Open Access Article
Open Access ArticleExploring condensable organic vapors and their co-occurrence with PM2.5 and O3 in winter in Eastern China†
Yuliang
Liu‡
 ab,
Chong
Liu‡
ab,
Wei
Nie
ab,
Chong
Liu‡
ab,
Wei
Nie
 *ab,
Yuanyuan
Li
ab,
Dafeng
Ge
ab,
Liangduo
Chen
ab,
Caijun
Zhu
ab,
Lei
Wang
ab,
Yuxuan
Zhang
ab,
Tengyu
Liu
*ab,
Yuanyuan
Li
ab,
Dafeng
Ge
ab,
Liangduo
Chen
ab,
Caijun
Zhu
ab,
Lei
Wang
ab,
Yuxuan
Zhang
ab,
Tengyu
Liu
 ab,
Ximeng
Qi
ab,
Jiaping
Wang
ab,
Dandan
Huang
c,
Zhe
Wang
ab,
Ximeng
Qi
ab,
Jiaping
Wang
ab,
Dandan
Huang
c,
Zhe
Wang
 d,
Chao
Yan
ab,
Xuguang
Chi
ab and
Aijun
Ding
ab
d,
Chao
Yan
ab,
Xuguang
Chi
ab and
Aijun
Ding
ab
aJoint International Research Laboratory of Atmospheric and Earth System Research, School of Atmospheric Sciences, Nanjing University, Nanjing, China. E-mail: niewei@nju.edu.cn
bNational Observation and Research Station for Atmospheric Processes and Environmental Change in Yangtze River Delta, Nanjing, Jiangsu Province, China
cState Environmental Protection Key Laboratory of Formation and Prevention of Urban Air Pollution Complex, Shanghai Academy of Environmental Sciences, Shanghai, China
dDivision of Environment and Sustainability, The Hong Kong University of Science and Technology, Hong Kong SAR, China
First published on 6th January 2023
Abstract
Oxygenated organic molecules (OOMs) are important oxidation products of volatile organic compounds (VOCs), and they act as key condensable vapors for new particle formation (NPF) and secondary organic aerosol (SOA) in the atmosphere. However, the large diversity and extremely low concentration make OOMs unmeasurable by conventional means, resulting in a poor understanding of OOMs, especially their formation. Herein, we observed OOMs with state-of-the-art mass spectrometry in a megacity in eastern China during the winter and characterized them by performing positive matrix factorization on binned mass spectra (binPMF). The binPMF analysis revealed 3 factors with clear precursor profiles (1 aromatic and 2 aliphatics), 2 ozone-related factors, 2 mixed-precursor-derived factors with unclear processes, and 4 factors dominated by nitrated phenols. We performed peak assignment on binPMF factors and identified over 1500 molecules with a mean total concentration of 4.7 × 107 molecules per cm3 with all nitrated phenols excluded. Most OOMs are organic nitrates produced by the oxidation of anthropogenic VOC with interactions between the derived RO2 and NOx. These molecules containing 3 to 7 effective oxygen atoms (excluding –NO2 in the nitrate moiety) introduced by autoxidation and multigenerational oxidation are less volatile, and hence, are susceptible to condensational loss. However, the observed OOM concentrations increase with the buildup of PM2.5. This can be explained by enhanced OOM photochemical production owing to accumulated VOCs and sustained oxidants that outcompete condensational loss. This suggests favored SOA production via OOM condensation during haze. Furthermore, the highest OOM concentrations occur when PM2.5 and O3 are coenhanced. Under this condition, OOMs mainly come from ozone-related factors that are generated jointly with ozone and from aliphatic-dominated factors that are closely associated with PM2.5. Overall, our results improve the understanding of OOM formation and its impact on the polluted atmosphere.
