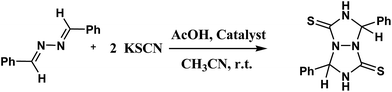
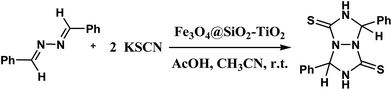
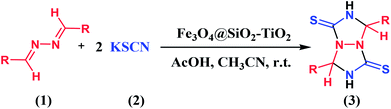An efficient synthesis of perhydro[1,2,4]triazolo[1,2-a][1,2,4]triazole-1,5-dithiones catalyzed by TiO2-functionalized nano-Fe3O4 encapsulated-silica particles as a reusable magnetic nanocatalyst†
Javad Safari* and
Leila Javadian
Laboratory of Organic Compound Research, Department of Organic Chemistry, Faculty of Chemistry, University of Kashan, P.O.Box: 87317-51167, Kashan, Iran. E-mail: Safari@kashanu.ac.ir; Tel: +98-31-55912320
First published on 26th November 2015
Abstract
Immobilization of a nano-TiO2 catalyst on the surface of a magnetic SiO2 support was performed through the reaction of a Fe3O4@SiO2 composite with Ti(OC4H9)4 via a simple process. The Fe3O4@SiO2–TiO2 nanocomposite was characterized using scanning electron microscopy (SEM), transmission electron microscopy (TEM), energy dispersive X-ray spectroscopy (EDS), X-ray diffraction (XRD), Fourier transform infrared spectra (FTIR), and a vibrating sample magnetometer (VSM). The Fe3O4@SiO2–TiO2 nanocomposite has been found to be an efficient catalyst for the synthesis of perhydro[1,2,4]triazolo[1,2-a][1,2,4]triazole-1,5-dithiones from the condensation of various aldazines and potassium thiocyanate in acetonitrile solvent at room temperature. It has been found that the nanocatalyst was recycled for up to 6 cycles with minimal loss in catalytic activity. The purpose of this research was to provide an easy method for the synthesis of perhydrotriazolotriazole derivatives by a robust and magnetically recoverable catalyst.
1. Introduction
1,3-Dipolar cycloaddition or [3 + 2] cycloaddition reactions are fundamental processes in organic chemistry that offer a powerful synthetic methodology to achieve five-membered heterocyclic systems in a stereo and regiocontrolled approach.1–4 The term “criss-cross” cycloaddition appeared in 1917 by Bailey and McPherson and the first paper described the cycloaddition of cyanic acid to benzalazine.5 Criss-cross cycloaddition reactions are a procedure for the synthesis of fused heterocyclic rings in a one pot arrangement that offers two fused five-membered rings.6,7 The formation of the corresponding products was described by Huisgen as the result of two following 1,3-dipolar cycloadditions in 1963.8 Since then, some papers have published listing examples of criss-cross cycloaddition reactions of aldazines and different dipolarophiles such as thiocyanate.9 In addition to aldazines, the current reactions have been reported for ketazines10 1,2-diazabuta-1,3-dienes,11,12 glyoxalimines,13 and hexafluoroacetonazine with many types of compounds including alkenes.14 The 1,3-dipolar ketazines and aldazines are 1,3-heterodiene compounds and have double 1,3-dipolar sites (Scheme 1).They adopt the s-trans conformation due to steric interaction of the alkyl or aryl substations. So, this conformation does not suffer the [4 + 2] cycloaddition known as the Diels–Alder reaction.15 Although, there are a few papers in the literatures that have described the synthesis of perhydrotriazolotriazoledithione derivatives, they have drawbacks such as high catalyst loading, drastic conditions, low yields and long reaction times.16,17
In recent decades, design of magnetically catalysts has attracted a great deal of attention due to simple separation of catalysts by a permanent magnetic field. A lot of materials such as FeCo, Fe2O3, NiFe2O4, Fe3O4, CoFe2O4 and black sand have the magnetic properties.18–23 Among them, Fe3O4 nanoparticles has been chosen because of its low toxicity and remarkable magneticproperties.24–26 Fe3O4 nanoparticles have emerged as supports for immobilization of catalyst which offer an easy separation of the catalyst without the need of conventional filtration method or tedious work-up processes. Beside the facile separation, a great feature of Fe3O4 nanoparticles is their surface modification that affords sometimes higher activity than their homogeneous systems. Fe3O4 core with shell of silica offers sites for surface modification with various compounds in catalyst application. Silica layer not only avoids the oxidation of the Fe3O4 by the outer atmosphere, but also can prevent the aggregation induced by the magnetic dipolar attraction between magnetic nanoparticles.27–29 Also, silica enhances a better dispersion of magnetic nanoparticles in suspension.30 Magnetic nanoparticles have been used as catalysts or catalyst supports in many organic reactions including oxidation, reductions, multicomponent reactions and C–C couplings with a high level of activity.31–34 It is anticipated that the magnetic properties of catalyst would be useful to improve the performance of the [3 + 2] cycloaddition reaction and provide a support for the solid acid catalyst. On the other side, extensive attention has been directed toward the application of solid acids in organic synthesis because such reagents help prevent release of reaction residues into the environment. In this regard, nanostructure solid acids show higher activity than their bulk materials due to their particular chemical and physical properties especially large surface to volume ratio.35 As an extension of our researches on the application of magnetic solid acids in organic transformations, functionalization of Fe3O4 nanoparticles with TiO2 has been studied to offer magnetic catalyst which combines the benefits of TiO2 and Fe3O4 to afford greater potential applications.
In this contribution, we hope to report an efficient method for the synthesis of perhydrotriazolotriazoledithions by the condensation of aldazines as 1,3-heterodienes with thiocyanate in [3 + 2] cycloaddition by a magnetically recyclable catalyst.
