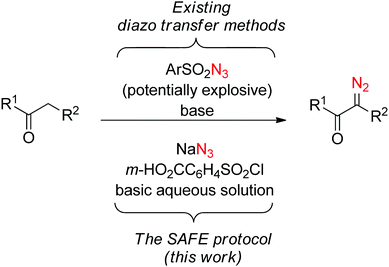 | ||
| Fig. 1 Comparison of previously employed diazo transfer methods with the SAFE protocol described herein. | ||
After relatively brief experimentation involving variations of reagent ratios, solvent volume and reaction time, we realized that a wealth of active-methylene substrates 1–49 can be conveniently transformed into their diazo counterparts 1′–49′ in good to excellent yields via a simple ‘mix-all’ protocol, mostly on 1.5 mmol scale, using m-carboxybenzenesulfonyl chloride (commercially available or conveniently prepared in >50 g batches – see the ESI†) and sodium azide in basic aqueous solution (Scheme 1). As it is evident from the substrate scope displayed by the new SAFE method, a diazo group can be transferred from sodium azide to give a wide variety of known as well as hitherto unreported (5′, 13′, 20′, 23′–24′, 30′, 33′–37′, 39′, 40′, 42′, 45′, 49′) diazo compounds. Only certain substrates required longer reaction time for the diazo transfer; otherwise the reaction was complete in 1–2 h. In addition to the R2 group being most commonly represented by another carbonyl group, it can include other carbanion-stabilizing moieties such as vinylogous (9) and phenylogous (10, 41–42) carbonyls (or variants thereof, cf.27–28), cyano (21–26), sulfone (33–38), phosphonate (31–32) or electron-deficient aromatic (29–30) groups. Some substrates (25–28) displayed incomplete conversion and suboptimal product yield under standard reaction conditions; both of these aspects were improved by doubling the amount of NaN3, the base and the sulfonyl chloride.
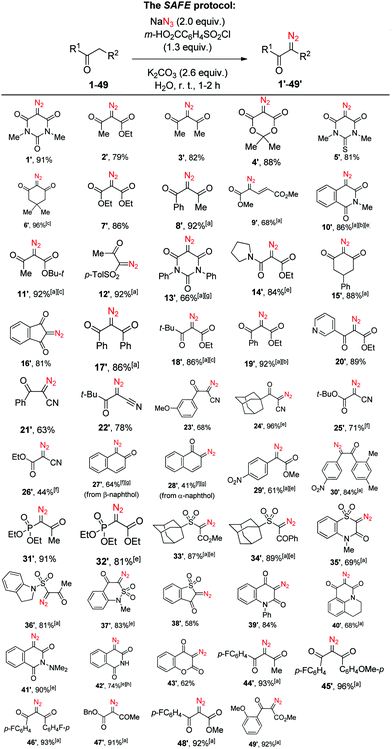 | ||
Scheme 1 Scope of the SAFE diazo transfer reaction. Yields of the isolated products. a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Acetonitrile (1–2 mL) was added to aid in the formation of a fine suspension or emulsion; b Acetonitrile (1–2 mL) was added to aid in the formation of a fine suspension or emulsion; b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.0 mmol scale; c 3.0 mmol scale; c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.0 mmol scale; d 6.0 mmol scale; d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9.0 mmol scale; e 9.0 mmol scale; e![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) increased reaction time (see the ESI,† for details); f increased reaction time (see the ESI,† for details); f![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) double amount of NaN3 and m-HO2CC6H4SO2Cl was used; g double amount of NaN3 and m-HO2CC6H4SO2Cl was used; g![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) purified by chromatography; h purified by chromatography; h![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) purified by crystallization. purified by crystallization. | ||
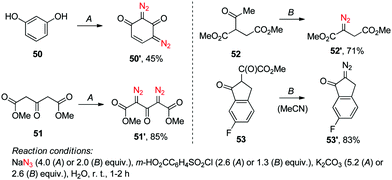
Naturally, increasing the amount of these reagents twofold was also needed to achieve double diazo transfer onto substrates 50–51 to produce respective bis-diazo compounds 50′–51′. Interestingly, the SAFE reaction conditions were found applicable to the variants of the Danheiser deacylative diazo transfer14 successfully producing hitherto undescribed diazo compounds 52′–53′ with the loss of acetyl and methoxalyl groups, respectively (Scheme 2). Altogether, except for a handful of examples (13′, 27′–28′, 42′), the crude diazo compounds produced in the course of the initial scope investigation had a purity of at least 95% as determined by 1H NMR. Moreover, the reaction displayed comparable yield on a 3.0–9.0 mmol scale.