Environmental significanceAtmospheric secondary organic aerosols, whose precursors are volatile organic compounds, significantly affect climate forcing and human health. The discovery of oxygenated organic molecules (OOMs), which act as crucial intermediates, provides an opportunity to understand organic gas oxidation and aerosol formation at the molecular level. However, comprehensive elucidation of the nature and formation of OOMs is far from achieved, particularly in densely populated areas suffering from severe air pollution. Herein, we investigated wintertime OOMs observed using advanced mass spectrometry in a typical urban environment. We found that OOMs are produced from anthropogenic organic gas oxidation and interacted strongly with nitrogen oxides. Importantly, the photochemical production of OOMs and their corresponding contributions to aerosol are amplified during haze. Some characteristic OOM formation processes are linked to the simultaneous enhancement of PM2.5 and ozone. |
1 Introduction
Secondary organic aerosol (SOA) is a ubiquitous and important component of atmospheric submicron particles,1 which deteriorate air quality,2,3 endanger human health4 and affect the earth's radiation balance.5 The oxidation of volatile organic compounds (VOCs) from anthropogenic and biogenic emissions ultimately leads to the formation of SOA,6,7 with oxygenated organic molecules (OOMs)8–10 being key intermediates in this convoluted process. Unlike traditional oxygenated VOCs, OOMs discovered using the latest developed mass spectrometric techniques11–13 are a subset of VOC oxidation products attaining multiple oxygenated functionalities; thus, they are considerably less volatile than their precursors. Generally, OOMs belonging to ultra-low-volatility VOC (ULVOC) can participate in particle nucleation (∼1 nm);14,15 OOMs belonging to extremely-low-volatility (ELVOC) can drive the initial growth (1–3 nm);16 and OOMs belonging to low-volatility VOC (LVOC) dominate the subsequent growth (3 to >50 nm).8 When particles contain organic components, OOMs belonging to semi-volatile VOC (SVOC) can partition into the particle phase. All these processes are responsible for SOA formation.17,18Currently, the formation of OOMs remains elusive.9 Conventional VOC oxidation mechanisms19 cannot account for OOMs with high O/C ratios. New ideas, including the autoxidation of organic peroxy radicals (RO2),20,21 dimerization of RO2,22 and multigenerational oxidation,23,24 have recently been proposed as efficient ways to produce these condensable organic vapors. However, the formation of OOMs in the atmosphere is expected to be an integrated result of numerous known and unknown processes, which are extremely complex and of high variability at different spatiotemporal scales. Thus, comprehensive field investigations are warranted to elucidate the origins, formation, and compositions of OOMs in diverse ambient environments.
In general, the concentration of atmospheric OOMs is extremely low (dozens ppqv to dozens pptv),25 making it difficult to detect. This leads to a scarcity of global observations of OOMs. Ambient OOM measurements were initially conducted in clean environments, such as forested or remote areas,26–29 and later sporadically in anthropogenically polluted regions.10,30,31 Moreover, many studies have focused on evaluating the contribution of OOMs to new particle formation (NPF) and SOA,26,32,33 whereas only few studies have specifically investigated OOM sources. Ozonolysis and OH-initiated oxidation of monoterpenes are considered to be the main sources of OOMs in the boreal forest.34,35 Isoprene and monoterpenes are identified as the main precursors of OOMs in the Southeastern US forest, and their oxidations are moderately influenced by anthropogenic emissions.36 Instead, OOMs are found to be dominantly generated from the oxidation processes of anthropogenic VOCs under the strong perturbation of nitrogen oxides (NOx) in urban cluster areas of eastern China.37,38 These limited observations have significantly improved our understanding of OOMs, but the properties and formation of OOMs in the polluted atmosphere are not sufficiently addressed and are the focus of this study.
The Yangtze River Delta (YRD) region in eastern China, as a global hotspot for anthropogenic emissions, suffers from severe air pollution.39,40 Although air pollution has been dramatically mitigated in recent years,41,42 fine particulate matter (PM2.5) and ozone (O3) pollution remain major challenges for continuously improving air quality in this region.43–45 Many studies have aimed to elucidate the scientific fundamentals behind the coordinated control of these two pollutants from the perspective of precursors (NOx and VOCs). It is noteworthy that OOMs are involved in chemical processes, resulting in PM2.5 and O3 pollution. Generally, VOCs are oxidized to produce RO2, which convert NO to NO2 and subsequently produce O3 by NO2 photolysis, meanwhile, RO2 is terminated to form OOMs and then accumulate SOA by gas-particle partitioning.
In this study, we report a comprehensive analysis to ascertain the formation of OOMs during winter in Nanjing, a megacity in the YRD region. OOMs were observed using a chemical ionization atmospheric pressure interface time-of-flight mass spectrometer with nitrate reagent ions (nitrate CI-APi-TOF).12 The characteristics of OOM sources or processes were explored by performing positive matrix factorization (PMF) on binned mass spectrometry data.46,47 Then, the concentration, molecular composition, and volatility of the OOMs were investigated in detail. Finally, the connections of OOM formation with PM2.5 and O3 were discussed prospectively.