2. Experimental
2.1. General
All the commercially available reagents were obtained from Aldrich or Merk and were used without further purification. 1H and 13C NMR spectra were recorded on a Bruker DRX-400 spectrometer. Chemical shifts are expressed in δ parts per million. The IR spectra of the compounds were recorded on a Perkin Elmer FT-IR 550 spectrophotometer. All melting points (mp) were determined on an ElectroMK3 apparatus, expressed in °C and are uncorrected. Analytical thin layer chromatography (TLC) on silica gel plates containing UV indicator was employed regularly to follow the course of reactions and to confirm the purity of products. The sonication was performed in Shanghai Branson-BUG40-06 ultrasonic cleaner. The obtained nanoparticles were characterized by XRD on a Bruker D8 Advance X-ray diffraction (XRD) diffractometer (CuK, radiation, k = 0.154056 nm and 40 kV voltage), at a scanning speed of 2° min−1 from 10° to 100° (2θ). Scanning electron microscope (SEM) was performed on a FEI Quanta 200 SEM operated at a 20 kV accelerating voltage. Magnetic properties were characterized by a vibrating sample magnetometer (VSM, MDKFD, University of kashan, Kashan, Iran) at room temperature. The samples for SEM were prepared by spreading a small drop containing nanoparticles onto a silicon wafer and being dried almost completely in air at room temperature for 2 h, and then were transferred onto SEM conductive tapes. The TEM images were recorded using a Ziess EM10C transmission electron microscope operated at a 80 KV accelerating voltage.2.2. Preparation of Fe3O4@SiO2 as a support
First, Fe3O4 nanoparticles were prepared by chemical co-precipitation of FeCl2 and FeCl3 [Fe2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe3+ = 1
Fe3+ = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2] in base solution as described in the literature.36,37 Then, Fe3O4–SiO2 composite were synthesized through the Stöber method using tetraethyl orthosilicate as a silica source in a basic water/ethanol mixture at room temperature under continuous mechanical stirring.38 Briefly, 0.5 g of the Fe3O4 nanoparticles was dispersed in 20 mL of distilled water and 50 mL of ethanol under ultrasound irradiation and then concentrated aqueous ammonia (1 mL) was added. Finally, 0.2 mL of tetraethyl orthosilicate diluted in ethanol (10 mL) was added drop-wisely under continuous mechanical stirring. After stirring for 18 h, Fe3O4@SiO2 nanoparticles were collected by magnetic separation and washed three times with water and ethanol. Finally, the magnetic product was dried at 70 °C for 6 h.
2] in base solution as described in the literature.36,37 Then, Fe3O4–SiO2 composite were synthesized through the Stöber method using tetraethyl orthosilicate as a silica source in a basic water/ethanol mixture at room temperature under continuous mechanical stirring.38 Briefly, 0.5 g of the Fe3O4 nanoparticles was dispersed in 20 mL of distilled water and 50 mL of ethanol under ultrasound irradiation and then concentrated aqueous ammonia (1 mL) was added. Finally, 0.2 mL of tetraethyl orthosilicate diluted in ethanol (10 mL) was added drop-wisely under continuous mechanical stirring. After stirring for 18 h, Fe3O4@SiO2 nanoparticles were collected by magnetic separation and washed three times with water and ethanol. Finally, the magnetic product was dried at 70 °C for 6 h.
2.3. Preparation of catalyst
A magnetically separable TiO2 catalyst was prepared by dissolving 1.5 mL of Ti(OC4H9)4 in 30 mL ethanol and adding this solution dropwise into a mixture containing 0.40 g of magnetic Fe3O4@SiO2 in 10 mL water/ethanol. The mixture was refluxed at 90 °C for about 2 h. Then, the magnetic sample was dried at 60 °C and calcined at 450 °C for 2 h to offer Fe3O4@SiO2–TiO2 nanocomposite.392.4. A typical procedure for the synthesis of aldazines
Aldazines were prepared by the method as described in the literature.40 A mixture of the aldehyde (2 mmol), hydrazine sulfate (1 mmol), and triethylamine (1 mmol) was heated at 60 °C for 3–5 min. The completion of the reaction was monitored by thin layer chromatography (petroleum ether![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 7
ethyl acetate = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). After the completion of the reaction, the mixture was cooled on ice. The aldazine was collected by suction filtration. Then, it was washed with water, and dried under vacuum. The yellow solid was recrystallized from ethanol to afford the desired aldazine as cream to orange crystals.
3). After the completion of the reaction, the mixture was cooled on ice. The aldazine was collected by suction filtration. Then, it was washed with water, and dried under vacuum. The yellow solid was recrystallized from ethanol to afford the desired aldazine as cream to orange crystals.
2.5. A typical procedure for the synthesis of tetrahydro-[1,2,4]triazolo[1,2-a][1,2,4]triazole-1,5-dithione derivatives
In a typical experiment, KSCN (2 mmol), AcOH (0.18 mL), CH3CN (6 mL) and catalytic amount of Fe3O4@SiO2–TiO2 nanocomposite (0.04 g) were stirred at room temperature. After stirring for 5 min, aldazine (1 mmol) was added to this mixture and the contents were stirred for an appropriate period while the progress of the reaction was followed by TLC (petroleum ether![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 7
ethyl acetate = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 as eluent). After completion of the reaction, the nanocatalyst was removed by an external magnet and reused. Then, cold water (50 mL) was added and the solid product was collected by filtration, was washed successively with CHCl3, dried and recrystallized from ethanol to afford the pure product. The products were identified by comparison of their spectroscopic and physical data with those reported in the literature.
3 as eluent). After completion of the reaction, the nanocatalyst was removed by an external magnet and reused. Then, cold water (50 mL) was added and the solid product was collected by filtration, was washed successively with CHCl3, dried and recrystallized from ethanol to afford the pure product. The products were identified by comparison of their spectroscopic and physical data with those reported in the literature.
