Open Access Article
Open Access ArticleMXenes vs. clays: emerging and traditional 2D layered nanoarchitectonics
Eduardo
Ruiz-Hitzky
 *a and
Cristina
Ruiz-Garcia
*a and
Cristina
Ruiz-Garcia
 b
b
aMaterials Science Institute of Madrid, CSIC, c/Sor Juana Inés de la Cruz 3, 28049 Madrid, Spain. E-mail: eduardo@icmm.csic.es
bChemical Engineering Department, Faculty of Science, c/Francisco Tomás y Valiente 7, Universidad Autónoma de Madrid, 28049 Madrid, Spain
First published on 8th November 2023
Abstract
Although MXene materials are considered an emerging research topic, they are receiving considerable interest because, like metals and graphene, they are good electronic conductors but with the particularity that they have a marked hydrophilic character. Having a structural organization and properties close to those of clay minerals (natural silicates typically with a lamellar morphology), they are sometimes referred to as “conducting clays” and exhibit colloidal, surface and intercalation properties also similar to those of clay minerals. The present contribution aims to inform and discuss the nature of MXenes in comparison with clay phyllosilicates, taking into account their structural analogies, outstanding surface properties and advanced applications. The current in-depth understanding of clay minerals may represent a basis for the future development of MXene-derived nanoarchitectures. Comparative examples of the preparation, and studies on the properties and applications of various nanoarchitectures based on clays and MXenes have been included in the present work.
1. Introduction
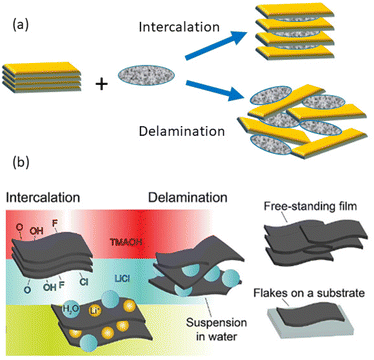
Research on 2D solids represents a very important area to achieve nanoscale functional materials with extraordinary properties capable of reaching advanced applications in many diverse research fields. In this way, inorganic layered solids such as transition metal oxides and chalcogenides (TMD), carbides and nitrides, phosphates and phosphonates, graphite and graphene-like materials, layered double hydroxides (LDHs), layered alkaline silicates and silicic acids, as well as a large group of phyllosilicates belonging to the family of clay minerals, have received much attention in recent years due to their outstanding physicochemical properties.The comparative physicochemical characteristics of a selection of 2D solids (MXenes, clay minerals, vanadium pentoxide xerogel, the lithiated phase of molybdenum disulphide and graphene/graphite) are grouped in Table 1. All of them have the ability to intercalate very diverse chemical species, as well as the capability to delaminate and/or exfoliate in single layers (nanosheets or monolayers) (Fig. 1), thus contributing to the assembly of diverse chemical species, including polymers and nanoparticles, generating a large family of nanoarchitectured materials.1–9 This characteristic of 2D solids makes it possible to prepare many diverse hybrid materials and nanoarchitectures by the intercalation of neutral and ionic species3,5,6,10–16 or by assembly with inorganic nanoparticles of a very distinct nature.17–21
| Physicochemical characteristics | MXenes | Clay minerals | V2O5·nH2O | LixMoS2 | Graphite/graphene |
|---|---|---|---|---|---|
| Inorganic layered (2D) solids | ✓ | ✓ | ✓ | ✓ | ✓ |
| Elemental layers of nanometric thickness | ✓ | ✓ | ✓ | ✓ | ✓ |
| Hydrophilic character | ✓ | ✓ | ✓ | ✓ | ✗ |
| Stable colloidal water dispersion | ✓ | ✓ | ✓ | ✓ | ✗ |
| Intercalation ability | ✓ | ✓ | ✓ | ✓ | ✓ |
| Electronic conductivity | ✓ | ✗ | * | ✓ | ✓ |
MXenes and 2D clay minerals are amenable for forming stable dispersions of delaminated solids in the form of flakes constituted by single layers or as few-layer materials showing remarkable characteristics that can be different from those in their condensed state.18 MXenes (Mn+1XnTx) discovered in the past decade22 represent a wide family of 2D solids derived from ternary transition metal carbides and/or carbonitrides named “MAX phases” of Mn+1AXn general formula where “M” represents a transition metal of elements in the periodic table from columns 3 and 7 (e.g., Ti) and “A” belongs to elements from columns 13 to 16 (e.g., Al). “X” represents carbon and/or nitrogen (carbides, nitrides or carbonitrides) and OH, O or F species resulting from HF treatment and located as surface terminations, called “T” groups, responsible for the hydrophilic properties possessed by MXenes (Fig. 2d and e).23 MXenes are receiving tremendous interest due to their exceptional physicochemical properties endowing advanced technological applications and constituting therefore marked progress in the field of new functional materials.22–27 MXenes are also known by the nickname of “conductive clays”,† apparently because of their similar characteristics to clay minerals.28 The appellation of clays as MXenes would be attributed to their similarity in terms of physicochemical properties like those of some 2D clays. In some cases, they have been called “carbide clays” (see for instance the paper by Ghidiu et al.28) which, in our opinion, is not entirely correct, since “clays” is a term mainly associated with silicates and other small-size particles present in the geosphere. Even more, publications on typical MXene topics have been recently presented at leading clay conferences, as for instance “The 2023 60th Clay Minerals Society Meeting”.29 From our perspective, it should be considered useful and enriching for clay scientists to know studies related to 2D solids of a different nature from clays, as well as other materials related to clay minerals, including also small colloidal particles, such as silica, iron oxides, etc., and, therefore, MXenes, in our opinion, may be included here.
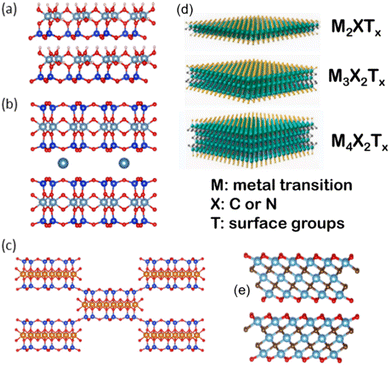 | ||
Fig. 2 Crystal structures of a selection of clay minerals (a–c) based on Al- and Mg-phyllosilicates of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 type such as kaolinite (a), 2 1 type such as kaolinite (a), 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 type such as montmorillonite (b) and sepiolite (c), and 2D MXenes (d and e) of Mn+1XnTx, as a general formula (M = early transition metal; X = C and/or N, and Tx = OH, F surface terminations) (d). The value n = 1–4 indicates the number of transition metal layers (and carbon and/or nitrogen layers), i.e., Ti3C2Tx (n = 2) (e). The (a), (b), (c) and (e) figures have been built using VESTA 3 software,33 and (d) has been adapted from Wyatt et al., 2D MXenes: Tunable Mechanical and Tribological Properties, Adv. Mater., 2021, 33, 2007973, Wiley. 1 type such as montmorillonite (b) and sepiolite (c), and 2D MXenes (d and e) of Mn+1XnTx, as a general formula (M = early transition metal; X = C and/or N, and Tx = OH, F surface terminations) (d). The value n = 1–4 indicates the number of transition metal layers (and carbon and/or nitrogen layers), i.e., Ti3C2Tx (n = 2) (e). The (a), (b), (c) and (e) figures have been built using VESTA 3 software,33 and (d) has been adapted from Wyatt et al., 2D MXenes: Tunable Mechanical and Tribological Properties, Adv. Mater., 2021, 33, 2007973, Wiley. | ||
The traditional term “clays” refers to raw materials or rocks from sedimentary deposits, but it is often used in the literature as an abbreviation for “clay minerals”, although the latter should be considered more appropriate for an accurate designation of this type of silicate.30 Clay minerals occur in nature as a result of the chemical weathering of silicate rocks, so the chemical composition of clays is not well defined in natural deposits. This is a limiting factor for their use in advanced applications, including the preparation of organic–inorganic hybrid materials and diverse functional nanoarchitectures. Despite their costs compared with natural clays, synthetic clay minerals with a well-designed composition and structure appear as an alternative to provide advanced functional materials for new applications that require well-defined and homogeneous samples.31 In addition, clay minerals are composed mainly of silica, alumina or magnesia or both and water, but iron or titanium could substitute for aluminum and/or magnesium in varying ratios, and appreciable quantities of inorganic ions like Na+, K+, Mg2+ and Ca2+ are frequently present as well.32
Typical clay minerals belong to several families that among other 2D silicates include from one side kaolinite and halloysite aluminosilicates, organized as a layered superposition, each composed of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 of two sheets of tetrahedral and octahedral components (SiO4 and Al2O3, respectively), as well as smectites (e.g., montmorillonite and hectorite) and vermiculites. These two latter groups are called 2
1 of two sheets of tetrahedral and octahedral components (SiO4 and Al2O3, respectively), as well as smectites (e.g., montmorillonite and hectorite) and vermiculites. These two latter groups are called 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 clays because they are structurally composed of layers comprising two tetrahedral sheets sandwiching one octahedral sheet. These 1
1 clays because they are structurally composed of layers comprising two tetrahedral sheets sandwiching one octahedral sheet. These 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 clay minerals are present under the general aspect of platelets, except halloysite clay that can also be present as nanotubes. Finally, there is a group of clay minerals such as sepiolite and palygorskite, with alternating structural blocks (2
1 clay minerals are present under the general aspect of platelets, except halloysite clay that can also be present as nanotubes. Finally, there is a group of clay minerals such as sepiolite and palygorskite, with alternating structural blocks (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 type) and tunnels showing a fibrous-like morphology. A representative selection of the structures of these types of clay is shown in Fig. 2.
1 type) and tunnels showing a fibrous-like morphology. A representative selection of the structures of these types of clay is shown in Fig. 2.
As far as their structural arrangement is concerned, it can be comparatively summarized that clays result from a combination of silica tetrahedra and aluminum or magnesium octahedra forming sheets. The isomorphic substitutions can generate negative charges compensated by exchangeable cations in the interlamellar region. On the other hand, MXenes have a completely different composition: they are aluminum carbides derived from the so-called MAX phases by the extraction of these atoms described above in arranged sheets with a delocalization of electrons in the lamellae that ensures an electrical conductivity comparable to that of graphene but absolutely different from that of clays.
Numerous review articles have been published to date dealing separately with very different aspects of the 2D traditional clays and the emerging MXenes. To the best of our knowledge, there is currently no publication dealing with the comparative aspects considered here with both types of layered solid.
The main goal of this critical review is to highlight the recent advances of MXenes in the development of new functional nanoarchitectured materials in connection with traditional clay minerals and their derivatives. Since the latter have been extensively studied in the past decades to develop advanced nanoarchitectured materials, this generated knowledge can be considered in many aspects as models to be applied in a useful way for the future development of new functional 2D MXene materials, based on the close relationship between these two types of solid.
2. Preparation and comparative physicochemical characteristics of clays and MXenes
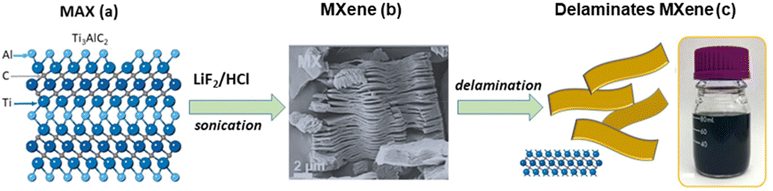
Clay minerals are naturally occurring silicates present in soils. Although some of them can also be synthesized at the laboratory and industrial scales, their most widespread use refers to minerals present in the abundant deposits available worldwide.31 The advantage of using synthetic clays should be mainly related to their potential use in advanced applications, facilitating the preparation of samples of controlled composition, as well as new crystalline phases. In addition, there is the possibility to modify them by introducing specific properties and also to prepare high-purity materials of interest in several fields, for example, in biomedicine.However, as already mentioned, the most economical and sustainable way to obtain clays with a certain degree of purity is to exploit their sedimentary deposits, located in countless places around the Earth. In fact, life on Earth is linked to the presence of clays and therefore of water, which is necessary for its geoformation. The use of clays for the preparation of advanced materials requires a previous purification of the raw clay, generally by sedimentation techniques, trying to eliminate common impurities such as quartz, mica, carbonates, iron oxides, organic matter, etc., which usually accompany natural clay silicates as minor components. In the frequent case of the purification of smectite clays (e.g. montmorillonite) it is important to proceed in a first step to the saturation of the silicate with Na+ ions by ion exchange processes to prepare the sodium homoionic clays, which are generally used with a known particle size distribution around the micrometer diameter.34 As for carbides, unlike the abundant clay silicates on Earth, only a few exoplanets appear to consist of a mantle dominated by carbides rather than silicates.35 Carbides related to the MXene family derive from the “MAX” phases (hexagonal ternary two-dimensional materials, vide supra), which in turn are usually synthesized by solid state reactions at high temperatures under an inert atmosphere, appearing as the most competitive method compared with alternative ways such as isostatic pressing, sputtering, or spark plasma sintering.36 For example, the typical procedure for the synthesis of the Ti3AlC2 typical MAX phase consists of the treatment of stoichiometric amounts of Ti, Al and C (as graphite), and grinding in ball mill equipment before reacting at 1400–1700 °C for 1–4 h in a tubular furnace under an Ar flux. This procedure is considered the best synthesis approach, as it represents a relatively low-cost and scalable process compared with the alternative methods.36,37Table 2 shows several strategies of MXene synthesis from MAX phases, mostly selected from the work of Chen and co-workers.38 The etching of “A” layers (e.g., Al in Ti3AlC2) by immersion in concentrated HF at room temperature was the first method for MXene preparation reported by Naguib and co-workers in 2011.22
| Synthesis strategies | Involved reagents | Materials characteristics | Advantages | Drawbacks |
|---|---|---|---|---|
| HF etching | HF | • Accordion-like structure | • Effective for most MAX | • Dangerous operation |
| • Abundant F terminations | • High yield | • Cannot be peeled | ||
| Fluoride-based acid etching | LiF/NaF/KF + HCl NH4HF2 | • Clay-like MXene | • Relatively safe | • Long etching time |
| • Large interlayer spacing | • Direct ultrasonic peeling | • Introducing fluoride salt impurities | ||
| • Few –F terminations | ||||
| Alkaline solution etching | NaOH or TMAOH | • Accordion-like structure only with –O, –OH terminations | • No risk of acid corrosion | • NaOH: severe etching conditions |
| • Free of F terminations | • TMAOH: HF pretreatment | |||
| HCl-based hydrothermal etching | HCl (aq) | • Layered structure with –Cl and –O terminations | • Easy experimental operation | • Severe etching conditions |
| • Fluorine-free | • Rely on the DFT predictions | |||
| Lithiation-expansion: microexplosion mechanism | Lithium ions | • Single- or few-layer structure without –F terminations | • Easy and safe synthesis | • Low productivity |
| • Free of –F terminations | • Consume resources | |||
| Water free etching | NH4HF2 in organic solvents | • Accordion-like structure | • Compatible with organic systems | • Long etching time |
| • Abundant –F terminations | • Delamination by ultrasonication | • Tedious washing steps | ||
| Molten salts | CuCl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NaCl NaCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KCl KCl |
• Delamination with TBAOH | • Fluorine-free process | • High temperature (ca. 700°) required |
| • Abundant –Cl terminations | • Free of –F terminations | |||
| Ionic liquids | Imidazolium-based ionic liquids | • Accordion-like morphologies but different from Ti3C2Tx produced by HF etching | • Fast and HF-free process avoiding the use of any acid | • High cost and toxicity of ionic liquids |
The resulting suspension was washed with water followed by centrifugation to separate the remaining solids, which after ultrasonication in methanol gave rise to flakes containing the carbide nanosheets by exfoliation of the MXenes remaining in suspension (Fig. 3).
