Open Access Article
Open Access ArticleMussel-inspired hydrogel tissue adhesives for wound closure
Maedeh Rahimnejad and
Wen Zhong *
*
Department of Biosystem Engineering, University of Manitoba, Canada. E-mail: wen.zhong@umanitoba.ca
First published on 9th October 2017
Abstract
Tissue adhesives have been introduced as a promising alternative for the traditional wound closure method of suturing. Design and development of tissue adhesives for biomedical applications has been inspired by outstanding examples in nature. This review covers the adhesive mechanisms, applications and characterizations of various biomimetic tissue adhesives reported during the past decade, with a focus on the mussel-inspired dopamine-based adhesives, which have attracted extensive interest due to their promising adhesive performance in a wet environment.
1. Introduction
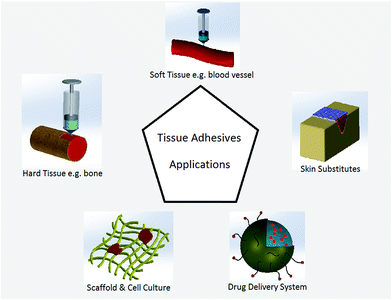
The advent of different wound closure methods has provided promising alternatives for such traditional wound closure methods as suturing. The knot tying procedure for sutures may cause tissue distortion, residual force, blocking of blood perfusion, and consequently impede wound healing. Foreign body reaction is another adverse outcome of sutures, especially at a site of knotting which represents a major mass of foreign body.1–6 A primary closure of wounds, suturing sometimes may not be feasible because of the inadequacy of the skin flaps, especially after trauma or following excision of lesions.1 In this regard, alternative methods for surgical wound closure such as biological glues and tissue adhesives have gained more attentions recently. Tissue adhesives offer various advantages over traditional wound closure methods include easiness to apply, causing less pain and no need for removal in a further visit.Tissue adhesives are becoming a common adjunct in surgical practice and are applied in tissue adhesion, hemostasis, and sealing of air and body fluids leakage during surgical procedure. Both biologic adhesives (e.g. fibrin-based adhesives) and synthetic adhesives (e.g. cyanoacrylate derivatives) are available for such applications.7,8 Comparing tissue adhesives and other wound closure techniques has been studied to determine different outcomes including cosmetic effect in the under study population.9–11 For cosmetic reasons, tissue adhesives have gained increasing popularity as an alternative method of wound closure.12,13 In recent years, tissue adhesives have commercialized with significant advantages such as decreasing foreign body reaction during wound healing procedure and no need for further removal.14,15 Tissue adhesive systems have been used in dentistry,16–18 orthopedics,19–21 cardiovascular22,23 as well as in ophthalmology,24 traumatology, and otorhinolaryngology and also for plastic and maxillofacial surgical applications.25,26 The advantages of hard tissue (e.g. bone or dent) gluing as compared to nailing or plating includes good fixation of small fragments, more homogenous weight bearing distribution within the fracture site. In the treatment of articular fractures, tissue adhesives act as subchondral spacer to compensate joint surface displacement.27 The applications of adhesives in soft tissues include skin12 and ocular wounds closures.28 Adhesion is critical in hemostatic biomaterials, therefore tissue adhesives play an important role to improve conventional sealing methods22 (Fig. 1). Chen et al. reported a mussel inspired hydrogel adhesive composed of poly(γ-glutamic acid) and dopamine, which showed 41.2% reduction in bleeding as compared to fibrin glue.29
Researchers have also become interested in using tissue adhesives in other areas, such as artificial soft tissues and controlled drug and bioagents delivery systems for releasing antibiotic and antibacterial drugs in the buccal or nasal cavity, for intestinal or rectal delivery, and even for application in urinary bladder.30–32 Injectable tissue adhesives loaded with cells can be a promising technique in cartilage impairment treatments.27 Tissue adhesives also showed appealing properties and functionality in cell culture systems and scaffolds modifications for appropriate cells responses, such as biospecific cell adhesion for better cell migration and viability. Ku et al. modified a microchannel by poly(dopamine) (PDA) as a simple and effective approach to maintain cell morphology in tissue engineering.33 Dopamine was also used as a coating material on another material like poly(ethylene glycol) to prepare a surface with better cell adhesive34 (Fig. 1).
Design and development of high-performance and environmentally friendly tissue adhesives has been inspired by many outstanding and promising examples in nature. One good example is the dopamine-based tissue adhesives inspired by the marine mussels that have the capacity to adhere to wet or moisture surfaces, which is an essential requirement for any adhesives to be used in the field of surgery and medicine.35–41
Herein, we make a concise review of tissue adhesives with unique chemical properties and their recent applications and developments. Specifically, mussel-inspired dopamine-based tissue adhesives for biomedical application will be the focus of discussion in terms of their key properties, mechanism and applications. Other types of tissue adhesives will also be explained and compared with the dopamine-based adhesives.
2. Mechanisms of tissue adhesives
2.1 Ideal properties of a tissue adhesive
Wound healing is a complex and dynamic process of replacing devitalized and dead cellular structures and tissue layers that normally occurs through the scar tissue formation. Tissue adhesives as one of the promising alternatives in primary wound closure affect wound healing procedure from different aspects,42–46 although existing tissue adhesives still have certain limitations in their performance that call for further efforts in research and development. For example, there have been reports indicated that tissue adhesives may lead to inappropriate wound edges apposition and hence delay wound healing procedure; and the breaking strength of wounds closed with tissue adhesives may be remarkably low in comparison with taped wounds.47–49 In addition, the tissue adhesives may potentially contribute to the infection in contaminated wounds. For example, in Staphylococcus aureus contaminated wounds, the occurrence rate of gross infection was considerably greater in wounds closed with tissue adhesives in compared to wounds closed with tapes.50There are several ideal properties that would generally be expected for a tissue adhesive developed for wound healing: both the tissue adhesive and its degradation products should be compatible with tissues. Therefore, researchers are confronted with the challenge of developing a reactive monomer that provides rapid polymerization, and using biocompatible initiators. The tissue adhesive should adhere to a wet or moisture surface at approximately body temperature. Furthermore, surgical tissue adhesives should provide proper applying time, biodegradability, strong and flexible bonding, spreading capacity and wettability.51
Mussel-inspired dopamine-based tissue adhesives were well known for their remarkable water resistance characteristic in addition to its biocompatibility, which makes them a potential ideal tissue adhesive for both inside and cutaneous wound closure and healing. Dopamine as a small molecule compound containing catechol and amine groups confers functionality of highly adhesion in wet conditions such as human body.52
2.2 Mussel inspired biomimetic tissue adhesives
Mussel adhesive proteins secreted from marine mussels' feet are responsible for their ability to from strong adhesive interaction with different type of substrates in a wet condition. Studies suggest that these proteins are highly rich in lysine and modified amino acid, 3,4-dihydroxyphenyl-L-alanine (DOPA) which contain catechol functional group and that these components may confer the observed adhesive properties.52,53 Although the detailed and accurate crosslinking mechanism is still unknown, it is suggested that oxidation of DOPA by means of metal ions or enzymes forms quinone which is essential for the adhesion to organic/inorganic surfaces or tissues.54 Thus, during maturation in adhesive plaque of mussel, the quinone moieties crosslink with other quinones using radical mechanism, thiols and/or amines using Michael addition.40,55 It is believed that the strong adhesion of mussels to a surface is based upon the strong covalently and noncovalently bonding with surfaces procreated by DOPA's phenolic hydroxyl or quinone groups. Moreover, lysine plays a significant role in crosslinking that resulted in solidification of secreted liquid protein adhesive. Enhancing the catechol content provides too much crosslinking, extra cohesion in system and hence lower surface attachment.56,57 The abundant amino and hydroxyl groups in these catechol and amine groups also allow them to be easily conjugated to a wide range of material surfaces.Recently, dopamine has piqued substantial interest in chemistry and biomedical research owing to a similar chemical structure to that of combined lysine and DOPA, as well as its adhesive potential.57 Dopamine constitutes one of the most abundant catecholamines in the human body that has long been investigated as a hormone or neurotransmitter, and abnormal levels have been shown to contribute to serious brain-related disorders. Under alkaline conditions, the catechol functional group oxidizes to quinone in order to provide condition for dopamine self-polymerizing and forming a thin film on support surfaces via covalent bonding and other strong intermolecular interactions like hydrogen bonding, metal chelation, and π–π interactions.58 The chemical structures of DOPA and dopamine are shown schematically in Fig. 2.
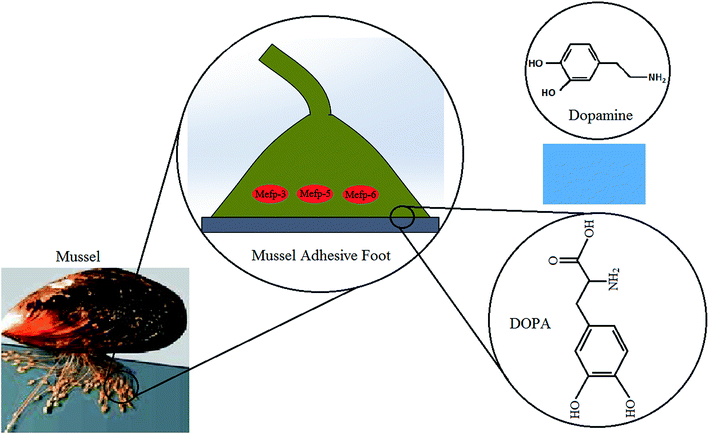 | ||
| Fig. 2 Molecular structures of DOPA and dopamine (the mussel picture63 reproduced by permission of the Company of Biologists). | ||
Adhesive proteins have been extracted from mussels and examined for biomedical applications. There have been extensive studies on materials surface functionalization using DOPA as a spacer to enhance their adhesion capacity. In recent works, both dopamine and DOPA have been added to different type of polymeric structures to develop highly functional biocompatible tissue adhesives suitable for wet and dry conditions.53,58–60 Many researchers also explore the applications of dopamine-based tissue adhesives by mimicking mussel DOPA.40,61,62
3. Formats of tissue adhesives
3.1 Tissue glues
Existing tissue glues on market generally are not perfect in terms of biocompatibility and biostability. For instance, cyanoacrylate-based adhesives not only cause compatibility issues by releasing formaldehyde degradation products, but also have low mechanical properties; hence they produce inflammation and tissue necrosis. Fibrin glues may show poor adhesion and generate a viral infection risk. In addition, tissue glues are more costly in compared to traditional wound closure methods such as suturing.As an effort to improve the performance of tissue adhesives, nature inspired tissue adhesives have been developed to provide highly biocompatible yet affordable products.64–67 Among them, DOPA or dopamine compounds have attracted extensive attention toward the fabrication of highly efficient tissue adhesives.
