Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Lights on! A significant photoenhancement effect on ATRP by ambient laboratory light†
Tao
Zhang
,
Dan
Gieseler
and
Rainer
Jordan
*
Chair of Macromolecular Chemistry, School of Science, Technische Universität Dresden, Mommsenstr. 4, 01069 Dresden, Germany. E-mail: Rainer.Jordan@tu-dresden.de
First published on 7th December 2015
Abstract
Recently we reported on photoinduced ATRP using a household fluorescent lamp as the light source (T. Zhang, et al., Polym. Chem., 2014, 5, 4790). Results implied that typical laboratory light might have a considerable impact on the ATRP reaction as fluorescent lamps are commonly used for ceiling and fume hood illumination. Here, we show the influence of ambient laboratory light on AGET, ARGET, and classical ATRP reactions. Except for ARGET ATRP, for all other ATRP types a significant photoenhancement effect by light originating only from fluorescent lamps was observed. A standard fume hood illumination caused the strongest influence.
Since the introduction of atom transfer radical polymerization (ATRP) by Matyjaszewski,1,2 this facile and versatile method is frequently used for the synthesis of well-defined polymers from a broad variety of monomers. The usefulness of ATRP was further augmented by the development of activator (re-)generation by electron transfer (A(R)GET) ATRP.3 Yagci and coworkers demonstrated that this can also be realized in situ by a photochemical process without the need of an additional photoactivator (photoinduced ATRP or CRP).4–6 Several other groups demonstrated the versatility of photoATRP for the facile preparation of defined polymers with well-defined architectures.7–13
Recently, we reported on the photoATRP in solution as well as photoinduced surface-initiated ATRP (PSI-ATRP) using a simple household fluorescent lamp as the only light source.14 The photoinduced ATRP was found to be highly controlled and it only converted monomers when CuII was steadily reduced to CuI by irradiation with the fluorescent lamp. The used ligand, PMDETA, plays an important role as it forms a photoredox active copper complex. Since, many other copper complexes can mediate photoredox reactions and fluorescent lamps are typical light sources in a chemical fume hood, we stated that “it most probably will make a difference if one is performing an ATRP reaction with the hood lights on or off.”14
This initiated a recent study by Matyjaszewski et al.15 on the contribution of photochemistry in activator regeneration in ATRP. Specifically, they investigated the contributions of the photochemical and the chemical processes on the regeneration of the CuI species. The system they investigated was the so-called “initiators for continuous activator regeneration” (ICAR) ATRP using a photoreactor at λ = 392 nm and a light intensity of 0.3 mW cm−2 which equals the light intensity of one fluorescent lamp at a distance of 1 m. The ICAR ATRP was performed with AIBN as the source of free radicals to regenerate the CuI activator, methyl acrylate (MA) as the monomer, EBiB as the alkyl halide initiator, TPMA as the ligand and CuBr2 at a ratio of [MA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [EBiB]
[EBiB]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [TPMA*2]
[TPMA*2]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [CuBr2]
[CuBr2]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [AIBN] = 300
[AIBN] = 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.12
0.12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.03
0.03![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 in anisole 50% (v/v) at 60 °C. For this system, only a negligible influence of visible light on the monomer conversion was observed. However, a large excess of AIBN was employed to continuously regenerate the CuI species which is otherwise consumed in termination reactions which allow the use of such low Cu concentrations. Furthermore, photolysis of AIBN is almost negligible because of its very low extinction coefficient at the used wavelength.16 Therefore, the reported results are, in our view, correct but very specific on this ICAR ATRP system investigated in the study by Matyjaszewski et al.15 and may not be applicable in general to the photochemical contribution to activator regeneration ATRP. Our previously reported results suggest that for many other ATRP types, the influence of ambient laboratory light mostly originating from sunlight and/or fluorescent lamps at the laboratory ceiling and in the chemical fume hood should be significant.14
0.2 in anisole 50% (v/v) at 60 °C. For this system, only a negligible influence of visible light on the monomer conversion was observed. However, a large excess of AIBN was employed to continuously regenerate the CuI species which is otherwise consumed in termination reactions which allow the use of such low Cu concentrations. Furthermore, photolysis of AIBN is almost negligible because of its very low extinction coefficient at the used wavelength.16 Therefore, the reported results are, in our view, correct but very specific on this ICAR ATRP system investigated in the study by Matyjaszewski et al.15 and may not be applicable in general to the photochemical contribution to activator regeneration ATRP. Our previously reported results suggest that for many other ATRP types, the influence of ambient laboratory light mostly originating from sunlight and/or fluorescent lamps at the laboratory ceiling and in the chemical fume hood should be significant.14
Here, we report on our results of a follow-up study to elucidate the effect of non-standardized but rather typical laboratory light settings on a series of frequently used ATRP types including AGET, ARGET, and classical ATRP. A significant influence of ambient laboratory light from fluorescent lamps on all ATRP reactions was observed except for ARGET ATRP.
