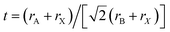Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Incorporation of potassium halides in the mechanosynthesis of inorganic perovskites: feasibility and limitations of ion-replacement and trap passivation†
Yousra El Ajjouri a,
Vladimir S. Chirvonyab,
Michele Sessolo
a,
Vladimir S. Chirvonyab,
Michele Sessolo a,
Francisco Palazon
a,
Francisco Palazon *a and
Henk J. Bolink
*a and
Henk J. Bolink a
a
aInstituto de Ciencia Molecular, ICMol, Universidad de Valencia, C/ Catedrático J. Beltrán 2, 46980 Paterna, Spain. E-mail: Francisco.Palazon@uv.es
bUMDO (Unidad de Materiales y Dispositivos Optoelectrónicos), Instituto de Ciencia de los Materiales, Universidad de Valencia, Valencia 46071, Spain
First published on 12th December 2018
Abstract
Potassium halides (KX; X = I, Br, or Cl) were incorporated as partial replacements of CsBr in the mechanosynthesis of CsPbBr3. This led to partial substitution of both monovalent ions forming mixed Cs1−xKxPbBr3−yXy perovskites. Longer photoluminescence lifetimes were also observed, possibly linked to the formation of a non-perovskite KPb2X5 passivating layer.
In the past few years, organic metal halide perovskites (OHPs) have drawn considerable attention as promising materials for optoelectronic devices.1–5 However, it is generally known that these materials make the development of stable solar cells and light emitting diodes rather difficult, due to their environmental instability related with the use of organic compounds.6–8 Thus, fully inorganic halide perovskites, such as cesium-based perovskites are sought after for their increased stability.9–13 The known poor solubility of cesium halides in common solvents may be bypassed by synthesizing inorganic perovskites in an all-dry manner such as by mechanosynthesis (e.g., grinding or ball-milling)14–18 and/or thermal vacuum deposition.19 Recently, halide perovskites with an increasing complexity in formulation containing up to 6 or 7 different ions have proven to be beneficial for device performance.20–24 In this context, mechanosynthesis by ball-milling represents an ideal platform to test different precursors or additives in a simple manner. Multi-cation perovskites enable tuning of the bandgap of the material23 as well as its Goldschmidt tolerance factor (t),25,26 which is calculated as follows:
where rA, rB and rX respectively stand for the ionic radiuses of the cation A, metal B and the anion X in the ABX3 perovskite.
To obtain a stable cubic ABX3 perovskite, it is generally accepted that the Goldschmidt tolerance factor should not be lower than 0.8 nor exceed a value of 1. A t-value outside of this range usually results in non-perovskite structures. Examples of such crystalline structures are orthorhombic (so-called “yellow phase”) cesium lead iodide (CsPbI3) and formamidinium lead iodide (FAPbI3). In the case of CsPbI3, the tolerance factor is too small whereas in the case of FAPbI3 the tolerance factor is too large to result in a stable cubic phase at room temperature. However, the multi-cation cesium formamidinium lead iodide perovskite ((Cs:FA)PbI3) was shown to be stable.23,27 This is only an example of the interest of multi-cation perovskites. Among other cations, potassium has been recently used as an additive in perovskites, with different conclusions.22,28–33 Some reports show a benefit from the presence of potassium in mixed (KCs)PbI3, where the guest cation is capable of stabilizing the perovskite structure.29 Others, based on the small tolerance factor of such structure, have concluded that incorporating potassium halides in the synthesis does not lead to the effective incorporation of potassium as replacement of the “A” cation within the perovskite structure. As a result, potassium stays at the grain boundaries and indirectly contributes to surface passivation by providing additional halides (bound to K+), partially compensating the halide vacancies. The halide vacancies are believed to be one of the main quenching traps which need to be passivated to improve the optoelectronic properties of the perovskite.28,34 On the contrary, other reports have concluded that addition of potassium halides leads to the formation of different separate phases.30,31 These discrepancies might originate from the different perovskite crystallization processes used, which can result in different morphology, phase purity or stoichiometry of the final compound. Therefore, dry mechanosynthesis is an ideal preparation method, as it does not involve solvents, it avoids the formation of intermediate species, and eliminates the need of thermal treatments to foster the perovskite crystallization.