Vollenweider et al. used DOPA as a crosslinking precursor to enhance the wet surface adhesion of an adhesive composed of polyethylene glycol (PEG) and polycaprolactone (PCL).68 Shao and Stewart fabricated medical glue suitable for open surgery wound closure with a composition of dopamide, poly-phosphate, poly-aminated gelatin, and divalent cations.69 Choi et al. produced recombinant mussel adhesive protein fp-5 (MAP fp-5) to develop bulk tissue adhesive via various vector systems and cDNA codon optimization.66
Recently, a mussel-inspired nano-composite tissue adhesive for sternal bone closure under wet environment was developed to consist of hyper-branched poly(β-amino ester) (PAE)/(polydopamine-co-acrylate) (PDA) and hydroxyapatite nanoparticles with increased wet adhesion and reinforced structure.70 Another research team developed an adhesive precursor composed of hyper-branched PAE polymer modified by catechol groups to improve commercial cyanoacrylate and fibrin bioglues.71 Zhou et al. investigated adhesion characteristics of amino acid-based poly(ester urea) (PEU) copolymers functionalized by catechols, demonstrating that the chemical and physical properties of the copolymer can be controlled via varying the amount of diols and amino acids, which is an desirable property for bioglue.72 Ahn et al. synthetized low molecular weight catecholic zwitterionic adhesive with a simpler structure as compared to mfp-5 and mfp-3. The adhesive was shown to have higher adhesion energy than mfp-5.73 Jeon's team used a dityrosine photo crosslinking technique in recombinant mussel adhesive protein DOPA, in which tyrosine residues crosslink with each other assisting photo-oxidation reaction.49 Lee et al. developed a biomimetic dental adhesive composed of poly dopamine methacrylate–methacryloyl chloride (PDMA–MEA) as the backbone and catechol and methoxyethyl groups in the side chains. The adhesives were demonstrated to have high adhesion, shear strength and biocompatibility due to catechol crosslinking.74
Ji et al. created bioadhesives comprised of poly(ethylene oxide) (PEO) and 1,8-octanediol, which have varying capacity from water-soluble to low in swollen by regulating the ratio of PEO to 1,8-octanediol.75 Hong et al. showed two metallo-bioadhesives, gelatin/Fe and dopamine-modified gelatin (GelDA/Fe) for wound closure. Based on the characterizations, Gel/Fe showed a greater strength whilst GelDA/Fe performed highly acceptable sealing property and seemed to be a better option as tissue glue. Both adhesives were found to have the capacity to induce cells adhesion and growth on the materials surfaces.76
Dopamine-based synthetic adhesives have been demonstrated to provide strong bonding between tissue and glue, appropriate strength in mechanical interlocking of glue with underlying tissue and improved cosmetic outcomes. On the other hand, they show slow degradation within 7 to 10 days and released histotoxic components from degraded polymer. These drawbacks can be usually improved by introducing hydrophilic polymers with biocompatible degradation products such as lactic acid and PEG. However, irradiation for polymerization which damages cells and tissue integrated with glue, weak internal strength and cohesion, large swelling in wet condition and inducing foreign body reaction still limited their medical applications.62,77
3.2 Hydrogel adhesives
Hydrogels exhibit capabilities that make them promising alternatives to be used as tissue adhesives in biomedical applications, including wound closure and injectable drug release systems. The hydrophilic and crosslinked structure endows hydrogels soft tissue like characteristics. In addition, hydrogels have high permeability to oxygen, nutrients, and other water-soluble metabolites.78,79 Therefore, hydrogels are becoming a popular format of tissue adhesives. Biomimetic, mussel-inspired tissue adhesive hydrogels were shown to have good healing capacity in the byssal threads of marine mussels80 and promising potential to be used as biomedical adhesives.81–84 There have been different strategies to incorporate the DOPA or dopamine functional groups onto the materials (Fig. 3) and to further endow additional functions to the tissue adhesives, as discussed in this section.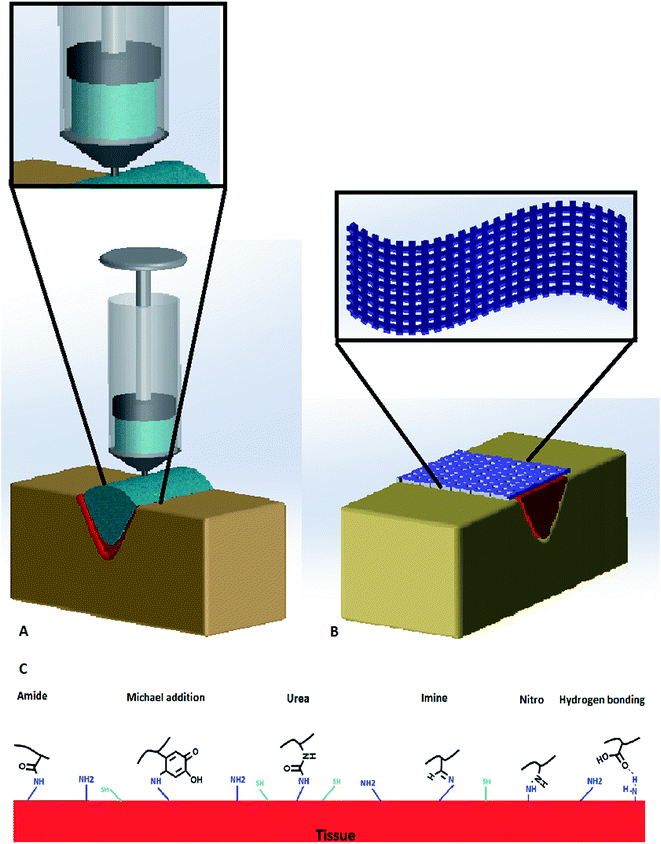 | ||
| Fig. 3 (A) Dopamine-based injectable adhesive hydrogels in wound closure, (B) dopamine-based nanofibrous adhesive mat in wound closure, and (C) dopamine functional groups reaction with tissue. | ||
In addition to surface modification for surface bioadhesion, various studies have been focused on conjugation of dopamine or catechol functional groups onto both natural and synthetic polymers. Such modifications not only affect the bioadhesive properties, but also the mechanical strength of the hydrogels. The most frequently used polymers for such purposes are discussed below.
Chitosan is a natural polymer that has been used to develop adhesive hydrogels. Xu et al. reported three chitosan/catechol hydrogels with difference types of catechols, including DOPA, hydrocaffeic acid (HCA), and dopamine to compare their swelling ratio and adhesion to the mucus. Results demonstrated that the swelling ratio and adhesion can be altered by the nature of catechol compounds, with HCA/chitosan hydrogels showed the lowest swelling ratio and highest mucus adhesion.87
Gelatin is a popular natural polymer for hydrogel fabrication. The adhesive capacity of an injectable gelatin hydrogel was improved by using DOPA. Upon addition of Fe3+ ions, the gelatin modified by DOPA can be converted into an adhesive hydrogel via DOPA–Fe3+ coordination and quinone coupling. After injection to a hemorrhaging site of a rat liver, the adhesive demonstrated good hemostasis by producing adhesive obstacle on site of bleeding. Also this adhesive hydrogel showed appropriate mechanical strength and good biocompatibility to be used in medicine and surgery.88 Zhu et al. developed dopamine based tissue adhesive containing gelatin as a backbone, genipin and FeCl3 as the slow and fast crosslinker, respectively. Its effect on mastectomy surgery in a rat model was examined. Acceptable tissue adhesion, mechanical strength, biocompatibility and minimized formation of seroma were reported.89 Dual crosslinking method was used to prepare dopamine based tissue adhesive with gelatin polymer backbone. Fan et al. created a gelatin adhesive which is capable to be used in both external (skin) and internal (cartilage) wound closure applications. The results showed high wet adhesion, promising biocompatibility and biodegradability.90
Another research group designed hyaluronic acid (HA) sticky hydrogels modified by a catecholamine cross linker for cell therapy. Catecholamine consisting of catechol group could improve HA functionality and cell adhesion. The catechol group oxidized to o-quinone was operated in alkaline pH (>7.5) condition to crosslink chemically catechol–catechol compositions, then the catechol moieties were conjugated to the HA hydrogel. The modified HA gel structure exhibited higher tissue adhesion and biocompatibility in comparison with HA hydrogel.91
Polyethylene glycol (PEG) or polyethylene oxide (PEO) is a very popular synthetic polymer for developing adhesive hydrogels. Brubaker et al. examined the use of PEG hydrogel with catechol functional group on extrahepatic islet transplantation which had potential application for diabetic patients. Direct immobilization of islets onto intra-abdominal tissue surface through the hydrogel adhesive in mice was achieved.92 Barret et al. developed mussel protein based amphiphilic poly(propylene oxide) (PPO)–poly(ethylene oxide) (PEO) polymer hydrogels that can be formed in situ for surgical wound treatment. The hydrogel demonstrated stresses of 3.4–4.3 MPa and 90% strain without any rupture and damage. It can tolerate 90% compression at body temperature in an equilibrium swollen phase. Higher roughness and negative swelling ratio can be achieved by modification of PEO using PPO and catechol functional groups.93 A fast curable hydrogel made of DOPA, dextran and three-arm PEG was prepared by linking DOPA to PEG–TA (tetraacrylate) by Michael reaction between acrylate double bonds and amine groups. Usage of PEG–DOPA increased the adhesion strength of the dextran hydrogels from 2.7 to 4.0 MPa.94 Also, Liu et al. modified and improved adhesion and bio interaction properties of PEG based tissue adhesive by applying dopamine, catechol groups and nanoparticles. They indicated that the nanocomposite demonstrated enhanced mechanical properties, rate of curation and bioadhesion because of strong conjugation between LAPONITE® and dopamine.95
Skelton et al. developed polyacrylamide (PAAm)/dopamine methacrylate (DMA) hydrogels incorporated with nano silicate and LAPONITE®. Photo polymerization enabled covalent linkage of catechol with hydrogel of PAAm containing nano silicate. The final hydrogels showed improved rigidity and viscosity that affect the rheology involving storage and loss moduli.96 Jenkins et al. carried out a study to characterize the effect of molecular weight of biomimetic proteins on adhesion of polymer. It was suggested that a higher adhesion stems from higher molecular weight. Though achieving high adhesion strength ranging in MPa is usually difficult for dopamine-based hydrogels due to their high water content.97
Zhou et al. assessed adhesion characteristics of mussel inspired dopamine based PEU gel. The findings confirmed phenylalanine, tyrosine, and catechol functional groups affect adhesion through contact angle measurements and Johnson–Kendall–Roberts (JKR) method: cross-linking oxidation of catechol remarkably increased lap-shear adhesion strength and catechol modified PEU showed better adhesive strength compared with fibrin glue on porcine skin.72
An adhesive hydrogel used for peripheral nerve regeneration contained of chitosan and ε-polylysine (PL) was prepared by Zhou et al. that was able to form in situ and simulate chemical structure of natural epineurium matrices which increases its nerves biocompatibility. Maleimide and thiol functional groups were allowed to react with each other by Michael-type addition, to form a cross linker to speed up curing time and eliminate further damages to nerves. Rapid gelation in 10 s prompted sealing of transected nerves. The morphology of the repaired nerve of sealed with the adhesive hydrogel after 8 weeks was similar to that of the normal nerve. Its functionality was evaluated in vivo on rat models, hence demonstrated potential application for nerve surgery.98
There have been plenty of reports on DOPA or dopamine incorporated hydrogels that combine both natural and synthetic polymers. Burke and colleagues developed a PEG/silk fibroin adhesive hydrogel in which dopamine was chemically coupled to silk fibroin enriched with carboxyl groups. PEG controlled the hydrophobicity of the fibroin backbone and the gel solubility that was incorporated.99 Li et al. focused on dopamine-modified poly(ethylene glycol) (PEGDM) adhesives incorporating gelatin microgel in order to increase adhesion and bioactivity. They compared the PEGDM hydrogel which combined with gelatin microgel properties with gelatin microgel. PEGDM containing gelatin microgel adhesive properties improved from 1.5 to 2 fold, promoted attachment of fibroblasts and cell viability regarding it implanted in rat, and improved rheology parameters such as enhanced loss moduli. The loss modulus and storage modulus that represent adhesive elasticity enhanced by gelatin microgel content increasing which indicated reversible physical bonding.100 A citrate based tissue adhesive hydrogel (POECd) was fabricated using melt polycondensation of four agents of 1,8-octanediol, PEO, citric acid (CA) and dopamine. Effects of PEO on swelling, mechanical and biocompatibility of adhesive hydrogel was also examined. The adhesive strength and viability demonstrated that the lap shear adhesion strength, in the range of 21.7–33.7 kPa was higher than commercial fibrin glue lap shear adhesion strength which was in the range of 9–15 kPa and lower toxicity compared to fibrin glue.75 Citrate polymer and its derivatives showed intrinsic antimicrobial behavior. Guo et al. synthetized citrate based antimicrobial bioadhesives composed of citric acid and catechol functional group.101 Such adhesives demonstrated 13 times higher adhesion strength as compared to fibrin glue. They also showed antimicrobial properties due to dual crosslinking procedures including click chemistry crosslinking and DOPA crosslinking.102 Methods of incorporation of DOPA or dopamine functional group into mussel inspired tissue adhesives and their property features are summarized in Table 1.
