Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
‘AND’-based fluorescence scaffold for the detection of ROS/RNS and a second analyte†
Maria L.
Odyniec‡
 a,
Adam C.
Sedgwick‡
a,
Adam C.
Sedgwick‡
 *a,
Alexander H.
Swan
*a,
Alexander H.
Swan
 a,
Maria
Weber
a,
Maria
Weber
 a,
T. M. Simon
Tang
a,
T. M. Simon
Tang
 a,
Jordan E.
Gardiner
a,
Jordan E.
Gardiner
 a,
Miao
Zhang
b,
Yun-Bao
Jiang
a,
Miao
Zhang
b,
Yun-Bao
Jiang
 b,
Gabriele
Kociok-Kohn
a,
Robert B. P.
Elmes
b,
Gabriele
Kociok-Kohn
a,
Robert B. P.
Elmes
 c,
Steven D.
Bull
c,
Steven D.
Bull
 *a,
Xiao-Peng
He
*a,
Xiao-Peng
He
 *d and
Tony D.
James
*d and
Tony D.
James
 *ae
*ae
aDepartment of Chemistry, University of Bath, Bath, BA2 7AY, UK. E-mail: t.d.james@bath.ac.uk; s.d.bull@bath.ac.uk
bDepartment of Chemistry, College of Chemistry and Chemical Engineering, the MOE Key Laboratory of Spectrochemical Analysis and Instrumentation, and iChEM, Xiamen University, Xiamen 361005, China
cDepartment of Chemistry, Maynooth University, Maynooth, Co. Kildare, Ireland
dKey Laboratory for Advanced Materials & Feringa Nobel Prize Scientist Joint, Research Center, East China University of Science and Technology, 130 Meilong Rd., Shanghai 200237, P. R. China
eDepartment of Materials and Life Sciences, Faculty of Science and Technology Sophia University, 7-1 Kioi-cho, Chiyoda-ku, Tokyo 102-8554, Japan
First published on 27th June 2018
Abstract
Traditionally, fluorescence probes have focused on the detection of a single biomarker for a specific process. In this work, we set out to develop a number of fluorescence probes that enable the detection of a chosen analyte in the presence of reactive oxygen/nitrogen species (ROS/RNS). These fluorescence probes when activated result in the formation of the highly fluorescent pink dye, resorufin. Therefore, we have labelled these fluorescent probes as ‘Pinkments’. Our first ‘Pinkment’ was shown to detect biologically relevant concentrations of ONOO− and have an excellent selectivity against other ROS/RNS. Pinkment-OH was developed to provide a core unit which could be easily functionalised to produce a range of ‘AND’ based fluorescence probes for the detection of ROS/RNS and a second analyte. For proof of concept, we synthesised Pinkment-OTBS and Pinkment-OAc. These ‘AND’-based probes were successfully shown to detect ROS/RNS and F− or esterase, respectively.
Since the discovery of the first fluorescence-based probe in 1867, fluorescence probes have revolutionised the understanding of biological systems.1–4 Historically, these probes have focused on the detection of a single analyte or biomarker. However, biological systems are complex with more than one chemical species being released/present during any biological processes. For example, glutathione (GSH) accumulates at the nucleus during the cell cycle to aid transcription factors binding to DNA5 and the pathological role of Zn2+ is believed to be associated with the glutamate system.6 Furthermore, the sensitivity of a cell towards peroxynitrite (ONOO−) largely depends on the concentration of intracellular GSH.7–10 Therefore, in order to further understand cellular functions and the root causes of disease it is important to be able to study biologically important species simultaneously.