In this work we synthesized halide perovskites by ball milling equimolar mixtures of PbBr2 and ABr, where A = K0.2Cs0.8 and compared the resulting powders with the pure cesium reference (A = Cs). High resolution X-ray diffraction (XRD) patterns as well as optical characterization are presented in Fig. 1. The main diffraction peaks from the resulting powder are presented in Fig. 1b, c and e. These peaks correspond to the orthorhombic APbBr3 perovskite (see Fig. S1† for the full diffractograms and reference pattern ICSD 97851). Hence, XRD confirms the formation of the perovskite phase from ball-milling of precursors. Furthermore, the high-resolution signals presented in Fig. 1b, c and e reveal a shift towards higher angles (smaller interatomic distances) when CsBr is partly replaced by KBr. This means that K+, which has a smaller ionic radius than Cs+, is effectively incorporated in the perovskite crystal structure, leading to mixed-cation (KCs)PbBr3. Such a cation-replacement is not trivial, as potassium is thought to be too small to occupy the “A” site in APbBr3.28,30,31 Indeed, concomitant to the shift of the main perovskite peaks, we also note that new peaks appear in the diffractogram (see Fig. S1† and 1a, d, f). These peaks are consistent with the non-perovskite APb2Br5 phase. For potassium-based lead halide compounds, this phase is the most commonly reported.35,36 We also ball-milled pure KBr and PbBr2 mixtures (without CsBr) in different ratios and found that KPb2Br5 was the dominant phase – along with unreacted KBr – even in KBr-rich conditions, see Fig. S2.† Therefore, we can conclude that the use of KBr as a source of K+ to replace Cs+ in inorganic perovskites is possible but limited by the higher stability of KPb2Br5 as compared to KPbBr3. When we reduced the amount of KBr to 5% (A = K0.05Cs0.95) we also observed similar perovskite peak shifts and formation of KPb2Br5, although to a lesser extent (see Fig. S3†). This suggests that the amount of Cs+ that can be replaced by K+ in the perovskite structure (without leading to the formation of KPb2Br5) is below 5%. This value is lower than previously reported by others.29 Other characterization methods such as high-resolution transmission electron microscopy and energy dispersive X-ray spectroscopy could possibly further elucidate the amount of potassium that is present under each form (included in the perovskite lattice or as separated KPb2Br5 compound). The optical characterization of the powders resulting from ball-milling equimolar ABr:PbBr2 mixtures with A = K0.2Cs0.8 is presented in Fig. 1g–i. Absorption (g) and photoluminescence (h) spectra are mostly unchanged with respect to the reference sample (A = Cs). Photoluminescence spectra (Fig. 1h) evidently consist of two sub-bands with maxima at about 522 and 540 nm (see deconvolution of the PL spectra as a sum of two Gaussian contours in ESI, Fig. S4†). Because of the broad and asymmetric nature of the PL spectra, it is not possible to unambiguously evaluate the impact of potassium incorporation on the optical bandgap. Indeed, it could be expected that the observed shrinkage of the lattice would affect the optical bandgap of the material and result in a shift of the PL peak. However, the origin of the two bands observed in both PL spectra might be due to different reasons. In a previous report on mechanosynthesis of CsPbBr3 via ball-milling of CsBr and PbBr2 a similar asymmetric spectrum was obtained and attributed to the presence of bulk and nano-sized CsPbBr3.16 Another possible explanation is linked to the emission from free electrons in conducting band and trap-localized carriers.37 In this second hypothesis, it is possible that an exchange of a part of Cs atoms by K decreases the relative contribution of the PL emission from free electrons at 522 nm and, respectively, increases contribution of the emission from trap states at 540 nm. Following the delayed luminescence model,38 trap-assisted luminescence should be longer lived than the emission of free electrons from the conducting band, as is indeed observed in Fig. 1i. However, we cannot exclude other possible origins of this longer lifetime such as trap passivation by molecular KBr which fills halide vacancies at the surface28 or by the other two mechanisms that our data prove to happen concomitantly: (i) replacement of Cs+ by K+ as monovalent cation in the perovskite structure, and (ii) formation of KPb2Br5 which might act as passivating layer on top of CsPbBr3. This passivation (independently on the exact mechanism from which it originates) should result in a higher photoluminescence quantum yield (PLQY). However, the absolute PLQY of these powder samples is too low for us to conduct reliable measurements.