2 Methodology
2.1 Study site
The measurement was conducted at the Station for Observing Regional Processes of the Earth System (SORPES; 32°07′14′′ N, 118°57′10′′ E; 62 m a.s.l.)48 from November 28, 2020 to January 3, 2021. The SORPES station is located in the northeastern suburbs of Nanjing, a megacity in the YRD region of eastern China. The station captures urban-scale emission activity49 and is frequently impacted by polluted air masses from the North China Plain and the YRD urban agglomeration.40,50 Detailed descriptions of the station can be found in previous studies.51–552.2 Instrumentation
A chemical ionization atmospheric pressure interface time-of-flight mass spectrometer with nitrate reagent ions (nitrate CI-APi-TOF; Aerodyne Research Inc. and Tofwerk AG) was used to detect ambient sulfuric acid and OOMs. The working principle2,13 and sampling configuration37 of this instrument were described in previous studies. Because standards for OOM measurable by the nitrate CI-APi-TOF are yet to be established, the concentrations of OOMs were quantified by an empirical method as described below:15,16,56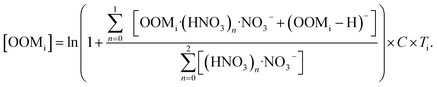 | (1) |
VOCs were detected by applying a proton transfer reaction time-of-flight mass spectrometer (PTR-TOF-MS, Ionicon Analytik). PM2.5 was measured using a combined technique of light scattering photometry and beta radiation attenuation (Thermo Scientific SHARP Monitor Model 5030). The chemical compositions of PM2.5 were obtained from a time-of-flight aerosol chemical speciation monitor (TOF-ACSM, Aerodyne Research Inc.). The number of concentrations of particles was measured using a differential mobility particle spectrometer (DMPS), covering a size range from 6 to 800 nm. Trace gases, including NOx, NOy, O3, SO2 and CO, were monitored by applying Thermo Environmental Instruments (Models 42i-TL, 42i-Y, 49i, 43i-TLE, and API T300, respectively). J(O1D) was measured using an ultra-fast CCD detector spectrometer, UVB enhanced (Meteorologieconsult Gmbh, Germany). Meteorological parameters were recorded by the Automatic Weather Station (CAMPEEL Co., AG1000).
2.3 BinPMF analysis
Positive matrix factorization (PMF)59 was applied to binned mass spectra (binPMF46) to separate various sources or processes related to OOMs. Briefly, the raw mass spectra were divided into narrow bins with a width of 0.006 Th after mass calibration. Then, the PMF model inputs, including data and error matrices, were prepared based on an idea from Zhang et al.46 The binPMF can separate the complex overlapping peaks and retain high resolution (HR) information as much as possible. This can minimize the uncertainty of HR peak fitting affecting the interpretation of PMF solutions. The PMF analysis in this study uses the IGOR-based analyzing interface SoFi (solution finder, version 6.8) and ME-2.60 Details of the preparation and diagnostics of binPMF are provided in Section S2 in the ESI.†3 Results and discussions
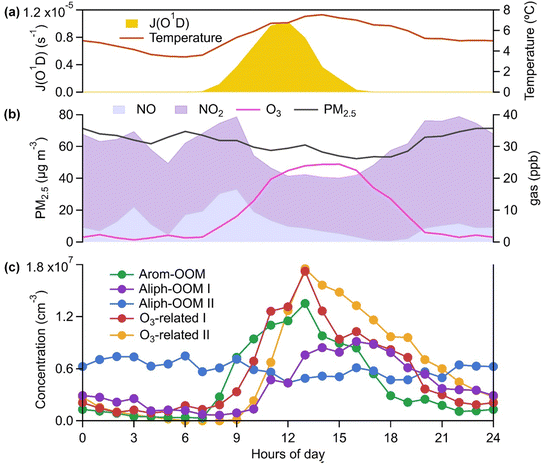