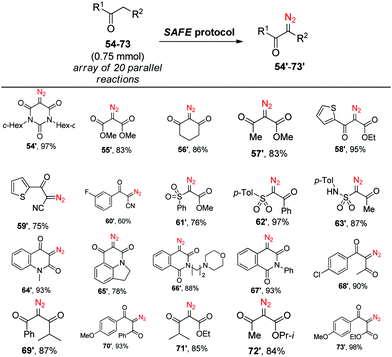
Having established the efficiency of the newly developed SAFE protocol, we sought to apply it to simultaneous generation of different diazo compounds from their active-methylene precursors in an array format. To test this possibility, we prepared an aqueous solution of sodium azide, m-carboxybenzenesulfonyl chloride and potassium carbonate and added it in proportionate aliquots to an array of 20 reaction vessels containing different substrates (54–73) followed by the addition of 50% v/v of MeCN. After 2 h of stirring at r.t., parallel extraction with chloroform gave, after drying and evaporation of the solvent, diazo compounds 54′–73′ all of which were judged to be at least 95% pure by 1H NMR (Scheme 3). Thus, the SAFE method was found suitable for producing structurally diverse sets of diazo compounds in a parallel fashion.
The established amenability of the SAFE protocol for array diazo compound synthesis motivated us to explore the possibility of modifying compounds 1′–73′ in a combinatorial fashion, so as to obtain the products of subsequent diazo chemistry directly from their active-methylene precursors, without a need to purify the SAFE diazo transfer products in the interim. Such a possibility was indeed realized for a small set of substrates (7–8, 12, 35 and 52) which were prepared in a parallel fashion and used directly in reactions involving Rh2(esp)2-catalyzed decomposition15 and Rh carbene insertion into an O–H or N–H bond of a carboxylic acid, an alcohol or a carboxamide (Scheme 4).
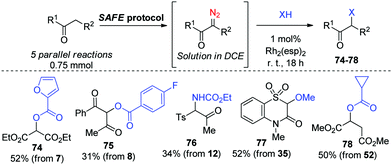 | ||
| Scheme 4 Combinatorial format for the SAFE diazo transfer and subsequent Rh carbene X–H insertion reactions. | ||
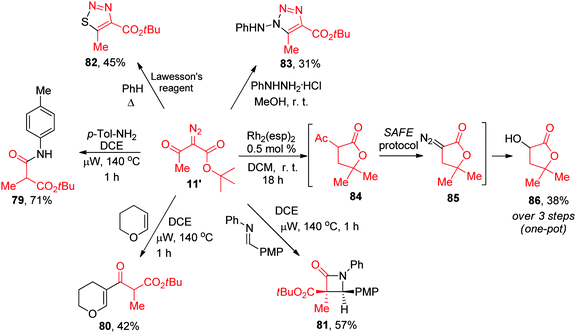
The latter finding demonstrated that the purity of diazo compounds obtained via the SAFE protocol was adequate for RhII-catalyzed X–H insertion chemistry. Considering the versatility of diazo compounds in generating diverse molecular skeletons,16 we aimed to confirm that 11′ (an exemplary compound prepared, without purification, on a 9 mmol scale, vide supra) can be considered a starting point for generating skeletally diverse compounds thereby validating the SAFE protocol for future use in diversity-oriented synthesis. This compound was split into six 1.38 mmol batches which were individually subjected to a range of reactions hallmark for diazo compounds. In particular, microwave-assisted Wolff rearrangement17 of compound 11′ generated the respective ketene which was subsequently trapped with an aniline (to give 79), or enol ether (to give 80)18 or underwent a [2+2] Staudinger cycloaddition with an imine19 to produce β-lactam 81 whose structure was confirmed by single-crystal X-ray analysis (ESI†). Reactions with the Lawesson's reagent20 and phenyl hydrazine21 