For all the following ATRP experiments, the same initiator (EBiB), monomer (MMA), ligand (PMDETA), solvent (DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol, 2
methanol, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), type of reaction vial (Schlenk flask made of Duran glass) and reaction conditions ([M], reaction temperature) were used. Under strict exclusion of light, the reaction solution for each ATRP type was prepared in one vial under stirring, degassed by freeze–thaw cycles and then divided into two or three equal portions for the polymerization reactions under different light conditions at room temperature. This allowed for a direct comparison of the results for each respective ATRP type. Three different light conditions were used. First, under strict exclusion of light (D) with the aid of aluminum foil wrapped around the glassware, second, ambient light with two fluorescent lamps (type L58W/880 “SKYWHITE” from Osram®) 1 m away from the reaction vial and the laboratory ceiling light consisting of 6 fluorescent lamps (type Master TL-D 58W/840 from Philips® at a distance of approx. 3–5 m from the vial) (L, Fig. 1a) and finally, under hood light illumination with the ceiling lights and two fluorescent lamps in the hood on (type Master TL-D 58W/840, Philips® at a distance of 1.4 m to the reaction vial) (H, Fig. 1b). These illumination scenarios were chosen to reflect non-standardized but common light settings in an average chemical laboratory and to allow for comparison with previous reports on photoinduced ATRP14 and ICAR ATRP.15 With a digital lux meter (Luzchem) a light intensity of 5.35 ± 0.15 mW cm−2 for illumination scenario “L” and 6.19 ± 0.29 mW cm−2 for “H” was determined.
1), type of reaction vial (Schlenk flask made of Duran glass) and reaction conditions ([M], reaction temperature) were used. Under strict exclusion of light, the reaction solution for each ATRP type was prepared in one vial under stirring, degassed by freeze–thaw cycles and then divided into two or three equal portions for the polymerization reactions under different light conditions at room temperature. This allowed for a direct comparison of the results for each respective ATRP type. Three different light conditions were used. First, under strict exclusion of light (D) with the aid of aluminum foil wrapped around the glassware, second, ambient light with two fluorescent lamps (type L58W/880 “SKYWHITE” from Osram®) 1 m away from the reaction vial and the laboratory ceiling light consisting of 6 fluorescent lamps (type Master TL-D 58W/840 from Philips® at a distance of approx. 3–5 m from the vial) (L, Fig. 1a) and finally, under hood light illumination with the ceiling lights and two fluorescent lamps in the hood on (type Master TL-D 58W/840, Philips® at a distance of 1.4 m to the reaction vial) (H, Fig. 1b). These illumination scenarios were chosen to reflect non-standardized but common light settings in an average chemical laboratory and to allow for comparison with previous reports on photoinduced ATRP14 and ICAR ATRP.15 With a digital lux meter (Luzchem) a light intensity of 5.35 ± 0.15 mW cm−2 for illumination scenario “L” and 6.19 ± 0.29 mW cm−2 for “H” was determined.
In Table 1 the results for the various ATRP reaction types are summarized. The recipes were derived from typical ATRP experiments as referenced below and were only slightly adjusted to allow for a direct comparison. Please note that all the ATRP reaction conditions are not optimized to yield maximum monomer conversion or best polymerization control.