The observations were conducted during winter (low temperature and low radiation, as shown in Fig. S1† and 1a, respectively) with intensive anthropogenic emissions and their resulting aerosol pollution (Fig. S1† and 1b). The OOMs unambiguously exhibited time-dependent mass spectra and concentration differences (Fig. S1†). To deconvolute the complex dynamic processes of OOM formation, we performed a binPMF analysis and found that the 13-factor solution could explain the data variation (Fig. S5 and S6†). The selected solution includes 3 factors from the oxidation of a single anthropogenic VOC family (Fig. 1c), 2 factors related to ozone chemistry (Fig. 1c), 2 factors derived from mixed precursors with unclear processes (Fig. S14†), 4 factors dominated by nitrated phenols (NPs, Fig. S17†) and 2 factors dominated by fluorinated contaminations. NPs that are usually volatile have been broadly investigated.61–64 Fluorinated contaminations come from Teflon tube volatiles and perfluoric acid for transmission efficiency calibrations. These two types of compounds are not our concern. The other 7 factors are dominated by organic nitrates (Fig. 2 and S14†), which are expected to contribute significantly to the particulate organic nitrates observed at this site.65 Detailed discussions of these OOM factors are given below (Table 1).| Factor | Average concentration (cm−3) | Effective formulas | MW (g mol−1) | OSc | O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C C |
N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C C |
DBE | log10(C*(μg m−3)) in 300 K |
|---|---|---|---|---|---|---|---|---|
a MW is the molecular weight, OSc is the carbon oxidation state, O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C is the oxygen to carbon ratio, N C is the oxygen to carbon ratio, N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C is the nitrogen to carbon ratio, DBE is the double bond equivalent, C* the saturation concentration and log10(C*) is the volatility. C is the nitrogen to carbon ratio, DBE is the double bond equivalent, C* the saturation concentration and log10(C*) is the volatility.
|
||||||||
| Arom-OOM | 6.70 × 106 | C8.4H13.4O6.1N1.0 | 226.0 | −0.68 | 0.78 | 0.13 | 2.2 | 0.9 |
| Aliph-OOM I | 7.02 × 106 | C8.2H13.8O6.2N1.2 | 228.3 | −0.79 | 0.84 | 0.16 | 1.6 | 1.2 |
| Aliph-OOM II | 8.00 × 106 | C7.4H13.1O6.9N1.7 | 235.4 | −1.02 | 1.00 | 0.25 | 1.0 | 1.4 |
| O3-related I | 8.01 × 106 | C7.6H11.9O7.0N1.2 | 232.5 | −0.40 | 0.99 | 0.17 | 2.1 | 0.5 |
| O3-related II | 7.17 × 106 | C7.0H10.4O6.1N0.9 | 205.4 | −0.29 | 0.94 | 0.14 | 2.4 | 1.6 |
| MT-mixed-OOM | 6.19 × 106 | C8.4H13.4O5.7N1.0 | 219.6 | −0.77 | 0.74 | 0.13 | 2.2 | 1.3 |
| Mixed-OOM | 5.65 × 106 | C7.4H11.2O5.8N0.9 | 205.6 | −0.51 | 0.86 | 0.14 | 2.4 | 1.8 |
3.1 Anthropogenic VOC chemistry
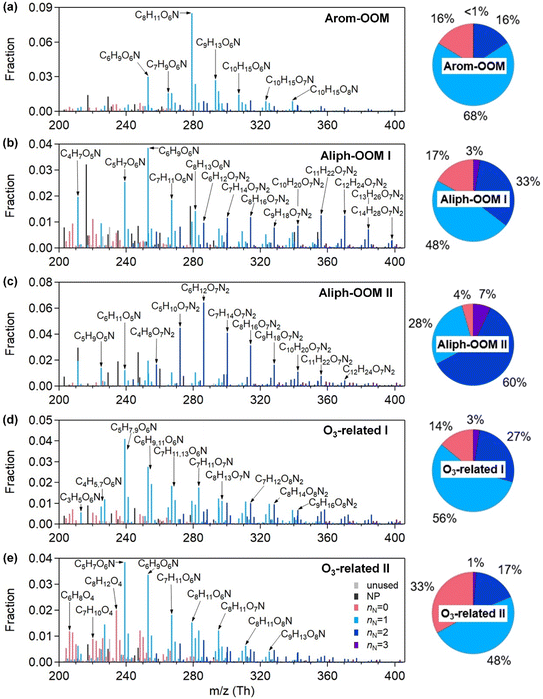
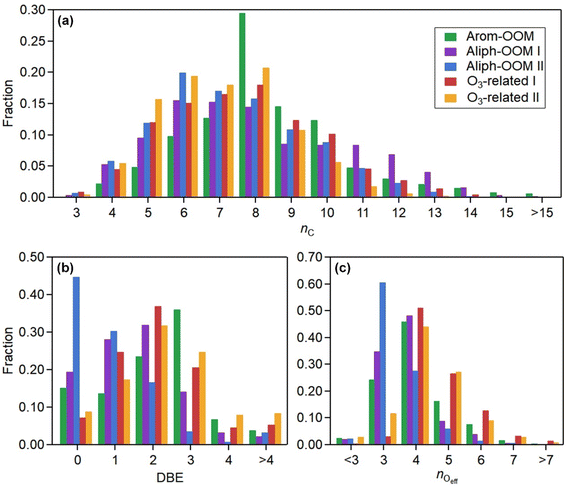
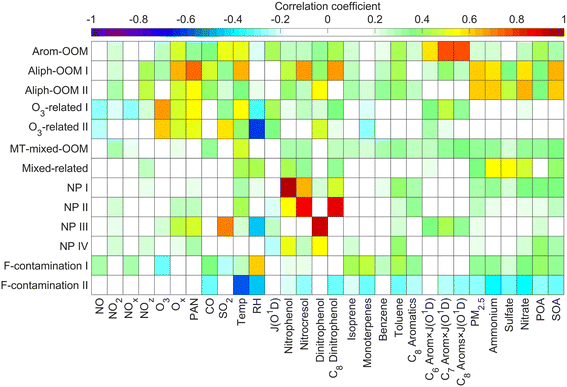