Alongside the development of the field of fluorescence probes, the field of molecular logic gates has developed.11 Molecular logic gates are molecules that have the ability to bind or react with multiple analytes (input) and turn it into a measurable optical output. Consequently, these attractive molecules are now emerging in the literature demonstrating the ability to simultaneously detect multiple analytes in biological systems.12–21 Within our research group, we are interested in developing reaction-based fluorescence probes including ‘AND’-based fluorescence probes for the detection of biologically important analytes.22–24 Dual responsive (‘AND’) fluorescence probes require both analytes being present to produce a fluorescence response. In this work, we identified a previously reported boronate-based fluorescence probe developed by Chang et al.PR1, with a free amino group attached (Fig. 1). Boronates have well-known reactivity towards ONOO− and hydrogen peroxide (H2O2),25 therefore we believed PR1 could provide a suitable scaffold for the development of ‘AND’-based systems for the detection of ROS/RNS and an second analyte.26 In this work, we initially functionalised PR1 with an additional ROS/RNS trigger, benzyl boronic acid ester, which afforded a selective ONOO− probe known as Pinkment (Fig. 1).
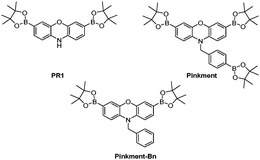 | ||
| Fig. 1 PR1 and Pinkment fluorescence probes for the detection of reactive oxygen and reactive nitrogen species (ROS/RNS). Pinkment-Bn – demonstrates the requirement elimination to produce a free N–H. | ||
The current literature route to obtain PR1 involves a very low yielding first step (10% overall yield 7%), which uses harsh conditions (hydrobromic acid (HBr)).26 Therefore, we alkylated commercially available phenoxazine using NaH and 4-bromobenzyl bromide to afford 1 in excellent yield (78%). 1 was then subsequently dissolved in CHCl3 and NBS was added portion wise to afford dibrominated intermediate 2 in good yield (48%), which required only trituration (EtOAc) for purification. Lastly, Suzuki–Miyaura conditions were applied to 2 using bis(pinacolato)diboron (B2pin2) as a boron transfer agent to furnish Pinkment in 36% yield (see ESI† – Scheme S4). In the presence of ONOO−, all three boronates were oxidised on Pinkment, forming the highly fluorescent pink dye, resorufin (see ESI† – Scheme S1) and a large fluorescence increase was observed at 590 nm (see ESI† – Fig. S1). Pinkment was able to detect biologically relevant concentrations of ONOO−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27,28 and displayed excellent selectivity towards ONOO−(see ESI† – Fig. S2–S4). To demonstrate the requirement of an ‘eliminating’ benzyl group on the free N–H, Pinkment-Bn (Fig. 1) was synthesised (see ESI† – Scheme S5) and as expected no increase in fluorescence intensity was observed with the addition of ONOO− (10 μM) (see ESI† – Fig. S5).
27,28 and displayed excellent selectivity towards ONOO−(see ESI† – Fig. S2–S4). To demonstrate the requirement of an ‘eliminating’ benzyl group on the free N–H, Pinkment-Bn (Fig. 1) was synthesised (see ESI† – Scheme S5) and as expected no increase in fluorescence intensity was observed with the addition of ONOO− (10 μM) (see ESI† – Fig. S5).
From these results, we believed we could develop a range of ‘AND’-based Pinkment fluorescence probes by altering the functionality on the benzyl group to react with a specific target analyte. To achieve this, we developed a synthesis for Pinkment-OH (Fig. 2), which provides a novel fluorescence core probe that can be functionalised with any chosen reactive chemical trigger for the detection of a chosen analyte in the presence of ROS/RNS (Fig. 2a).