To avoid the use of concentrated HF, which is a toxic and aggressive reagent that requires careful safety precautions, alternative MXene synthesis approaches have been developed up to the present. In this way, Ghidiu et al.28 reported in 2014 the first synthesis of an MXene using LiF dissolved in concentrated HCl at moderate temperature. Although extended periods of reaction time were required, the use of concentrated HF solutions was avoided. Despite the fact that the latter procedure is safer than the former, it still produces small amounts of HF, so it is of great interest to develop alternative methods that completely avoid the use of fluorinated compounds, preventing the formation of terminations “T” as F bonded to the resulting MXene sheets. In this regard, Li et al.42 used a hydrothermal etching route by autoclaving at 270 °C in highly concentrated NaOH. Following this approach, a lot of work has been developed but nevertheless according to Chen et al.38 the advantages of the use of NaOH at elevated temperatures, which involves potential high safety risks too, are not clear. Thus a safer organic alkali etching procedure was proposed by Xuan et al. using TMAOH to successfully etch MAX phases like Ti3AlC2.43 In this case a previous step is required, applying a first treatment of the MAX phase with HF at low concentration followed by Al etching by the TMAOH, leading to MXenes with Al(OH)4− species as terminal groups. The fact that the use of HF remains necessary here, coupled with the caustic and toxic nature associated with alkylammonium hydroxides, again points to the potential risks associated with current MXene synthesis procedures. In this context numerous researchers around the world continue studying safer and more sustainable synthesis methods.
It should be noted that fluorine is present in MXenes from HF or LiF treatments whereas the F occasionally detected in natural clays is due to its geoformation or introduced during its synthesis in the laboratory or in industry. Fluorine in clay minerals structurally substitutes hydroxyl groups very strongly bound to Mg2+ or Al3+ ions located in the octahedral layers of the clays, where it is immobilized. For example, the mineral hectorite, as well as the synthetic clay marketed as LAPONITE®, have a relatively high fluorine content, which is not capable of participating in interactions with substances placed in contact with clays,44 again making these silicates safe for humans.
To address experimental conditions that avoid the use of HF for MXene synthesis, alternative methods such as HCl-based hydrothermal etching, halogen etching, in situ electrochemical synthesis, CVD processes, molten salts, waterless etching, lithium insertion, ionic liquids, etc., have been proposed.38 For instance, etching of Ti3AlC2 with ammonium bifluoride (NH4HF2) in organic solvents of high polarity, such as dimethyl sulfoxide (DMSO), N,N-dimethyl formamide (DMF), propylene carbonate, etc., appears to be a safer procedure resulting in hybrid materials with increased interlayer spacing and high –F terminations.45 A drawback of the latter procedure is the long etching time (196 h) required at present to generate MXenes. An alternative fluorine-free procedure for the synthesis of MXenes consisting of treatments with CuCl2 in molten salts has been recently proposed by Liu et al.40 In this way, the Ti3AlC2 MAX phase reacts with molten salts (CuCl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NaCl
NaCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KCl), facilitating the Al etching in a redox process with the formation of copper metal. The AlCl3 formed in gas phase at the high temperature of the molten salts (ca. 700 °C) produces the expansion of the MXene layers (eqn (1)), which can be easily delaminated in a further treatment with tetrabutylammonium hydroxide (TBAOH) under ultrasonication.
KCl), facilitating the Al etching in a redox process with the formation of copper metal. The AlCl3 formed in gas phase at the high temperature of the molten salts (ca. 700 °C) produces the expansion of the MXene layers (eqn (1)), which can be easily delaminated in a further treatment with tetrabutylammonium hydroxide (TBAOH) under ultrasonication.
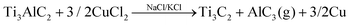 | (1) |
A different synthetic procedure for the preparation of MXenes avoiding the use of any acid, including HF, has been recently reported by Husmann et al. using fluorine-containing ionic liquids and water.46 The use of imidazolium-based ionic liquid containing BF4− or PF6− anions in the presence of water leads to the selective removal of Al, the ionic liquid intercalating between the MXene layers favoring their delamination in a very fast process compared with etching processes involving acidic reagents.
Finally, another interesting approach for the synthesis of fluorine-free MXenes is the so-called “lithiation–expansion–microexplosion mechanism” recently reported by Sun et al.47 As an example, Li from a lithium foil as a cathode is electrochemically inserted into Ti3AlC2, and the further ultrasonication in water generates a microexplosion reaction producing the etching and generation of Ti3C2Tx MXene. In our opinion, the latter mechanism could be compared with the formation of the lithiated phases of transition metal dichalcogenides (TMDs), where Li is spontaneously inserted into the van der Waals gap of the TMD.48 Interestingly, 2D dichalcogenides, such as MoS2, can be easily intercalated through a redox reaction with lithium reagents (e.g. n-butyllithium) in anhydrous solvents (e.g. n-hexane), giving rise to LixTMD compounds.48 Lithiated phases like LixMoS2 react violently with water, generating H2 and inducing TMD delamination in d-MoS2 single layers, which could re-stack entrapping diverse oxyethylene compounds such as crown-ethers and cryptand macrocycles, as well as poly(ethylene oxide) (PEO), leading to nanocomposites possessing controlled ion-mobility.15,49 Interestingly, these approaches could be of interest for possible application to MAX phases, trying to develop new procedures for the preparation of MXene functional nanoarchitectures. More recently, Rasamani and co-workers enlarged this approach, reporting MoS2 intercalation through the chemical insertion of Li+ following exchange with NH3 and NH4+ taking place in the interlayer region, with a second step of TMD exfoliation followed by restacking in the presence of diverse guest compounds.50 From these results, it may be considered that, in theory, MAX phases could foreseeably show a similar behavior to TMDs against lithiation processes. However, to date no reference to this effect has been identified by searching databases such as WoS-Clarivate.
Regarding clay minerals, swelling of smectites and vermiculites followed by delamination is also well known in these charged 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 phyllosilicates, particularly in homoionic samples exchanged with high hydration energy cations, such as Li+, Na+ and other alkaline ions. The extent of the swelling mainly depends on the location and density charge in the silicate microcrystals, as well as on the nature of the interlayer exchangeable cations. The swelling of smectites (montmorillonite, hectorite, etc.) exchanged with Li+ and Na+ ions leads to colloidal dispersions when water is spontaneously incorporated, provoking the delamination/exfoliation of individual clay layers (nanosheets) leading to stable colloidal dispersions.34 Vermiculites, which are similar in structure to smectites but have a higher density charge and often occur as macroscopic crystals, are also typical swelling clay minerals, especially when exchanged with Li+ or alkylammonium cations (i.e., n-propyl and n-butyl-ammonium)51 or even with certain amino acids such as L-ornithine.52 They give rise to macroscopic 2D swollen materials that generate visible vermicular (accordion-like) arrangements and lead to gels by delamination when they are dispersed in water or in solutions of very low salt concentration. These accordion-like morphologies, typically observed in the early swelling phases produced by vermiculites, are also observed (vide supra) to some extent in the lithiated MAX phases (Fig. 3), appearing as intermediate in the formation of delaminated MXenes.53
1 phyllosilicates, particularly in homoionic samples exchanged with high hydration energy cations, such as Li+, Na+ and other alkaline ions. The extent of the swelling mainly depends on the location and density charge in the silicate microcrystals, as well as on the nature of the interlayer exchangeable cations. The swelling of smectites (montmorillonite, hectorite, etc.) exchanged with Li+ and Na+ ions leads to colloidal dispersions when water is spontaneously incorporated, provoking the delamination/exfoliation of individual clay layers (nanosheets) leading to stable colloidal dispersions.34 Vermiculites, which are similar in structure to smectites but have a higher density charge and often occur as macroscopic crystals, are also typical swelling clay minerals, especially when exchanged with Li+ or alkylammonium cations (i.e., n-propyl and n-butyl-ammonium)51 or even with certain amino acids such as L-ornithine.52 They give rise to macroscopic 2D swollen materials that generate visible vermicular (accordion-like) arrangements and lead to gels by delamination when they are dispersed in water or in solutions of very low salt concentration. These accordion-like morphologies, typically observed in the early swelling phases produced by vermiculites, are also observed (vide supra) to some extent in the lithiated MAX phases (Fig. 3), appearing as intermediate in the formation of delaminated MXenes.53
The delamination of certain 2D solids into single-sheet or few-layer materials remaining as stable colloids in aqueous dispersions facilitates the formation of diverse nanoarchitectures following restacking processes. This approach was initially applied in clay-derivatives, the entrapment of molecules, polymers and nanoparticles being mainly characterized by spectroscopy, microscopy and diffraction techniques, including small-angle X-ray scattering and high-angle neutron diffraction. Compared with 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 phyllosilicates (i.e., smectite and vermiculite clays), the family of kaolinite 1
1 phyllosilicates (i.e., smectite and vermiculite clays), the family of kaolinite 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 aluminosilicates (kaolinite, nacrite, dickite and halloysite) can also be exfoliated/delaminated but with great difficulty. In this case, partial delamination can be reached by applying different routes from the usual ones for smectite and vermiculite. In fact, 1
1 aluminosilicates (kaolinite, nacrite, dickite and halloysite) can also be exfoliated/delaminated but with great difficulty. In this case, partial delamination can be reached by applying different routes from the usual ones for smectite and vermiculite. In fact, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 clay minerals can be hardly delaminated by mechanical exfoliation by grinding and assisted sonication.54
1 clay minerals can be hardly delaminated by mechanical exfoliation by grinding and assisted sonication.54
Table 3 compares various physicochemical characteristics of both smectite clay minerals and MXenes. They show many similar aspects, although decisive details related to their inherent properties, such as chemical composition and electronic conductivity, indicate that these two types of 2D material could be considered for similar or complementary applications.
| Chemical composition | Silicates | Carbides/nitrides/carbonitrides |
|---|---|---|
| Aspect ratio | High | High |
| 2D inorganic solids | √ | √ |
| Elemental layers of nanometric thickness | √ | √ |
| Hydrophilic character | √ | √ |
| Stable water dispersions | √ | √ |
| Ion-exchange capacity | √ | √ |
| Swelling and intercalation ability | √ | √ |
| Delamination ability | √ | √ |
| Electronic conductivity | No | √ |
| Biocompatibility | √ | √ |
| Natural origin | √ | No |
| Synthesis preparation | √ | √ |
| Cost | Low | High |
Certain physicochemical properties of clays highlighted above have led to their traditional and historical use since antiquity in pottery, ceramics and glass, building, paper making, polymer reinforcement, medical and pharmaceutical uses, decoration and artistic developments, among other applications. Clay minerals are nowadays receiving a wide range of uses in industry, engineering and the environment and more recently in nanotechnology, as summarized in Table 4, including a comparison with some recent MXene applications. In fact, clays have been used for thousands of years, even in daily life applications, while MXenes have only been known for a few years, but there have been rapidly developed high-tech advances in functional materials and nanoarchitectonic fields.