The first three factors possess clear anthropogenic precursor features, including one mainly from the oxidation of aromatic VOCs (denoted as Arom-OOM) and the other two from the oxidation of aliphatic VOCs (Aliph-OOMs I and II). The Arom-OOM factor is characterized by molecules with a double bond equivalent (DBE) of 3 (Fig. 2a), in which the most prominent ones are CxH2x−5O6N (x = [6, 12], Table S2†). They are produced from the reactions of a homologous series of OH-initiated aromatic bicyclic peroxy radicals with NO.66 C8-OOMs are the most abundant in this factor (Fig. 3a), which coincides with the OH reactivity distribution of aromatics (Fig. S8a†). This factor has a positive correlation (Fig. 4) and similar daily variation (Fig. S8b†) with the proxies of aromatic photo-oxidation (aromatics × J(O1D)), with its concentration reaching a peak plateau after 10:00 local time (LT), declining after 14:00 LT, and staying low throughout the night (Fig. 1c). The CxH2x−5O8N (x = [7, 11]) series (Table S2†) potentially formed through autoxidation (Fig. S9†) is still easy to observe although the abundances of NOx measured here are significantly higher than the set conditions of previous laboratory studies.67–69 In addition, products with DBE < 3 (Fig. 3b) in this factor are supposed to be produced by multigenerational OH oxidations. These pathways can efficiently make aromatic-derived OOMs more oxidized, but more studies are needed to clarify their persistence under such heavily polluted conditions.Unlike the Arom-OOM factor, the two factors from aliphatic oxidation comprise more saturated compounds (Fig. 1b, c and 3b), particularly a series of multi-nitrates (Tables S3 and S4†), such as CxH2x−2O7−8N2 (x = [5, 13]) and CxH2xO7N2 (x = [4, 13]). In the case of alkane oxidation under high NOx, one OH attack can only add one nitrate group or one carbonyl group to the product molecule (Fig. S10†). According to the method proposed by Liu et al.,37 the two Aliph-OOM factors are dominated by second- and third-generation products (Fig. S10†). This observation validates the substantial amount of aliphatic dinitrates and trinitrates measured in recent laboratory experiments.70,71 It seems that aromatic-derived OOMs contain more oxygen atoms than aliphatic-derived OOMs (Fig. 3c), and the latter are more prone to contain nitrate groups than the former (Fig. 1a–c). Thus, we speculate that aliphatics and aromatics play distinct roles in the budgets of reactive nitrogen oxides.72
Both Aliph-OOM factors are correlated with PM2.5 (Fig. 4), suggesting their close connections with aerosol pollution. However, the two Aliph-OOM factors differ obviously in their temporal variation. The Aliph-OOM I factor has a later daytime peak (about 13:00–18:00 LT, Fig. 1c), suggesting that these multi-generational products are formed locally and require enough photochemistry. In contrast, the Aliph-OOM-II factor shows unclear diurnal variation (Fig. 1c) and presents many accumulation episodes in the time series, such as PM2.5 (Fig. S1†). Thus, it