| Light intensity [mW cm−2] | MMA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EBiB EBiB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CuI CuI![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CuII CuII![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PMDETA PMDETA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sn(EH)2 Sn(EH)2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ascorbic acid ascorbic acid |
T [° C] | t [h] |
M
n,theo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a [g mol−1] a [g mol−1] |
M n,GPC [g mol−1] | Đ | Conversionb [%] | ||
|---|---|---|---|---|---|---|---|---|---|
| a M n,theo = ([MMA]0/[EBiB]0 × conversion × Mmonomer) + Minitiator. b The conversion was determined by 1H NMR spectroscopy from the ratio of the OCH3 signal intensity of the polymer (3.60 ppm) and the monomer (3.75 ppm). | |||||||||
| ARGET ATRP | 1D | 0 | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1: 1:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) – –![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01 0.01![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) – – |
r.t. | 22 | 3706 | 67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 780 780 |
1.35 | 17.53 |
| 1L | 5.35 ± 0.15 | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) – –![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01 0.01![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) – – |
r.t. | 22 | 3974 | 68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 065 065 |
1.35 | 18.23 | |
| 1H | 6.19 ± 0.29 | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) – –![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01 0.01![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) – – |
r.t. | 22 | 3846 | 68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 326 326 |
1.34 | 18.87 | |
| Classical ATRP (CuI/CuII) | 2D | 0 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01 0.01![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) – –![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) – – |
r.t. | 45 | 813 | — | — | 6.17 |
| 2L | 5.35 ± 0.15 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01 0.01![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) – –![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) – – |
r.t. | 45 | 1458 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 826 826 |
1.16 | 12.62 | |
| 3D | 0 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.35 0.35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.15 0.15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) – –![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) – – |
55 | 10 | 6686 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 476 476 |
1.15 | 64.83 | |
| 3H | 6.19 ± 0.29 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.35 0.35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.15 0.15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) – –![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) – – |
55 | 10 | 6865 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
1.15 | 66.68 | |
| 4D | 0 | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.35 0.35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.15 0.15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) – –![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) – – |
55 | 45 | 7885 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 651 651 |
1.14 | 38.41 | |
| 4H | 6.19 ± 0.29 | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.35 0.35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.15 0.15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) – –![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) – – |
55 | 45 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 149 149 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 195 195 |
1.17 | 59.75 | |
| Classical ATRP (CuI) | 5D | 0 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) – –![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) – –![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) – – |
r.t. | 24 | 737 | — | — | 5.41 |
| 5L | 5.35 ± 0.15 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) – –![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) – –![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) – – |
r.t. | 24 | 1658 | 9799 | 1.18 | 14.61 | |
| 6D | 0 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) – –![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) – –![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.015 0.015 |
r.t. | 8 | 2385 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 260 260 |
1.13 | 21.87 | |
| 6L | 5.35 ± 0.15 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) – –![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) – –![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.015 0.015 |
r.t. | 8 | 3977 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 949 949 |
1.17 | 37.77 | |
| AGET | 7D | 0 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) – –![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) – –![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.05 0.05 |
r.t. | 7 | 4546 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 344 344 |
1.17 | 43.45 |
| 7L | 5.35 ± 0.15 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) – –![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) – –![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.05 0.05 |
r.t. | 7 | 5336 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 786 786 |
1.26 | 51.34 | |
| ATRP | 8D | 0 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) – –![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.05 0.05![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) – – |
r.t. | 24 | 5164 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 559 559 |
1.24 | 49.63 |
| 8L | 5.35 ± 0.15 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) – –![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.05 0.05![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) – – |
r.t. | 24 | 5755 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 437 437 |
1.27 | 55.53 | |
1. Activator regenerated by electron transfer (ARGET) ATRP17
In ARGET ATRP very low copper concentrations are used ([I]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Cu] = 1
[Cu] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01) along with an excess of the reducing agent to constantly regenerate the CuI complex by chemical means. Analogous to the findings of Matyjaszewski et al.15 for ICAR ATRP, the influence of visible light on ARGET ATRP is not significant and monomer conversions are almost identical for the different light scenarios (entry #1D, 1L, 1H). This is expectable, as the efficient chemical reduction of CuII to CuI is ensured by the excess of the reducing agent under light as well as under dark conditions.
0.01) along with an excess of the reducing agent to constantly regenerate the CuI complex by chemical means. Analogous to the findings of Matyjaszewski et al.15 for ICAR ATRP, the influence of visible light on ARGET ATRP is not significant and monomer conversions are almost identical for the different light scenarios (entry #1D, 1L, 1H). This is expectable, as the efficient chemical reduction of CuII to CuI is ensured by the excess of the reducing agent under light as well as under dark conditions.