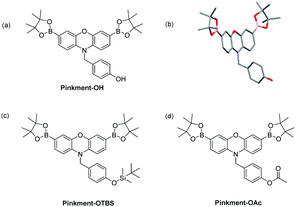 | ||
| Fig. 2 (a) Pinkment-OH – a core fluorescence unit that enables the synthesis of ‘AND’ based fluorescence probes for the detection of (ROS/RNS) and a second analyte. (b) Molecular structure of Pinkment-OH with thermal ellipsoids shown at 50% probability. Hydrogen atoms are omitted for clarity except for the OH hydrogen atom. (c) Pinkment-OTBS (d) Pinkment-OAc for the detection of ROS/RNS ‘AND’ fluoride or esterase, respectively. See ESI† – Fig. S4. CCDC 1844385 contain the supplementary crystallographic data for Pinkment-OH.† | ||
For the synthesis of Pinkment-OH, the phenol of 4-hydroxybenzyl alcohol was selectively piv protected to afford 5 using pivaloyl chloride and NEt3 in DCM. 5 was then converted to its corresponding bromide 6 using MsCl and NEt3 followed by the addition of LiBr. Phenoxazine was then alkylated with 6 to produce 7 using NaH and DMF in satisfactory yield (51%). 7 was then dibrominated using NBS in CHCl3 to afford 8, which was subsequently deprotected using DIBAL-H to afford 9 in good yield (67%). The same Suzuki–Miyaura conditions were then applied to furnish Pinkment-OH in excellent yield (83%) (see ESI† – Scheme S6). To illustrate the stability of Pinkment-OH, an X-ray structure was obtained (Fig. 2b). Interestingly, Pinkment-OH formed dimers via intermolecular hydrogen bonding (see ESI† – Fig. S20).
Pinkment-OH was shown to have a high sensitivity and excellent selectivity towards ONOO− making it suitable for cellular imaging experiments (see ESI† – Fig. S6–S9). As proof of concept for ‘AND’-based pinkment sensors, we chose to synthesise Pinkment-OTBS and Pinkment-OAc (Fig. 2c and d), which can be used to detect ROS/RNS and fluoride (F−) or esterase, respectively.
To afford Pinkment-OTBS, Pinkment-OH was silyl protected using standard silyl protection conditions, however, the reaction was found to be very slow (3 d) and Pinkment-OTBS was isolated in very low yield (11%) (see ESI† – Scheme S7). We then evaluated the ability of Pinkment-OTBS to detect F− ‘AND’ ONOO− in a buffer solution (52% w/w MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
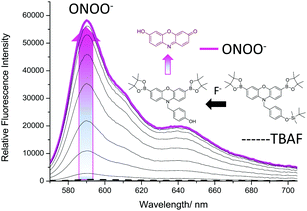
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O). This MeOH/H2O buffer solution was required for the silyl-ether deprotection reaction to proceed effectively. As shown in ESI† – Fig. S10, 100% PBS produced a much smaller response when both analytes were added, which is consistent with literature reported TBS fluorescence probes.29,30 As shown in Fig. 3, addition of tert-butylammonium fluoride (TBAF – source of F−) led to no increase in fluorescence intensity, however, subsequent additions of ONOO− (0–20 μM) led to a significant increase in fluorescence intensity.
H2O). This MeOH/H2O buffer solution was required for the silyl-ether deprotection reaction to proceed effectively. As shown in ESI† – Fig. S10, 100% PBS produced a much smaller response when both analytes were added, which is consistent with literature reported TBS fluorescence probes.29,30 As shown in Fig. 3, addition of tert-butylammonium fluoride (TBAF – source of F−) led to no increase in fluorescence intensity, however, subsequent additions of ONOO− (0–20 μM) led to a significant increase in fluorescence intensity.
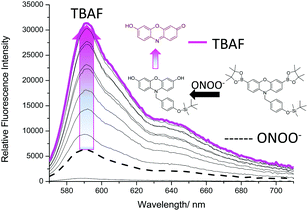
To ensure both analytes were required, the addition of TBAF and ONOO− were then repeated in reverse order. Due to the high reactivity of ONOO−,8,31 a small increase in fluorescence intensity was observed on addition to Pinkment-OTBS (20 μM). However, like Fig. 3, a large increase in fluorescence intensity was only observed after the subsequent addition of TBAF (0–10 mM) (Fig. 4). As shown in ESI† – Fig. S12 and S13, Pinkment-OTBS displayed an excellent selectivity towards ONOO− against other ROS in the presence of TBAF. Pinkment-OTBS was then screened against other halide sources (TBAB, TBAC and TBAI) in the presence of ONOO− (20 μM) see ESI† – Fig. S14. As expected Pinkment-OTBS displayed excellent selectivity towards TBAF in the presence of ONOO−.