is more likely to characterize OOMs in aged air masses transported to this site. The properties of OOMs from the two different processes described above are distinct. The Aliph-OOM-I factor has a larger proportion of heavy (C10–14) molecules than the Aliph-OOM-II factor does (Fig. 3a), which is probably because long-chain aliphatic nitrates are less volatile and more easily consumed by condensation onto particle surfaces during transport. Moreover, the Aliph-OOM-I factor has a higher fraction of potential carbonyl groups (Fig. 3b), such as the CxH2x−3O6N (x = [5, 13]) series with a DBE of 2, while the Aliph-OOM-II factor has a higher proportion of nitrate groups (Fig. 2b and c), such as the CxH2x−1O9−10N3 (x = [5, 10]) series. Both carbonyl and nitrate moieties are vulnerable to loss through heterogeneous uptake on particles.72,73 The difference in chemical composition between the two factors and their high concentration at high particle loadings may be attributed to their generation processes. Future laboratory studies are urgently needed to reveal the unknown part of aliphatic oxidation in a typical urban atmosphere.
3.2 Ozone-related chemistry
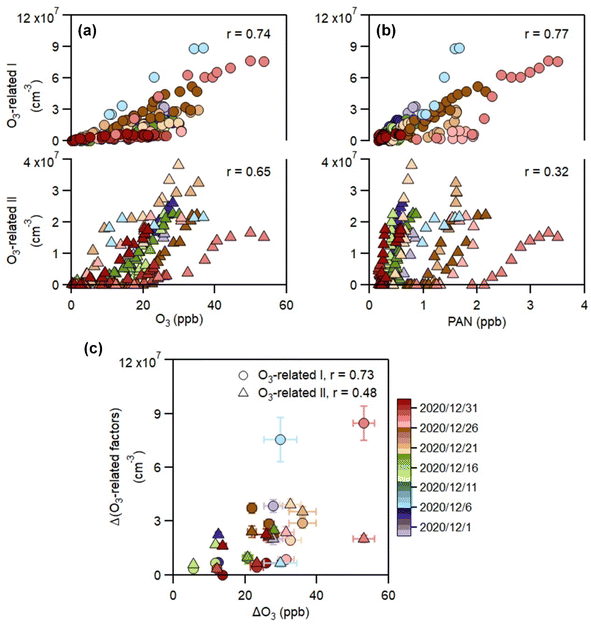
In contrast to the above factors distinguished by precursors, the following two factors are driven by photochemistry. The O3-related I factor is well correlated with ozone and peroxyacetyl nitrate (PAN) in 17 ozone production cases (Fig. 5a and b). These cases are selected by the following criteria: (a) good correlation between O3 and time (r > 0.9), i.e., O3 is continuously produced without substantial interruptions; (b) the maximum hourly J(O1D) for the day exceeds 1 × 10−5 s−1; and (c) the duration of the case exceeds 2 hours. It follows that this factor exhibiting significant daytime peaks (Fig. 1c) is likely produced jointly with ozone and PAN by tropospheric photochemistry involving VOCs and NOx.74 The fingerprint molecules of the O3-related-I factor (Fig. 2d and Table S5†) are the CxH2x−3O6N (x = [4, 12]) and CxH2x−1O6N (x = [4, 11]) series, which presumably contain a peroxy-acyl-nitrate moiety but one more carbonyl or hydroxyl than PAN, respectively. The O3-related I factor has the lowest volatility (Table 1), resulting from the outstanding proportion of OOMs with an effective oxygen number (nOeff = nO − 2 × nN) over 4 (Fig. 3c). This indicates that this specific VOC oxidation process linked to ozone production facilitates the production of condensable organic vapors and, hence, the accumulation of SOA (Fig. S12†).The O3-related II factor, which is dominated by the CxH2x−3O6N (x = [4, 10]) and CxH2x−1O6N (x = [4, 9]) series (Fig. 2e and Table S6†), is moderately correlated with ozone and weakly correlated with PAN (Fig. 5a and b). This shows that the O3-related II factor is partially homologous to the O3-related-I factor. Both aliphatic-derived (DBE = 0, 1) and aromatic-derived OOMs (DBE = 3, 4) are present in these two factors (Fig. 3b), illustrating