| Polymer | Methods of incorporation of DOPA/dopamine | Property features | Ref. |
|---|---|---|---|
| Chitosan | Mixed hydrogels of chitosan and a catechol-containing compound: DOPA, hydrocaffeic acid (HCA), or dopamine | Mucoadhesion of chitosan hydrogels increased by the incorporation of catechol-containing compounds | 87 |
| Chitosan & ε-polylysine (PL) | Catechol groups conjugated onto PL molecules in a nerve adhesive hydrogel composed of chitosan and PL | Incorporation of catechol groups was shown to enhance both mechanical and nerve adhesion properties of the hydrogels | 98 |
| Citrate polymer and derivatives | Polycondensation of citric acid (CA), PEG and catechol-containing compound | Higher adhesion strength and lower toxicity compared to fibrin glue | 101–103 |
| Gelatin | Adhesive hydrogel containing gelatin modified by DOPA via DOPA–Fe3+ coordination and quinone coupling | Good hemostasis, appropriate mechanical strength and good biocompatibility for applications in surgery | 88–90 |
| Hyaluronic acid (HA) | HA hydrogels functionalized by catechol via oxidative crosslinking | Modified HA gels with higher tissue adhesion and biocompatibility | 91 |
| Polyethylene glycol (PEG) or polyethylene oxide (PEO) | PEG, PEO or their derivative poly (ethylene glycol) dimethacrylate (PEGDMA) conjugated with catechol groups | Enhanced mechanical properties and bioadhesion, negative swelling ratio, promoted attachment and viability of cells | 92, 93, 95 and 100 |
| PEG (three-arm) & dextran | DOPA linked to PEG–TA (tetraacrylate) by Michael reaction between acrylate double bonds and amine groups | Enhanced adhesion strength | 94 |
| Polyacrylamide (PAAm) | Copolymerization of DMA (dopamine methacrylamide) into PAAm hydrogels | Improved mechanical and rheological properties | 96 |
| Polyaspartamide (PolyAspAm) | Material surface modified by being immersed in a coating solution containing PolyAspAm conjugated with dopamine | Bioadhesive surface achieved on various substrates including metals, oxides, synthetic polymers and ceramics | 86 |
| Poly(ester urea) (PEU) | Incorporation with phenylalanine, tyrosine, and catechol groups | Increased adhesion | 72 |
| Silk fibroin | Dopamine chemically coupled to silk fibroin enriched with carboxyl groups | β-Sheet structures of silk fibroin not affected | 99 |
| Polymers | Mechanisms of self-healing and/or environmental sensitivity | Property features | Ref. |
|---|---|---|---|
| Chitosan & Pluronic F-127 | Catechol conjugated chitosan crosslinked with thiolated Pluronic F-127 | Temperature sensitive wet adhesion and hemostasis | 22 |
| Hyaluronic acid (HA) | HA–catechol conjugation via 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide hydrochloride (EDC) | Biocompatible with neural stem cell and pH sensitive adhesion | 111 |
| HA & Pluronic F-127 | Dopamine conjugated HA and thiol-terminated Pluronic F-127 crosslinked by catechol–thiol reaction | Temperature sensitive rapid and reversible sol–gel transition and excellent tissue adhesion | 117 |
| Polyacrylate & polymethacrylate | Self-healing in catechol-functionalized acrylic polymers initiated by catechol-mediated hydrogen bonding | Metal-free underwater self-healing | 105 |
| Polyallylamine | DOPA/iron coordination in response to pH in DOPA-functionalized polyallylamine | High mechanical strength and fast self-repair behavior in alkaline condition | 107 |
| Poly(ethylene glycol) (PEG) | pH-induced metal–ligand (catechol–Fe3+) cross-linking in DOPA-modified PEG | Crosslinked networks with elastic moduli approaching covalently cross-linked gels and with self-healing | 106 |
| Phenyl borate–catechol complexation in hydrogels containing dopamine functionalized 4-armed PEG and phenylboronic acid modified 4-armed PEG | Self-healing hydrogels with pH, glucose, and dopamine triple responsiveness and biocompatibility | 110 | |
| Four-armed PEG end-capped with dopamine | pH dependent adhesive performance | 113 and 114 | |
| Hyperbranched PEG incorporated with catechol and photocrosslinkable methacrylate groups | Provide on-demand photocuring and strong adhesion | 118 | |
| Poly(dopamine methacrylate-co-N-isopropylacrylamide) | Boronate ester linkages provide self-healing properties at alkaline condition | Rapid self-repair adhesive polymer and multiple stimuli-responsive behavior | 109 |
| Polyurethanes (PU) | PU hydrogels containing hexamethylene diisocyanate as hard segment, PEG as soft segment, and lysine–dopamine as side chain | Rapid pH sensitive gelation through Fe–catechol complexes | 112 |
Some studies not only concentrated on wet adhesion of mussel inspired tissue adhesive hydrogels, but also considered their self-healing properties. The metals and mussel foot proteins' coordinative bonding which is able to be reversed, attribute mussel byssus self-healing properties. This metal induced protein complexation mechanism motivated creation of self-repair polymer hydrogels with controlled features. In this regard, DOPA–Fe3+ noncovalently cross-linked hydrogels were extensively studied. Holten-Andersen et al. synthetized a PEG based adhesive hydrogel containing catechol groups in which the mechanical properties and self-healing behavior could be controlled by pH changes. This study exhibited that the catechol groups can produce a mono-complex with ferric ions in pH <5.6, bis-complex in pH between 5.6 and 9.1 and tris-complex in pH greater than 9.1. Hydrogels with covalent cross-linking at high pH resulted in catechol–Fe3+ cross-linked hydrogels, show elastic moduli. The hydrogels cross-linked by tris-catechol–Fe3+ were shown to rapidly restore to its stiffness and cohesiveness, that may be ascribed to the broken catechol–Fe3+ crosslinks rehabilitation.106 A pH responsive hydrogel was developed through bonding DOPA to an intrinsic pH responsive polymer which was functionalized by amine groups. The hydrogels that reacted with ferric ions, demonstrated high mechanical strength and fast self-repair behavior in alkaline condition.107
Another self-repair metal–catechol based adhesive hydrogel was prepared utilizing dynamically reversible B3+–catechol complexation. Catechol group is able to create stable covalent conjugation with boronic acid structure at neutral and alkaline condition and dissociate in an acidic pH. He et al. fabricated a self-repair hydrogel using the complexation of catechol cross-linking, PEG derivatives and 1,3-benzenediboronic acid.108 The boronate ester linkages could provide self-healing behavior and completely mechanical deformation recovery at alkaline condition in hydrogels. Poly(dopamine methacrylate-co-N-isopropylacrylamide) and boric acid (H3BO3) were used to develop a rapid self-repair adhesive polymer with multiple stimuli-responsive behavior, which approves strong pH and catechol concentration dependent functionality of B3+–catechol coordinated in the hydrogels.109 A multi-sensitive catechol based adhesive hydrogel was fabricated by Shan et al. via catechol and phenylborate incorporation. Such adhesives demonstrated self-healing property and responsiveness to pH, glucose and dopamine. Cytocompatibility and high adhesion strength were reported, indicating its potential application in drug delivery systems and tissue regeneration.110
There have been research on the influence of environmental pH on curing rate and tissue adhesion of dopamine based PEG hydrogels. It was suggested that an acidic condition decreases curing rate and adhesive strength, while an alkaline environment lessen tissue adhesion due to reduced concentration of catechol. Formulated hydrogel showed good curing rate, improved mechanical properties and adhesion at pH 7.4.113 Nitro-group functionalized dopamine-based four-arm PEG was analyzed to achieve optimum adhesion, strength and rate of curing in presence of different concentration of NaIO4 and pH. Nitrodopamine-based PEG hydrogel had much more constant adhesive strength in pH ranges from 5.7 to 8, while dopamine-based ones had higher adhesive strength in neutral (body normal pH) and alkaline pH ranges.114 Kim et al. fabricated an adhesive hydrogel by using DOPA-comprising recombinant mussel adhesive proteins to provide strong adhesion strength, flexibility, appropriate viscoelasticity and self-healing properties. The adhesive strength of the Fe3+-mediated noncovalent crosslinked hydrogel was measured to be around 130 kPa in alkaline pH environment, whilst the quinone-mediated covalent hydrogel adhesive strength improved with curing time up to close 200 kPa, after 30 min NaIO4 treatment. The recombinant mussel adhesive proteins produced from traditional biosynthesis system that do not have DOPA, thus the subsequent procedure of tyrosine residues transformation into DOPA molecules suffers from a low modification yield.115 To find a solution for this problem, an in vivo residue-specific incorporation strategy was adopted by Yang et al. to develop engineered mussel adhesive proteins in Escherichia coli with a very high amount of DOPA which was 16.5 mol% in comparison with natural ones (20–25 mol%). This protein exhibited enhanced wet adhesion capacity as demonstrated via surface forces apparatus (SFA) measurements and quartz crystal microbalance (QCM) analysis, showing potential application as bioadhesives in biomedicine.116
Despite of improved biocompatibility and biodegradability of tissue adhesives with hydrogel based structures; they usually demonstrate poor mechanical properties, particularly at loaded parts of the body such as knee meniscus and heart valves. Dopamine based structures provide wet adhesion and strong cohesion between adhesive hydrogel and tissue, thus the robust mechanical strength remains the main concern for researchers. Toxic oxidizing agents like periodate and iron ion utilizing and enhanced degradation time due to strong crosslinking are other disadvantages of hydrogel adhesives.88,126 Nevertheless, DOPA functionalized hydrogel polymers show great performance to be used as tissue adhesives, though no clinical studies have been done to date.