2. Classical ATRP (CuI/CuII)2,18
In classical ATRP, a higher copper concentration ([I]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [CuI]
[CuI]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [CuII] = 1
[CuII] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1–1
0.1–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01–0.1) has to be used in order to maintain the control of the radical polymerization throughout the reaction to cope with the persistent radical effect (PRE).19 As expected, at room temperature and in organic solvents the polymerization is controlled but is extremely slow. After 45 h under light exclusion only 6% of the initial monomer was converted, and not enough polymer was formed for a reliable analysis of molar mass and dispersity by gel permeation chromatography (GPC) (#2D). However, under ambient laboratory light conditions (#2L) the monomer conversion almost doubled to a value of 12.6%. GPC analysis of the resulting PMMA revealed good control of the polymerization (Đ = 1.16).
0.01–0.1) has to be used in order to maintain the control of the radical polymerization throughout the reaction to cope with the persistent radical effect (PRE).19 As expected, at room temperature and in organic solvents the polymerization is controlled but is extremely slow. After 45 h under light exclusion only 6% of the initial monomer was converted, and not enough polymer was formed for a reliable analysis of molar mass and dispersity by gel permeation chromatography (GPC) (#2D). However, under ambient laboratory light conditions (#2L) the monomer conversion almost doubled to a value of 12.6%. GPC analysis of the resulting PMMA revealed good control of the polymerization (Đ = 1.16).
Although a significant photoeffect was observed, one may easily argue that for an optimized ATRP recipe at elevated temperatures, the light effect may be less pronounced or even negligible. Thus, we performed ATRP with MMA as the monomer at 55 °C with a quite standard or “classical” ATRP recipe (MMA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EBiB
EBiB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CuI
CuI![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CuII
CuII![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PMDETA = 200
PMDETA = 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.35
0.35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.15
0.15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in a common chemical fume hood with the standard installed lights on (Table 1 entry #4H) as shown in Fig. 1b, and the same reaction was performed under exclusion of light (vial wrapped with aluminum foil, Table 1 entry #4D). Both reaction tubes were placed in the same heating and stirring apparatus. After 45 h polymerization time, the polymers from both reactions were isolated and analyzed with identical procedures and instrumentation. The low dispersity (Đ) values for both ATRP reactions (#4D, #4H) indicate good control of the polymerization with good agreement between the theoretical and experimental molar mass (Mn). However, the ATRP under light exclusion only gave 38% monomer conversion after 45 h while for the same ATRP with irradiation with the fume hood lights, the monomer conversion exceeded 59%. Thus, the contribution of photochemical processes to classical ATRP has to be considered as significant even at high temperatures. Interestingly, for an MMA
1) in a common chemical fume hood with the standard installed lights on (Table 1 entry #4H) as shown in Fig. 1b, and the same reaction was performed under exclusion of light (vial wrapped with aluminum foil, Table 1 entry #4D). Both reaction tubes were placed in the same heating and stirring apparatus. After 45 h polymerization time, the polymers from both reactions were isolated and analyzed with identical procedures and instrumentation. The low dispersity (Đ) values for both ATRP reactions (#4D, #4H) indicate good control of the polymerization with good agreement between the theoretical and experimental molar mass (Mn). However, the ATRP under light exclusion only gave 38% monomer conversion after 45 h while for the same ATRP with irradiation with the fume hood lights, the monomer conversion exceeded 59%. Thus, the contribution of photochemical processes to classical ATRP has to be considered as significant even at high temperatures. Interestingly, for an MMA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) initiator = 100
initiator = 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the ATRP proceeds fast with a conversion of around 65% within 10 h polymerization time. As in this case the initial activator is sufficient to carry out the ATRP over the entire polymerization time, the activator photoregeneration by ambient light is of minor importance and almost no difference between irradiated and non-irradiated reactions was found (Table 1, entries #3D & #3H).
1, the ATRP proceeds fast with a conversion of around 65% within 10 h polymerization time. As in this case the initial activator is sufficient to carry out the ATRP over the entire polymerization time, the activator photoregeneration by ambient light is of minor importance and almost no difference between irradiated and non-irradiated reactions was found (Table 1, entries #3D & #3H).