the crucial effect of anthropogenic VOCs on ozone production. Compared with the O3-related-I factor, the O3-related-II factor has a lower concentration in the morning (high NO) and a higher concentration in the afternoon (high temperature). Importantly, the O3-related-II factor contains the most non-nitrates, which are related to chemical processes influenced by lower NO and higher temperature. It could be that the branching ratio of the RO2 + NO reaction to nitrate decreases as the temperature increases,72 or perhaps the contribution of the RO2 + HO2 reaction increases as NO decreases and temperature increases.37
3.3 Other OOM factors
Here are two OOM factors of mixed precursor origin without distinctive features. The MT-mixed-OOM factor contains potential monoterpene-derived OOMs (Fig. S14a†), such as C10H15,17OxN (x = [5, 8]) and C10H16OxN2 (x = [7, 10]). Monoterpenes are likely to stem from anthropogenic emissions in urban areas during the cold season,75 and thus the co-presence of many oxidation products of anthropogenic precursors in this factor is understandable. The mixed-OOM factor comprising aliphatic-derived and aromatic-derived OOMs (Fig. S14b†) shows no clear relationship with the external gas-phase and particulate tracers (Fig. 4). It is noteworthy that these mixed factors cannot be further split by increasing the number of factors (Fig. S15 and S16†), indicating that they do not suffer from incomplete separations. Instead, they may come from complex nonlinear formation processes that are real in the atmosphere but cannot be interpreted well based on current understandings.3.4 Connections of OOMs with PM2.5 and O3
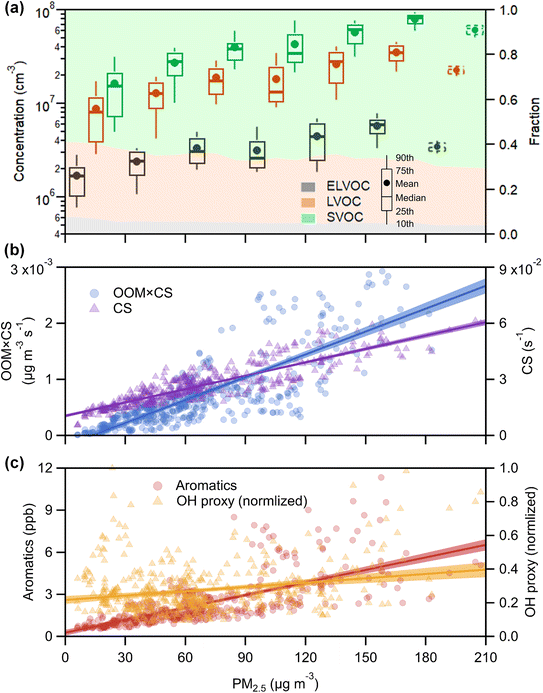
Using binPMF to separate overlapping peaks and provide useful chemical information, over 1500 non-nitro molecules were identified through HR fitting and then reconstructed from the selected binPMF solution (Fig. S18a†). The mean total concentration is 4.7 × 107 molecules per cm3, of which about 81% is organic nitrate generated by the reaction of NOx with RO2 from anthropogenic-VOC oxidations (Fig. S18a†). Under such a strong influence of NOx, non-nitro OOMs can still acquire 3–7 effective oxygen atoms (even over 7 for a few molecules) (Fig. S18b†), which benefit from multigenerational oxidation and autoxidation discussed in the aforementioned binPMF factors. Interestingly, the total OOM concentration is found to be correlated with PM2.5 and O3 (Fig. S19†). Understanding the formation of OOMs in relation to PM2.5 and O3 is an intriguing issue in the context of VOCs acting as fuels for atmospheric chemistry.76First, the observed concentrations of OOMs from ELVOC to SVOC show unexpected upward trends with the accumulation of PM2.5 (Fig. 6a). However, this suggests that OOM condensation (roughly represented by OOMs × CS, Fig. 6b) remains a crucial pathway of SOA formation during haze when the multiphase reactions are proposed to be significant.2,73,77 This efficient gas-to-phase conversion