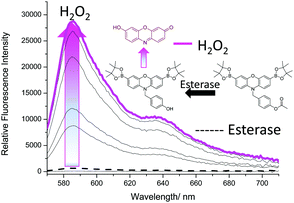
We then turned our attention towards the synthesis of Pinkment-OAc. Despite the poor reactivity with TBS-Cl, Pinkment-OH reacted with AcCl smoothly and produced Pinkment-OAc in excellent yield (71%) (see ESI† – Scheme S8). Due to the known ability of ONOO− to react with carbonyls, its addition to Pinkment-OAc was avoided (see ESI† – Fig. S15 and S16). Therefore, as proof of concept, we evaluated Pinkment-OAc with H2O2 ‘AND’ esterase. As shown in Fig. 5, the addition of porcine liver esterase, PLE (0.6 U) led to no increase in fluorescence intensity. However, subsequent additions of H2O2 (0–1 mM) led to a large increase in fluorescence intensity.
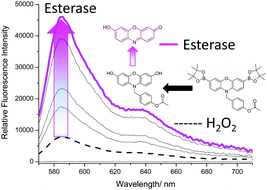
Again, to ensure that both analytes were required, the concentration of H2O2 was kept constant (1 mM) and the amount of PLE was varied. Due to the known ability of H2O2 to hydrolyse carbonyl containing compounds, it was no surprise that there was a small increase in fluorescence intensity.32,33 However, with increasing additions of PLE much larger increases in fluorescence intensity were observed (Fig. 6). Interestingly, concentrations >0.6 U led to a smaller increase in fluorescence. This is believed to be the result of the excess esterase reacting with the added H2O2 (see ESI† – Fig. S17).
In summary, several fluorescence-based probes have been synthesised that when activated result in the formation of the highly fluorescent dye, resorufin. As the dye is pink in colour, we have labelled these fluorescent probes as ‘pinkments’. The original Pinkment was shown to detect biologically relevant concentrations of ONOO− and have an excellent selectivity against other ROS/RNS making it suitable for cellular imaging experiments. Pinkment provided a suitable platform for the development of ‘AND’-based fluorescent probes for the detection of ROS/RNS and another analyte. Pinkment-OH is a core building block that enables easy functionalisation in order to produce a range of ‘AND’ based fluorescence probes for the detection of (ROS/RNS) and a second analyte. As proof of concept, we developed Pinkment-OTBS and Pinkment-OAc for the detection of ROS/RNS and F− and esterase, respectively. Both Pinkment-OTBS and Pinkment-OAc were demonstrated to be successful ‘AND’-based fluorescence probes. However, both probes demonstrated slight reactivity to ROS/RNS alone, due to both silyl ether and acetate functional groups being unstable towards nucleophiles. We are now turning our attention to the attachment of palladium ion (Pd2+) and mercury ion (Hg2+) reactive triggers, previously developed by Ahn et al. and Koide et al.34,35 It is believed that the alkyl ethers will provide the appropriate stability towards ROS/RNS and Hg and Pd nanoparticles produce ROS in biological systems promoting apoptosis.36–39
We would like to thank the EPSRC and the University of Bath for funding for ACS, MLO, JEG for studentships. MW would like to thank the EPSRC for funding (i) EP/L016354/1 and CDT in Sustainable Chemical Technologies. TDJ wishes to thank the Royal Society for a Wolfson Research Merit Award and Sophia University for a visiting professorship. MZ and YBJ were supported by the NSF of China under grants 21435005 and 21521004. XPH wishes to thank the National Natural Science Foundation of China (21722801), the Science and Technology Commission of Shanghai Municipality (15540723800) and the Shanghai Rising-Star Program (16QA1401400) for financial support. TDJ, MZ and YBJ thank the Royal Society for funding an International Joint Project (IE121564). NMR characterisation facilities were provided through the Chemical Characterisation and Analysis Facility (CCAF) at the University of Bath (http://www.bath.ac.uk/ccaf). The EPSRC UK National Mass Spectrometry Facility at Swansea University is thanked for analyses. All data supporting this study are provided as ESI† accompanying this paper.