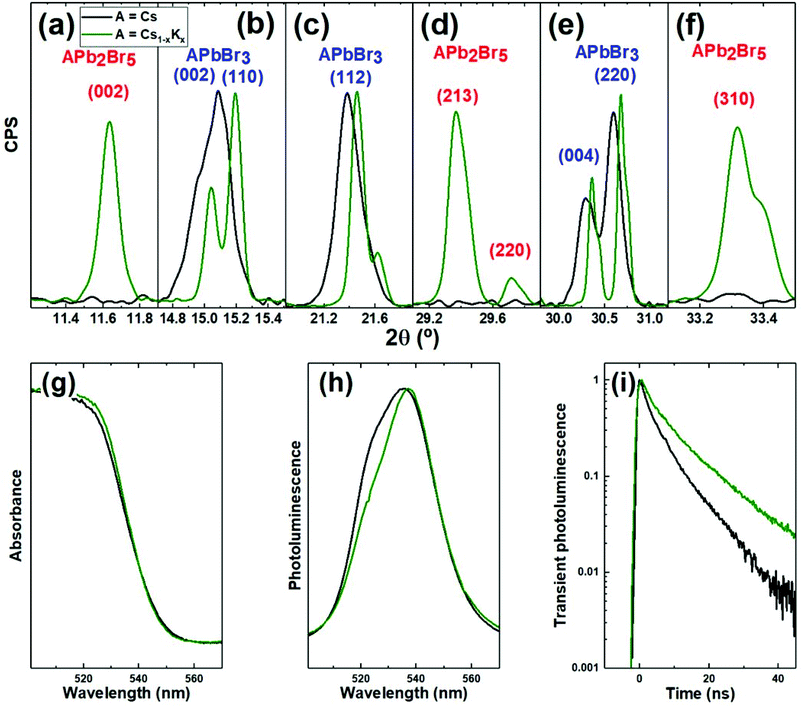 | ||
| Fig. 1 XRD (a–f) and optical (g–i) characterization of powders prepared from addition of PbBr2 to CsBr (REF; black lines) or Cs0.8K0.2Br (green lines). XRD peaks corresponding to APbBr3 perovskite (b, c, and e) present a shift upon addition of KBr. Panels (a, d, and f) present a rise in intensity linked to the formation of non-perovskite APb2Br5 phase. Full diffractograms are presented in Fig. S1.† Absorption (g) and photoluminescence (h) spectra remain mostly unchanged while photoluminescence lifetime (i) is increased. | ||
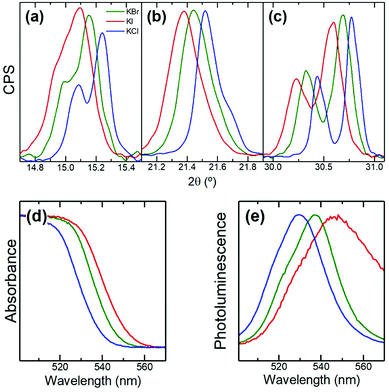
We also replaced KBr by KX (X = Cl or I) while keeping CsBr and PbBr2 as precursors in the mechanosynthesis. Fig. 2 shows XRD and optical characterization of the resulting powders. Fig. 2a–c demonstrate that the perovskite phase is formed in all cases and that the heteroanion (Cl or I) introduced via the potassium salt is replacing Br in the APbX3 structure. Indeed, when KI is used the main perovskite peaks shift towards lower diffraction angles consistent with the introduction of the larger I− anion compared to Br−. The opposite applies when KCl is used. As a result, we observe significant shifts in the bandgap of the perovskite as shown by absorption and photoluminescence (Fig. 2d, e). Hence, our results show that KX can also be used as a source of anions to tune the optical properties of the resulting inorganic perovskite. This means that the X halide does not only remain tightly bound to K+ at the surface of the perovskite material affecting only surface-related effects (surface quenching traps) but also enters the structure and thus affects bulk-related properties (bandgap).
In conclusion, we have shown that incorporating potassium halides in the mechanosynthesis of inorganic cesium lead halide perovskites leads to several chemical, structural and optical effects. First of all, potassium partly replaces cesium in the APbBr3 perovskite structure. Second, the potassium salt can also act as a source of heteroanions to tune the bandgap of the resulting perovskite. Third, KPb2X5 phase forms concomitantly with the perovskite phase. This phase may act as a surface passivation layer as longer lifetimes are observed on samples with added KBr with respect to pure CsPbBr3. These findings will aid to further optimize thin film perovskite based devices such as LEDs and solar cells that recently have shown beneficial effects of incorporating potassium halides.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The research leading to these results has received funding from the European Union Programme for Research and Innovation Horizon 2020 (2014-2020) under the Marie Skłodowska-Curie Grant Agreement PerovSAMs No. 747599. We also acknowledge financial support from the Spanish Ministry of Economy and Competitiveness (MINECO) via the Unidad de Excelencia María de Maeztu MDM-2015-0538, MAT2017-88821-R, and the Generalitat Valenciana (Prometeo/2016/135 and GRISOLIAP/2017/089). M. S. thanks the MINECO for his RyC contract.Notes and references
- N. K. Noel, A. Abate, S. D. Stranks, E. S. Parrott, V. M. Burlakov, A. Goriely and H. J. Snaith, ACS Nano, 2014, 8, 9815–9821 CrossRef CAS PubMed.