3. Classical ATRP (initial CuI only)
Additionally, we also investigated a variant of the classical ATRP in which initially only the activator CuI is added to the polymerization solution. It has been shown that in water, DMSO, DMF and methanol, CuI readily disproportionates to the (arguably) supplemental Cu0 activator and the deactivating CuII complex.20–22 From the data in Table 1, entries #5D and #5L, it is apparent that polymerization proceeds noticeably faster compared to ATRP with initial CuI/CuII addition. Under the exclusion of light (D), this ATRP did not yield sufficient polymer for reliable characterization, and monomer conversion after 24 h was only around 5–6% (#5D). Under ambient light conditions (#5L), however, the monomer conversion nearly tripled to 14–15% and narrowly distributed PMMA with Đ = 1.18 and Mn = 9799 g mol−1 was obtained. Additionally, we performed experiments with added ascorbic acid as a reducing agent at very low concentrations ([I]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [CuI]
[CuI]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [ascorbic acid] = 1
[ascorbic acid] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1
0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.015). As expected, this substantially improved the performance of polymerization under light exclusion conditions because of the chemical reduction of CuII to CuI (#6D). Interestingly, the same reaction performed under ambient light conditions (#6L), gave even higher monomer conversion (#6D: 22% vs. #6L: 38%). Apparently, the photoenhancement is substantial even in the presence of chemical reducing agents.
0.015). As expected, this substantially improved the performance of polymerization under light exclusion conditions because of the chemical reduction of CuII to CuI (#6D). Interestingly, the same reaction performed under ambient light conditions (#6L), gave even higher monomer conversion (#6D: 22% vs. #6L: 38%). Apparently, the photoenhancement is substantial even in the presence of chemical reducing agents.
4. Activator generated by electron transfer (AGET) ATRP3,23
Finally, the influence of laboratory light on AGET ATRP was investigated. In AGET ATRP, CuII and a reducing agent are added to the initial reaction solution at a typical ratio of [I]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [CuII]
[CuII]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [reducing agent] = 1
[reducing agent] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1
0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.05 in order to partially convert CuII to the CuI activator. As reducing agents for AGET ATRP, ascorbic acid3 and tin(II) 2-ethylhexanoate (Sn(EH)2)23 are commonly used. After 7 h reaction time under light exclusion (#7D), the polymerization with ascorbic acid reached 44% monomer conversion which was again slightly enhanced by ambient light to a value of 51% (#7L). With Sn(EH)2 as a milder reducing agent and after 24 h reaction time, the effect of light accounted for an increase of the monomer conversion by 6% (#8D vs. #8L). All AGET ATRP polymer products showed good to acceptable molar mass distributions of Đ = 1.17–1.27.
0.05 in order to partially convert CuII to the CuI activator. As reducing agents for AGET ATRP, ascorbic acid3 and tin(II) 2-ethylhexanoate (Sn(EH)2)23 are commonly used. After 7 h reaction time under light exclusion (#7D), the polymerization with ascorbic acid reached 44% monomer conversion which was again slightly enhanced by ambient light to a value of 51% (#7L). With Sn(EH)2 as a milder reducing agent and after 24 h reaction time, the effect of light accounted for an increase of the monomer conversion by 6% (#8D vs. #8L). All AGET ATRP polymer products showed good to acceptable molar mass distributions of Đ = 1.17–1.27.