| Advanced applications | Clay-based materials | MXene-based materials |
|---|---|---|
| • Adsorption and heterogeneous catalysis | • Air and water purification by adsorption of common pollutants14,69 | • MXene-hybrids for environmental remediation80 |
| • Molecular separation: water and air purification. Environmental protection and remediation | • Pillared-clay materials by insertion of Al137+ polycations: intercalated clay catalysts70,71 | • Al13-Ti3C2Tx pillared-MXene by insertion of Al137+ polycations |
| • Clay modifications by assembly of semiconducting NP (TiO2, ZnO…) for photocatalytic reactions20 | • MXenes-TiO2 for photocatalysis24,81,82 | |
| • Uptake of heavy and radioactive metal ions using nanoscale modified clay minerals72,73 | • MXene-nanocomposites for heavy metal and radionucleid removal83,84 | |
| • Magnetic adsorbents based on ferrofluid infiltration74 | • MXenes-magnetite with enhanced photothermal activity85 and radionucleid removal86 | |
| • Clay-based bionanocomposites for oil-spill remediation75 | • MXene-based membranes and sponges for oil-spills87,88 | |
| • Wastewater and sewage treatments involving agricultural, urban and industrial wastewater treatments76–79 | • MXene-based nanoarchitectures for wastewater disposal89,90 | |
| • Membranes, ionic and electronic conductors and sensor devices | • Membranes for ion-recognition and oxygen sensors91 | • Membranes for ion-exchange processes102 |
| • Corrosion protection | • Bionanocomposites for ion-detection92–94 | • Bionanocomposites for ion-detection103 |
| • Organoclays as components of (bio)sensors16,95,96 | • Ionic and electronic conductors based on polymer-MXene nanocomposites104,105 | |
| • Ionic and electronic conductors based on polymer-clay nanocomposites11,95,97,98 | • Carbon-MXenes composites53,106 | |
| • Carbon-clay conductive materials16,96,99,100 | • Non-volatile memory devices107 | |
| • Protective, anticorrosion and barrier coatings101 | • EMI shielding108,109 | |
| • High-performance corrosion inhibition110,111 | ||
| • Electroactive and electrochemical materials | • Clay-based materials as electrical insulators (dielectrics)112,113 | • Components for supercapacitors120–122 |
| • Energy production and storage | • Electrical resistances by assembly of clays/graphite114 | • Use in photovoltaic devices123 |
| • Fuel-cell electrodes115 | • Thermoelectric power generation124 | |
| • Carbon-clay and conducting polymer-clay composites for energy storage99,116–119 | • Electrodes in Zn–air batteries, Li/Na ion batteries, Li–S batteries25,121,125–127 | |
| • Photoactive materials | • Photodegradation of organic pollutants128,129 | • Photocatalytic degradation of organic pollutants in water, such as dye waste132,133 |
| • Protection from solar and UV-radiation130 | • Photocatalytic fuel production, such as hydrogen H2 evolution from water splitting, and CO2 reduction133,134 | |
| • Protection from the photodegradation of labile pesticides131 | ||
| • Polymer nanocomposites | • Enhanced mechanical and thermal properties of nylon, epoxy, unsaturated polyester, engineering resins, etc.5,7,135 | • MXene-polymer nanocomposites with improved mechanical properties143,144 |
| • Improved flame retardancy of thermoplastics and thermosets136 | • MXene compounds for flame retardancy145 | |
| • Materials applied in the automotive, medical and healthcare, adhesive, building and construction sectors137–139 | • NH3 sensing and monitoring devices146 | |
| • Bionanocomposites as biocompatible materials139–141 | • MXene-based nanocomposites for piezoresistive sensors147,148 | |
| • Packaging and barrier films (food, cosmetics, drugs, electronics…)99,142 | • Photothermal and photodynamic properties149,150 | |
| • Biomedical applications53,127,151–153 | ||
| • Gas barrier143 | ||
| • Food, agriculture and farming | • Clay-organic derivatives for controlled release of agrochemical formulations154,155 | • MXenes in controlled pesticide release160 |
| • Crop-protection formulations156 | • MXene applications in sustainable agriculture161 | |
| • Processing of edible oils157 | ||
| • Mycotoxin sequesters158 | ||
| • Antifouling paints159 | ||
| • Pharmaceutical, biomedical and biotechnological applications | • Clay minerals and related solids for controlled drug release162 | • MXene nanocomposites for controlled drug delivery166,167 |
| • Clay derivatives as carriers of vaccines163 | • MXene-based nanoarchitectures for wound healing and hemostatic applications53,168 | |
| • Clay-based composites for wound healing and hemostatic applications164,165 | • MXene nanostructures for cancer therapy85,169,170 | |
| • Clay derivatives for tissue engineering139 | • Biofunctionalized MXenes for cancer detection171 | |
| • Clay-based bionanocomposites for non-viral gene transfection and cancer treatment | • MXene polymeric composites for tissue engineering172 | |
A common feature of MXenes and clays is their marked hydrophilic character, forming stable colloids (Table 3). Clay particles are easily dispersed in water, salt and molecular solutions, producing stable hydrogels with interesting rheological properties. In particular, smectite clay minerals such as montmorillonite exchanged with high hydration energy ions (i.e., Na+, Li+) give rise to delaminated silicate dispersions in water, which can self-organize via face–edge aggregation forming reversible thixotropic gel networks useful for a wide range of applications, for instance paints, petrochemicals, pesticides, food, cosmetics, pharmaceuticals and regenerative medicine.55–59 Applications related to food, human and animal health are possible due to the biocompatible character of clay minerals, which have been extensively used for medicinal purposes since prehistoric times.60–62 Fibrous clay minerals such as sepiolite could also be disaggregated in water by the application of high-speed shear forces or sonomechanical irradiation, giving rise to high-viscosity clay hydrogels that can be used to stabilize many diverse nanocomposites and nanoparticles, resulting in functional nano/micro-architectured solids.63 In turn, MXenes are produced as colloids after etching of the “A” elements from the MAX phase leading to the Mn+1XnTx compounds in water dispersion. Unlike clay minerals, these colloidal phases of MXenes are altered over time due to oxidation processes mainly attributed to their reaction with water molecules. The nature of the atmosphere (inert or oxygen-containing) adopted for their storage is an important factor in the chemical evolution of MXene flakes formed in aqueous media. For example, Ti3C2Tx prepared by Al etching in Ti3AlC2 in aqueous dispersion is decomposed to anatase after hours or days depending on the nature of the atmosphere in which it is placed.64 Dispersions in anhydrous solvents even in the presence of O2 preserve these MXenes against decomposition.64 In contrast, the colloids generated by the dispersion of clays are very stable and do not undergo appreciable alteration due to their prolonged contact with water and exposure to the atmosphere, even with strong variations of the ambient temperature. The chemical alterations of clay minerals can only take place in the presence of aggressive acidic or basic media and high temperatures. Clay modifications occur naturally through geochemistry in the Earth, generally taking place under hydrothermal conditions that lead to mineralogical, chemical, and textural changes in clays, affected by temperature and the chemical environment, and always over very long periods of time.65
Layered materials, and particularly clay minerals as well as the composites derived from them, have been extensively investigated from the point of view of molecular modeling and solid simulations. According to Cygan et al.66 computational chemistry techniques based on classical force field and quantum chemistry methods that are commonly employed to evaluate electronic structures offer a practical, very useful and affordable approach to evaluate the structure and dynamics of 2D solids at the atomic scale. Molecular dynamics (MD), Monte Carlo (MC) techniques and quantum methods could provide accurate models for computational studies of layered materials.66 Similar computational studies on MXenes appear very useful in determining their structure–properties relationships. In particular, aspects related to atomic structure, the nature and role of terminal groups, as well as surface chemistry and electronic structural features offer the most important atomistic insights.67 Computational tools are of particular interest to study more complex materials derived from MXenes, such as artificial nacre prepared by assembling MXenes and polymers arranged as nanocomposites.68
3. Clay intercalation vs. MXene intercalation: towards nanoarchitectured functional materials
Both 2D materials, clays and MXenes, have the ability to intercalate diverse organic and inorganic compounds acceding into their intracrystalline nanospaces following ion-exchange processes and other mechanisms. Clays and MXenes integrate in their structure nanolayers negatively charged with compensating cations in the interlayer space. The first well-defined organic–inorganic hybrid materials on record were developed by the intercalation of alkylammonium cations into smectites such as montmorillonite and related phyllosilicates (e.g. vermiculites) via cation exchange reactions.173–175 In fact, the interlayer cations present in smectites (exchangeable cations) can be replaced by many positively charged inorganic or organic compounds in aqueous solution. In addition to ion-exchange process, the interlayer insertion can be controlled by diverse mechanisms such as van der Waals forces, hydrogen bonding and water bridges, electrostatic bonds, ion–dipole and coordination, proton and electron transfer, and eventually by covalent bonding (i.e. grafting reactions).176,177 It can be postulated in a first approximation that MXene carbonitrides could also interact with compounds of a very different nature following processes similar to clays.Fig. 4 shows a comparison between clays and MXenes regarding their ability to intercalate neutral molecules such as H2O, N2H2, DMSO, DMF, metal and organic cations including bulky polyhydroxy cations such as Al137+ (“Al13” Keggin-cage, vide infra) and surfactants (e.g. cetyltrimethylammonium bromide, CTAB), polymers leading to polymer–clay nanocomposites, bionanocomposites, 3D silica networks, and small NPs (e.g., carbon nanomaterials), in some cases assisted by intercalation–delamination processes.
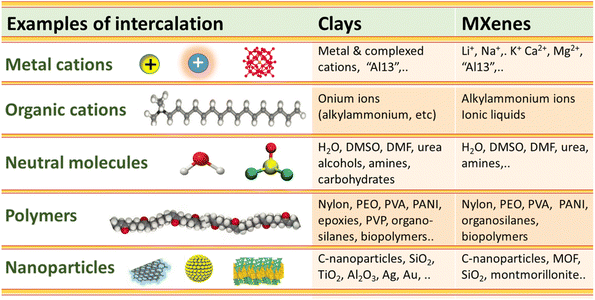 | ||
| Fig. 4 Scheme comparing the intercalation capacity of various chemical species in the interlayer space of clay minerals and MXene 2D solids, leading to their corresponding derivatives. | ||
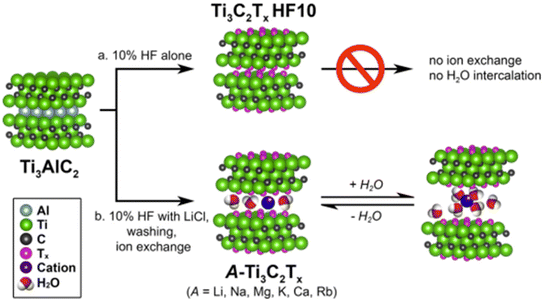
For instance, similarly to smectite clays, Ti3C2Tx MXenes lead to the spontaneous intercalation of neutral organic molecules such as hydrazine hydrate, N-methylformamide (NMF), DMF, DMSO, urea, etc.39,178,179 Cations of diverse nature, such as Li+, Na+, K+, NH4+, Mg2+ and Al3+, could be easily incorporated to the MXenes following ion-exchanging processes.120 The accessibility to the interlayer space depends on the experimental conditions adopted during the etching of the MAX phase (Fig. 5). However, when the etching process was carried out in the absence of lithium salts the ion-exchanges were disfavored. In fact, the presence of Li+ ions in the interlamellar space attained by HF/LiCl treatments of the MAX phase facilitates the easy exchange by alkali and alkaline earth cations (e.g., Na+, K+, Rb+, Mg2+ and Ca2+). As occurs in clay minerals, the basal spacing values of these 2D carbonitrides varies with the nature of the interlamellar cation as well as the relative humidity.
Compared with MXenes, 2D charged clay minerals (e.g. smectites and vermiculites) show relatively weak interactions between consecutive structural nanolayers. In fact, MXenes have a higher surface charge density than lamellar clays, so that to promote their exfoliation these carbides may require the application of shear forces, following a process similar to that proposed by Ma and Sasaki for transition metal oxides and hydroxides.9
3.1. Pillared clays and MXenes
Intercalations based on ion-exchange mechanisms occurring in 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 charged silicates (e.g. smectites and vermiculite clay minerals) may involve bulky positively charged inorganic species such as highly charged cage-like polyhydroxy cations, e.g. [Al13O4(OH)24(H2O)12]7+, the Keggin cage abbreviated as Al137+ or even “Al13”. These polycations have been used to prepare the so-called “pillared clays” intensely studied in the last 40 years.21,70,180–183 This type of bulky cation produces an expansion between the layers of the 2D solid of approximately 1 nm, in which after a calcination step, typically by heating at 300–500 °C, the Al13 is transformed into alumina pillars that bonded to the silicate layers. The resulting pillared clay materials are provided with a large specific surface area associated with a permanent micro- and meso-porosity and showing molecular sieving functions. The reproducible generation of pillared clays in large quantities regarding various industrial applications, particularly adsorption and heterogeneous catalysis, was the objective of big projects developed in the past decades.184
1 charged silicates (e.g. smectites and vermiculite clay minerals) may involve bulky positively charged inorganic species such as highly charged cage-like polyhydroxy cations, e.g. [Al13O4(OH)24(H2O)12]7+, the Keggin cage abbreviated as Al137+ or even “Al13”. These polycations have been used to prepare the so-called “pillared clays” intensely studied in the last 40 years.21,70,180–183 This type of bulky cation produces an expansion between the layers of the 2D solid of approximately 1 nm, in which after a calcination step, typically by heating at 300–500 °C, the Al13 is transformed into alumina pillars that bonded to the silicate layers. The resulting pillared clay materials are provided with a large specific surface area associated with a permanent micro- and meso-porosity and showing molecular sieving functions. The reproducible generation of pillared clays in large quantities regarding various industrial applications, particularly adsorption and heterogeneous catalysis, was the objective of big projects developed in the past decades.184
The interlayer insertion of inorganic clusters or nanoparticles including Al137+ polycations is highly interesting to prepare functional nanoarchitectures useful as efficient membranes, heterogeneous catalysts and energy storage components.37,185
Following a similar way to that reported several decades ago for clay minerals (Fig. 6), Al137+ polycations can be strongly and homogeneously bonded onto the surface of the Ti3C2Tx nanosheets through electrostatic mechanisms showing variable d-spacing values (0.27–1.12 nm) depending on the adopted experimental conditions and the nature of the initial mixture of components.37,185 In this way, by tuning the preparation conditions of the Al13-MXenes, 2D arrangements with basal spacings that can be adjusted over a wide range of high-precision d-spacing values (with a scale accuracy of 0.1 nm) are achieved. Thus, the properties of the pillared Ti3C2Tx-based MXenes are suitable for the fabrication of membranes with marked selectivity towards cations depending on their valence and showing wonderful performance in monovalent ion rejection.37,185 The resulting material derived from the Ti3C2Tx MXene was named “Al13-Ti3C2Tx clay”, but we must clarify that it differs significantly from typical Al13 pillared clays. In fact, in the latter, Al137+ polycations are transformed into alumina pillars by calcination at temperatures above 300 °C, which, to our knowledge, has not been applied in Al13-MXenes to date. These thermal treatments can be carried out both by conventional heating and by microwave irradiation181 with the aim to consolidate the Al13 pillars which, in this way, are transformed into protonated alumina pillars useful for acid catalysis processes.
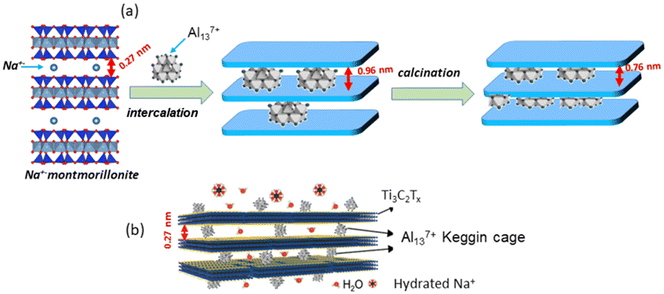 | ||
| Fig. 6 Schematic representation of the preparation of Al13 pillared clays based on homoionic (Na+) montmorillonite (a) with d-spacing values from the reference,181 and the structural arrangement showing Al13-Ti3C2Tx pillared MXene (b) formed by the assembly of the Al137+ polycations with Ti3C2Tx nanosheets in a colloidal solution (reprinted with permission from Zhu et al., ACS Nano, 2020, 14(11), 15306–15316. Copyright 2020 American Chemical Society). | ||
Silica-pillared clays have been prepared at the end of the last century by diverse intercalation approaches using smectites like montmorillonite and diverse pillaring precursors, such as tris(acetylacetonato)-silicon(IV) cations, i.e. Si(acac)3+ (ref. 186) and alkoxysilanes,187,188 allowing a permanent separation of the silicate nanosheets and enlarging the specific surface area of the clay mineral. The attempts to intercalate the hydrolyzed silica sol in montmorillonite by direct treatments were also reported by Moini and Pinnavaia,189 obtaining silica-clay solids possessing a relatively large surface area (250–460 m2 g−1). According to the XRD data, these latter materials do not exhibit regular intercalation phases as is typically observed in other pillared clay materials.