3.3 Micro/nano fibrous films, sheets & patches
To explore the application of tissue adhesives in care for different wounds such as surgical, burn wounds and diabetic ulcers, improve mechanical properties and to increase strengths of tissue adhesives, there have been recent studies on the development of tissue adhesives in the form of film, mat or sheet.127,128 To date, a few studies concentrate on nature bioinspired particularly dopamine based nanofibrous tissue adhesive sheet or film as advanced materials for wound closure (Fig. 3B).Lee et al. studied performance and functionalities of mussel and gecko inspired tissue adhesives in wet and dry conditions. They prepared a bio inspired adhesive including of nanostructure polymer pillars which were coated by a polymer that contains mussel derived adhesive proteins. The mussel proteins increased wet adhesion of the nanostructure film for several contact cycles in both dry and wet surfaces, which showed reversible adhesion on different surfaces.58 Later, DOPA based metallopolymer film was created containing Fe3+ and mfp-1, in order to improve hardening and adhesion. Although, healing of byssal threads of marine mussels can rare be achieved by artificial materials typically due to lack of dynamic migration of their building blocks or molecular components across the defects to restore bonds after damage and rupture, they concluded that interactions between DOPA and Fe3+ produce opportunity to engineer self-healing and strong adhesive polymer.129 A novel triazine based adhesive was synthetized as primers for sealing of bone fractures in surgeries. The study assessed various phenol functional groups as primers in fabricating fibrous patches. Poly(parahydroxylstyrene) (PHS) and phenolic dopamine was used to form adhesive patches with elevated bioadhesion in wet condition for bone fracture fixations in vitro.19 Murphy et al. developed tissue adhesive polymer made of DOPA derivatives and PEG and PCL, providing wet environment friendly biodegradable and biocompatible adhesive film for hernia repair. The film structure was shown to promote rapid tissue ingrowth, to decrease inflammation and enhance lap shear strength as applied on bovine pericardium.130
Sparks et al. developed an adhesive polymer, in which dopamine acrylamide (DAm) was used to provide adhesion by catechol oxidation. Catechol was incorporated with pentaerythritol triallyl ether (APE) and pentaerythritol tetra(3-mercaptopropionate) (PETMP) that provided thiol-ene functional groups. Oxidized catechol was found to result in enhanced adhesion of the coatings to different type of substrates such as glass, aluminum, steel, and marble by varying the DAm amount in the network, as an increase in DAm concentration remarkably decreases the crosslinking density.131 Kim and coworkers incorporated catechol onto water insoluble chitosan and found that catechol conjugation changes the solubility of chitosan and create an adhesive film.132 Oh et al. also worked on modification of chitosan to provide films with good wet adhesive capacity by incorporating an oxidant, sodium periodate into mix of chitosan and DOPA. Compared with chitosan film, the resulted film showed 7 fold higher stiffness and lower swelling ratio.133 A cell adhesive patch was fabricated through coating of PDA on poly(lactide-co-caprolactone) (PLCL) fibrous patch by Shin et al. for cardiac cell delivery application. The RGD peptide immobilization was confirmed via surface wettability measurement. Further, they investigated the patch biocompatibility, myoblast cells adhesion, proliferation and differentiation in vitro.134
Zhang et al. reported synthetizing of PDA and poly(N-isopropylacrylamide) (PNIPAM) adhesive coating and a drug delivery system. The adhesive surface contains liposomes that adsorb hepatocytes, macrophages, and myoblasts to the films.135 PDA can be used for metal and metal oxide surfaces modification, and utilized to modify reduced graphene oxide (RGO) through dopamine self-polymerization. Guo et al. fabricated RGO adhesive sheet coated by PDA which was used as a metal ions reducing agent for the cell immobilization and also improvement of surface adhesion.136 Cheng et al. studied surface modification of graphene oxide (GO) sheet by dopamine in which the morphology was controllable with high capacity of drug loading. They used carbodiimide chemistry technique to graft dopamine onto heparin. Then the dopamine-grafted heparin was adhered to the RGO surface. The developed materials showed good biocompatibility, controlled 2-dimentional morphology and significant drug loading.137
Ge et al. used layer by layer (LbL) electrospinning method to fabricate dopamine based nanofibrous structure of PCL/gelatin and increase adipose cell adhesion in bone tissue engineering. The mussel inspired electrospun structure not only improved mechanical strength of the scaffold, but also increased cell attachment through enhancing surface hydrophilicity.138 Dopamine based coating on HA polymer and multilayer films by using LbL of chitosan and dopamine/HA was done by Neto and coworkers. Films presented cell viability, appropriate biocompatibility and high adhesion mechanics.139 Another research group used a solvent free and simple technique to fabricate a thin, flexible and strong polysaccharide film which has potential to be applied in biomedical fields. Catecholamine was used as a crosslinking agent and chitosan as polymer. The tensile strength of the film is approximately 10 MPa, which is higher than that of an A4 paper (3.5 MPa).140 Jiang et al. devised gelatin/PCL nanofibrous sheet modified with dopamine which was used in surgical wound closure. Dopamine was crosslinked to the gelatin backbone via amino group using photo initiating method. Final adhesive sheet demonstrated efficient suture-less surgical wound sealing in stomach.141 Poly(L-lysine) (PLL) was incorporated with PDA to prepare an adhesive film as a coating for endothelial cells support improvement. PLL provided highly integration with PDA because of high amount of amine functional groups, subsequently excellent interaction with endothelial cells and enzymatically biodegradation.142
Recently, Zieger et al. introduced catechol based surface modification with a new catechol derivatives family containing cyclic catechol oligomers to generate homogeneous layer with preserved topography. This dopamine based thin film had potential to prepare multi layered structure through covalent binding of catechol with various substrates such as Au, TiO2, and SiO2.143 To fix and stabilize bone fracture, Olofsson and colleagues reported fibrous adhesive patch using primers made of dopamine derivatives that consist of different allyl, thiol and methacrylamide groups. A thin primer layer was capable to improve the bone and adhesive interaction. On the other hand, these groups increased shear strength in adhesion. They resulted in non-toxic, remarkable adhesion and 0.3 MPa shear adhesive strength.144
To maximize the catechol agents' presence, catechol based nanoparticles have been used because of their high surface-volume ratio. Dopamine oxidative self-polymerization forms the nanoparticles. This procedure is similar to the melanin formation pathway, thus these catechol based nanoparticles have been named as melanin-like nanoparticles. Scognamiglio et al. fabricated an adhesive membrane coated145 with melanin-like nanoparticles for surgical wound closure. In this study, alginate/hyaluronan membrane was created via molding method145 and coated by dopamine.146 Han's research team fabricated tissue adhesive nanosheet using PDA. Catechol groups were oxidized and embedded within the sheets which contain free catechol functional groups, and acrylamide was allowed to interact with the sheets via free radical polymerization. They showed that the adhesives had good adhesive strengths of 120, 80.8, 80.7, and 28.5 kPa on glass, Ti, polyethylene (PE), and porcine skin, respectively. They demonstrated durable adhesiveness to both hydrophilic and hydrophobic substrates. They also provided repeatable adhesiveness onto skin over 20 cycles of peeling off. However the adhesive sheet did not adhere in a wet environment.147
The major problem of applying adhesives in a form of film or sheet and patch to the wound site is mass production. The fabrication methods are complicated in some cases and need specific devices and techniques. However, this form of adhesives is able to be used in high tension sites of body because of high mechanical strength.128,135,148
4. Evaluation of tissue adhesives
4.1 Macroscopic approaches
The tissue adhesives were usually characterized in terms of their mechanical strength, biocompatibility and biodegradability.Different methods have been used to evaluate the adhesive and cohesion strengths of the tissue adhesives using compression and tensile tests, lap shear and rheological analysis. Lap shear test can be conducted to evaluate the shear strength and modulus required to detach two materials of the same or different compositions adhered to each other by an adhesive. It can determine the adhesion strength at the interface in both wet and dry conditions. The type and thickness of the adhesives influence the lap shear strengths. In lap shear analyzes, the failure strain in tension for brittle adhesives ranged from 1.3% to 44% for the most ductile adhesives.100,149 Studies showed that the strength of lap-joint enhances when the bond line becomes thinner, thickness minimizes or in tough adhesives. The highest strength was obtained for bond lines in the range of 0.05 to 0.5 mm. The adhesive strength may also be affected by other parameters, such as the type of loading including shear, peel, or cleavage, the adherent elastic or plastic behavior, and the adhesive ductility or brittleness. For instance, the failure load enhanced as the bond line became thicker in peel joints of ductile adhesives due to the adhesive load distribution over a larger area.149 Dopamine incorporation in adhesive networks enhanced lap shear in wet condition. Stress–strain behavior in catechol-based tissue hydrogels showed up to 90% strain without rupture.93 Real-time photo crosslinking rheology can be carried out with a UV light source or rheometer to show viscoelastic property. The samples were exposed to UV light for 2 minute after the first minute of data collection. G′ and G′′ demonstrate value of storage and loss modulus of adhesive, respectively.118 For all structures, when the storage modulus values were higher than the loss modulus values, it indicated that the adhesive was crosslinked covalently.100 The importance of sacrificial bond within the bioadhesive network was that it can dissipated energy of fracture under mechanical loads.115 Universal Testing Machines (UTM) analysis has been used for measuring such mechanical properties as adhesion strength to the tissue, tensile and compressive strengths of adhesive materials. The primary and secondary geometry of adhesive species can be recorded before and after force loading, respectfully.91
To characterize biodegradability of tissue adhesives, in vitro and in vivo tests have been conducted. For degradation evaluation in vitro, the hydrogel is usually swollen in solution of PBS and sodium azide containing neutrophil elastase. After certain number of days, the degradation components in the solution can be evaluated. In vivo degradation characterization of samples can be conducted in mice or rats for a certain amount of time, then the samples be removed from the mice or rat for assessment. Depending on the type and application of the adhesives, the adhesive samples can be implanted into artery, skin or other tissues/organs.