2.5.1.1. Tetrahydro-3,7-diphenyl-[1,2,4]triazolo[1,2-a][1,2,4]triazole-1,5-dithione (3a). White solid, mp = 188–189 °C; Rf (petroleum ether
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethylacetate = 7
ethylacetate = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (v/v)) = 0.26; IR (KBr)/ν (cm−1): 3385, 3180, 1500, 1251; 1H NMR (acetone-d6, 400 MHz)/δ ppm: 6.81 (s, 2H, CH), 7.38–7.42 (m, 10H, Ar-H), 11.42 (s, 2H, NH); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 73 (CH), 126.25 (CH), 127.76 (CH), 128.31 (CH), 129.73 (CH), 184.1 (C
3 (v/v)) = 0.26; IR (KBr)/ν (cm−1): 3385, 3180, 1500, 1251; 1H NMR (acetone-d6, 400 MHz)/δ ppm: 6.81 (s, 2H, CH), 7.38–7.42 (m, 10H, Ar-H), 11.42 (s, 2H, NH); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 73 (CH), 126.25 (CH), 127.76 (CH), 128.31 (CH), 129.73 (CH), 184.1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S) ppm; CHNcalculated (%): C (58.89), H (4.29), N (17.18), S (19.63); CHNfound (%): C (58.82), H (4.39), N (17.34), S (19.73).17
S) ppm; CHNcalculated (%): C (58.89), H (4.29), N (17.18), S (19.63); CHNfound (%): C (58.82), H (4.39), N (17.34), S (19.73).17
2.5.1.2. Tetrahydro-3,7-bis(3-nitrophenyl)-[1,2,4]triazolo[1,2-a][1,2,4]triazole-1,5-dithione (3b). White solid, mp = 190–191 °C; Rf (petroleum ether
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethylacetate = 7
ethylacetate = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (v/v)) = 0.15; IR (KBr)/ν (cm−1): 3215, 1616, 1529, 1491, 1352, 1247, 1161; 1H NMR (acetone-d6, 400 MHz)/δ ppm: 7.25 (s, 2H, CH), 7.80 (t, J = 7.0 Hz, 2H, ArH), 8.00 (d, J = 7.0 Hz, 2H, ArH), 8.31 (d, J = 7.0 Hz, 2H, ArH), 8.39 (s, 2H, ArH), 10.38 (s, 2H, NH) ppm; 13C NMR (acetone-d6, 100 MHz) δ (ppm): 72.0 (CH), 119.43 (CH), 122.55 (CH), 129.88 (CH), 133.76 (CH), 145.50 (CH), 148.60 (C), 158.09 (C), 184.11 (C
3 (v/v)) = 0.15; IR (KBr)/ν (cm−1): 3215, 1616, 1529, 1491, 1352, 1247, 1161; 1H NMR (acetone-d6, 400 MHz)/δ ppm: 7.25 (s, 2H, CH), 7.80 (t, J = 7.0 Hz, 2H, ArH), 8.00 (d, J = 7.0 Hz, 2H, ArH), 8.31 (d, J = 7.0 Hz, 2H, ArH), 8.39 (s, 2H, ArH), 10.38 (s, 2H, NH) ppm; 13C NMR (acetone-d6, 100 MHz) δ (ppm): 72.0 (CH), 119.43 (CH), 122.55 (CH), 129.88 (CH), 133.76 (CH), 145.50 (CH), 148.60 (C), 158.09 (C), 184.11 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S) ppm; CHNcalculated (%): C (46.02), H (2.2.87), N (20.13), S (15.62), O (15.36); CHNfound (%): C (46.45), H (3.01), N (19.18), S (15.35), O (16.01).
S) ppm; CHNcalculated (%): C (46.02), H (2.2.87), N (20.13), S (15.62), O (15.36); CHNfound (%): C (46.45), H (3.01), N (19.18), S (15.35), O (16.01).
2.5.1.3. Tetrahydro-3,7-bis(4-methoxyphenyl)-[1,2,4]triazolo[1,2-a][1,2,4]triazole-1,5-dithione (3c). White solid, mp = 160–162 °C; Rf (petroleum ether
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethylacetate = 7
ethylacetate = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (v/v)) = 0.18; IR (KBr)/ν (cm−1): 3390, 3157, 2960, 1613, 1508, 1249, 1173, 1028; 1H NMR (acetone-d6, 400 MHz)/δ ppm: 3.72 (s, 6H, OCH3), 6.75 (s, 2H, CH), 6.99 (s, 4H, Ar-H), 7.28–7.30 (d, J = 6.8 Hz, 4H, Ar-H), 11.31 (s, 2H, NH) ppm; 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 55.73 (CH3), 77.03 (CH), 114.76 (CH), 127.67 (CH), 130.0 (C), 160.35 (C), 184.0 (C
3 (v/v)) = 0.18; IR (KBr)/ν (cm−1): 3390, 3157, 2960, 1613, 1508, 1249, 1173, 1028; 1H NMR (acetone-d6, 400 MHz)/δ ppm: 3.72 (s, 6H, OCH3), 6.75 (s, 2H, CH), 6.99 (s, 4H, Ar-H), 7.28–7.30 (d, J = 6.8 Hz, 4H, Ar-H), 11.31 (s, 2H, NH) ppm; 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 55.73 (CH3), 77.03 (CH), 114.76 (CH), 127.67 (CH), 130.0 (C), 160.35 (C), 184.0 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S); ppm; CHNcalculated (%): C (55.96), H (4.66), N (14.51), S (16.58); CHNfound (%): C (55.93), H (4.76), N (14.72), S (16.68).17
S); ppm; CHNcalculated (%): C (55.96), H (4.66), N (14.51), S (16.58); CHNfound (%): C (55.93), H (4.76), N (14.72), S (16.68).17
2.5.1.4. Tetrahydro-3,7-bis(2-chlorophenyl)-[1,2,4]triazolo[1,2-a][1,2,4]triazole-1,5-dithione (3d). White solid, mp = 197 °C; Rf (petroleum ether
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethylacetate = 7
ethylacetate = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (v/v)) = 0.32; IR (KBr) ν (cm−1): 3433, 3183, 2929, 1498, 1251; 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 7.16 (s, 1H, CH), 7.34–7.36 (m, 1H, Ar-H), 7.43–7.47 (m, 2H, Ar-H), 7.54–7.61 (m, 1H, Ar-H), 11.48 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 74.86 (CH), 128.14 (CH), 128.63 (CH), 130.62 (CH), 131.87 (CH), 132.48 (C), 134.01 (C), 185.19 (C
3 (v/v)) = 0.32; IR (KBr) ν (cm−1): 3433, 3183, 2929, 1498, 1251; 1H NMR (DMSO-d6, 400 MHz) δ (ppm): 7.16 (s, 1H, CH), 7.34–7.36 (m, 1H, Ar-H), 7.43–7.47 (m, 2H, Ar-H), 7.54–7.61 (m, 1H, Ar-H), 11.48 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 74.86 (CH), 128.14 (CH), 128.63 (CH), 130.62 (CH), 131.87 (CH), 132.48 (C), 134.01 (C), 185.19 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S) ppm; CHNcalculated (%): C (48.60), H (3.04), N (14.18), S (16.20), Cl (17.72); CHNfound (%): C (47.99), H (3.58), N (14.76), S (16.09), Cl (17.58).