Letaïef and Ruiz-Hitzky described a new family of silica-clay materials that could be assimilated to inorganic–inorganic nanocomposites, as they consist of silicate nanosheets from delaminated clays dispersed in silica matrices (Fig. 7a).190 These silica-clay porous materials are produced through sol–gel processes using silicon alkoxides as precursors that react with smectites and vermiculites expanded by the previous intercalation of CTAB and other alkylammonium species.21,191
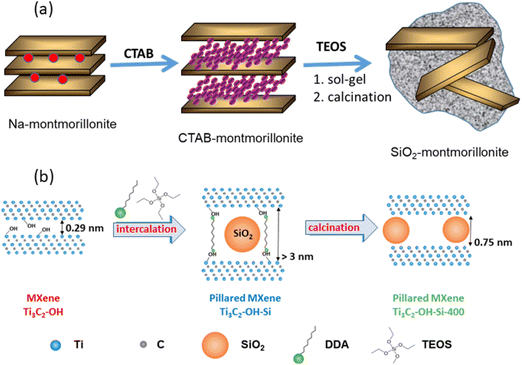 | ||
Fig. 7 Scheme showing: (a) the assembly of silica to delaminated 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 charged clay minerals through previous intercalation of CTAB and further reaction with an alkoxysilane (e.g., TEOS), as reported by Letaief and Ruiz-Hitzky,190 and (b) pillaring of Ti3C2Tx-MXene by silica introduced by the intercalation of TEOS in the presence of alkylammonium species (figure from ref. 192 licensed under CC-BY). 1 charged clay minerals through previous intercalation of CTAB and further reaction with an alkoxysilane (e.g., TEOS), as reported by Letaief and Ruiz-Hitzky,190 and (b) pillaring of Ti3C2Tx-MXene by silica introduced by the intercalation of TEOS in the presence of alkylammonium species (figure from ref. 192 licensed under CC-BY). | ||
Like clay minerals, MXenes can also be pillared with silica and even delaminated by inclusion in silica matrices. In this way, SiO2-pillared Ti3C2Tx MXenes have been recently synthesized in a first step by the co-intercalation of amines such as dodecyl- and octyl-amine in the presence of silicon alkoxides used as a silica precursor (e.g., tetraethyl orthosilicate (TEOS)). In a second step the resulting materials are calcined by heating under an argon atmosphere at temperatures ranging from 300 to 500 °C (Fig. 7b).192 A shift to lower angles is observed in the XRD patterns, particularly the (002) diffraction peak, indicating the increase of the interlayer spacing in the MXene reaching a maximum value of 3.2 nm. The resulting open structure due to the pillaring effect shows an extraordinary increase of the specific surface area showing values of 235 m2 g−1, which is twice the maximum surface area achieved by MXene-based materials.192 Interestingly, the presence of electroactive components in MXenes, unlike clays, can be used in the development of electrode materials for electrochemical devices. SiO2-pillared Ti3C2Tx materials have been successfully used as electrodes for Na+-ion batteries, showing superior capacity, rate capability, and stability compared with those of pillar-free materials.192
The original works based on clays could be taken nowadays as an example for future developments of new MXene-based pillar materials. Thus, other precursor agents for pillar formation, already applied in the preparation of silica-clays, such as Si(acac)3+ as proposed by Endo et al.186 or such as mixtures of SiO2 and metal-oxides as investigated by Han et al.,188 could be potentially adopted for the preparation of new silica-MXene pillared solids. In addition, the insertion of pillars of different compositions, including for instance Ti, Zr, Zn and Fe oxides, have been developed in layered clays,193–197 representing potential interest for the development of functional MXene-based pillared materials. In this context, pillars such as SnS with an electroactive response have recently been assembled to MXenes. The resulting solids have been successfully applied to the fabrication of Li+-battery electrodes with an improved capacity in successive cycles, which has been attributed to the “pillar effect” of the SnS/Ti3C2Tx pillared MXenes.198
Qin et al. have recently described the fabrication of silicate-based nanocomposites incorporating MXenes prepared by the ultrasonic treatment of clay dispersions in the presence of MXene.199 To explain the observed protective effect of clay against spontaneous MXene oxidation, a mechanism of Ti3C2Tx MXene intercalation within the montmorillonite layers has been proposed by this author and collaborators. However, it is difficult to explain such a structural organization because both 2D solids present negatively charged surfaces, pointing to their electrical charge incompatibility as both components exhibit negatively charged surfaces. Furthermore, the XRD patterns of the resulting materials do not agree with the expected increase of the basal spacing of montmorillonite by intercalated MXene nanolayers. In our opinion, the assembly of the montmorillonite clay mineral with Ti3C2Tx could be tentatively explained by a steric hindrance preventing the restacking of the interpenetrating clay and MXene flakes. A comparable assembly between a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 layered clay, such as kaolinite, and sepiolite fibrous clay has been also attempted by ultrasonication of the corresponding silicate dispersions.63 Interestingly, the MXene-based composite materials integrate the photothermal properties inherent in the presence of the titanium carbide, being efficiently activated by NIR laser (e.g., 808 nm) irradiation, reaching temperatures in the order of 70 °C within a few minutes. These clay-MXene materials can include Ag+ ions that are reduced in situ to metallic Ag NP, and the resulting materials show marked and long-lasting antibacterial activity.199
1 layered clay, such as kaolinite, and sepiolite fibrous clay has been also attempted by ultrasonication of the corresponding silicate dispersions.63 Interestingly, the MXene-based composite materials integrate the photothermal properties inherent in the presence of the titanium carbide, being efficiently activated by NIR laser (e.g., 808 nm) irradiation, reaching temperatures in the order of 70 °C within a few minutes. These clay-MXene materials can include Ag+ ions that are reduced in situ to metallic Ag NP, and the resulting materials show marked and long-lasting antibacterial activity.199
Besides that, multilayer MXene-clay nanoarchitectures have been prepared by layer-by-layer (LbL) procedures leading to freestanding thin films resulting from the assembly of Ti3C2 MXene, poly(vinyl alcohol) (PVA) and montmorillonite.200 These nanocomposites show a very high tensile strength imparted by the clay mineral, the PVA acts as a flexible polymer binder and the MXene provides suitable performance as a lightweight EMI shielding material.
3.2. Clay- and MXene-based carbon nanocomposites
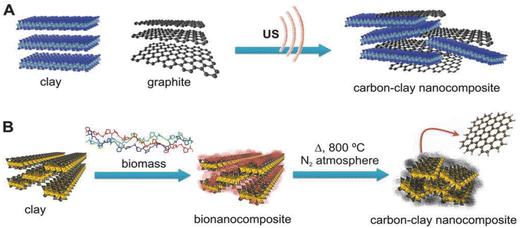
Although carbon (e.g. graphite) and clay minerals (e.g. kaolinite) are very different types of material, their combination by mixing both finely divided components has long been described for use as electrical resistors201 or as pencil cores.96 Since the 1980s, these materials have been used to prepare diverse functional carbon-clay nanocomposites by modifying their initial properties, such as the texture and the surface chemistry characteristics, paving the way for key fields of application related to energy storage,117 heterogeneous catalysis,202 food packaging,96 sensing devices96 and environmental remediation.202 In these composites the presence of the clay increases the hydrophilicity introduced by the carbon moiety, allowing the incorporation of other components, for example hydrogel polymers96,203 and metal nanoparticles202,204 among other constituents, thus enlarging their applicability.Two general approaches can be described for preparing carbon-clay materials (Fig. 8): (i) top-down processes, by simply and directly assembling the two individual components, and (ii) bottom-up procedures, growing the carbon on the clay surface. Top-down approaches (Fig. 8) use as starting materials small graphite particles or graphene nanoplatelets (GNP), which, by grinding205,206 or by dispersion in water under high shear or ultrasonication treatments in the presence of the clay minerals, give rise to carbon-clay nanocomposites.96,207,208 The role of clay minerals in their assembly with GNP and MWCNT is crucial, particularly in the case of sepiolite since it can generate high-viscosity dispersions63 leading to the steric stabilization of carbon nanoparticles, preventing their rebound and maintaining the carbon-sepiolite in homogeneous dispersion for a long time (several years).208 Water dispersion processes allow the incorporation of soluble or easily dispersible compounds such as hydrogels like PVA and biopolymers like chitosan, alginate or gelatin, leading to bionanocomposites that can be doped with MWCNT, enhancing their electrical conductivity while maintaining their mechanical properties.96
Concerning the bottom-up approach, Kyotani's group was the pioneer in the application of this procedure, intercalating polyacrylonitrile (PAN) as a carbon precursor in the interlayer space of montmorillonite, followed by a thermal treatment in the absence of oxygen.209–211 These processes were carried out at high temperature (>1000 °C), resulting in well-crystallized graphite that remains intercalated between the clay silicate layers.209 Related studies were conducted employing diverse clay minerals such as lamellar smectites and vermiculites,212,213 and fibrous silicates such as sepiolite.117,213 Diverse carbon precursors including PAN,214 sucrose (table sugar),212,215,216 biopolymers,216 asphaltenes217 and caramel117,213 among others give rise to carbon-clay nanocomposites based on graphene-like materials supported on these silicates under treatments at temperatures much lower (450–750 °C) than those initially adopted by Kyotani, saving energy and avoiding structural alterations of the silicate fraction. The proposed mechanism to explain the generation of graphene-like materials under these experimental conditions, for instance in the case of sucrose-sepiolite (Fig. 8) as starting components, points to the role of the intrinsic porosity of the silicate that could retain volatile intermediate compounds (e.g. furaldehydes) generated during the pyrolysis of the carbohydrate, facilitating their subsequent polycondensation towards graphene-like compounds (unpublished results). The resulting conductive composites exhibit a reduced conductivity compared with graphene materials but they can merit application as active components of electrochemical devices, including batteries, supercapacitors and sensors100 and pulsed electric field (PEF) processes for “cold sterilization”, of great interest in food packaging.218
The combination of intrinsically insulating solids such as clays and graphene materials results in a decrease of electronic conductivity whereas conductive MXenes could enhance the resulting conductivity. In this way, replacing clay by MXenes in carbon-clay materials by applying top-down and bottom-up methodologies, it is expected to obtain higher conducting materials. In this way, MXene-carbon composites can be prepared from polymers such as PAN219 and block copolymer P123/melamine–formaldehyde resin,122 or from carbon gases such as ethene,220 obtaining carbon nanofibers, mesoporous carbons and CNT, respectively, assembled on the MXenes. As well, these composites have been obtained from the intimate mixture of the carbon particles and the MXenes, as a top-down process, by dispersion in the liquid phase of graphene221,222 or CNT223 and MXenes, or by direct grinding of these components.224 The synergetic effect of carbon and MXenes is boosted and improves the electronic properties of these materials, presenting them as excellent candidates as electrodes in electrochemical devices, for instance supercapacitors.219,222
In addition, carbon-MXene composites show attractive surface properties with a potential impact as new materials for environmental remediation. For instance, delaminated Ti3C2Tx MXenes could be assembled to porous carbon microspheres, impeding their restacking and producing solids of elevated specific surface area (>800 m2 g−1) acting as superadsorbents of pollutants.225 It can be assumed that the incipient results shown by these emerging MXene-carbon composites have a promising future for the preparation of new advanced systems of interest in critical fields of application. The key is the combination of the metallic character with electrical conductivity, porosity and surface properties with high adsorption capacity, together with their hydrophilic character allowing stable colloidal dispersions and co-assembly with many diverse components of polar nature.
3.3. Clay- and MXene-based polymer nanocomposites
In the world of organic–inorganic hybrid materials, the discovery of polymer-clay nanocomposites by Fukushima and other Toyota researchers, by the synthesis of an intercalated compound of montmorillonite and 6-polyamide,226,227 had an enormous impact from the fundamental and application points of view. Thus, the preparation in this way of polymer-clay nanocomposites, a new class of composites where the dispersed phase (i.e. the silicate) is constituted by particles that have at least one of their dimensions in the nanoscale, represents an important advancement in the composite materials field.177,228,229 These revolutionary hybrid materials are achieved by a delamination of the silicate in the polymer matrix, allowing extraordinary enhancement of the mechanical properties with a very low content of reinforcing clay (<5% w/w). In the example proposed by Toyota in the 1980s regarding the 6-polyamide/montmorillonite, an increase of ca. 70% in the Young modulus and 126% in the flexural modulus was reached by incorporation of ca. 4% w/w of clay over the polyamide in comparison with the pure polymer.Analogous systems related to polymer-clay nanocomposites were prepared using various 2D solids such as layered transition metal oxides (e.g. V2O5 xerogel), layered phosphates (e.g. Zr-phosphate), transition metal chalcogenides (e.g. MoS2) and graphene-based solids. In this context, more recently, MXenes have been used as the disperse phase of diverse polymer matrices.230 In a similar way to clay minerals, Ti3C2Tx nanosheets have been successfully dispersed in polyamide-6 by ε-caprolactam intercalation followed by reaction with 12-aminolauric acid.231 In addition to these hybrid materials and exactly as in the case of clay minerals, other polymer-MXene nanocomposites have been prepared with polymeric matrices of a very diverse nature, such as poly(acrylic acid) (PAA), of interest as structural materials, conductive polymers, such as PEO, polyaniline (PANI) and polypyrrole (PPy), as well as biopolymers such as chitosan and nanocellulose. Conducting polymers such as PEO and PANI were pioneering intercalation compounds using layered clay minerals.11,15,232–235 The resulting clay-polymer nanocomposites exhibit electronic conductivity when PANI or PPy is the intercalated polymer168 and ionic conductivity when they incorporated PEO that facilitates the ion-mobility of interlayer cations.236–238 These nanoarchitectures have properties of interest in electrical and electrochemical devices, but the presence of clay components with insulating characteristics reduces their application capacity where ideally the conductivity should have outstanding values.