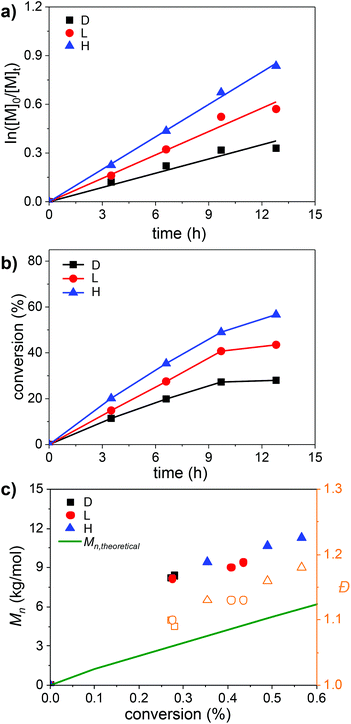
The acceptable monomer conversion of AGET ATRP even in the dark allowed for a study on the polymerization kinetics under these three illumination conditions. The first-order kinetic plots for AGET ATRP under the three light scenarios (D, L, H) clearly show the significant influence of ambient laboratory light on the polymerization (Fig. 2a). While for all reactions a strict linear dependency of ln([M]0/[M]t) with the reaction time indicates good control of the radical polymerization, the remarkable effect of ambient light upon AGET ATRP is apparent. Initially, we were surprised by the significant acceleration of AGET ATRP under fume hood illumination (H, Fig. 1b), which was found to be even higher compared to the ambient light irradiation (L, Fig. 1a). However, the measurement of the respective light intensities at the location of the reaction vials gave 5.35 ± 0.15 mW cm−2 for ambient light (L) and 6.19 ± 0.29 mW cm−2 for hood light (H). Thus, the results are reasonable as the acceleration of ATRP scales well with the light intensity. Our findings are in agreement with earlier reports by Guan and Smart24 who discovered that visible light significantly affects the ATRP by using a 275 W sunlight lamp. Moreover, under exclusion of light (D) or ambient light conditions (L) a leveling of the first order kinetic plot is noticeable and it indicates an increase of chain termination at longer reaction times. This, however, is not the case if the ATRP is running under fume hood illumination (H). This is further corroborated by the development of the average polymer molar mass as a function of the polymerization time (Fig. 2b). For AGET ATRP under exclusion of light (D), monomer conversion stopped after 10 h at 28%. Illuminated by the fume hood lights (H), the same reaction proceeded well to a conversion of 56% within 13 h. Under the ambient light conditions (L), the monomer conversion only slightly increases after 10 h.
Finally, the development of the number average molar mass with the monomer conversion for all three reaction settings (Fig. 2c) shows again a linear dependency but also the low initiator efficiency of EBiB,9,25 especially at room temperature as is apparent from the discrepancy of Mn and Mn,theo. The dispersity remains narrow with Đ = 1.09–1.20 indicating good control of the AGET ATRP under all three conditions.
In normal ATRP, CuII deactivating species can accumulate through the PRE,19 which increases continuously with the polymerization time. The photochemical effect may be through the reactivation of the CuII complex by an in situ reduction process.14,26 If lower CuI concentrations or longer reaction times were used, the influence of a photochemical process could be greater. Additionally, if higher CuI concentrations were used, the generation of the deactivating complex by PRE can be neglected and the polymerization itself (in the dark) would also be in good control. In the case of ARGET ATRP and ICAR ATRP, both the methods employ chemical reducing agents such as ascorbic acid (ARGET) or a radical initiator (ICAR) to regenerate the CuI activating species. The contribution of the photochemical process is therefore negligible. Furthermore, the additional amine ligand is essential to the photochemical pathway in ATRP, since it is a good electron-donor in the photoreduction of CuII to the CuI complex.11,14
5. Conclusion
The influence of non-standardized but typical laboratory light scenarios on various ATRP reactions was investigated. Except for ICAR ATRP, as reported previously by Matyjaszewski et al.15 and ARGET ATRP in this study, all other ATRP types showed a remarkable photoenhancement effect by ambient light, originating from common fluorescent lamps. The influence of ambient light on the monomer conversion is stronger when less Cu complex is used in the respective ATRP recipe. Even in the presence of other reducing agents, this effect is significant. In general, it can be stated that the slower the ATRP reaction proceeds (low propagation rates), the more pronounced can be the effect of ambient laboratory light.As previously assumed,14 we can now conclude that it makes a difference if one is performing an ATRP reaction with the hood lights on or off. Surely, more detailed studies are needed to better understand the mechanism of the effect of ambient light on ATRP with various catalysts, ligands, solvents, monomers and additives. However, the influence of ambient laboratory light can no longer be neglected. It would therefore be helpful to report the light conditions during ATRP experiments to ensure reproducibility.
Acknowledgements
T. Z. acknowledges support by the China Scholarship Council (CSC) by a PhD stipend. R. J. acknowledges financial support by the Cluster of Excellence “Center for Advancing Electronics Dresden” (cfAED) and by the “Excellence Initiative by the German Federal and State Governments” (Institutional Strategy, measure “support the best”).References
- K. Matyjaszewski and J. Xia, Chem. Rev., 2001, 101, 2921 CrossRef CAS PubMed.