S) ppm; CHNcalculated (%): C (48.60), H (3.04), N (14.18), S (16.20), Cl (17.72); CHNfound (%): C (47.99), H (3.58), N (14.76), S (16.09), Cl (17.58).
2.5.1.5. Tetrahydro-3,7-bis(3-chlorophenyl)-[1,2,4]triazolo[1,2-a][1,2,4]triazole-1,5-dithione (3e). White solid, mp = 194–195 °C; Rf (petroleum ether
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethylacetate = 7
ethylacetate = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (v/v)) = 0.35; IR (KBr)/ν (cm−1): 3427, 3191, 2927, 1501, 1247; 1H NMR (acetone-d6, 400 MHz)/δ ppm: 7.07 (s, 1H, CH), 7.47–7.51 (m, 3H, Ar-H), 7.55 (s, 1H, Ar-H), 10.14 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 76.62 (CH), 124.88 (CH), 126.39 (CH), 128.12 (CH), 131.84 (CH), 134.15 (C), 139.76 (C), 184.47 (C
3 (v/v)) = 0.35; IR (KBr)/ν (cm−1): 3427, 3191, 2927, 1501, 1247; 1H NMR (acetone-d6, 400 MHz)/δ ppm: 7.07 (s, 1H, CH), 7.47–7.51 (m, 3H, Ar-H), 7.55 (s, 1H, Ar-H), 10.14 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 76.62 (CH), 124.88 (CH), 126.39 (CH), 128.12 (CH), 131.84 (CH), 134.15 (C), 139.76 (C), 184.47 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S) ppm; CHNcalculated (%): C (48.60), H (3.04), N (14.18), S (16.20), Cl (17.72); CHNfound (%): C (48.32), H (3.13), N (14.31), S (16.29), Cl (17.81).17
S) ppm; CHNcalculated (%): C (48.60), H (3.04), N (14.18), S (16.20), Cl (17.72); CHNfound (%): C (48.32), H (3.13), N (14.31), S (16.29), Cl (17.81).17
2.5.1.6. Tetrahydro-3,7-bis(4-chlorophenyl)-[1,2,4]triazolo[1,2-a][1,2,4]triazole-1,5-dithione (3f). White solid, mp = 201–203 °C; Rf (petroleum ether
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethylacetate = 7
ethylacetate = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (v/v)) = 0.31; IR (KBr)/ν (cm−1): 3438, 3168, 2925, 1629, 1492, 1249, 1157; 1H NMR (acetone-d6, 400 MHz)/δ ppm: 6.84 (s, 2H, CH), 7.39 (s, 4H, ArH), 7.51 (s, 4H, ArH), 11.48 (s, 2H, NH) ppm; 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 75.50 (CH), 128.4 (CH), 130.45 (CH), 133.21 (CH), 133.8 (CH), 184.1 (C
3 (v/v)) = 0.31; IR (KBr)/ν (cm−1): 3438, 3168, 2925, 1629, 1492, 1249, 1157; 1H NMR (acetone-d6, 400 MHz)/δ ppm: 6.84 (s, 2H, CH), 7.39 (s, 4H, ArH), 7.51 (s, 4H, ArH), 11.48 (s, 2H, NH) ppm; 13C NMR (DMSO-d6, 100 MHz) δ (ppm): 75.50 (CH), 128.4 (CH), 130.45 (CH), 133.21 (CH), 133.8 (CH), 184.1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S) ppm; CHNcalculated (%): C (48.60), H (3.04), N (14.18), S (16.20), Cl (17.72); CHNfound (%): C (58.82), H (4.39), N (17.34), S (19.73).17
S) ppm; CHNcalculated (%): C (48.60), H (3.04), N (14.18), S (16.20), Cl (17.72); CHNfound (%): C (58.82), H (4.39), N (17.34), S (19.73).17
2.5.1.7. Tetrahydro-3,7-bis(3-hydroxyphenyl)-[1,2,4]triazolo[1,2-a][1,2,4]triazole-1,5-dithione (3g). White solid, mp = 165–167 °C; Rf (petroleum ether
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethylacetate = 7
ethylacetate = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (v/v)) = 0.12; IR (KBr)/ν (cm−1): 3423, 3177, 2958, 1601, 1505, 1250; 1H NMR (acetone-d6, 400 MHz) δ (ppm): 6.95 (s, 4H, Ar-H), 7.24 (s, 1H, CH), 8.62 (s, 1H, OH), 9.97 (s, 1H, NH) ppm; 13C NMR (acetone-d6, 100 MHz) δ (ppm): 73 (CH), 112.01 (CH), 113.88 (CH), 119.03 (CH), 130.0 (CH), 145.50 (CH), 158.09 (C), 184.0 (C
3 (v/v)) = 0.12; IR (KBr)/ν (cm−1): 3423, 3177, 2958, 1601, 1505, 1250; 1H NMR (acetone-d6, 400 MHz) δ (ppm): 6.95 (s, 4H, Ar-H), 7.24 (s, 1H, CH), 8.62 (s, 1H, OH), 9.97 (s, 1H, NH) ppm; 13C NMR (acetone-d6, 100 MHz) δ (ppm): 73 (CH), 112.01 (CH), 113.88 (CH), 119.03 (CH), 130.0 (CH), 145.50 (CH), 158.09 (C), 184.0 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S) ppm; CHNcalculated (%): C (53.70), H (3.88), N (15.04), S (17.68), O (9.70); CHNfound (%): C (53.76), H (3.58), N (15.34), S (17.74), O (9.66).