Semiconducting 2D solids, such as certain metal oxides and dichalcogenides of transition metals (e.g., V2O5 xerogel and MoS2) used as hosts for the intercalation of conducting polymers, show an enhancement of the electronic conductivity, demonstrating mixed ionic-electronic conductivity when PEO is incorporated in these systems.3,239–241 The recent use of MXenes provided with elevated electrical conductivity represents an attractive way to improve the properties of the intercalated electroactive polymers, leading to new functional organic–inorganic nanoarchitectures dealing with applications in diverse devices.242
The intercalation of PEO in smectite clays gives rise to materials showing anisotropic ion-conductivity, typically in the order of 10−7–10−4 S cm−1 in the layer plane, which is much higher than the value measured in the perpendicular direction.11,233 MXene-PEO nanocomposites prepared by the incorporation of lithium bis(trifluoromethanesulfonyl)imide (LiTFSI), a lithium salt, show low ionic conductivity (around 10−7 S cm−1),243 and the 2D MXene arranged as disorganized platelets with a random distribution of delaminated carbide layers (Fig. 9). The electronic conductivity of PEO-MXene nanocomposites ascribed to the MXene component is very low (in the order of 10−10 S cm−1), probably due to the presence of insulating PEO chains involving a structural organization unable to form a favorable percolation pathway.
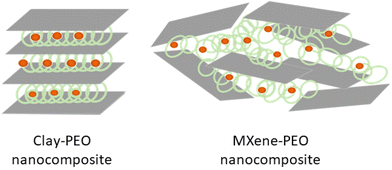 | ||
| Fig. 9 Structural arrangements of clay-PEO based on montmorillonite layered clay mineral, described by Ruiz-Hitzky and Aranda;233 and MXene-PEO based on Ti3C2Tx, described by Pan et al.243 | ||
3.4. Biohybrid nanoarchitectonics based on clays and MXenes
Biohybrid materials result from the nanoscale combination of whole living cells or some of their fragments and inorganic components, giving rise to complex systems that can be endowed with extraordinary bioactivity.176,244 Among these types of material, bionanocomposites have been defined as biohybrids that involve the assembly of natural polymers with inorganic nanoparticles,245 including clay minerals and more recently MXenes interacting at the nanoscale. The diverse processing strategies have been reviewed by Ojijo and Ray, discussing the methods used in the preparation of bionanocomposites.246 Bionanocomposites based on clay minerals have been intensively studied to produce hybrid materials whose properties are defined both by the nature of the inorganic part and by the properties of the biopolymers involved.129,140,247 These materials are attracting an increase of interest since the participation of natural polymers can offer additional properties to the clay components, resulting in sustainable composite materials that also show biodegradability/biocompatibility, thus widening the field of their applications. The main envisaged applications of clay-based bionanocomposites are aimed at bioplastics, packaging, sensors, tissue engineering and environmental protection. Typical clay minerals used as the inorganic component are montmorillonite, halloysite, sepiolite, palygorskite, kaolinite, hectorite (LAPONITE®) and vermiculite. The 2D charged phyllosilicates such as montmorillonite, hectorite and vermiculite can act as a host for the intercalation of biopolymers or to assemble them through delamination processes, whereas nanotubular halloysite and nanofibrous sepiolite interact through their external surfaces.248,249 With regard to the biopolymers involved, most are polysaccharides such as chitosan, alginate, starch cellulose and chitosan and, to a lesser extent, some proteins such as gelatin and zein and nucleic acids such as DNA and RNA, as well as certain biocompounds produced by microorganisms (e.g., polylactic acid, PLA).248,249The chitosan-montmorillonite bionanocomposite is produced by intercalation involving ion-exchange processes that displace the interlayer cations of the clay because the involved chitosan is present in its protonated form. These bionanocomposites show a well-ordered structural arrangement together with anion exchange properties12 that allow the development of ion-selective sensors specially towards monovalent anions and particularly towards nitrate species, whose recognition could be controlled by automatic systems using artificial intelligence.94,250 Sepiolite-chitosan also exhibits ion-selectivity, the mechanical properties of the resulting bionanocomposite being enhanced with respect to the starting biopolymer.93,247,251
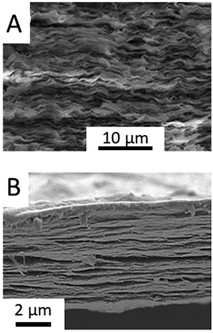
Illustrative examples of recently reported clay-based bionanocomposites include biopolymers such as synthetic nanocellulose and nucleic acids (DNA, etc.). The preparation of artificial nacre by the intercalation of organic compounds in clay minerals was first reported by Kotov's team252 and more recently studied by Sung et al. (Fig. 10A).253 Nanocellulose-clay composites represent an important group of bionanohybrids with excellent mechanical properties that can be also involved in the synthesis of artificial nacre by assembling to MXenes (Fig. 10B).143
Clay-based biohybrids incorporating nanocellulose have been recently used for the preparation of multifunctional nanoarchitectures by the further assembly of nanocomponents such as carbon nanomaterials (e.g., MWCNT), magnetic nanoparticles (e.g., Fe3O4) and nanoparticulate semiconductors (e.g., TiO2, ZnO).254
As first stated by Choy and collaborators,6 clay-DNA bionanocomposites are of great interest in biomedicine for gene transfection as well as in future advanced information storage. Sepiolite-DNA has recently been used as an efficient nanoplatform for DNA transfer into mammalian cells.255,256
MXenes have been also assembled to biopolymers mainly by delamination/re-stacking processes allowing the preparation of transparent conductive films of functional bionanocomposites, pointing to their applications for supercapacitors, Li-ion batteries, EMI shielding, sensors, electrocatalysis, biomedicine and environmental remediation among other fields of interest.257 For instance, chitosan and other polysaccharides assembled to MXenes (e.g. Ti3C2Tx) give multifunctional biohybrid nanoarchitectures tested in diverse biomedical applications.258
For certain applications, bionanocomposites could be prepared as foams or sponges by gas foaming or conventional freeze-drying processes involving hydrophilic biopolymers, such as polysaccharides (e.g., cellulose and chitosan) or proteins (e.g., gelatin), which disperse easily in water together with the inorganic counterpart.259
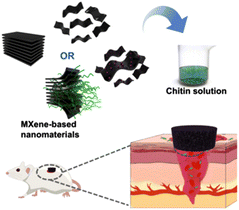
As a selected comparative example of clay minerals and MXene nanoarchitectures, chitosan-clay nanocomposites based on rectorite layered silicate have been successfully applied as efficient hemostatic agents (in vitro porcine skin model), confirming that viscous injectable clay/chitosan nanocomposites act as a facile and sustainable biomaterial for skin hemostasis, as they can stably adhere to skin and impede bleeding.260 Chitin-MXene composites based on Ti3C2Tx have been prepared as sponges showing excellent behavior for hemostasis as well as antibacterial infected wound healing (Fig. 11).53 It is here assumed that the photothermal effect inherent in MXene incorporated into these bionanocomposites efficiently contributes to their antibacterial activity. MXenes (e.g., Ti3C2) are biocompatible nanosheets, showing a high photothermal-conversion efficiency allowing in vitro/in vivo photothermal ablation of tumors, representing a great potential for diverse biomedical applications and particularly in cancer therapy. For instance, Lin et al. reported on MXene materials presenting high absorption in the NIR region for highly effective photothermal ablation of tumors in mouse models in vivo.170
Other uses of MXene bionanoarchitectures deal with a broad field related to health, from theranostics261 to piezoelectric sensors for the real-time monitoring of certain human activities.148
4. Concluding remarks
Why should clay scientists be interested in MXenes? Why should experts on MXenes be interested in clay minerals? The answer to these questions could be found above as we have shown in this critical review, a comparison between both types of solid, which share essential characteristics such as structural features and certain physical and chemical properties, particularly those related to the nature of their surface and their rheological behaviors, as well as their metallic or insulating character. It should be noted that the electrical conductivity properties are decisive in finding applications, which can be much wider in MXenes with high conductivity compared with clays, which are insulators or ionic conductors with very low electrical conductivity values.Among the common properties of both types of solid, MXenes and 2D clay minerals belonging to the smectites and vermiculites show intercalation properties as well as the ability towards exfoliation or delamination into single layers. These characteristics allow the development of new functional nanoarchitectures prepared from their assembly with diverse entities such as nanoparticles, biopolymers, or even microorganisms such as viral particles and living cells.
A less studied aspect of MXenes refers to their rheological properties, which have been widely studied in clays, especially regarding the microfibrous sepiolite. This behavior can represent an example of inspiration for further rheological studies involving the MXenes.
The structural analogies between clays and MXenes lead to exceptional common surface and rheological properties and thus enable advanced applications in various fields, such as powerful selective membranes, adsorbents and absorbents, heterogeneous catalysts and insulating materials used in building sector among other uses, which could be applicable to both types of 2D solid. The additional characteristic of the MXenes in terms of their electronic conductivity allows the inclusion of important further applications249 such as energy production and storage,25,106,120 sensor devices,103 “cold sterilization” based on PEF processes,219 and bioactive materials of interest in diverse biomedical applications.53,167,168
In addition, hollow nanotubular halloysite clay presents a molecular storage capacity allowing loading with diverse functional agents, which can also serve as inspiration for new applications of MXenes or partially etched MAX carbides. The tendency of MXene flakes to roll up, creating equivalent nanotubular conformations, should be considered. On the other hand, the ability to intercalate and delaminate forming nanosheet dispersions, already well known for V2O5 xerogel and other 2D solids such as transition metal oxides and chalcogenides (e.g., MoS2) showing electrical conductivity typical of semiconductors, could be profitable for the equivalent studies of MXenes.
Moreover, it should also be considered that computational chemistry studies not only appear to be of enormous theoretical interest in predicting and determining the structural characteristics and derived properties of 2D-based nanoarchitectures resulting from both clay minerals and MXenes, but also that these studies represent a powerful tool for the rational advancement of new applications of these emerging materials.
Finally, it could be also concluded that the terminology recently observed in publications on MXenes does not seem fully accurate regarding the use of the term “clay” to refer to these carbides/carbonitrides. However, the MXene topic can be considered as an inspiring subject for clay scientists and, in the same way, the studies related to clay minerals are of great interest to researchers working on MXenes. In fact, it would be considered that important characteristics of these transition-metal carbides and nitrides are closely related to other 2D solids showing the ability to intercalate, exfoliate and form colloidal systems in a similar way to clay minerals. Therefore, the deep knowledge so widely achieved in the science and technology of clays in recent decades may have great potential to contribute to the rapidly advancing research on MXenes and related materials currently underway.
Author contributions
E. R-H: conceptualization, supervision, writing – original draft and review & editing; C. R-G: visualization, writing – original draft and review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge financial support from the PID2019-105479RB-I00 (MCIN/AEI/10.13039/501100011033) project. We acknowledge Prof. M. Camblor for assistance in the graphics of clays and MXenes structural models.References
- R. Schöllhorn, Phys. B + C, 1980, 99, 89–99 CrossRef.
- R. Schöllhorn, Chem. Mater., 1996, 8, 1747–1757 CrossRef.
- E. Ruiz-Hitzky, R. Jimenez, B. Casal, V. Manriquez, A. S. Ana and G. Gonzalez, Adv. Mater., 1993, 5, 738–741 CrossRef CAS.
- G. Alberti, M. Casciola and U. Costantino, J. Colloid Interface Sci., 1985, 107, 256–263 CrossRef CAS.
- S. Sinha Ray and M. Okamoto, Prog. Polym. Sci., 2003, 28, 1539–1641 CrossRef.
- J. Choy, S. Choi, J. Oh and T. Park, Appl. Clay Sci., 2007, 36, 122–132 CrossRef CAS.
- A. Usuki, Y. Kojima, M. Kawasumi, A. Okada, Y. Fukushima, T. Kurauchi and O. Kamigaito, J. Mater. Res., 2011, 8, 1179–1184 CrossRef.
- A. Gupta, T. Sakthivel and S. Seal, Prog. Mater. Sci., 2015, 73, 44–126 CrossRef CAS.
- M. Terrones, A. R. Botello-Mendez, J. Campos-Delgado, F. Lopez-Urias, Y. I. Vega-Cantu, F. J. Rodriguez-Macias, A. L. Elias, E. Munoz-Sandoval, A. G. Cano-Marquez, J.-C. Charlier and H. Terrones, Nano Today, 2010, 5, 351–372 CrossRef.
- E. Ruiz-Hitzky and B. Casal, Nature, 1978, 276, 596–597 CrossRef CAS.
- P. Aranda and E. Ruiz-Hitzky, Chem. Mater., 1992, 4, 1395–1403 CrossRef CAS.
- M. Darder, M. Colilla and E. Ruiz-Hitzky, Chem. Mater., 2003, 15, 3774–3780 CrossRef CAS.
- M. Faustini, L. Nicole, E. Ruiz-Hitzky and C. Sanchez, Adv. Funct. Mater., 2018, 28, 1704158 CrossRef.
- M. Ogawa and K. Kuroda, Chem. Rev., 1995, 95, 399–438 CrossRef CAS.
- E. Ruiz-Hitzky, Adv. Mater., 1993, 5, 334–340 CrossRef CAS.
- E. Ruiz-Hitzky, P. Aranda, M. Darder and M. Ogawa, Chem. Soc. Rev., 2011, 40, 801–828 RSC.
- K. Ariga, Beilstein J. Nanotechnol., 2023, 14, 434–453 CrossRef CAS PubMed.
- V. Nicolosi, M. Chhowalla, M. G. Kanatzidis, M. S. Strano and J. N. Coleman, Science, 2013, 340, 1226419 CrossRef.
- E. Ruiz-Hitzky and P. Aranda, J. Sol-Gel Sci. Technol., 2013, 70, 307–316 CrossRef.
- E. Ruiz-Hitzky, P. Aranda, M. Akkari, N. Khaorapapong and M. Ogawa, Beilstein J. Nanotechnol., 2019, 10, 1140–1156 CrossRef CAS PubMed.
- E. Ruiz-Hitzky, P. Aranda and C. Belver, in Manipulation of Nanoscale Materials, ed. K. Ariga, The Royal Society of Chemistry, 2012, 10.1039/9781849734158-00087.
- M. Naguib, M. Kurtoglu, V. Presser, J. Lu, J. Niu, M. Heon, L. Hultman, Y. Gogotsi and M. W. Barsoum, Adv. Mater., 2011, 23, 4248–4253 CrossRef CAS PubMed.
- B. Anasori and Y. Gogotsi, 2D Metal Carbides and Nitrides (MXenes): Structure, Properties and Applications, Springer International Publishing, Cham, 2019 Search PubMed.
- M. Naguib, V. N. Mochalin, M. W. Barsoum and Y. Gogotsi, Adv. Mater., 2014, 26, 992–1005 CrossRef CAS PubMed.
- B. Anasori, M. R. Lukatskaya and Y. Gogotsi, Nat. Rev. Mater., 2017, 2, 9224 Search PubMed.
- S. Ramanavicius and A. Ramanavicius, Int. J. Mol. Sci., 2020, 21, 9224 CrossRef CAS PubMed.