delivered 1,2,3-thiadiazole 82 and 1,2,3-triazole 83, respectively. Finally, the RhII carbene insertion into the C–H bond of the tert-butyl moiety22 produced γ-lactone 84 which was, without isolation, subjected to the deacetylative SAFE diazo transfer (realized in a two-phase format by adding the alkaline aqueous diazo transfer cocktail to the crude DCM solution of 84). However, instead of diazo compound 85, we obtained α-hydroxy γ-lactone 86 formed, presumably, via the metal carbene insertion into the HO–H bond catalyzed by the leftover Rh2(esp)2 present in the biphasic mixture (Scheme 5).
To conclude, we developed the first ‘sulfonyl-azide-free’ (SAFE) protocol for diazo transfer in an aqueous medium.23 It has been found workable for 73 structurally diverse, active-methylene substrates and produced the respective diazo compounds (22 of them new) in high product yields and purities. The SAFE method was found to be applicable to producing diazo compounds in an array format and the products thus obtained can be conveniently used in subsequent chemistry conducted in a combinatorial fashion. Moreover, the range of chemistries applied to the evolution of the diazo compound scaffold can be expanded so as to enable diversity-oriented synthesis of skeletally unique compounds. Efforts are underway in our laboratories to adapt the SAFE protocol to a continuous flow format. The results of these studies will be reported in due course.
We are grateful to the Russian Foundation for Basic Research for financial support (grant #19-03-00775). We thank the Research Centre for Magnetic Resonance, the Center for Chemical Analysis and Materials Research, and the Centre for X-ray Diffraction Methods of Saint Petersburg State University Research Park for obtaining the analytical data.
Conflicts of interest
The authors declare no conflict of interest.Notes and references
- A. Ford, H. Miel, A. Ring, C. N. Slattery, A. R. Maguire and M. A. McKervey, Chem. Rev., 2015, 115, 9981 CrossRef CAS PubMed
.
- K. A. Mix, M. R. Aronoff and R. T. Raines, ACS Chem. Biol., 2016, 11, 3233 CrossRef CAS PubMed
.
- W. Regitz, Angew. Chem., Int. Ed. Engl., 1967, 6, 733 CrossRef
.
- F. W. Bollinger and L. D. Tuma, Synlett, 1996, 407 CrossRef CAS
.
- T. J. Curphey, Org. Prep. Proced. Int., 1981, 13, 112 CrossRef CAS
.
- J. S. Baum, D. A. Shook, H. M. L. Davies and H. D. Smith, Synth. Commun., 1987, 17, 1709 CrossRef CAS
.
- F. W. B. G. G. Hazen, F. E. Roberts, W. K. Russ, J. J. Seman and S. Staskiewicz, Org. Synth., 1996, 73, 144 CrossRef
.
- G. T. Potter, G. C. Jayson, G. J. Miller and J. M. Gardiner, J. Org. Chem., 2016, 81, 3443 CrossRef CAS PubMed
.
- E. Tarrant, C. V. O'Brien and S. G. Collins, RSC Adv., 2016, 6, 31202 RSC
.
- M. K. Muthyala, S. Choudhary and A. Kumar, J. Org. Chem., 2012, 77, 8787 CrossRef CAS PubMed
.