S) ppm; CHNcalculated (%): C (53.70), H (3.88), N (15.04), S (17.68), O (9.70); CHNfound (%): C (53.76), H (3.58), N (15.34), S (17.74), O (9.66).
2.5.1.8. Tetrahydro-3,7-bis(3-hydroxy-4-methoxyphenyl)-[1,2,4]triazolo[1,2-a][1,2,4]triazole-1,5-dithione (3h). White solid, mp = 177–180 °C; Rf (petroleum ether
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethylacetate = 7
ethylacetate = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (v/v)) = 0.06; IR (KBr)/ν (cm−1): 3422, 2929, 1510, 1278, 1133; 1H NMR (acetone-d6, 400 MHz)/δ ppm: 3.81 (s, 6H, OCH3), 6.86 (s, 2H, CH), 6.96–7.40 (m, 6H, ArH), 7.89 (s, 2H, OH), 9.88 (s, 2H, NH) ppm; 13C NMR (acetone-d6, 100 MHz) δ (ppm): 55.40 (OCH3), 77.09 (CH), 111.54 (CH), 112.79 (CH), 117.32 (CH), 129.98 (C), 146.91 (C), 148.37 (C), 185.00 (C
3 (v/v)) = 0.06; IR (KBr)/ν (cm−1): 3422, 2929, 1510, 1278, 1133; 1H NMR (acetone-d6, 400 MHz)/δ ppm: 3.81 (s, 6H, OCH3), 6.86 (s, 2H, CH), 6.96–7.40 (m, 6H, ArH), 7.89 (s, 2H, OH), 9.88 (s, 2H, NH) ppm; 13C NMR (acetone-d6, 100 MHz) δ (ppm): 55.40 (OCH3), 77.09 (CH), 111.54 (CH), 112.79 (CH), 117.32 (CH), 129.98 (C), 146.91 (C), 148.37 (C), 185.00 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S); CHNcalculated (%): C (51.52), H (4.29), N (13.35), S (15.55), O (15.29); CHNfound (%): C (51.34), H (4.83), N (12.88), S (14.98), O (15.97).
S); CHNcalculated (%): C (51.52), H (4.29), N (13.35), S (15.55), O (15.29); CHNfound (%): C (51.34), H (4.83), N (12.88), S (14.98), O (15.97).
3. Results and discussion
3.1. Characterization of magnetic nanocatalyst
The synthetic path to the magnetic nanocatalyst is shown in Scheme 2.Iron-oxide nanoparticles were prepared via co-precipitation and modified with a thin layer of amorphous silica by Stöber method through sol–gel method. The Fe3O4@SiO2-supported TiO2 magnetic catalyst obtained after addition of Ti(OC4H9)4 into the Fe3O4@SiO2 magnetic composite. Silica layer was utilized to encapsulate the Fe3O4 NPs to prevent any decrease in catalytic property when it was incorporated into the TiO2 structure. Because silica as a shell can decrease the electronic interactions at the point of contact.19
The magnetic nanoparticles were characterized by X-ray diffraction (XRD), Fourier transform infrared (FT-IR), scanning electron microscopy (SEM), energy-dispersive X-ray spectrometry (EDX), and vibrating sample magnetometer (VSM).
The phase and purity of three samples (Fe3O4, Fe3O4@SiO2 and Fe3O4@SiO2–TiO2) were plotted by the X-ray diffraction patterns (Fig. 1).
The XRD pattern of Fe3O4 MNPs exhibit the peaks at 2θ = 30.1, 35.5, 43.2, 53.5, 57.0, 62.8 and 74.3° (Fig. 1a). It is in agreement with the JCPDS card no. 19-0629.36 The coating of amorphous phase SiO2 does not change the structure of Fe3O4 nanoparticles (Fig. 1b). Also, the position and relative intensities of peaks indicate that the structure of Fe3O4@SiO2 can be remained after the surface modification with TiO2. The XRD pattern of the Fe3O4@SiO2–TiO2 MNPs shows peaks at 2θ = 25.2, 30.3, 35.6, 43.4, 48.2, 53.5, 57.2 63.1 and 74.4° (Fig. 1c). The peaks at around 25° and 48° conform to the (1 0 1) and (2 0 0) Bragg reflection planes which indicates the existence of tetragonal anatase TiO2.
The elemental composition is calculated from the energy dispersive X-ray. The EDAX image confirmed the existence of Fe, Si, O and Ti elements in the magnetic catalyst (Fig. 2). The elemental compositions of Fe3O4@SiO2–TiO2 nanocatalyst were 14.9, 70.3, 6.7, and 7.9 wt% for Fe, O, Si, and Ti, respectively. The presence of Ti in the composites indicates that the titanium dioxide nanoparticles have been deposited on the surface of Fe3O4@SiO2.
Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) were recorded to understand morphological changes occurring on the magnetic catalyst and also the size and shape of the nanoparticles.
The SEM and TEM images of synthesized samples are shown in Fig. 3. The SEM image of Fe3O4 MNPs have a mean diameter lower than 20 nm a nearly spherical shape (Fig. 3a). Fig. 3b shows that Fe3O4@SiO2 nanoparticles keep the morphological properties of Fe3O4 MNPs except for a larger particle size and smoother surface. The TEM image shown in Fig. 3d demonstrates that silica is successfully coated on the Fe3O4 NPs with a thin layer of different phase to form silica shell. So, Fe3O4@SiO2 is nearly in core–shell structure.
 | ||
| Fig. 3 SEM image of the (a) Fe3O4, (b) Fe3O4@SiO2 and (c) Fe3O4@SiO2–TiO2. TEM image of the (d) Fe3O4@SiO2 and (e) Fe3O4@SiO2–TiO2. | ||
Then, the chemical nature of the magnetic catalyst was surveyed. The SEM and TEM images in Fig. 3c and e show that Fe3O4@SiO2–TiO2 nanocatalyst have a larger particle size than Fe3O4@SiO2 nanoparticles (about 30 nm in size) and nearly spherical shape. The nanocatalyst shows some aggregation, which was due to calcination at 450 °C.39
In order to explore the molecular structures of Fe3O4, Fe3O4@SiO2, and Fe3O4@SiO2–TiO2, the FT-IR analysis was investigated. Fig. 4 shows the FT-IR spectra recorded in the range of 4000–400 cm−1. The main absorption peaks 3423 and 570 cm−1 were assigned to O–H and Fe–O stretching vibration modes of pure Fe3O4 NPs, respectively (Fig. 4a). The absorption peak of SiO2-coated sample at 450 cm−1 is due to the Si–O–Fe bond.