- Y. Wang, Z. Niu, Y. Dai, P. Mu and J. Li, Nanoscale, 2023, 15, 4170–4194 RSC.
- M. Ghidiu, M. R. Lukatskaya, M. Q. Zhao, Y. Gogotsi and M. W. Barsoum, Nature, 2014, 516, 78–81 CrossRef CAS PubMed.
- M. Naguib, Engineering Electrically Conductive Clay-Like MXenes for Energy Storage, in Program and Abstracts Book, 60th Annual Meeting, The Clay Minerals Society, Austin, TX, USA, 2023 Search PubMed.
- F. Bergaya and G. Lagaly, in Developments in Clay Science, ed. F. Bergaya and G. Lagaly, Elsevier, 2013, vol. 1, ch. General Introduction: Clays, Clay Minerals, and Clay Science Search PubMed.
- D. Zhang, C.-H. Zhou, C.-X. Lin, D.-S. Tong and W.-H. Yu, Appl. Clay Sci., 2010, 50, 1–11 CrossRef CAS.
- H. Kodama and R. E. Grim, Encyclopedia Britannica, “clay mineral”, https://www.britannica.com/science/clay-mineral, (accessed 22nd June 2023).
- K. Momma and F. Izumi, J. Appl. Crystallogr., 2011, 44, 1272–1276 CrossRef CAS.
- R. A. de Freitas and F. Wypych, in Developments in Clay Science, ed. F. Bergaya and G. Lagaly, Elsevier, 2022, vol. 10, pp. 253–275 Search PubMed.
- D. Kim, R. F. Smith, I. K. Ocampo, F. Coppari, M. C. Marshall, M. K. Ginnane, J. K. Wicks, S. J. Tracy, M. Millot, A. Lazicki, J. R. Rygg, J. H. Eggert and T. S. Duffy, Nat. Commun., 2022, 13, 2260 CrossRef CAS PubMed.
- C. E. Shuck, K. Ventura-Martinez, A. Goad, S. Uzun, M. Shekhirev and Y. Gogotsi, ACS Chem. Health Saf., 2021, 28, 326–338 CrossRef CAS.
- A. Maughan, Porous two-dimensional materials (MXenes) for high capacity energy storage, PhD thesis, University of Lancaster, 2019.
- J. Chen, Y. Ding, D. Yan, J. Huang and S. Peng, SusMat, 2022, 2, 293–318 CrossRef CAS.
- J. Xu, T. Peng, X. Qin, Q. Zhang, T. Liu, W. Dai, B. Chen, H. Yu and S. Shi, J. Mater. Chem. A, 2021, 9, 14147–14171 RSC.
- L. Liu, M. Orbay, S. Luo, S. Duluard, H. Shao, J. Harmel, P. Rozier, P.-L. Taberna and P. Simon, ACS Nano, 2022, 16, 111–118 CrossRef CAS PubMed.
- N. H. Solangi, R. R. Karri, S. A. Mazari, N. M. Mubarak, A. S. Jatoi, G. Malafaia and A. K. Azad, Coord. Chem. Rev., 2023, 477, 214965 CrossRef CAS.
- T. Li, L. Yao, Q. Liu, J. Gu, R. Luo, J. Li, X. Yan, W. Wang, P. Liu, B. Chen, W. Zhang, W. Abbas, R. Naz and D. Zhang, Angew. Chem., Int. Ed., 2018, 57, 6115–6119 CrossRef CAS PubMed.
- J. Xuan, Z. Wang, Y. Chen, D. Liang, L. Cheng, X. Yang, Z. Liu, R. Ma, T. Sasaki and F. Geng, Angew. Chem., Int. Ed., 2016, 55, 14569–14574 CrossRef CAS PubMed.
- J. Santaren, J. Sanz and E. Ruiz-Hitzky, Clays Clay Miner., 1990, 38, 63–68 CrossRef CAS.
- V. Natu, R. Pai, M. Sokol, M. Carey, V. Kalra and M. W. Barsoum, Chem, 2020, 6, 616–630 CAS.
- S. Husmann, Ö. Budak, H. Shim, K. Liang, M. Aslan, A. Kruth, A. Quade, M. Naguib and V. Presser, Chem. Commun., 2020, 56, 11082–11085 RSC.
- Z. Sun, M. Yuan, L. Lin, H. Yang, C. Nan, H. Li, G. Sun and X. Yang, ACS Mater. Lett., 2019, 1, 628–632 CrossRef CAS.
- D. Voiry, A. Mohite and M. Chhowalla, Chem. Soc. Rev., 2015, 44, 2702–2712 RSC.
- E. Ruiz-Hitzky, B. Casal, P. Aranda and J. C. Galvan, Rev. Inorg. Chem., 2001, 21, 125–159 CAS.
- K. D. Rasamani, F. Alimohammadi and Y. G. Sun, Mater. Today, 2017, 20, 83–91 CrossRef CAS.
- G. D. Williams, N. T. Skipper, M. V. Smalley, A. K. Soper and S. M. King, Faraday Discuss., 1996, 104, 295–306 RSC.
- J. A. Rausell-Colom, J. Saez-Auñón and C. H. Pons, Clay Miner., 2018, 24, 459–478 CrossRef.
- S. Li, B. Gu, X. Li, S. Tang, L. Zheng, E. Ruiz-Hitzky, Z. Sun, C. Xu and X. Wang, Adv. Healthcare Mater., 2022, 11, 2102367 CrossRef CAS PubMed.
- X. Zuo, D. Wang, S. Zhang, Q. Liu and H. Yang, Minerals, 2018, 8, 112 CrossRef.
- J. I. Dawson, J. M. Kanczler, X. B. Yang, G. S. Attard and R. O. C. Oreffo, Adv. Mater., 2011, 23, 3304–3308 CrossRef CAS PubMed.
- A. Gholamipour-Shirazi, M. S. Carvalho, M. F. G. Huila, K. Araki, P. Dommersnes and J. O. Fossum, Sci. Rep., 2016, 6, 37239 CrossRef CAS PubMed.
- Y. Lvov, W. Wang, L. Zhang and R. Fakhrullin, Adv. Mater., 2016, 28, 1227–1250 CrossRef CAS PubMed.
- P. Mongondry, J. F. Tassin and T. Nicolai, J. Colloid Interface Sci., 2005, 283, 397–405 CrossRef CAS PubMed.
- J.-M. Oh, D.-H. Park, S.-J. Choi and J.-H. Choy, Recent Pat. Nanotechnol., 2012, 6, 200–217 CrossRef CAS PubMed.
- G. M. Bekaroğlu and S. İşçi, ACS Omega, 2022, 7, 38825–38831 CrossRef PubMed.
- M. Rautureau, C. d. S. Figueiredo Gomes, N. Liewig and M. Katouzian-Safadi, Clays and Health: Properties and Therapeutic Uses, Springer Cham, 2017 Search PubMed.
- E. Ruiz-Hitzky, M. Darder, B. Wicklein, F. A. Castro-Smirnov and P. Aranda, Clays Clay Miner., 2019, 67, 44–58 CrossRef CAS.
- E. Ruiz-Hitzky, C. Ruiz-Garcia, F. M. Fernandes, G. Lo Dico, L. Lisuzzo, V. Prevot, M. Darder and P. Aranda, Front. Chem., 2021, 9, 733105 CrossRef CAS PubMed.
- S. Huang and V. N. Mochalin, Inorg. Chem., 2019, 58, 1958–1966 CrossRef CAS PubMed.
- P. Fulignati, Minerals, 2020, 10, 919 CrossRef CAS.
- R. T. Cygan, J. A. Greathouse, H. Heinz and A. G. Kalinichev, J. Mater. Chem., 2009, 19, 2470–2481 RSC.
- T. Wu and D.-e. Jiang, MRS Bull., 2023, 48, 253–260 CrossRef.
- S. Srivatsa, P. Paćko, L. Mishnaevsky, T. Uhl and K. Grabowski, Minerals, 2020, 13, 5189 CAS.
- B. Biswas, B. Sarkar, R. Rusmin and R. Naidu, Environ. Int., 2015, 85, 168–181 CrossRef CAS PubMed.
- T. J. Pinnavaia, Science, 1983, 220, 365–371 CrossRef CAS PubMed.
- J. F. Wang, J. Merino, P. Aranda, J. C. Galvan and E. Ruiz-Hitzky, J. Mater. Chem., 1999, 9, 161–168 RSC.
- B. K. Singh and W. Um, Minerals, 2023, 13, 239 CrossRef CAS.
- N. Boukhalfa, M. Darder, M. Boutahala, P. Aranda and E. Ruiz-Hitzky, Bull. Chem. Soc. Jpn., 2021, 94, 122–132 CrossRef CAS.
- Y. González-Alfaro, P. Aranda, F. M. Fernandes, B. Wicklein, M. Darder and E. Ruiz-Hitzky, Adv. Mater., 2011, 23, 5224–5228 CrossRef PubMed.
- A. Sanguanwong, A. E. Flood, M. Ogawa, R. Martín-Sampedro, M. Darder, B. Wicklein, P. Aranda and E. Ruiz-Hitzky, J. Hazard. Mater., 2021, 417, 126068 CrossRef CAS PubMed.
- M. B. Ahmed, J. L. Zhou, H. H. Ngo and W. Guo, Sci. Total Environ., 2015, 532, 112–126 CrossRef CAS PubMed.
- J. Herney-Ramirez, M. A. Vicente and L. M. Madeira, Appl. Catal., B, 2010, 98, 10–26 CrossRef CAS.
- G. Rytwo, Sci. World J., 2012, 1–7, DOI:10.1100/2012/498503.
- G. Rytwo, Abstracts of Papers of the American Chemical Society, 2012, p. 243 Search PubMed.
- A. Khosla, H. T. A. A. Sonu, K. Singh, Gaurav, R. Walvekar, Z. Zhao, A. Kaushik, M. Khalid and V. Chaudhary, Adv. Sci., 2022, 9, 2203527 CrossRef CAS PubMed.
- C. Peng, X. Yang, Y. Li, H. Yu, H. Wang and F. Peng, ACS Appl. Mater. Interfaces, 2016, 8, 6051–6060 CrossRef CAS PubMed.
- J. Low, L. Zhang, T. Tong, B. Shen and J. Yu, J. Catal., 2018, 361, 255–266 CrossRef CAS.
- Y. Sun and Y. Li, Environ. Pollut., 2021, 278, 116861 CrossRef CAS PubMed.
- L. Wang and Y.-L. Liu, Crystals, 2023, 13(5), 804 CrossRef CAS.
- E. A. Hussein, M. M. Zagho, G. K. Nasrallah and A. A. Elzatahry, Int. J. Nanomed., 2018, 13, 2897–2906 CrossRef CAS PubMed.
- F. Liu, Z. Hu, M. Xiang and B. Hu, Appl. Surf. Sci., 2022, 601, 154227 CrossRef CAS.
- Z.-K. Li, Y. Liu, L. Li, Y. Wei, J. Caro and H. Wang, J. Membr. Sci., 2019, 592, 117361 CrossRef.
- P.-L. Wang, C. Ma, Q. Yuan, T. Mai and M.-G. Ma, J. Colloid Interface Sci., 2022, 606, 971–982 CrossRef CAS PubMed.
- Y. Ibrahim, A. Kassab, K. Eid, A. M. Abdullah, K. I. Ozoemena and A. Elzatahry, Nanomaterials, 2020, 10, 885 CrossRef CAS PubMed.
- M. Tawalbeh, S. Mohammed, A. Al-Othman, M. Yusuf, M. Mofijur and H. Kamyab, Environ. Res., 2023, 228, 115919 CrossRef CAS PubMed.
- T. Zhang, H. Bai, Y. Zhao, B. Ren, T. Wen, L. Chen, S. Song and S. Komarneni, ACS Nano, 2022, 16, 4930–4939 CrossRef CAS PubMed.
- M. Darder, P. Aranda, A. I. Ruiz, F. M. Fernandes and E. Ruiz-Hitzky, Mater. Sci. Technol., 2008, 24, 1100–1110 CrossRef CAS.
- M. Darder, M. Colilla and E. Ruiz-Hitzky, Chem. Mater., 2003, 15, 3774–3780 CrossRef CAS.
- M. Darder, M. Colilla and E. Ruiz-Hitzky, Appl. Clay Sci., 2005, 28, 199–208 CrossRef CAS.
- E. Ruiz-Hitzky, Chem. Rec., 2003, 3, 88–100 CrossRef CAS PubMed.
- E. Ruiz-Hitzky, M. M. C. Sobral, A. Gomez-Aviles, C. Nunes, C. Ruiz-Garcia, P. Ferreira and P. Aranda, Adv. Funct. Mater., 2016, 26, 7394–7405 CrossRef CAS.
- S. Letaïef, P. Aranda and E. Ruiz-Hitzky, Appl. Clay Sci., 2005, 28, 183–198 CrossRef.
- S. Letaïef, P. Aranda, R. Fernández-Saavedra, J. C. Margeson, C. Detellier and E. Ruiz-Hitzky, J. Mater. Chem., 2008, 18, 2227–2233 RSC.
- A. Barra, C. Nunes, E. Ruiz-Hitzky and P. Ferreira, Int. J. Mol. Sci., 2022, 23, 1848 CrossRef CAS PubMed.
- M. Darder, P. Aranda, C. Ruiz-Garcia, F. M. Fernandes and E. Ruiz-Hitzky, Adv. Funct. Mater., 2018, 28, 1704323 CrossRef.
- J.-M. Yeh, S.-J. Liou, C.-Y. Lai, P.-C. Wu and T.-Y. Tsai, Chem. Mater., 2001, 13, 1131–1136 CrossRef CAS.
- M. Ghidiu, J. Halim, S. Kota, D. Bish, Y. Gogotsi and M. W. Barsoum, Chem. Mater., 2016, 28, 3507–3514 CrossRef CAS.
- E. Wang, Y. Cao, Y. Bai, Y. Gai, Y. Shan, Q. Li, T. Jiang, H. Feng and Z. Li, J. Micromech. Microeng., 2022, 32, 044003 CrossRef.
- Z. Huang, S. Wang, S. Kota, Q. Pan, M. W. Barsoum and C. Y. Li, Polymer, 2016, 102, 119–126 CrossRef CAS.
- U. Noor, M. F. Mughal, T. Ahmed, M. F. Farid, M. Ammar, U. Kulsum, A. Saleem, M. Naeem, A. Khan, A. Sharif and K. Waqar, Nanotechnology, 2023, 34, 262001 CrossRef PubMed.