Conflicts of interest
No conflicts of interest.Notes and references
- D. Wu, A. C. Sedgwick, T. Gunnlaugsson, E. U. Akkaya, J. Yoon and T. D. James, Chem. Soc. Rev., 2017, 46, 7105–7123 RSC.
- J. Chan, S. C. Dodani and C. J. Chang, Nat. Chem., 2012, 4, 973–984 CrossRef PubMed.
- Y. M. Yang, Q. Zhao, W. Feng and F. Y. Li, Chem. Rev., 2013, 113, 192–270 CrossRef PubMed.
- X. P. He and H. Tian, Chem, 2018, 4, 246–268 Search PubMed.
- J. Markovic, C. Borras, A. Ortega, J. Sastre, J. Vina and F. V. Pallardo, J. Biol. Chem., 2007, 282, 20416–20424 CrossRef PubMed.
- B. Pochwat, G. Nowak and B. Szewczyk, Neural Plast., 2015, 2015, 591563 Search PubMed.
- K. A. Marshall, R. Reist, P. Jenner and B. Halliwell, Free Radical Biol. Med., 1999, 27, 515–520 CrossRef PubMed.
- P. Pacher, J. S. Beckman and L. Liaudet, Physiol. Rev., 2007, 87, 315–424 CrossRef PubMed.
- J. P. Bolanos, S. J. R. Heales, J. M. Land and J. B. Clark, J. Neurochem., 1995, 64, 1965–1972 CrossRef PubMed.
- M. Nakamura, V. H. Thourani, R. S. Ronson, D. A. Velez, X. L. Ma, S. Katzmark, J. Robinson, L. S. Schmarkey, Z. Q. Zhao, N. P. Wang, R. A. Guyton and J. Vinten-Johansen, Circulation, 2000, 102, 332–338 CrossRef.
- S. Erbas-Cakmak, S. Kolemen, A. C. Sedgwick, T. Gunnlaugsson, T. D. James, J. Yoon and E. U. Akkaya, Chem. Soc. Rev., 2018, 47, 2228–2248 RSC.
- C. Y. Ang, S. Y. Tan, S. J. Wu, Q. Y. Qu, M. F. E. Wong, Z. Luo, P. Z. Li, S. T. Selvan and Y. L. Zhao, J. Mater. Chem. C, 2016, 4, 2761–2774 RSC.
- S. Debieu and A. Romieu, Org. Biomol. Chem., 2015, 13, 10348–10361 RSC.
- G. C. Van de Bittner, C. R. Bertozzi and C. J. Chang, J. Am. Chem. Soc., 2013, 135, 1783–1795 CrossRef PubMed.
- F. B. Yu, P. Li, B. S. Wang and K. L. Han, J. Am. Chem. Soc., 2013, 135, 7674–7680 CrossRef PubMed.
- X. F. Yang, Q. Huang, Y. G. Zhong, Z. Li, H. Li, M. Lowry, J. O. Escobedo and R. M. Strongin, Chem. Sci., 2014, 5, 2177–2183 RSC.
- S. Resa, A. Orte, D. Miguel, J. M. Paredes, V. Puente-Munoz, R. Salto, M. D. Giron, M. J. Ruedas-Rama, J. M. Cuerva, J. M. Alvarez-Pez and L. Crovetto, Chem. – Eur. J., 2015, 21, 14772–14779 CrossRef PubMed.
- X. P. He, X. L. Hu, T. D. James, J. Yoon and H. Tian, Chem. Soc. Rev., 2017, 46, 6687–6696 RSC.
- L. Yu, S. L. Wang, K. Z. Huang, Z. G. Liu, F. Gao and W. B. Zeng, Tetrahedron, 2015, 71, 4679–4706 CrossRef.