The bands at 1073 cm−1 and 803 cm−1 are characteristic peaks of the symmetrical and asymmetrical vibrations of Si–O–Si (Fig. 4b). The peak at 451 cm−1 is an indication of the presence of Si–O–Fe. Fig. 4c show the IR spectra of TiO2 modified Fe3O4@SiO2 NPs. From this spectrum, the appearance of a new peak at 636 cm−1 indicates the formation of Si–O–Ti surface structures and successful linking of the TiO2 onto the Fe3O4@SiO2 MNPs. There was no evidence for interaction between Ti and Fe in the IR spectra. It indicates that Fe3O4 is encapsulated by the SiO2 layer well. So, the catalytic property of Fe3O4@SiO2–TiO2 is not reduced by this interaction.
The magnetization curves of Fe3O4, Fe3O4@SiO2 and Fe3O4@SiO2–TiO2 were recorded in Fig. 5. The magnetic property of the Fe3O4@SiO2–TiO2 magnetic catalyst were examined and were compared with the magnetic properties of Fe3O4 and Fe3O4@SiO2. VSM study indicated a decrease in the magnetic saturation of Fe3O4@SiO2–TiO2 composite due to the non-magnetic nature of the shell NPs.
 | ||
| Fig. 5 Magnetization curves for the Fe3O4 (red line), Fe3O4@SiO2 (blue line) and Fe3O4@SiO2–TiO2 (green line) at room temperature. | ||
Room temperature specific magnetization versus applied magnetic field curve measurements of the Fe3O4@SiO2–TiO2 indicated the saturation magnetization value (Ms) of 31 emu g−1, which is slightly lower than of the Fe3O4@SiO2 (46.94 emu g−1) and uncoated Fe3O4 MNPs (55.70 emu g−1).
3.2. Application of Fe3O4@SiO2–TiO2 nanocomposite as a heterogeneous catalyst in the synthesis of tetrahydro-[1,2,4]triazolo[1,2-a][1,2,4]triazole-1,5-dithione derivatives
In this research, we tried to prepare tetrahydro-[1,2,4]triazolo[1,2-a][1,2,4]triazole-1,5-dithione derivatives from the reaction between aldazine derivatives and potassium thiocyanate under mild conditions. Titanium dioxide anchored on the surface of the Fe3O4@SiO2 nanoparticles and carried out successfully the cycloaddition reaction. Initially, in order to optimize the reaction conditions, the model reaction was proceeded from the condensation of benzaldazine and potassium isothiocyanate in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio at room temperature for the synthesis of compound 3a using TiO2, Fe3O4 or Fe3O4@SiO2–TiO2 as a catalyst. Fe3O4@SiO2–TiO2 acted as a highly efficient magnetic catalyst to synthesize perhydro[1,2,4]triazolo[1,2-a][1,2,4]triazole-1,5-dithione (3a) and afforded the product in higher yield and lower reaction time compared with other catalysts (Table 1). Also, other advantages of the current catalyst are simple separation and recoverable of Fe3O4@SiO2–TiO2 compared with reported catalysts. With notice to above results, the importance of Fe3O4@SiO2–TiO2 composite nanoparticles as a heterogeneous catalyst was revealed in this study and therefore it was selected as the efficient catalyst for further work.
2 ratio at room temperature for the synthesis of compound 3a using TiO2, Fe3O4 or Fe3O4@SiO2–TiO2 as a catalyst. Fe3O4@SiO2–TiO2 acted as a highly efficient magnetic catalyst to synthesize perhydro[1,2,4]triazolo[1,2-a][1,2,4]triazole-1,5-dithione (3a) and afforded the product in higher yield and lower reaction time compared with other catalysts (Table 1). Also, other advantages of the current catalyst are simple separation and recoverable of Fe3O4@SiO2–TiO2 compared with reported catalysts. With notice to above results, the importance of Fe3O4@SiO2–TiO2 composite nanoparticles as a heterogeneous catalyst was revealed in this study and therefore it was selected as the efficient catalyst for further work.
The obtained results of the reaction to determine the optimum amount of magnetic catalyst are shown in Table 2. The reaction slowly proceeded in low yield without catalyst. The higher yield of the corresponding product was obtained in shorter time with an increase in the amount of magnetic nanocatalyst. As can be seen from Table 1, comparison of the results shows a better yield using 0.04 g of catalyst to synthesize of tetrahydro-[1,2,4]triazolo[1,2-a][1,2,4]triazole-1,5-dithiones in the presence of Fe3O4@SiO2–TiO2. While a higher amount of the catalyst did not show any change in reaction time and yield of the corresponding product.
| Entry | Catalyst loading (g) | Time (min) | Yield (%) |
|---|---|---|---|
| a Reaction condition: aldazine (1 mmol), KSCN (2 mmol) AcOH (0.18 mL) and CH3CN (6 mL) at room temperature. | |||
| 1 | Blank | 60 | 32 |
| 2 | 0.01 | 35 | 79 |
| 3 | 0.02 | 23 | 96 |
| 4 | 0.03 | 17 | 97 |
| 5 | 0.04 | 17 | 98 |
| 6 | 0.05 | 17 | 98 |
To demonstrate the advantage of the present work, we compared the results of Fe3O4@SiO2–TiO2 magnetic catalyst as a catalyst in the synthesis of tetrahydro-[1,2,4]triazolo[1,2-a][1,2,4]triazole-1,5-dithiones in the presence of various solvents (Table 3). It can be seen from Table 2 that the present CH3CN was found to be the most efficient solvent among tested solvents in term of reaction time of the desired products in the presence of Fe3O4@SiO2–TiO2.