- Z. Zhou, W. Panatdasirisuk, T. S. Mathis, B. Anasori, C. Lu, X. Zhang, Z. Liao, Y. Gogotsi and S. Yang, Nanoscale, 2018, 10, 6005–6013 RSC.
- X. Feng, J. Huang, J. Ning, D. Wang, J. Zhang and Y. Hao, Carbon, 2023, 205, 365–372 CrossRef CAS.
- F. Shahzad, M. Alhabeb, C. B. Hatter, B. Anasori, S. Man Hong, C. M. Koo and Y. Gogotsi, Science, 2016, 353, 1137–1140 CrossRef CAS PubMed.
- J. Liu, H.-B. Zhang, R. Sun, Y. Liu, Z. Liu, A. Zhou and Z.-Z. Yu, Adv. Mater., 2017, 29, 1702367 CrossRef PubMed.
- H. Yan, L. Zhang, H. Li, X. Fan and M. Zhu, Carbon, 2020, 157, 217–233 CrossRef CAS.
- H. Zhao, J. Ding, M. Zhou and H. Yu, ACS Appl. Nano Mater., 2021, 4, 3075–3086 CrossRef CAS.
- P. D. Kaviratna, T. J. Pinnavaia and P. A. Schroeder, J. Phys. Chem. Solids, 1996, 57, 1897–1906 CrossRef CAS.
- V. Tomer, G. Polizos, C. A. Randall and E. Manias, J. Appl. Phys., 2011, 109, 074113 CrossRef.
- D. T. Rathnayake, K. S. P. Karunadasa, A. S. K. Wijekoon, C. H. Manoratne and R. M. G. Rajapakse, Chem. Pap., 2022, 76, 6653–6658 CrossRef CAS.
- Y. S. Choi, T. K. Kim, E. A. Kim, S. H. Joo, C. Pak, Y. H. Lee, H. Chang and D. Seung, Adv. Mater., 2008, 20, 2341–2344 CrossRef CAS.
- C. Tang, K. Hackenberg, Q. Fu, P. M. Ajayan and H. Ardebili, Nano Lett., 2012, 12, 1152–1156 CrossRef CAS PubMed.
- C. Ruiz-Garcia, R. Jimenez, J. Perez-Carvajal, A. Berenguer-Murcia, M. Darder, P. Aranda, D. Cazorla-Amoros and E. Ruiz-Hitzky, Sci. Adv. Mater., 2014, 6, 151–158 CrossRef CAS.
- S. Maiti, A. Pramanik, S. Chattopadhyay, G. De and S. Mahanty, J. Colloid Interface Sci., 2016, 464, 73–82 CrossRef CAS PubMed.
- S.-S. Chai, W.-B. Zhang, J.-L. Yang, L. Zhang, X.-W. Han, M. M. Theint and X.-J. Ma, J. Rare Earths, 2023, 41, 728–739 CrossRef CAS.
- M. R. Lukatskaya, O. Mashtalir, C. E. Ren, Y. Dall'Agnese, P. Rozier, P. L. Taberna, M. Naguib, P. Simon, M. W. Barsoum and Y. Gogotsi, Science, 2013, 341, 1502–1505 CrossRef CAS PubMed.
- R. B. Rakhi, B. Ahmed, M. N. Hedhili, D. H. Anjum and H. N. Alshareef, Chem. Mater., 2015, 27, 5314–5323 CrossRef CAS.
- A. Enaiet Allah, RSC Adv., 2023, 13, 9983–9997 RSC.
- S. Qamar, K. Fatima, N. Ullah, Z. Akhter, A. Waseem and M. Sultan, Nanoscale, 2022, 14, 13018–13039 RSC.
- S. Sarikurt, D. Çakır, M. Keçeli and C. Sevik, Nanoscale, 2018, 10, 8859–8868 RSC.
- Z. Fan, Y. Wang, Z. Xie, X. Xu, Y. Yuan, Z. Cheng and Y. Liu, Nanoscale, 2018, 10, 9642–9652 RSC.
- J. Pang, R. G. Mendes, A. Bachmatiuk, L. Zhao, H. Q. Ta, T. Gemming, H. Liu, Z. Liu and M. H. Rummeli, Chem. Soc. Rev., 2019, 48, 72–133 RSC.
- D. Parajuli, N. Murali, K. C. Devendra, B. Karki, K. Samatha, A. A. Kim, M. Park and B. Pant, Polymers, 2022, 14, 3433 CrossRef CAS PubMed.
- B. Szczepanik, Appl. Clay Sci., 2017, 141, 227–239 CrossRef CAS.
- E. Ruiz-Hitzky, P. Aranda, M. Darder and G. Rytwo, J. Mater. Chem., 2010, 20, 9306–9321 RSC.
- T. Hoang-Minh, T. L. Le, J. Kasbohm and R. Gieré, Appl. Clay Sci., 2010, 48, 349–357 CrossRef CAS.
- L. Margulies, H. Rozen and E. Cohen, Nature, 1985, 315, 658–659 CrossRef CAS.
- K. Gopalram, A. Kapoor, P. S. Kumar, A. Sunil and G. Rangasamy, Ind. Eng. Chem. Res., 2023, 62, 6559–6583 CrossRef CAS.
- H. Jiang, S. Zhang, X. Feng, S. Yin and W. Zhao, Processes, 2023, 11, 1413 CrossRef.
- Z. Guo, J. Zhou, L. Zhu and Z. Sun, J. Mater. Chem. A, 2016, 4, 11446–11452 RSC.
- T. Lan and T. J. Pinnavaia, Chem. Mater., 1994, 6, 2216–2219 CrossRef CAS.
- P. Kiliaris and C. D. Papaspyrides, Prog. Polym. Sci., 2010, 35, 902–958 CrossRef CAS.
- J. M. Garcés, D. J. Moll, J. Bicerano, R. Fibiger and D. G. McLeod, Adv. Mater., 2000, 12, 1835–1839 CrossRef.
- S. Gul, A. Kausar, B. Muhammad and S. Jabeen, Polym.-Plast. Technol. Eng., 2016, 55, 684–703 CrossRef CAS.
- M. Okamoto and B. John, Prog. Polym. Sci., 2013, 38, 1487–1503 CrossRef CAS.
- M. Darder, P. Aranda and E. Ruiz-Hitzky, Adv. Mater., 2007, 19, 1309–1319 CrossRef CAS.
- S. Sinha Ray, P. Maiti, M. Okamoto, K. Yamada and K. Ueda, Macromolecules, 2002, 35, 3104–3110 CrossRef.
- M. Wasim, F. Shi, J. Liu, H. Zhang, K. Zhu and Z. Tian, J. Polym. Res., 2022, 29, 423 CrossRef CAS.
- S. Tang, Z. Wu, X. Li, F. Xie, D. Ye, E. Ruiz-Hitzky, L. Wei and X. Wang, Carbohydr. Polym., 2023, 299, 120204 CrossRef CAS PubMed.
- H. Zhang, L. Wang, Q. Chen, P. Li, A. Zhou, X. Cao and Q. Hu, Mater. Des., 2016, 92, 682–689 CrossRef CAS.
- L. Li, X. Liu, J. Wang, Y. Yang, Y. Cao and W. Wang, Composites, Part A, 2019, 127, 105649 CrossRef CAS.
- V. Chaudhary, A. Gautam, Y. K. Mishra and A. Kaushik, Nanomaterials, 2021, 11, 2496 CrossRef CAS PubMed.
- Y. Wang, Y. Yue, F. Cheng, Y. Cheng, B. Ge, N. Liu and Y. Gao, ACS Nano, 2022, 16, 1734–1758 CrossRef CAS PubMed.
- L. Wei, Z. Wu, S. Tang, X. Qin, Y. Xiong, J. Li, E. Ruiz-Hitzky and X. Wang, Carbon, 2023, 203, 386–396 CrossRef CAS.
- S. Tang, Z. Wu, G. Feng, L. Wei, J. Weng, E. Ruiz-Hitzky and X. Wang, Chem. Eng. J., 2023, 454, 140457 CrossRef CAS.
- R. Li, L. Zhang, L. Shi and P. Wang, ACS Nano, 2017, 11, 3752–3759 CrossRef CAS PubMed.
- S. M. George and B. Kandasubramanian, Ceram. Int., 2020, 46, 8522–8535 CrossRef CAS.
- H. Riazi, S. K. Nemani, M. C. Grady, B. Anasori and M. Soroush, J. Mater. Chem. A, 2021, 9, 8051–8098 RSC.
- Y. Shen, W. Yang, F. Hu, X. Zheng, Y. Zheng, H. Liu, H. Algadi and K. Chen, Adv. Compos. Hybrid Mater., 2022, 6, 21 CrossRef.
- T. Undabeytia, U. Shuali, S. Nir and B. Rubin, Minerals, 2021, 11, 9 CrossRef CAS.
- T. Undabeytia, S. Nir, E. Tel-Or and B. Rubin, J. Agric. Food Chem., 2000, 48, 4774–4779 CrossRef CAS PubMed.
- M. Nuruzzaman, M. M. Rahman, Y. Liu and R. Naidu, J. Agric. Food Chem., 2016, 64, 1447–1483 CrossRef CAS PubMed.
- G. Rytwo, R. Lavi, Y. Rytwo, H. Monchase, S. Dultz and T. N. König, Sci. Total Environ., 2013, 442, 134–142 CrossRef CAS PubMed.
- S. L. Lemke, P. G. Grant and T. D. Phillips, J. Agric. Food Chem., 1998, 46, 3789–3796 CrossRef CAS.
- Y. Fu, W. C. Wang, L. Q. Zhang, V. Vinokurov, A. Stavitskaya and Y. Lvov, Materials, 2019, 12, 4195 CrossRef CAS PubMed.
- S. Song, X. Jiang, H. Shen, W. Wu, Q. Shi, M. Wan, J. Zhang, H. Mo and J. Shen, ACS Appl. Bio Mater., 2021, 4, 6912–6923 CrossRef CAS PubMed.
- J. Singhal, S. Verma and S. Kumar, Sci. Total Environ., 2022, 837, 155669 CrossRef CAS PubMed.
- C. Aguzzi, P. Cerezo, C. Viseras and C. Caramella, Appl. Clay Sci., 2007, 36, 22–36 CrossRef CAS.
- E. Ruiz-Hitzky, M. Darder, B. Wicklein, F. M. Fernandes, F. A. Castro-Smirnov, M. A. M. del Burgo, G. del Real and P. Aranda, Advanced biohybrid materials based on nanoclays for biomedical applications, Incheon, South Korea, 2012.
- E. E. Gaskell and A. R. Hamilton, Future Med. Chem., 2014, 6, 641–655 CrossRef CAS PubMed.
- M. Long, Y. Zhang, P. Huang, S. Chang, Y. H. Hu, Q. Yang, L. F. Mao and H. M. Yang, Adv. Funct. Mater., 2018, 28, 1704452 CrossRef.
- P. Iravani, S. Iravani and R. S. Varma, Micromachines, 2022, 13, 1383 CrossRef PubMed.
- M. Soleymaniha, M.-A. Shahbazi, A. R. Rafieerad, A. Maleki and A. Amiri, Adv. Healthcare Mater., 2019, 8, 1801137 CrossRef PubMed.
- H. Li, J. Dai, X. Yi and F. Cheng, Biomater. Adv., 2022, 140, 213055 CrossRef CAS PubMed.
- G. Liu, J. Zou, Q. Tang, X. Yang, Y. Zhang, Q. Zhang, W. Huang, P. Chen, J. Shao and X. Dong, ACS Appl. Mater. Interfaces, 2017, 9, 40077–40086 CrossRef CAS PubMed.
- H. Lin, X. Wang, L. Yu, Y. Chen and J. Shi, Nano Lett., 2017, 17, 384–391 CrossRef CAS PubMed.
- S. Kumar, Y. Lei, N. H. Alshareef, M. A. Quevedo-Lopez and K. N. Salama, Biosens. Bioelectron., 2018, 121, 243–249 CrossRef CAS PubMed.
- L. S. Pires, F. D. Magalhães and A. M. Pinto, Polymers, 2022, 14, 1464 CrossRef CAS PubMed.
- C. R. Smith, J. Am. Chem. Soc., 1934, 56, 1561–1563 CrossRef CAS.
- J. E. Gieseking, Soil Sci., 1939, 47, 1–14 CrossRef CAS.
- S. B. Hendricks, J. Phys. Chem., 1941, 45, 65–81 CrossRef CAS.
- E. Ruiz-Hitzky, P. Aranda and M. Darder, in Tailored Organic–Inorganic Materials, ed. J. L. C. A. C. E. Brunet, 2015, ch. 6, pp. 245–297, DOI:10.1002/9781118792223.ch6.
- E. Ruiz-Hitzky, P. Aranda and M. J. Serratosa, in Handbook of Layered Materials, ed. S. M. Auerbach, K. A. Carrado and P. K. Dutta, Taylor & Francis Group, New York, 2004, ch. 3, pp. 91–154 Search PubMed.
- M. Naguib, V. N. Mochalin, Y. Dall’Agnese, M. Heon, M. W. Barsoum and Y. Gogotsi, Nat. Commun., 2013, 4, 1716 CrossRef PubMed.
- S. H. Overbury, A. I. Kolesnikov, G. M. Brown, Z. Y. Zhang, G. S. Nair, R. L. Sacci, R. Lotfi, A. C. T. van Duin and M. Naguib, J. Am. Chem. Soc., 2018, 140, 10305–10314 CrossRef CAS PubMed.
- G. W. Brindley and R. E. Sempels, Clay Miner., 1977, 12, 229–237 CrossRef CAS.
- A. M. de Andres, J. Merino, J. C. Galvan and E. Ruiz-Hitzky, Mater. Res. Bull., 1999, 34, 641–651 CrossRef CAS.
- D. Plee, F. Borg, L. Gatineau and J. J. Fripiat, J. Am. Chem. Soc., 1985, 107, 2362–2369 CrossRef CAS.
- A. Schutz, W. E. E. Stone, G. Poncelet and J. J. Fripiat, Clays Clay Miner., 1987, 35, 251–262 CrossRef CAS.
- W. Jones, G. Poncelet, E. Ruiz-Hitzky, P. Pomonis, H. Van Damme, F. Bergaya and N. Papayannakos, Synthesis, Characterisation and Application of Pillared Clays Produced in Large QuantitiesBrite, https://www-jmg.ch.cam.ac.uk/resources/pages/wj/index.html (accessed 4th of March, 2023).
- J. Zhu, L. Wang, J. Wang, F. Wang, M. Tian, S. Zheng, N. Shao, L. Wang and M. He, ACS Nano, 2020, 14, 15306–15316 CrossRef CAS PubMed.