4.2 Microscopic approaches
Microscopic assessments have been conducted to study chemical bonding, material surface morphology and roughness. Fourier transform infrared spectroscopy (FTIR) has been used to confirm the incorporation of amine/thiol groups into polymers. The DOPA dipping and dopamine crosslinking can be determined by specific peaks. FTIR peaks at the range of 1611 cm−1 suggest N–H functional group in DOPA.94 In PEG and dopamine hydrogel, the peaks of 1000–1150 cm−1, 2880 cm−1 and 1729 cm−1 assign to the ether, alkyl and carbonyl functional groups in PEG–dopamine chemical interaction, which is an indicating of crosslinking.95 1H nuclear magnetic resonance spectroscopy (1H NMR) and ultraviolet-visible (UV) spectrophotometer analysis have also been employed to characterize the structure. Interpolation of catechol to polymers is known by catechol concentration confirmed the degree of substitution of catechol.99 The chemical shift in 1H NMR diagram provides information about the molecule structure. In a NMR spectrum, a peak at the 6.41–6.67 ranges shows the benzene ring protons.94 In addition, a study proved catechol chemical bonding through 1H NMR characterization in which 6.6–6.8 ppm signal demonstrated crosslinking between catechol and polylysine hydrogel.98 In this study, UV analysis represented catechol group moiety UV absorbance at 280 nm.98 UV is also used for the quantitative study of transition metal ions and highly conjugated organic compounds such as DOPA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe3+ and DOPA
Fe3+ and DOPA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IO4− in different pH conditions.88,115 For an example, variation of pH changed the color of DOPA
IO4− in different pH conditions.88,115 For an example, variation of pH changed the color of DOPA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe3+ to the green, purple and pink which demonstrated mono-, bis-, and tris-complexes, respectively. Covalent gelation in DOPA
Fe3+ to the green, purple and pink which demonstrated mono-, bis-, and tris-complexes, respectively. Covalent gelation in DOPA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IO4− and noncovalent reaction in DOPA
IO4− and noncovalent reaction in DOPA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe3+ have been investigated by color changes using UV.115
Fe3+ have been investigated by color changes using UV.115
Surface chemistry of tissue adhesives can be characterized using X-ray photoelectron spectroscopy (XPS). The surface of tissue adhesives before and after modification can be investigated specifically in the experiments that dopamine and its derivatives are applied in the form of coatings or surface modification. The most significant feature of polydopamine is its ability to be coated onto almost any substrate, effectively serving as an adlayer to change the surface property of the coated substrate and allowing further functionalization. XPS characterization of the substrate surfaces after coating revealed the complete disappearance of original substrate signals.52
Atomic force microscopy (AFM) has been used to characterize the surface morphology of the adhesives films, sheets and patches.148 Higher surface roughness suggests enhanced surface area, thus more surface absorption.139 In nanofibrous structures, surface roughness is related to the available surface coated by polydopamine.138 In addition, the adhesive property of tissue adhesives can be evaluates by AFM force analysis method. The adhesive forces can be measured at nanoscale by allowing the contact between the AFM cantilever tip and the adhesive surface, followed by a forced separation of the tip from the surface upon retraction. The force required for separation can be used as an indicator for adhesive strength at the surface/interface.58,150
As to the characterization of biocompatibility, primary in vitro tests like the methlythiazoletetrazolium (MTT) assay, hemostasis analysis and histological analysis provide functionality and biocompatibility evaluations. The viability of cells exposed to the tissue adhesive samples determines cytotoxicity, with cell viability less than 70% being considered as non-biocompatible adhesives.94,151 In some studies, quantitative real-time polymerase chain reaction (qRT-PCR) analysis was done to exhibit gene expression that assist in understanding cytotoxicity and cell viability.91
Despite of the benefits and advantages of tissue adhesives discussed in this review, dopamine based tissue adhesives do show some limitations including low mechanical strength under loading in hard tissues repair,144 poor adhesion in highly wet environment in which co-closure with suture has been required.147 It is still a challenge to develop a perfect bioadhesive that can adhere to tissues and stop bleeding completely, be biocompatible, non-toxic and biodegradable, be cost effective and simple to use in order to attract surgeons for applications in complicated surgical situations. Furthermore, the synthetic techniques are complex for the majority of mussel inspired tissue adhesives.152 Several strategies have been done in recent years to face the current demands in tissue gluing; however, more characterizations and different side effects assessment in short or long terms use are needed.144
5. Conclusion
The use of traditional methods for wound closure like sutures, staples and flaps has certain disadvantages, particularly for delicate and vulnerable tissues. Tissue adhesives have been identified as a promising alternative for these purposes. They can also be used in combination with sutures in highly wet environment when the tissue glue by itself cannot hold tissues together. To tackle the problem of wet adhesion, dopamine-based adhesives in recently years have attracted extensive interests as a promising material to improve wet adhesive performance. Tissue adhesives also have applications in hard tissue gluing, drug or cell delivery systems and cell culture. Their potential in improvement of cell adhesion onto the surfaces is undeniable.This review summarizes the recent advances in dopamine-based tissue adhesives that have been employed in biomedical applications. Different formats of dopamine-based adhesives, including glues, hydrogels and films used in the development of tissue adhesives with robust adhesiveness and excellent mechanical properties in biological and physiological environments, have been extensively explained. Tissue adhesives have potential to be grafted or added to different types of polymers, synthetic or natural, in order to provide various functionalities. In addition, tissue adhesives evaluation methods from both microscopic and macroscopic perspectives are discussed.
The biomimetic tissue adhesives are a rapidly expanding research field. Future work on the dopamine-based adhesives is expected to lead to the development of adhesive materials with excellent tissue compatibility, predictable biodegradability, appropriate mechanical properties and high wet adhesive capacities. Extra features such as self-healing, environmental responsive and loading of bioactive molecules, can be introduced to the adhesive materials to enhance their performance for different biomedical applications. The mechanisms of the outstanding adhesive capacity of dopamine or DOPA based materials have not been fully understand and therefore need further investigation. Other chemical, physical or surface characteristics of adhesive materials that may have an impact on their adhesive capacities are also worth extra research efforts.
Conflicts of interest
We confirm that none of the authors have any competing interests in the manuscript.List of abbreviations
| ECM | Extracellular matrix |
| AFM | Atomic force microscopy |
| ALP | Alkaline phosphatase |
| APE | Pentaerythritol triallyl ether |
| AspAm | Aspartamide |
| CA | Citric acid |
| cPEG | Catechol-terminated PEG |
| DAm | Dopamine acrylamide |
| DDS | Drug delivery system |
| DMA | Dopamine methacrylate |
| DOPA | 3,4-Dihydroxyphenylalanine |
| ECM | Extra cellular matrix |
| FDA | Food and drug administration |
| FTIR | Fourier transform infrared spectroscopy |
| GRF | Gelatin–resorcinol–formaldehyde |
| GRFG | Gelatin–resorcinol–formaldehyde–glutaraldehyde |
| HA | Hyaluronic acid |
| HCA | Hydrocaffeic acid |
| HEAA | 2-Hydroxyethyl acrylamide |
| JKR | Johnson–Kendall–Roberts |
| LbL | Layer by layer |
| MEA | Methacryloyl chloride |
| Mefp's | Mytilus edulis foot proteins |
| MTT | Methlythiazoletetrazolium |
| 1H NMR | 1H nuclear magnetic resonance spectroscopy |
| PAAm | Polyacrylamide |
| PAE | Poly(β-amino ester) |
| PCL | Polycaprolactone |
| PDA | Polydopamine-co-acrylate |
| PEG | Polyethylene glycol |
| PEGDM | Dopamine-modified poly(ethylene glycol) |
| PEI | Poly(ethylene imine) |
| PEO | Poly(ethylene oxide) |
| PEU | Poly(ester urea) |
| PHS | Poly(parahydroxylstyrene) |
| PNIPAM | Poly(N-isopropylacrylamide) |
| HD | Hexamethylenediamine |
| PL | ε-Polylysine |
| PLL | Poly(L-lysine) |
| PMA | Poly(methacrylic acid) |
| PPO | Poly(propylene oxide) |
| PU | Polyurethanes |
| HEPES | 4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid |
| PHS | Poly(parahydroxylstyrene) |
| PLCL | Poly(lactide-co-caprolactone) |
| RGO | Reduced graphene oxide |
| GO | Graphene oxide |
| PE | Polyethylene |
| QCM | Quartz crystal microbalance |
| qRT-PCR | Quantitative real-time polymerase chain reaction |
| RAFT | Reversible addition fragmentation chain transfer polymerization |
| RGD | Arginine_glycine_aspartic acid |
| PSI | Polysuccinimide |
| SFA | Surface forces apparatus |
| UTM | Universal testing machine |
| UV | Ultraviolet-visible |
| XPS | X-ray photoelectron spectroscopy |
Acknowledgements
The authors want to acknowledge the support from NSERC Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery grant. Authors would like to thank Mohammadreza Kazemian for his contribution in figures preparation.References
- S. L. Basha, et al., Randomized controlled trial of wound complication rates of subcuticular suture vs staples for skin closure at cesarean delivery, Am. J. Obstet. Gynecol., 2010, 203(3), 285 CrossRef PubMed.