| Entry | Solvent | Time (min) | Yield (%) |
|---|---|---|---|
| a The catalyst sonicated for 20 min before utilization. | |||
| 1 | DMSO | 20 | 92 |
| 2 | CH3CN | 17 | 98 |
| 3 | H2O | 25 | 63 |
| 4 | EtOH | 25 | 89 |
| 5 | CHCl3 | 37 | 45 |
After establishing the optimal conditions, the scope of the cycloaddition reaction between potassium thiocyanate and several aldazine derivatives were carried out at ambient temperature according to the general experimental procedure (Table 4). As can be seen from this table, benzaldazine bearing ortho substituent (Table 4, entry 4) slightly afford lower yield than benzaldazines bearing meta or para substituents. This is possibly due to the steric effect. There is more steric hindrance for the 2-substituted benzaldazine on the product formation than the 3- or 4-substituted benzaldazines.
| Entry | R | Product | Time (min) | Yield (%) | Mp (°C) |
|---|---|---|---|---|---|
| a Reaction conditions: aldazine (1 mmol), KSCN (2 mmol), AcOH (0.18 mL), Fe3O4–SiO2–TiO2 (0.04 g) and CH3CN (6 mL) at room temperature. | |||||
| 1 | C6H5 | 3a | 17 | 98 | 189–190 |
| 2 | 3-NO2C6H4 | 3b | 30 | 88 | 197–199 |
| 3 | 4-OCH3C6H4 | 3c | 15 | 98 | 165–166 |
| 4 | 2-ClC6H4 | 3d | 43 | 77 | 190–192 |
| 5 | 3-ClC6H4 | 3e | 20 | 92 | 194–196 |
| 6 | 4-ClC6H4 | 3f | 16 | 94 | 197–200 |
| 7 | 3-OHC6H4 | 3g | 25 | 90 | 167–169 |
| 8 | 3-OH, 4-OCH3C6H3 | 3h | 19 | 95 | 170–173 |
| 9 | 2-OH, 4-CH3C6H3 | — | 240 | — | — |
| 10 | 2-OHC6H4 | — | 240 | — | — |
In the case of aldazine derivatives with a hydroxyl group at 2-position (Table 4, entries 9 and 10), the desired products were not synthesized. This is possibly due to tautomeric phenomena of these compounds.
Tetrahydro-[1,2,4]triazolo[1,2-a][1,2,4]triazole-1,5-dithiones as products in a green method were prepared using of potassium thiocyanate and various aldazines in the presence of catalytic amount of Fe3O4–SiO2–TiO2 MNPs (0.04 g) at room temperature. The structure of the described products was confirmed by physical and spectroscopic data. The infrared spectra of the tetrahydro-[1,2,4]triazolo[1,2-a][1,2,4]triazole-1,5-dithiones exhibits a band at about 3400 cm−1 and another band at about 1250 cm−1 due to NH and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S related to the fused five membered rings, respectively. In the 1H NMR spectra the signal around δ = 8–11 ppm is assigned to NH hydrogen atom.
S related to the fused five membered rings, respectively. In the 1H NMR spectra the signal around δ = 8–11 ppm is assigned to NH hydrogen atom.
From a green chemistry perspective, the stability of Fe3O4–SiO2–TiO2 magnetic catalyst has been investigated by the possibility of reusability. Upon completion of the reaction, to evaluate the catalytic stability of the catalyst, the magnetic catalyst recycled readily by an external magnet, was rinsed with ethyl acetate several times and was dried. It was utilized to a second run of the reaction. The prepared nanocatalyst was stable without the need for surfactant stabilizers. Although the yield decreased from 98 to 96% after six runs, the catalytic activity was still acceptable (Table 5).
| Run | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Yield (%) | 98 | 98 | 97 | 97 | 97 | 96 | 93 |
3.3. The proposed reaction mechanism
One of the significant advantage of the present catalyst is a large number of empty d-orbitals of the TiO2 and the low amount of nanocatalyst used in the reaction. The magnetically separable TiO2 catalyst show great catalytic activity. The features of the Ti4+ centres on the surface of TiO2 nanoparticles play an important role in increasing activation of thiocyanate. In fact, titanium dioxide grafted onto the Fe3O4@SiO2 could catalyze the reaction by the coordination of the unfilled orbitals of TiO2. Thus, it is obvious that Fe3O4@SiO2–TiO2 catalytic system increases the rate of reaction.The formation of the corresponding products can be explained by a proposed mechanism (Scheme 3). According to above reason, enhancing the electrophilic property of the thiocyanate has occurred using titanium dioxide supported on Fe3O4@SiO2. Then, aldazine react as dipoles with two molecules of activated thiocyanate as a dipolarophile to form tetrahydro-[1,2,4]triazolo[1,2-a][1,2,4]triazole-1,5-dithiones.
 | ||
| Scheme 3 The proposed mechanism for the synthesis of tetrahydro-[1,2,4]triazolo[1,2-a][1,2,4]triazole-1,5-dithiones using Fe3O4@SiO2–TiO2. | ||
4. Conclusions
In this research, we have been described the synthesis of tetrahydro-[1,2,4]triazolo[1,2-a][1,2,4]triazole-1,5-dithiones via condensation of different kinds of aldazine derivatives with potassium isothiocyanate using Fe3O4–SiO2–TiO2 composite nanoparticles as a magnetic nanocatalyst. The reaction in the presence of recent magnetic catalyst indicated a lot of significant advantages such as eco-friendly and recyclable catalyst, excellent product yields, low reaction times and simplicity of work-up.Acknowledgements
The authors are grateful to University of Kashan for supporting this work by Grant 256722/XVI.References
- Q. Lu, G. Song, J. P. Jasinski, A. C. Keeley and W. Zhang, Green Chem., 2012, 14, 3010–3012 RSC.
- K. V. Gothelf, Cycloaddition Reactions in Organic Synthesis, ed. S. Kobayashi and K. A. Jørgensen, Wiley-VCH, Weinheim, 2002, pp. 211–247 Search PubMed.
- S. Karlsson and H. E. Hogberg, Org. Prep. Proced. Int., 2001, 33, 103–172 CrossRef CAS.