- T. Endo, M. M. Mortland and T. J. Pinnavaia, Clays Clay Miner., 1980, 28, 105–110 CrossRef CAS.
- J. Ahenach, P. Cool, R. E. N. Impens and E. F. Vansant, J. Porous Mater., 2000, 7, 475–481 CrossRef CAS.
- Y.-S. Han, H. Matsumoto and S. Yamanaka, Chem. Mater., 1997, 9, 2013–2018 CrossRef CAS.
- A. Moini and T. J. Pinnavaia, Solid State Ionics, 1988, 26, 119–123 CrossRef CAS.
- S. Letaief and E. Ruiz-Hitzky, Chem. Commun., 2003, 2996–2997, 10.1039/b310854f.
- S. Letaïef, M. A. Martín-Luengo, P. Aranda and E. Ruiz-Hitzky, Adv. Funct. Mater., 2006, 16, 401–409 CrossRef.
- P. A. Maughan, V. R. Seymour, R. Bernardo-Gavito, D. J. Kelly, S. Shao, S. Tantisriyanurak, R. Dawson, S. J. Haigh, R. J. Young, N. Tapia-Ruiz and N. Bimbo, Langmuir, 2020, 36, 4370–4382 CrossRef CAS PubMed.
- E. Manova, P. Aranda, M. A. Martin-Luengo, S. Letaief and E. Ruiz-Hitzky, Microporous Mesoporous Mater., 2010, 131, 252–260 CrossRef CAS.
- S. Yamanaka, T. Nishihara, M. Hattori and Y. Suzuki, Mater. Chem. Phys., 1987, 17, 87–101 CrossRef CAS.
- E. G. Rightor, M. S. Tzou and T. J. Pinnavaia, J. Catal., 1991, 130, 29–40 CrossRef CAS.
- M. Akkari, P. Aranda, H. Ben Rhaiem, A. Ben Haj Amara and E. Ruiz-Hitzky, Appl. Clay Sci., 2016, 131, 131–139 CrossRef CAS.
- G. W. Brindley and S. Yamanaka, Am. Mineral., 1979, 64, 830–835 CAS.
- S. Zhang, H. Ying, P. Huang, J. Wang, Z. Zhang, T. Yang and W.-Q. Han, ACS Nano, 2020, 14, 17665–17674 CrossRef CAS PubMed.
- X. Qin, Z. Wu, J. Fang, S. Li, S. Tang and X. Wang, Appl. Surf. Sci., 2023, 616, 156521 CrossRef CAS.
- J. Lipton, G.-M. Weng, M. Alhabeb, K. Maleski, F. Antonio, J. Kong, Y. Gogotsi and A. D. Taylor, Nanoscale, 2019, 11, 20295–20300 RSC.
- A. C. Longden, Phys. Rev., 1902, 15, 355–365 Search PubMed.
- C. Ruiz-García, F. Heras, M. Á. Gilarranz, P. Aranda and E. Ruiz-Hitzky, Appl. Clay Sci., 2018, 161, 132–138 CrossRef.
- W. D. Zhang, I. Y. Phang and T. X. Liu, Adv. Mater., 2006, 18, 73–77 CrossRef CAS.
- P. Aranda and E. Ruiz-Hitzky, Chem. Rec., 2018, 18, 1125–1137 CrossRef CAS PubMed.
- S.-N. Ding, C.-L. Zheng, N. Wan and S. Cosnier, Monatsh. Chem. – Chem. Mon., 2014, 145, 1389–1394 CrossRef CAS.
- Y.-F. Lan and J.-J. Lin, J. Phys. Chem. A, 2009, 113, 8654–8659 CrossRef CAS PubMed.
- Z. Wang, X. Meng, J. Li, X. Du, S. Li, Z. Jiang and T. Tang, J. Phys. Chem. C, 2009, 113, 8058–8064 CrossRef CAS.
- F. M. Fernandes and E. Ruiz-Hitzky, Carbon, 2014, 72, 296–303 CrossRef CAS.
- T. Kyotani, N. Sonobe and A. Tomita, Nature, 1988, 331, 331–333 CrossRef.
- N. Sonobe, T. Kyotani, Y. Hishiyama, M. Shiraishi and A. Tomita, J. Phys. Chem., 1988, 92, 7029–7034 CrossRef CAS.
- N. Sonobe, T. Kyotani and A. Tomita, Carbon, 1988, 26, 573–578 CrossRef CAS.
- M. Darder and E. R. Ruiz-Hitzky, J. Mater. Chem., 2005, 15, 3913–3918 RSC.
- C. Ruiz-Garcia, J. Perez-Carvajal, A. Berenguer-Murcia, M. Darder, P. Aranda, D. Cazorla-Amoros and E. Ruiz-Hitzky, Phys. Chem. Chem. Phys., 2013, 15, 18635–18641 RSC.
- R. Fernández-Saavedra, P. Aranda and E. Ruiz-Hitzky, Adv. Funct. Mater., 2004, 14, 77–82 CrossRef.
- A. Gomez-Aviles, M. Darder, P. Aranda and E. Ruiz-Hitzky, Angew. Chem., Int. Ed., 2007, 46, 923–925 CrossRef CAS PubMed.
- E. Ruiz-Hitzky, M. Darder, F. M. Fernandes, E. Zatile, F. J. Palomares and P. Aranda, Adv. Mater., 2011, 23, 5250–5255 CrossRef CAS PubMed.
- C. Xu, G. Ning, X. Zhu, G. Wang, X. Liu, J. Gao, Q. Zhang, W. Qian and F. Wei, Carbon, 2013, 62, 213–221 CrossRef CAS.
- A. Barra, J. D. C. Santos, M. R. F. Silva, C. Nunes, E. Ruiz-Hitzky, I. Gonçalves, S. Yildirim, P. Ferreira and P. A. A. P. Marques, Nanomaterials, 2020, 10, 2077 CrossRef CAS PubMed.
- A. S. Levitt, M. Alhabeb, C. B. Hatter, A. Sarycheva, G. Dion and Y. Gogotsi, J. Mater. Chem. A, 2019, 7, 269–277 RSC.
- X. Li, X. Yin, M. Han, C. Song, H. Xu, Z. Hou, L. Zhang and L. Cheng, J. Mater. Chem. C, 2017, 5, 4068–4074 RSC.
- B. Dai, B. Zhao, X. Xie, T. Su, B. Fan, R. Zhang and R. Yang, J. Mater. Chem. C, 2018, 6, 5690–5697 RSC.
- J. Rajendran, T. S. Kannan, L. S. Dhanasekaran, P. Murugan, R. Atchudan, Z. A. Alothman, M. Ouladsmane and A. K. Sundramoorthy, Chemosphere, 2022, 287, 132106 CrossRef CAS PubMed.
- X. Xie, M.-Q. Zhao, B. Anasori, K. Maleski, C. E. Ren, J. Li, B. W. Byles, E. Pomerantseva, G. Wang and Y. Gogotsi, Nano Energy, 2016, 26, 513–523 CrossRef CAS.
- P. Gao, H. Shi, T. Ma, S. Liang, Y. Xia, Z. Xu, S. Wang, C. Min and L. Liu, ACS Appl. Mater. Interfaces, 2021, 13, 51028–51038 CrossRef CAS PubMed.
- Z. Wu, W. Deng, S. Tang, E. Ruiz-Hitzky, J. Luo and X. Wang, Chem. Eng. J., 2021, 426, 130776 CrossRef CAS.
- Y. Fukushima and S. Inagaki, J. Inclusion Phenom., 1987, 5, 473–482 CrossRef CAS.
- A. Okada, Y. Fukushima, M. Kawasumi, S. Inagaki, A. Usuki, S. Sugiyama, T. Kurauchi and O. Kamigaito, USA Pat., 4739007, 1988 Search PubMed.
- E. Ruiz-Hitzky, P. Aranda and M. J. Serratosa, in Handbook of Layered Materials, ed. S. M. Auerbach, K. A. Carrado and P. K. Dutta, Marcel Dekker, New York, 2004, p. 91 Search PubMed.
- A. Okada and A. Usuki, Macromol. Mater. Eng., 2006, 291, 1449–1476 CrossRef CAS.
- M. Carey and M. W. Barsoum, Mater. Today Adv., 2021, 9, 100120 CrossRef CAS.
- M. Carey, Z. Hinton, M. Sokol, N. J. Alvarez and M. W. Barsoum, ACS Appl. Mater. Interfaces, 2019, 11, 20425–20436 CrossRef CAS PubMed.
- Y. J. Liu, D. C. Degroot, J. L. Schindler, C. R. Kannewurf and M. G. Kanatzidis, Chem. Mater., 1991, 3, 992–994 CrossRef CAS.
- E. Ruiz-Hitzky and P. Aranda, Adv. Mater., 1990, 2, 545–547 CrossRef CAS.
- E. Ruiz-Hitzky, P. Aranda, B. Casal and J. C. Galvan, Adv. Mater., 1995, 7, 180–184 CrossRef CAS.
- V. Mehrotra and E. P. Giannelis, Solid State Commun., 1991, 77, 155–158 CrossRef.
- M. Boota, B. Anasori, C. Voigt, M.-Q. Zhao, M. W. Barsoum and Y. Gogotsi, Adv. Mater., 2016, 28, 1517–1522 CrossRef CAS PubMed.
- K. Li, J. Zhao, A. Zhussupbekova, C. E. Shuck, L. Hughes, Y. Dong, S. Barwich, S. Vaesen, I. V. Shvets, M. Möbius, W. Schmitt, Y. Gogotsi and V. Nicolosi, Nat. Commun., 2022, 13, 6884 CrossRef CAS PubMed.
- K. Li, X. Wang, S. Li, P. Urbankowski, J. Li, Y. Xu and Y. Gogotsi, Small, 2020, 16, 1906851 CrossRef CAS PubMed.
- N. Lara and E. RuizHitzky, J. Braz. Chem. Soc., 1996, 7, 193–197 CrossRef CAS.
- M. G. Kanatzidis, C. G. Wu, H. O. Marcy and C. R. Kannewurf, J. Am. Chem. Soc., 1989, 111, 4139–4141 CrossRef CAS.
- E. Ruiz-Hitzky, P. Aranda and B. Casal, J. Mater. Chem., 1992, 2, 581–582 RSC.
- C.-F. Du, X. Zhao, Z. Wang, H. Yu and Q. Ye, Nanomaterials, 2021, 11, 166 CrossRef CAS PubMed.
- Q. Pan, Y. Zheng, S. Kota, W. Huang, S. Wang, H. Qi, S. Kim, Y. Tu, M. W. Barsoum and C. Y. Li, Nanoscale Adv., 2019, 1, 395–402 RSC.
- C. Wang, Z. Zhang, J. Wang, Q. Wang and L. Shang, Mater. Today Bio, 2022, 16, 100352 CrossRef CAS PubMed.
- E. Ruiz-Hitzky, P. Aranda and M. Darder, in Kirk-Othmer Encyclopedia of Chemical Technology, John Wiley & Sons, 2008, pp. 1–28. DOI:10.1002/0471238961.bionruiz.a01.
- V. Ojijo and S. Sinha Ray, Prog. Polym. Sci., 2013, 38, 1543–1589 CrossRef CAS.
- E. Ruiz-Hitzky, M. Darder, F. M. Fernandes, B. Wicklein, A. C. S. Alcântara and P. Aranda, Prog. Polym. Sci., 2013, 38, 1392–1414 CrossRef CAS.
- E. Ruiz-Hitzky, K. Ariga and Y. Lvov, Bio–inorganic Hybrid Nanomaterials, Wiley-VCH, 2007 Search PubMed.
- E. Ruiz-Hitzky, M. Darder and P. Aranda, J. Mater. Chem., 2005, 15, 3650–3662 RSC.
- V. J. Cadarso, A. Llobera, C. Fernandez-Sanchez, M. Darder and C. Dominguez, Sens. Actuators, B, 2009, 139, 143–149 CrossRef CAS.
- A. C. S. Alcântara, M. Darder, P. Aranda and E. Ruiz-Hitzky, Appl. Clay Sci., 2014, 96, 2–8 CrossRef.
- Z. Tang, N. A. Kotov, S. Magonov and B. Ozturk, Nat. Mater., 2003, 2, 413–418 CrossRef CAS PubMed.
- K. Sung, S. Nakagawa and N. Yoshie, ACS Omega, 2017, 2, 8475–8482 CrossRef CAS PubMed.
- M. M. González del Campo, M. Darder, P. Aranda, M. Akkari, Y. Huttel, A. Mayoral, J. Bettini and E. Ruiz-Hitzky, Adv. Funct. Mater., 2018, 28, 1703048 CrossRef.
- F. A. Castro-Smirnov, J. Ayache, J.-R. Bertrand, E. Dardillac, E. Le Cam, O. Piétrement, P. Aranda, E. Ruiz-Hitzky and B. S. Lopez, Sci. Rep., 2017, 7, 5586 CrossRef PubMed.
- F. A. Castro-Smirnov, O. Piétrement, P. Aranda, J.-R. Bertrand, J. Ayache, E. Le Cam, E. Ruiz-Hitzky and B. S. Lopez, Sci. Rep., 2016, 6, 36341 CrossRef CAS PubMed.
- V. M. Hong Ng, H. Huang, K. Zhou, P. S. Lee, W. Que, J. Z. Xu and L. B. Kong, J. Mater. Chem. A, 2017, 5, 3039–3068 RSC.
- C. I. Idumah, International Journal of Polymeric Materials and Polymeric Biomaterials, 2022, pp. 1–22, DOI:10.1080/00914037.2022.2098297.
- M. Darder, P. Aranda, M. L. Ferrer, M. C. Gutiérrez, F. del Monte and E. Ruiz-Hitzky, Adv. Mater., 2011, 23, 5262–5267 CrossRef CAS PubMed.
- X. Li, Y.-C. Li, M. Chen, Q. Shi, R. Sun and X. Wang, J. Mater. Chem. B, 2018, 6, 6544–6549 RSC.
- H. Lin, Y. Chen and J. Shi, Adv. Sci., 2018, 5, 1800518 CrossRef PubMed.
Footnote |
† See for instance the two most cited articles searching by “Clay* and Intercalation” as the topic entries in Clarivate-WoS, which are related to MXenes, both published in 2014 with a total of ca. 7000 citations. The total number of publications on clays is 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 680 and 9710 on MXenes in the 2019–2022 period. 680 and 9710 on MXenes in the 2019–2022 period. |
| This journal is © The Royal Society of Chemistry 2023 |