- R. T. Forsch, Essentials of skin laceration repair, Am. Fam. Physician, 2008, 78(8), 945–951 Search PubMed.
- P. Heger, et al., Aktuelle Studienlage zum Bauchdeckenverschluss, Der Chirurg, 2016, 87(9), 737–743 CrossRef CAS PubMed.
- A. Islam and A. Ehsan, Comparison of suture material and technique of closure of subcutaneous fat and skin in caesarean section, N. Am. J. Med. Sci., 2011, 3(2), 85 CrossRef PubMed.
- P. Sergeant, et al., Needle-to-suture ratio, as well as suture material, impacts needle-hole bleeding in vascular anastomoses, Interact. Cardiovasc. Thorac. Surg., 2016, 22(6), 813–816 CrossRef PubMed.
- N. Xu, et al., Light-activated sealing of skin wounds, Lasers Surg. Med., 2015, 47(1), 17–29 CrossRef PubMed.
- J. Hochberg, K. M. Meyer and M. D. Marion, Suture choice and other methods of skin closure, Surg. Clin. North Am., 2009, 89(3), 627–641 CrossRef PubMed.
- A. Duarte, et al., Surgical adhesives: systematic review of the main types and development forecast, Prog. Polym. Sci., 2012, 37(8), 1031–1050 CrossRef CAS.
- A. Lauto, D. Mawad and L. J. R. Foster, Adhesive biomaterials for tissue reconstruction, J. Chem. Technol. Biotechnol., 2008, 83(4), 464–472 CrossRef CAS.
- P. Coulthard, et al., Tissue adhesives for closure of surgical incisions, The Cochrane Library, 2010 Search PubMed.
- C. Yag-Howard, Sutures, needles, and tissue adhesives: a review for dermatologic surgery, Dermatol. Surg., 2014, 40, S3–S15 CrossRef CAS PubMed.
- A. L. Tajirian and D. J. Goldberg, A review of sutures and other skin closure materials, J. Cosmet. Laser Ther., 2010, 12(6), 296–302 CrossRef PubMed.
- M. Rahimnejad, S. Derakhshanfar and W. Zhong, Biomaterials and tissue engineering for scar management in wound care, Burns & Trauma, 2017, 5(1), 4 Search PubMed.
- S. Delibegović, et al., Tissue reaction to absorbable endoloop, nonabsorbable titanium staples, and polymer Hem-o-lok clip after laparoscopic appendectomy, J. Soc. Laparoendosc. Surg., 2011, 15(1), 70 CrossRef PubMed.
- W. D. Spotnitz and S. G. Burks, Hemostats, sealants, and adhesives II: update as well as how and when to use the components of the surgical toolbox, Clin. Appl. Thromb./Hemostasis, 2010, 497–514 CrossRef CAS PubMed.
- K. Yoshihara, et al., Nano-controlled molecular interaction at adhesive interfaces for hard tissue reconstruction, Acta Biomater., 2010, 6(9), 3573–3582 CrossRef CAS PubMed.
- P. Spencer, et al., Adhesive/dentin interface: the weak link in the composite restoration, Ann. Biomed. Eng., 2010, 38(6), 1989–2003 CrossRef PubMed.
- M. Cardoso, et al., Current aspects on bonding effectiveness and stability in adhesive dentistry, Aust. Dent. J., 2011, 56(s1), 31–44 CrossRef PubMed.
- A. Nordberg, et al., Highly adhesive phenolic compounds as interfacial primers for bone fracture fixations, ACS Appl. Mater. Interfaces, 2010, 2(3), 654–657 CAS.
- H.-S. Jeong, et al., Use of fibrin glue for open comminuted nasal bone fractures, J. Craniofac. Surg., 2010, 21(1), 75–78 CrossRef PubMed.
- J. Simson, et al., An orthopedic tissue adhesive for targeted delivery of intraoperative biologics, J. Orthop. Res., 2013, 31(3), 392–400 CrossRef CAS PubMed.
- J. H. Ryu, et al., Catechol-functionalized chitosan/pluronic hydrogels for tissue adhesives and hemostatic materials, Biomacromolecules, 2011, 12(7), 2653–2659 CrossRef CAS PubMed.
- N. Lang, et al., A blood-resistant surgical glue for minimally invasive repair of vessels and heart defects, Sci. Transl. Med., 2014, 6(218), 218ra6 CrossRef PubMed.
- H. C. Park, et al., Tissue adhesives in ocular surgery, Expert Rev. Ophthalmol., 2014, 631–655 Search PubMed.
- A. D. Mackeen, V. Berghella, and M. L. Larsen, Techniques and materials for skin closure in caesarean section, The Cochrane Library, 2012 Search PubMed.
- N. Annabi, et al., Surgical materials: current challenges and nano-enabled solutions, Nano Today, 2014, 9(5), 574–589 CrossRef CAS PubMed.
- B. Sharma, et al., Human cartilage repair with a photoreactive adhesive-hydrogel composite, Sci. Transl. Med., 2013, 5(167), 167ra6 Search PubMed.
- S. Vyas, S. Kamdar and P. Vyas, Tissue adhesives in ophthalmology, J. Clin. Ophthalmol. Res., 2013, 1(2), 107–112 CrossRef.
- W. Chen, et al., A mussel-inspired poly (γ-glutamic acid) tissue adhesive with high wet strength for wound closure, J. Mater. Chem. B, 2017, 5(28), 5668–5678 RSC.
- K. A. Vakalopoulos, et al., Tissue adhesives in gastrointestinal anastomosis: a systematic review, J. Surg. Res., 2013, 180(2), 290–300 CrossRef CAS PubMed.
- A. Sosnik, J. das Neves and B. Sarmento, Mucoadhesive polymers in the design of nano-drug delivery systems for administration by non-parenteral routes: a review, Prog. Polym. Sci., 2014, 39(12), 2030–2075 CrossRef CAS.
- P. P. Spicer and A. G. Mikos, Fibrin glue as a drug delivery system, J. Controlled Release, 2010, 148(1), 49–55 CrossRef CAS PubMed.
- S. H. Ku, J. S. Lee and C. B. Park, Spatial control of cell adhesion and patterning through mussel-inspired surface modification by polydopamine, Langmuir, 2010, 26(19), 15104–15108 CrossRef CAS PubMed.
- K. Sun, et al., Using self-polymerized dopamine to modify the antifouling property of oligo (ethylene glycol) self-assembled monolayers and its application in cell patterning, Langmuir, 2011, 27(10), 5709–5712 CrossRef CAS PubMed.
- H. J. Kim, et al., Mussel adhesion-employed water-immiscible fluid bioadhesive for urinary fistula sealing, Biomaterials, 2015, 72, 104–111 CrossRef CAS PubMed.
- Y. Ai, et al., Study on the synthesis and properties of mussel mimetic poly (ethylene glycol) bioadhesive, J. Photochem. Photobiol., B, 2013, 120, 183–190 CrossRef CAS PubMed.
- Y. Akdogan, et al., Intrinsic Surface-Drying Properties of Bioadhesive Proteins, Angew. Chem., 2014, 126(42), 11435–11438 CrossRef.
- B.-H. Choi, et al., Highly purified mussel adhesive protein to secure biosafety for in vivo applications, Microb. Cell Fact., 2014, 13(1), 1 CrossRef PubMed.
- N. Hiraishi, et al., Mussel mimetic, bioadhesive polymers from plant derived materials, J. Investig. Clin. Dent., 2015, 6(1), 59–62 CrossRef PubMed.
- N. Bandara, H. Zeng and J. Wu, Marine mussel adhesion: biochemistry, mechanisms, and biomimetics, J. Adhes. Sci. Technol., 2013, 27(18–19), 2139–2162 CrossRef CAS.
- S. Ornes, Mussels' sticky feet lead to applications, Proc. Natl. Acad. Sci. U. S. A., 2013, 110(42), 16697–16699 CrossRef CAS PubMed.
- K. A. Bielefeld, S. Amini-Nik and B. A. Alman, Cutaneous wound healing: recruiting developmental pathways for regeneration, Cell. Mol. Life Sci., 2013, 70(12), 2059–2081 CrossRef CAS PubMed.
- M. C. Bloemen, et al., Prevention and curative management of hypertrophic scar formation, Burns, 2009, 35(4), 463–475 CrossRef PubMed.
- J. Boateng and O. Catanzano, Advanced therapeutic dressings for effective wound healing—A Review, J. Pharm. Sci., 2015, 104(11), 3653–3680 CrossRef CAS PubMed.
- C. Profyris, C. Tziotzios and I. Do Vale, Cutaneous scarring: pathophysiology, molecular mechanisms, and scar reduction therapeutics: part I. The molecular basis of scar formation, J. Am. Acad. Dermatol., 2012, 66(1), 1–10 CrossRef CAS PubMed.
- C. Tziotzios, C. Profyris and J. Sterling, Cutaneous scarring: pathophysiology, molecular mechanisms, and scar reduction therapeutics: part II. Strategies to reduce scar formation after dermatologic procedures, J. Am. Acad. Dermatol., 2012, 66(1), 13–24 CrossRef CAS PubMed.
- M. Tschoi, E. A. Hoy and M. S. Granick, Skin flaps, Surg. Clin. North Am., 2009, 89(3), 643–658 CrossRef PubMed.
- M. Matsuda, M. Inoue and T. Taguchi, Enhanced bonding strength of a novel tissue adhesive consisting of cholesteryl group-modified gelatin and disuccinimidyl tartarate, J. Bioact. Compat. Polym., 2012, 27(1), 31–44 CrossRef CAS.
- E. Y. Jeon, et al., Rapidly light-activated surgical protein glue inspired by mussel adhesion and insect structural crosslinking, Biomaterials, 2015, 67, 11–19 CrossRef CAS PubMed.
- A. Chambers and M. Scarci, Is skin closure with cyanoacrylate glue effective for the prevention of sternal wound infections?, Interact. Cardiovasc. Thorac. Surg., 2010, 10(5), 793–796 CrossRef PubMed.
- J. W. Gooch, Biocompatible polymeric materials and tourniquets for wounds, Springer Science & Business Media, 2010 Search PubMed.
- H. Lee, et al., Mussel-inspired surface chemistry for multifunctional coatings, Science, 2007, 318(5849), 426–430 CrossRef CAS PubMed.
- B. P. Lee, et al., Mussel-inspired adhesives and coatings, Annu. Rev. Mater. Res., 2011, 41, 99 CrossRef CAS PubMed.