- S. D. Stranks, G. E. Eperon, G. Grancini, C. Menelaou, M. J. P. Alcocer, T. Leijtens, L. M. Herz, A. Petrozza and H. J. Snaith, Science, 2013, 342, 341–344 CrossRef CAS PubMed.
- W. Li, Z. Wang, F. Deschler, S. Gao, R. H. Friend and A. K. Cheetham, Nat. Rev. Mater., 2017, 2(3) DOI:10.1038/natrevmats.2016.99.
- T. M. Brenner, D. A. Egger, L. Kronik, G. Hodes and D. Cahen, Nat. Rev. Mater., 2016, 1(1) DOI:10.1038/natrevmats.2015.7.
- S. D. Stranks and H. J. Snaith, Nat. Nanotechnol., 2015, 10, 391–402 CrossRef CAS PubMed.
- J. S. Manser, M. I. Saidaminov, J. A. Christians, O. M. Bakr and P. V. Kamat, Acc. Chem. Res., 2016, 49, 330–338 CrossRef CAS.
- S.-H. Turren-Cruz, A. Hagfeldt and M. Saliba, Science, 2018, 3583, 1–9 Search PubMed.
- F. Palazon, D. Pérez-del-Rey, S. Marras, M. Prato, M. Sessolo, H. J. Bolink and L. Manna, ACS Energy Lett., 2018, 835–839 CrossRef CAS.
- F. Palazon, F. Chen, Q. A. Akkerman, M. Imran, R. Krahne and L. Manna, ACS Appl. Nano Mater., 2018, 1(10), 5396–5400 CrossRef CAS.
- R. F. Service, Science, 2016, 351, 113–114 CrossRef CAS PubMed.
- M. Kulbak, S. Gupta, N. Kedem, I. Levine, T. Bendikov, G. Hodes and D. Cahen, J. Phys. Chem. Lett., 2016, 7, 167–172 CrossRef CAS PubMed.
- L. Zhang, X. Yang, Q. Jiang, P. Wang, Z. Yin, X. Zhang, H. Tan, Y. M. Yang, M. Wei, B. R. Sutherland, E. H. Sargent and J. You, Nat. Commun., 2017, 8, 1–8 CrossRef.
- C. Y. Chen, H. Y. Lin, K. M. Chiang, W. L. Tsai, Y. C. Huang, C. S. Tsao and H. W. Lin, Adv. Mater., 2017, 29, 1–7 Search PubMed.
- D. Prochowicz, M. Franckevičius, A. M. Cieślak, S. M. Zakeeruddin, M. Grätzel and J. Lewiński, J. Mater. Chem. A, 2015, 3, 20772–20777 RSC.
- Z. Y. Zhu, Q. Q. Yang, L. F. Gao, L. Zhang, A. Y. Shi, C. L. Sun, Q. Wang and H. L. Zhang, J. Phys. Chem. Lett., 2017, 8, 1610–1614 CrossRef CAS PubMed.
- L. Protesescu, S. Yakunin, O. Nazarenko, D. N. Dirin and M. V. Kovalenko, ACS Appl. Nano Mater., 2018, 1, 1300–1308 CrossRef CAS PubMed.
- A. D. Jodlowski, A. Yépez, R. Luque, L. Camacho and G. de Miguel, Angew. Chem., Int. Ed., 2016, 55, 14972–14977 CrossRef CAS PubMed.
- Y. El Ajjouri, F. Palazon, M. Sessolo and H. J. Bolink, Chem. Mater., 2018, 30, 7423–7427 CrossRef CAS.
- J. Ávila, C. Momblona, P. P. Boix, M. Sessolo and H. J. Bolink, Joule, 2017, 1, 431–442 CrossRef.
- L. Gil-Escrig, C. Momblona, M. G. La-Placa, P. P. Boix, M. Sessolo and H. J. Bolink, Adv. Energy Mater., 2018, 8, 1–6 Search PubMed.
- D. Forgács, D. Pérez-del-Rey, J. Ávila, C. Momblona, L. Gil-Escrig, B. Dänekamp, M. Sessolo and H. J. Bolink, J. Mater. Chem. A, 2017, 5, 3203–3207 RSC.