- J.-S. Wang and K. Matyjaszewski, Macromolecules, 1995, 28, 7901 CrossRef CAS.
- K. Min, H. Gao and K. Matyjaszewski, J. Am. Chem. Soc., 2005, 127, 3825 CrossRef CAS PubMed.
- M. A. Tasdelen, M. Uygun and Y. Yagci, Macromol. Rapid Commun., 2011, 32, 58 CrossRef CAS PubMed.
- M. A. Tasdelen, M. Uygun and Y. Yagci, Macromol. Chem. Phys., 2010, 211, 2271 CrossRef CAS.
- S. Dadashi-Silab, M. Atilla Tasdelen and Y. Yagci, J. Polym. Sci., Part A: Polym. Chem., 2014, 52, 2878 CrossRef CAS.
- A. Anastasaki, V. Nikolaou, Q. Zhang, J. Burns, S. R. Samanta, C. Waldron, A. J. Haddleton, R. McHale, D. Fox, V. Percec, P. Wilson and D. M. Haddleton, J. Am. Chem. Soc., 2014, 136, 1141 CrossRef CAS PubMed.
- A. Anastasaki, V. Nikolaou, G. S. Pappas, Q. Zhang, C. Wan, P. Wilson, T. P. Davis, M. R. Whittaker and D. Haddleton, Chem. Sci., 2014, 5, 3536 RSC.
- J. Mosnacek and M. Ilcikova, Macromolecules, 2012, 45, 5859 CrossRef CAS.
- Y.-M. Chuang, A. Ethirajan and T. Junkers, ACS Macro Lett., 2014, 3, 732 CrossRef CAS.
- T. G. Ribelli, D. Konkolewicz, S. Bernhard and K. Matyjaszewski, J. Am. Chem. Soc., 2014, 136, 13303 CrossRef CAS PubMed.
- B. P. Fors and C. J. Hawker, Angew. Chem., Int. Ed., 2012, 51, 8850 CrossRef CAS PubMed.
- E. Frick, A. Anastasaki, D. M. Haddleton and C. Barner-Kowollik, J. Am. Chem. Soc., 2015, 137, 6889 CrossRef CAS PubMed.
- T. Zhang, T. Chen, I. Amin and R. Jordan, Polym. Chem., 2014, 5, 4790 RSC.
- T. G. Ribelli, D. Konkolewicz, X. Pan and K. Matyjaszewski, Macromolecules, 2014, 47, 6316 CrossRef CAS.
- P. Smith and A. M. Rosenberg, J. Am. Chem. Soc., 1959, 81, 2037 CrossRef CAS.
- W. Jakubowski and K. Matyjaszewski, Angew. Chem., Int. Ed., 2006, 45, 4482 CrossRef CAS PubMed.
- K. Matyjaszewski, A. K. Nanda and W. Tang, Macromolecules, 2005, 38, 2015 CrossRef CAS.
- W. Tang, N. V. Tsarevsky and K. Matyjaszewski, J. Am. Chem. Soc., 2006, 128, 1598 CrossRef CAS PubMed.
- A. Simula, V. Nikolaou, F. Alsubaie, A. Anastasaki and D. M. Haddleton, Polym. Chem., 2015, 6, 5940 RSC.
- S. Samanta, V. Nikolaou, S. Keller, M. Monteiro, D. A. Wilson, D. Haddleton and P. V. Percec, Polym. Chem., 2015, 11, 2084 RSC.
- B. M. Rosen and V. Percec, Chem. Rev., 2009, 109, 5069 CrossRef CAS PubMed.
- W. Jakubowski and K. Matyjaszewski, Macromolecules, 2005, 38, 4139 CrossRef CAS.
- Z. Guan and B. Smart, Macromolecules, 2000, 33, 6904 CrossRef CAS.
- T. Ando, M. Kamigaito and M. Sawamoto, Tetrahedron, 1997, 53, 15445 CrossRef CAS.
- A. Anastasaki, V. Nikolaou, G. Nurumbetov, N. P. Truong, G. S. Pappas, N. G. Engelis, J. F. Quinn, M. R. Whittaker, T. P. Davis and D. M. Haddleton, Macromolecules, 2015, 48, 5140 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5py01858g |
| This journal is © The Royal Society of Chemistry 2016 |