- S. C. Nicklisch and J. H. Waite, Mini-review: The role of redox in Dopa-mediated marine adhesion, Biofouling, 2012, 28(8), 865–877 CrossRef CAS PubMed.
- J. Yu, et al., Mussel protein adhesion depends on interprotein thiol-mediated redox modulation, Nat. Chem. Biol., 2011, 7(9), 588–590 CrossRef CAS PubMed.
- K. M. Gray, et al., Biomimetic fabrication of information-rich phenolic-chitosan films, Soft Matter, 2011, 7(20), 9601–9615 RSC.
- C. Ghobril and M. Grinstaff, The chemistry and engineering of polymeric hydrogel adhesives for wound closure: a tutorial, Chem. Soc. Rev., 2015, 44(7), 1820–1835 RSC.
- H. Lee, B. P. Lee and P. B. Messersmith, A reversible wet/dry adhesive inspired by mussels and geckos, Nature, 2007, 448(7151), 338–341 CrossRef CAS PubMed.
- C. R. Matos-Pérez, J. D. White and J. J. Wilker, Polymer composition and substrate influences on the adhesive bonding of a biomimetic, cross-linking polymer, J. Am. Chem. Soc., 2012, 134(22), 9498–9505 CrossRef PubMed.
- S. M. Kang, et al., One-step modification of superhydrophobic surfaces by a mussel-inspired polymer coating, Angew. Chem., Int. Ed., 2010, 49(49), 9401–9404 CrossRef CAS PubMed.
- S. Moulay, Dopa/catechol-tethered polymers: Bioadhesives and biomimetic adhesive materials, Polym. Rev., 2014, 54(3), 436–513 CrossRef CAS.
- M. Mehdizadeh and J. Yang, Design strategies and applications of tissue bioadhesives, Macromol. Biosci., 2013, 13(3), 271–288 CrossRef CAS PubMed.
- J. H. Waite, Mussel adhesion - essential footwork, J. Exp. Biol., 2017, 220(4), 517–530 CrossRef PubMed.
- B. Krishnamoorthy, et al., Randomized prospective study comparing conventional subcuticular skin closure with Dermabond skin glue after saphenous vein harvesting, Ann. Thorac. Surg., 2009, 88(5), 1445–1449 CrossRef PubMed.
- A. Dragu, et al., Foreign body reaction after usage of tissue adhesives for skin closure: a case report and review of the literature, Arch. Orthop. Trauma Surg., 2009, 129(2), 167–169 CrossRef PubMed.
- Y. S. Choi, et al., Recombinant mussel adhesive protein fp-5 (MAP fp-5) as a bulk bioadhesive and surface coating material, Biofouling, 2011, 27(7), 729–737 CrossRef CAS PubMed.
- T. Wang, J. Nie and D. Yang, Dextran and gelatin based photocrosslinkable tissue adhesive, Carbohydr. Polym., 2012, 90(4), 1428–1436 CrossRef CAS PubMed.
- L. Vollenweider, et al., Biomimetic adhesive coatings for soft tissue repair, 15th Int Coat Sci Technol Sym, 2010 Search PubMed.
- H. Shao and R. J. Stewart, Biomimetic underwater adhesives with environmentally triggered setting mechanisms, Adv. Mater., 2010, 22(6), 729–733 CrossRef CAS PubMed.
- H. Zhang, et al., A biomimetic hyperbranched poly (amino ester)-based nanocomposite as a tunable bone adhesive for sternal closure, J. Mater. Chem. B, 2014, 2(26), 4067–4071 RSC.
- H. Zhang, et al., Mussel-inspired hyperbranched poly (amino ester) polymer as strong wet tissue adhesive, Biomaterials, 2014, 35(2), 711–719 CrossRef CAS PubMed.
- J. Zhou, et al., Adhesion properties of catechol-based biodegradable amino acid-based poly (ester urea) copolymers inspired from mussel proteins, Biomacromolecules, 2014, 16(1), 266–274 CrossRef PubMed.
- B. K. Ahn, et al., High-performance mussel-inspired adhesives of reduced complexity, Nat. Commun., 2015, 6, 8663 CrossRef PubMed.
- S.-B. Lee, et al., Catechol-Functionalized Synthetic Polymer as a Dental Adhesive to Contaminated Dentin Surface for a Composite Restoration, Biomacromolecules, 2015, 16(8), 2265–2275 CrossRef CAS PubMed.
- Y. Ji, et al., Mussel-inspired soft-tissue adhesive based on poly (diol citrate) with catechol functionality, J. Mater. Sci.: Mater. Med., 2016, 27(2), 1–9 CrossRef PubMed.
- S. Hong, et al., Supramolecular Metallo-Bioadhesive for Minimally Invasive Use, Adv. Mater., 2016, 28(39), 8675–8680 CrossRef CAS PubMed.
- A. Kawabata, et al., A foreign body granuloma after the usage of polyglycolic acid mesh and fibrin glue for dural repair. A case report, J. Orthop. Sci., 2015, 22(2), 371–374 CrossRef PubMed.
- L. Yu and J. Ding, Injectable hydrogels as unique biomedical materials, Chem. Soc. Rev., 2008, 37(8), 1473–1481 RSC.
- C. T. Huynh, M. K. Nguyen and D. S. Lee, Injectable block copolymer hydrogels: achievements and future challenges for biomedical applications, Macromolecules, 2011, 44(17), 6629–6636 CrossRef CAS.
- M. D. Hager, et al., Self-Healing Materials, Adv. Mater., 2010, 22(47), 5424–5430 CrossRef CAS PubMed.
- L. Moroni and J. H. Elisseeff, Biomaterials engineered for integration, Mater. Today, 2008, 11(5), 44–51 CrossRef CAS.
- L. P. Bré, et al., Taking tissue adhesives to the future: from traditional synthetic to new biomimetic approaches, Biomater. Sci., 2013, 1(3), 239–253 RSC.
- J. I. Paez, et al., Gauging and tuning cross-linking kinetics of catechol-PEG adhesives via catecholamine functionalization, Biomacromolecules, 2015, 16(12), 3811–3818 CrossRef CAS PubMed.
- J. Sedó, et al., Catechol-Based Biomimetic Functional Materials, Adv. Mater., 2013, 25(5), 653–701 CrossRef PubMed.
- Y. Yang, et al., A biocompatible and functional adhesive amine-rich coating based on dopamine polymerization, J. Mater. Chem. B, 2015, 3(1), 72–81 RSC.
- J. H. An, et al., Surface modification using bio-inspired adhesive polymers based on polyaspartamide derivatives, Polym. Int., 2011, 60(11), 1581–1586 CrossRef CAS.
- J. Xu, et al., Mollusk glue inspired mucoadhesives for biomedical applications, Langmuir, 2012, 28(39), 14010–14017 CrossRef CAS PubMed.
- Y. C. Choi, et al., Human gelatin tissue-adhesive hydrogels prepared by enzyme-mediated biosynthesis of DOPA and Fe 3+ ion crosslinking, J. Mater. Chem. B, 2014, 2(2), 201–209 RSC.
- W. Zhu, et al., A mussel-inspired double-crosslink tissue adhesive on rat mastectomy model: seroma prevention and in vivo biocompatibility, J. Surg. Res., 2017, 215, 173–182 CrossRef CAS PubMed.
- C. Fan, et al., A mussel-inspired double-crosslinked tissue adhesive intended for internal medical use, Acta Biomater., 2016, 33, 51–63 CrossRef CAS PubMed.
- J. Shin, et al., Tissue Adhesive Catechol-Modified Hyaluronic Acid Hydrogel for Effective, Minimally Invasive Cell Therapy, Adv. Funct. Mater., 2015, 25(25), 3814–3824 CrossRef CAS.
- C. E. Brubaker, et al., Biological performance of mussel-inspired adhesive in extrahepatic islet transplantation, Biomaterials, 2010, 31(3), 420–427 CrossRef CAS PubMed.
- D. G. Barrett, G. G. Bushnell and P. B. Messersmith, Mechanically robust, negative-swelling, mussel-inspired tissue adhesives, Adv. Healthcare Mater., 2013, 2(5), 745–755 CrossRef CAS PubMed.
- C. Li, et al., Photocrosslinkable bioadhesive based on dextran and PEG derivatives, Mater. Sci. Eng. C, 2014, 35, 300–306 CrossRef CAS PubMed.
- Y. Liu, et al., Injectable dopamine-modified poly (ethylene glycol) nanocomposite hydrogel with enhanced adhesive property and bioactivity, ACS Appl. Mater. Interfaces, 2014, 6(19), 16982–16992 CAS.
- S. Skelton, et al., Biomimetic adhesive containing nanocomposite hydrogel with enhanced materials properties, Soft Matter, 2013, 9(14), 3825–3833 RSC.
- C. L. Jenkins, H. J. Meredith and J. J. Wilker, Molecular weight effects upon the adhesive bonding of a mussel mimetic polymer, ACS Appl. Mater. Interfaces, 2013, 5(11), 5091–5096 CAS.
- Y. Zhou, et al., Rapid gelling chitosan/polylysine hydrogel with enhanced bulk cohesive and interfacial adhesive force: mimicking features of epineurial matrix for peripheral nerve anastomosis, Biomacromolecules, 2016, 17(2), 622–630 CrossRef CAS PubMed.
- K. A. Burke, D. C. Roberts and D. L. Kaplan, Silk Fibroin Aqueous-Based Adhesives Inspired by Mussel Adhesive Proteins, Biomacromolecules, 2015, 17(1), 237–245 CrossRef PubMed.
- Y. Li, et al., Gelatin Microgel Incorporated Poly (Ethylene Glycol)-based Bioadhesive with Enhanced Adhesive Property and Bioactivity, ACS Appl. Mater. Interfaces, 2016, 8(19), 11980–11989 CAS.
- J. Guo, et al., Synthesis and characterization of anti-bacterial and anti-fungal citrate-based mussel-inspired bioadhesives, Biomaterials, 2016, 85, 204–217 CrossRef CAS PubMed.
- J. Guo, et al., Click chemistry improved wet adhesion strength of mussel-inspired citrate-based antimicrobial bioadhesives, Biomaterials, 2017, 112, 275–286 CrossRef CAS PubMed.
- Y. Ji, et al., Mussel-inspired soft-tissue adhesive based on poly (diol citrate) with catechol functionality, J. Mater. Sci.: Mater. Med., 2016, 27(2), 30 CrossRef PubMed.
- B. Yang, et al., Facilely prepared inexpensive and biocompatible self-healing hydrogel: a new injectable cell therapy carrier, Polym. Chem., 2012, 3(12), 3235–3238 RSC.