- E. S. Sattely, S. J. Meek, S. J. Malcolmson, R. R. Schrock and A. H. Hoveyda, J. Am. Chem. Soc., 2009, 131, 943 CrossRef CAS PubMed
.
-
(a) R. M. O'Mahony, D. Lynch, H. L. D. Hayes, E. N. Thuama, P. Donnellan, R. C. Jones, B. Glennon, S. G. Collins and A. R. Maguire, Eur. J. Org. Chem., 2017, 6533 CrossRef
; (b) B. J. Deadman, R. M. O'Mahony, D. Lynch, D. C. Crowley, S. G. Collins and A. R. Maguire, Org. Biomol. Chem., 2016, 14, 3423 RSC
.
-
(a) R. J. Moreau and E. J. Sorensen, Tetrahedron, 2007, 63, 6446 CrossRef CAS
; (b) D. Morton, A. Dick, D. Ghosh and H. M. L. Davies, Chem. Commun., 2012, 48, 5838 RSC
; (c) I. Rodríguez, M. I. Calaza, A. I. Jimenez and C. Cativiela, Tetrahedron, 2012, 68, 9578 CrossRef
; (d) D. Ghosh, J. Lo, D. I. Morton, D. Valette, J. Xi, J. Griswold, S. Hubbell, C. Egbuta, W. Jiang, J. An and H. M. L. Davies, J. Med. Chem., 2012, 55, 8464–8476 CrossRef CAS PubMed
; (e) C. Qin and H. M. L. Davies, Org. Lett., 2013, 15, 6152 CrossRef CAS PubMed
; (f) C. Doebelin, Y. He and T. M. Kamenecka, Tetrahedron Lett., 2016, 57, 5658 CrossRef CAS
; (g) R. M. O’Mahony, C. M. Broderick, D. Lynch, S. G. Collins and A. R. Maguire, Tetrahedron Lett., 2019, 60, 35 CrossRef
.
- R. L. Danheiser, R. F. Miller, R. G. Brisbois and S. Z. Park, J. Org. Chem., 1990, 55, 1959 CrossRef CAS
.
- C. Hunter, K. Chinthapally and I. Sharma, Eur. J. Org. Chem., 2016, 2260 CrossRef
.
- M. Ibbeson, L. Laraia, E. Alza, C. J. O'Connor, Y. S. Tan, H. M. L. Davies, G. McKenzie, A. R. Venkitaraman and D. R. Spring, Nat. Commun., 2014, 5, 3155 CrossRef PubMed
.
- M. Presset, Y. Coquerel and J. Rodrigez, J. Org. Chem., 2009, 74, 415 CrossRef CAS PubMed
.
- R. P. Pandit and Y. R. Lee, Org. Biomol. Chem., 2014, 12, 4407 RSC
.
- Y. Wang, Y. Liang, L. Jiao, D.-M. Du and J. Xu, J. Org. Chem., 2006, 71, 6983 CrossRef PubMed
.
- M. Caron, J. Org. Chem., 1986, 51, 4075 CrossRef CAS
.
- Z. Wang, X. Bi, P. Liao, R. Zhang, Y. Liang and D. Dong, Chem. Commun., 2012, 48, 7076 RSC
.
- M. P. Doyle, V. Bagheri, M. M. Pearson and J. D. Edwards, Tetrahedron Lett., 1989, 30, 7001 CrossRef CAS
.
-
The aqueous-phase waste should be disposed of according to the general guidelines for aqueous solutions containing inorganic azides: Bretherick's handbook of reactive chemical hazards, ed. P. G. Urben, Butterworth-Heinemann, Oxford, 6th edn, 1999 Search PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, full characterization data, crystallographic information, and copies of 13C and 1H NMR spectra. CCDC 1897286. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9cc02042j |
| This journal is © The Royal Society of Chemistry 2019 |