- J. L. Kolanowski, F. Liu and E. J. New, Chem. Soc. Rev., 2018, 47, 195–208 RSC.
- A. Romieu, Org. Biomol. Chem., 2015, 13, 1294–1306 RSC.
- A. C. Sedgwick, H. H. Han, J. E. Gardiner, S. D. Bull, X. P. He and T. D. James, Chem. Commun., 2017, 53, 12822–12825 RSC.
- A. C. Sedgwick, R. S. L. Chapman, J. E. Gardiner, L. R. Peacock, G. Kim, J. Yoon, S. D. Bull and T. D. James, Chem. Commun., 2017, 53, 10441–10443 RSC.
- A. C. Sedgwick, H. H. Han, J. E. Gardiner, S. D. Bull, X. P. He and T. D. James, Chem. Sci., 2018, 9, 3672–3676 RSC.
- A. Sikora, J. Zielonka, M. Lopez, J. Joseph and B. Kalyanaraman, Free Radical Biol. Med., 2009, 47, 1401–1407 CrossRef PubMed.
- E. W. Miller, A. E. Albers, A. Pralle, E. Y. Isacoff and C. J. Chang, J. Am. Chem. Soc., 2005, 127, 16652–16659 CrossRef PubMed.
- X. M. Chen, B. H. Zhou, T. T. Yan, H. Wu, J. H. Feng, H. S. Chen, C. Gao, T. Peng, D. Yang and J. G. Shen, Free Radical Biol. Med., 2018, 117, 158–167 CrossRef PubMed.
- H. Fujigaki, K. Saito, F. Lin, S. Fujigaki, K. Takahashi, B. M. Martin, C. Y. Chen, J. Masuda, J. Kowalak, O. Takikawa, M. Seishima and S. P. Markey, J. Immunol., 2006, 176, 372–379 CrossRef.
- X. F. Yang, S. J. Ye, Q. Bai and X. Q. Wang, J. Fluoresc., 2007, 17, 81–87 CrossRef PubMed.
- J. Cao, C. C. Zhao, P. Feng, Y. L. Zhang and W. H. Zhu, RSC Adv., 2012, 2, 418–420 RSC.
- D. Yang, Y. C. Tang, J. Chen, X. C. Wang, M. D. Bartberger, K. N. Houk and L. Olson, J. Am. Chem. Soc., 1999, 121, 11976–11983 CrossRef.
- M. Abo, Y. Urano, K. Hanaoka, T. Terai, T. Komatsu and T. Nagano, J. Am. Chem. Soc., 2011, 133, 10629–10637 CrossRef PubMed.
- J. Yang, X. L. Zhang, P. Yuan, Y. G. Xu, J. Grutzendler, Y. H. Shao, A. Moore and C. Z. Ran, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 12384–12389 CrossRef PubMed.
- M. Santra, S. K. Ko, I. Shin and K. H. Ahn, Chem. Commun., 2010, 46, 3964–3966 RSC.
- F. L. Song, S. Watanabe, P. E. Floreancig and K. Koide, J. Am. Chem. Soc., 2008, 130, 16460–16461 CrossRef PubMed.
- B. J. Shenker, T. L. Guo and I. M. Shapiro, Environ. Res., 2000, 84, 89–99 CrossRef PubMed.
- A. E. A. Moneim, Neural Regener. Res., 2015, 10, 881–882 CrossRef PubMed.
- A. E. A. Moneim, Metab. Brain Dis., 2015, 30, 935–942 CrossRef PubMed.
- S. Alarifi, D. Ali, S. Alkahtani and R. S. Almeer, Oxid. Med. Cell. Longevity, 2017 DOI:10.1155/2017/8439098.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 1844385. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8cc04316g |
| ‡ A. C. S. and M. L. O. contributed equally, A. C. S. – organic synthesis, M. L. O. – fluorescence analysis. |
| This journal is © The Royal Society of Chemistry 2018 |