- B. K. Ahn, et al., Surface-initiated self-healing of polymers in aqueous media, Nat. Mater., 2014, 13(9), 867–872 CrossRef CAS PubMed.
- N. Holten-Andersen, et al., pH-induced metal-ligand cross-links inspired by mussel yield self-healing polymer networks with near-covalent elastic moduli, Proc. Natl. Acad. Sci. U. S. A., 2011, 108(7), 2651–2655 CrossRef CAS PubMed.
- M. Krogsgaard, et al., Self-healing mussel-inspired multi-pH-responsive hydrogels, Biomacromolecules, 2013, 14(2), 297–301 CrossRef CAS PubMed.
- L. He, et al., pH responsive self-healing hydrogels formed by boronate–catechol complexation, Chem. Commun., 2011, 47(26), 7497–7499 RSC.
- M. Vatankhah-Varnoosfaderani, et al., Rapid self-healing and triple stimuli responsiveness of a supramolecular polymer gel based on boron–catechol interactions in a novel water-soluble mussel-inspired copolymer, Polym. Chem., 2014, 5(2), 512–523 RSC.
- M. Shan, et al., A pH, glucose, and dopamine triple-responsive, self-healable adhesive hydrogel formed by phenylborate–catechol complexation, Polym. Chem., 2017, 8(19), 2997–3005 RSC.
- S. Hong, et al., Hyaluronic Acid Catechol: A Biopolymer Exhibiting a pH-Dependent Adhesive or Cohesive Property for Human Neural Stem Cell Engineering, Adv. Funct. Mater., 2013, 23(14), 1774–1780 CrossRef CAS.
- P. Sun, et al., Facile Preparation of Mussel-Inspired Polyurethane Hydrogel and Its Rapid Curing Behavior, ACS Appl. Mater. Interfaces, 2014, 6(15), 12495–12504 CAS.
- M. Cencer, et al., Effect of pH on the rate of curing and bioadhesive properties of dopamine functionalized poly (ethylene glycol) hydrogels, Biomacromolecules, 2014, 15(8), 2861–2869 CrossRef CAS PubMed.
- M. Cencer, et al., Effect of nitro-functionalization on the cross-linking and bioadhesion of biomimetic adhesive moiety, Biomacromolecules, 2014, 16(1), 404–410 CrossRef PubMed.
- B. J. Kim, et al., Mussel-mimetic protein-based adhesive hydrogel, Biomacromolecules, 2014, 15(5), 1579–1585 CrossRef CAS PubMed.
- B. Yang, et al., In Vivo Residue-Specific Dopa-Incorporated Engineered Mussel Bioglue with Enhanced Adhesion and Water Resistance, Angew. Chem., Int. Ed., 2014, 53(49), 13360–13364 CrossRef CAS PubMed.
- Y. Lee, et al., Thermo-sensitive, injectable, and tissue adhesive sol–gel transition hyaluronic acid/pluronic composite hydrogels prepared from bio-inspired catechol-thiol reaction, Soft Matter, 2010, 6(5), 977–983 RSC.
- H. Zhang, et al., On-demand and negative-thermo-swelling tissue adhesive based on highly branched ambivalent PEG–catechol copolymers, J. Mater. Chem. B, 2015, 3(31), 6420–6428 RSC.
- B. Laulicht, et al., Bioinspired Bioadhesive Polymers: Dopa-Modified Poly (acrylic acid) Derivatives, Macromol. Biosci., 2012, 12(11), 1555–1565 CrossRef CAS PubMed.
- C. J. Kastrup, et al., Painting blood vessels and atherosclerotic plaques with an adhesive drug depot, Proc. Natl. Acad. Sci. U. S. A., 2012, 109(52), 21444–21449 CrossRef CAS PubMed.
- D. E. Fullenkamp, et al., Mussel-inspired silver-releasing antibacterial hydrogels, Biomaterials, 2012, 33(15), 3783–3791 CrossRef CAS PubMed.
- S. Lim, et al., Bioadhesive Nanoaggregates Based on Polyaspartamide-g-C18/DOPA for Wound Healing, Biomacromolecules, 2017, 18(8), 2402–2409 CrossRef CAS PubMed.
- C. Gong, et al., Injectable dopamine-modified poly (α, β-aspartic acid) nanocomposite hydrogel as bioadhesive drug delivery system, J. Biomed. Mater. Res., Part A, 2017, 105(4), 1000–1008 CrossRef CAS PubMed.
- L. S. M. Teixeira, et al., Enzyme-catalyzed crosslinkable hydrogels: emerging strategies for tissue engineering, Biomaterials, 2012, 33(5), 1281–1290 CrossRef PubMed.
- C. E. Brubaker and P. B. Messersmith, Enzymatically degradable mussel-inspired adhesive hydrogel, Biomacromolecules, 2011, 12(12), 4326–4334 CrossRef CAS PubMed.
- B. P. Lee and S. Konst, Novel hydrogel actuator inspired by reversible mussel adhesive protein chemistry, Adv. Mater., 2014, 26(21), 3415–3419 CrossRef CAS PubMed.
- A. B. South and L. A. Lyon, Autonomic Self-Healing of Hydrogel Thin Films, Angew. Chem., 2010, 122(4), 779–783 CrossRef.
- W. Liu, S. Thomopoulos and Y. Xia, Electrospun nanofibers for regenerative medicine, Adv. Healthcare Mater., 2012, 1(1), 10–25 CrossRef CAS PubMed.
- H. Zeng, et al., Strong reversible Fe3+-mediated bridging between dopa-containing protein films in water, Proc. Natl. Acad. Sci. U. S. A., 2010, 107(29), 12850–12853 CrossRef CAS PubMed.
- J. L. Murphy, et al., Adhesive performance of biomimetic adhesive-coated biologic scaffolds, Biomacromolecules, 2010, 11(11), 2976–2984 CrossRef CAS PubMed.
- B. J. Sparks, et al., Mussel-inspired thiol–ene polymer networks: Influencing network properties and adhesion with catechol functionality, Chem. Mater., 2012, 24(18), 3633–3642 CrossRef CAS.
- K. Kim, et al., Bio-inspired catechol conjugation converts water-insoluble chitosan into a highly water-soluble, adhesive chitosan derivative for hydrogels and LbL assembly, Biomater. Sci., 2013, 1(7), 783–790 RSC.
- D. X. Oh and D. S. Hwang, A biomimetic chitosan composite with improved mechanical properties in wet conditions, Biotechnol. Prog., 2013, 29(2), 505–512 CrossRef CAS PubMed.
- Y. M. Shin, et al., Bio-inspired Immobilization of Cell-Adhesive Ligands on Electrospun Nanofibrous Patches for Cell Delivery, Macromol. Mater. Eng., 2013, 298(5), 555–564 CrossRef CAS.
- Y. Zhang, et al., Highly-branched poly (N-isopropylacrylamide) as a component in poly (dopamine) films, J. Phys. Chem. B, 2013, 117(36), 10504–10512 CrossRef CAS PubMed.
- L. Guo, et al., A mussel-inspired polydopamine coating as a versatile platform for the in situ synthesis of graphene-based nanocomposites, Nanoscale, 2012, 4(19), 5864–5867 RSC.
- C. Cheng, et al., General and biomimetic approach to biopolymer-functionalized graphene oxide nanosheet through adhesive dopamine, Biomacromolecules, 2012, 13(12), 4236–4246 CrossRef CAS PubMed.
- L. Ge, et al., Polydopamine-coated paper-stack nanofibrous membranes enhancing adipose stem cells' adhesion and osteogenic differentiation, J. Mater. Chem. B, 2014, 2(40), 6917–6923 RSC.
- A. I. Neto, et al., Nanostructured Polymeric Coatings Based on Chitosan and Dopamine-Modified Hyaluronic Acid for Biomedical Applications, Small, 2014, 10(12), 2459–2469 CrossRef CAS PubMed.
- J. H. Ryu, et al., Bio-Inspired, Water-Soluble to Insoluble Self-Conversion for Flexible, Biocompatible, Transparent, Catecholamine Polysaccharide Thin Films, Adv. Funct. Mater., 2014, 24(48), 7709–7716 CrossRef CAS.
- J. Jiang, et al., Mussel-inspired nanofibrous sheet for suture-less stomach incision surgery, Chem. Commun., 2015, 51(41), 8695–8698 RSC.
- Y. Zhang, et al., Mixed poly (dopamine)/poly (l-lysine)(composite) coatings: from assembly to interaction with endothelial cells, Biomater. Sci., 2015, 3(8), 1188–1196 RSC.
- M. M. Zieger, et al., Ultrathin Monomolecular Films and Robust Assemblies Based on Cyclic Catechols, Langmuir, 2017, 33(3), 670–679 CrossRef CAS PubMed.
- K. Olofsson, et al., Activated dopamine derivatives as primers for adhesive-patch fixation of bone fractures, RSC Adv., 2016, 6(31), 26398–26405 RSC.
- F. Scognamiglio, et al., Enhanced bioadhesivity of dopamine-functionalized polysaccharidic membranes for general surgery applications, Acta Biomater., 2016, 44, 232–242 CrossRef CAS PubMed.
- F. Scognamiglio, et al., Adhesive coatings based on melanin-like nanoparticles for surgical membranes, Colloids Surf., B, 2017, 155, 553–559 CrossRef CAS PubMed.
- L. Han, et al., Mussel-Inspired Adhesive and Tough Hydrogel Based on Nanoclay Confined Dopamine Polymerization, ACS Nano, 2017, 11(3), 2561–2574 CrossRef CAS PubMed.
- J. Longo, et al., Stable Bioactive Enzyme-Containing Multilayer Films Based on Covalent Cross-Linking from Mussel-Inspired Adhesives, Langmuir, 2015, 31(45), 12447–12454 CrossRef CAS PubMed.
- H. Yuk, et al., Tough bonding of hydrogels to diverse non-porous surfaces, Nat. Mater., 2016, 15(2), 190–196 CrossRef CAS PubMed.
- M. Xing, et al., Adhesion force studies of nanofibers and nanoparticles, Langmuir, 2010, 26(14), 11809–11814 CrossRef CAS PubMed.
- M. Mehdizadeh, et al., Injectable citrate-based mussel-inspired tissue bioadhesives with high wet strength for sutureless wound closure, Biomaterials, 2012, 33(32), 7972–7983 CrossRef CAS PubMed.
- P. Kord Forooshani and B. P. Lee, Recent approaches in designing bioadhesive materials inspired by mussel adhesive protein, J. Polym. Sci., Part A: Polym. Chem., 2016, 55(1), 9–33 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2017 |