- C. Najera and J. M. Sansano, Curr. Org. Chem., 2003, 7, 1105–1150 CrossRef CAS.
- J. R. Bailey and A. T. McPherson, J. Am. Chem. Soc., 1917, 39, 1322–1338 CrossRef CAS.
- H. Zachová, S. Man, J. Taraba and M. Potáček, Tetrahedron, 2009, 65, 792–797 CrossRef.
- J. Verner and M. Potáček, Molecules, 2006, 11, 34–42 CrossRef CAS.
- R. Huisgen, Angew. Chem., Int. Ed. Engl., 1963, 2, 565–598 CrossRef.
- T. Wagner-Jauregg, Synthesis, 1976, 349–373 CrossRef CAS.
- J. G. Schantl, H. Gstach, P. Hebeisen and N. Lanznaster, Tetrahedron, 1985, 41, 5525–5528 CrossRef CAS.
- J. G. Schantl and P. Nadenik, Synlett, 1998, 7, 786–788 CrossRef.
- H. Kumar, R. Goyal, S. Kaur, R. D. Anand, A. Parmar and B. Kumar, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2002, 2182–2184 CAS.
- K. Burger, H. Schickaneder, F. Hein, A. Gieren, V. Lamm and H. Engelhardt, Liebigs Ann. Chem., 1982, 5, 845–852 CrossRef.
- F. J. Hoogesteger, R. W. A. Havenith, J. W. Zwikker, L. W. Jenneskens, K. Huub, N. Veldman and A. L. Spek, J. Org. Chem., 1995, 60, 4375–4384 CrossRef CAS.
- S. Evans, R. C. Gearhart and E. E. Schweizer, J. Org. Chem., 1977, 42, 452–458 CrossRef CAS.
- J. Safari, S. Gandomi-Ravandi and M. Ghotbinejad, J. Korean Chem. Soc., 2012, 56, 78–81 CrossRef CAS.
- J. Safari, S. Gandomi-Ravandi and M. Ghotbinejad, J. Saudi Chem. Soc., 2012 DOI:10.1016/j.jscs.2012.02.009.
- T. T. Baby and S. Ramaprabhu, Talanta, 2010, 80, 2016–2022 CrossRef CAS.
- A. H. Lv, C. Hu, Y. L. Nie and J. H. Qu, Appl. Catal., B, 2010, 100, 62–67 CrossRef CAS.
- M. L. Luo, D. Bowden and P. Brimblecombe, Appl. Catal., B, 2009, 87, 1–8 CrossRef CAS.
- Y. M. Ren, Q. Dong, J. Feng, J. Ma, Q. Wen and M. L. Zhang, J. Colloid Interface Sci., 2012, 382, 90–96 CrossRef CAS PubMed.
- S. H. Sun, H. Zeng, D. B. Robinson, S. Raoux, P. M. Rice, S. X. Wang and G. X. Li, J. Am. Chem. Soc., 2004, 126, 273–279 CrossRef CAS.
- Z. D. Zhang, J. G. Zheng, I. Skorvanek, G. H. Wen, J. Kovac, F. W. Wang, J. L. Yu, Z. J. Li, X. L. Dong, S. R. Jin, W. Liu and X. X. Zhang, J. Phys.: Condens. Matter, 2001, 13, 1921–1929 CrossRef CAS.
- S. H. Xuan, F. Wang, X. L. Gong, S. K. Kong, J. C. Yu and K. C. Leung, Chem. Commun., 2011, 47, 2514–2516 RSC.
- A. L. Morel, S. I. Nikitenko, K. Gionnet, A. Wattiaux, J. Lai-Kee-Him, C. Labrugere, B. Chevalier, G. Deleris, C. Petibois, A. Brisson and M. Simonoff, ACS Nano, 2008, 2, 847–856 CrossRef CAS.
- L. Zhou, C. Gao and W. J. Xu, Langmuir, 2010, 26, 11217–11225 CrossRef CAS.
- Z. Q. He, T. M. Hong, J. M. Chen and S. Song, Sep. Purif. Technol., 2012, 96, 50–57 CrossRef CAS.
- G. H. Du, Z. L. Liu, X. Xia, Q. Chu and S. M. Zhang, J. Sol-Gel Sci. Technol., 2006, 39, 285–291 CrossRef CAS.
- C. Q. Yang, G. Wang, Z. Y. Lu, J. Sun, J. Q. Zhuang and W. S. Yang, J. Mater. Chem., 2005, 15, 4252–4257 RSC.
- Q. Dai, J. Wang, J. Yu, J. Chen and J. Chen, Appl. Catal., B, 2014, 144, 686–693 CrossRef CAS.
- M. Shokouhimehr, Y. Z. Piao, J. Y. Kim, Y. J. Jang and T. Hyeon, Angew. Chem., 2007, 119, 7169–7173 CrossRef.
- T. Cheng, D. Zhang, H. Li and G. Liu, Green Chem., 2014, 16, 3401–3427 RSC.
- L. L. Chng, N. Erathodiyil and J. Y. Ying, Acc. Chem. Res., 2013, 46, 1825–1837 CrossRef CAS PubMed.
- B. Karimi, F. Mansouri and H. Vali, Green Chem., 2014, 16, 2587–2596 RSC.
- A. Bamoniri and N. Moshtael-Arani, RSC Adv., 2015, 5, 16911–16920 RSC.
- J. Safari and L. Javadian, C. R. Chim., 2013, 16, 1165–1171 CrossRef CAS.
- J. Safari and L. Javadian, Ultrason. Sonochem., 2015, 22, 341–348 CrossRef CAS PubMed.
- H. Naeimi and Z. S. Nazifi, J. Nanopart. Res., 2013, 15, 2026–2036 CrossRef PubMed.
- W. Liyan, W. Hongxia, W. Aijie and L. Min, Chin. J. Catal., 2009, 30, 939–944 CrossRef.
- J. Safari and S. Gandomi-ravandi, Synth. Commun., 2011, 41, 645–651 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra23457c |
| This journal is © The Royal Society of Chemistry 2015 |