- T. Bu, X. Liu, Y. Zhou, J. Yi, X. Huang, L. Luo, J. Xiao, Z. Ku, Y. Peng, F. Huang, Y.-B. Cheng and J. Zhong, Energy Environ. Sci., 2017, 10(12), 2509–2515 RSC.
- M. Saliba, T. Matsui, K. Domanski, J. Seo, A. Ummadisingu, S. M. Zakeeruddin, J. P. Correa-Baena, W. R. Tress, A. Abate, A. Hagfeldt and M. Gratzel, Science, 2016, 5557, 1–8 Search PubMed.
- B. Philippe, M. Saliba, J. P. Correa-Baena, U. B. Cappel, S. H. Turren-Cruz, M. Grätzel, A. Hagfeldt and H. Rensmo, Chem. Mater., 2017, 29, 3589–3596 CrossRef CAS.
- Z. Li, M. Yang, J. S. Park, S. H. Wei, J. J. Berry and K. Zhu, Chem. Mater., 2016, 28, 284–292 CrossRef CAS.
- C. J. Bartel, C. Sutton, B. R. Goldsmith, R. Ouyang, C. B. Musgrave, L. M. Ghiringhelli and M. Scheffler, arXiv Prepr. arXiv, 2018, 6, 1–13.
- D. P. McMeekin, G. Sadoughi, W. Rehman, G. E. Eperon, M. Saliba, M. T. Hörantner, A. Haghighirad, N. Sakai, L. Korte, B. Rech, M. B. Johnston, L. M. Herz and H. J. Snaith, Science, 2016, 351, 151–155 CrossRef CAS PubMed.
- M. Abdi-Jalebi, Z. Andaji-Garmaroudi, S. Cacovich, C. Stavrakas, B. Philippe, J. M. Richter, M. Alsari, E. P. Booker, E. M. Hutter, A. J. Pearson, S. Lilliu, T. J. Savenije, H. Rensmo, G. Divitini, C. Ducati, R. H. Friend and S. D. Stranks, Nature, 2018, 555, 497–501 CrossRef CAS PubMed.
- J. K. Nam, S. U. Chai, W. Cha, Y. J. Choi, W. Kim, M. S. Jung, J. Kwon, D. Kim and J. H. Park, Nano Lett., 2017, 17, 2028–2033 CrossRef CAS PubMed.
- D. J. Kubicki, D. Prochowicz, A. Hofstetter, S. M. Zakeeruddin, M. Grätzel and L. Emsley, J. Am. Chem. Soc., 2018, 140, 7232–7238 CrossRef CAS PubMed.
- D. J. Kubicki, D. Prochowicz, A. Hofstetter, S. M. Zakeeruddin, M. Grätzel and L. Emsley, J. Am. Chem. Soc., 2017, 139, 14173–14180 CrossRef CAS.
- Z. Tang, T. Bessho, F. Awai, T. Kinoshita, M. M. Maitani, R. Jono, T. N. Murakami, H. Wang, T. Kubo, S. Uchida and H. Segawa, Sci. Rep., 2017, 7, 1–7 CrossRef.
- S. Huang, B. Wang, Q. Zhang, Z. Li, A. Shan and L. Li, Adv. Opt. Mater., 2018, 5, 1701106 CrossRef.
- M. Abdi-Jalebi, Z. Andaji-Garmaroudi, A. J. Pearson, G. Divitini, S. Cacovich, B. Philippe, H. Rensmo, C. Ducati, R. H. Friend and S. D. Stranks, ACS Energy Lett., 2018, 3(11), 2671–2678 CrossRef CAS.
- A. Y. Tarasova, L. I. Isaenko, V. G. Kesler, V. M. Pashkov, A. P. Yelisseyev, N. M. Denysyuk and O. Y. Khyzhun, J. Phys. Chem. Solids, 2012, 73, 674–682 CrossRef CAS.
- L. I. Isaenko, I. N. Ogorodnikov, V. A. Pustovarov, A. Y. Tarasova and V. M. Pashkov, Opt. Mater., 2013, 35, 620–625 CrossRef CAS.
- P. Ščajev, C. Qin, R. Aleksiejūnas, P. Baronas, S. Miasojedovas, T. Fujihara, T. Matsushima, C. Adachi and S. Juršėnas, J. Phys. Chem. Lett., 2018, 9, 3167–3172 CrossRef.
- V. S. Chirvony, S. González-Carrero, I. Suárez, R. E. Galian, M. Sessolo, H. J. Bolink, J. P. Martínez-Pastor and J. Pérez-Prieto, J. Phys. Chem. C, 2017, 121, 13381–13390 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra08823c |
| This journal is © The Royal Society of Chemistry 2018 |