has been revealed to be prevalent in major urban cluster areas in eastern China under highly polluted atmospheric conditions.10 It is noteworthy that the percentage of SVOC in OOMs increases with PM2.5 whereas the fractions of ELVOC and LVOC decrease (Fig. 6a), which demonstrates that less volatile OOMs are more susceptible to condensation loss. However, the source of OOMs is supposed to increase much faster and outperforms the enhanced condensational loss of OOMs. With the buildup of haze, the emitted precursors (e.g., aromatics) are enriched (Fig. 6c) owing to stagnant wind fields coupled with a shallow boundary layer.78,79 At the high OH consumption rate induced by these reactive gases, the abundance of OH (represented by the OH proxy,80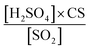 ) does not decline (Fig. 6c) most likely owing to the rapid OH recycling from peroxyl radicals81 and the atmospheric oxidation capability amplified by heterogeneous reactions on aerosol surfaces.49,82,83 The intense photochemistry can also be deduced from the co-accumulation of PAN and Ox with PM2.5 (Fig. S20†). Consequently, the formation of OOMs and the contribution of OOMs to SOA are enhanced during haze (Fig. 6b).
) does not decline (Fig. 6c) most likely owing to the rapid OH recycling from peroxyl radicals81 and the atmospheric oxidation capability amplified by heterogeneous reactions on aerosol surfaces.49,82,83 The intense photochemistry can also be deduced from the co-accumulation of PAN and Ox with PM2.5 (Fig. S20†). Consequently, the formation of OOMs and the contribution of OOMs to SOA are enhanced during haze (Fig. 6b).
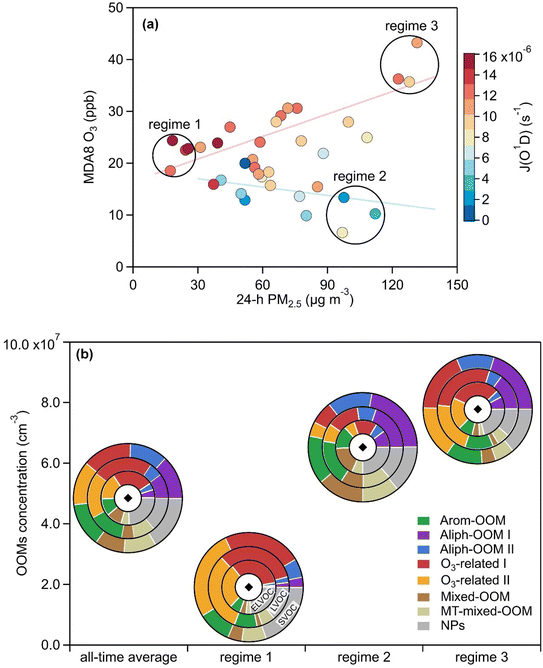
Further, such strong photochemical production of OOMs is accompanied by efficient ozone production, which is usually masked by the fast removal caused by heavily emitted NO.81 As shown in Fig. 7a, under moderate radiation conditions (the maximum daily J(O1D) > 1 × 10−5 s−1), the maximum daily 8 h average (MDA8) ozone follows 24 h average PM2.5 and is elevated even on haze days. It has recently been found that ozone can benefit from active photochemistry to exceed the air quality standard during cold seasons in some areas with high VOC emissions84,85 although ozone is generally considered to be a summertime pollutant.86 Here, we pick three regimes on the scatter plot of MDA8 O3 and 24 h average PM2.5 (Fig. 7a) and then compare the formation of OOMs in different regimes (Fig. 7b). Under relatively clean conditions (regime 1: low 24 h average PM2.5 and mid MDA8 O3), OOMs with different volatilities are mainly assigned to ozone-related factors (Fig. 7b). When shifting to normal winter haze conditions (regime 2: high 24 h average PM2.5 and low MDA8 O3), the major sources of OOMs are more extensive with clearly increased contributions from the two Aliph-OOM factors and the Arom-OOM factor (Fig. 7b), which ultimately increases OOM concentrations (Fig. 7b). The highest concentrations of OOMs are present in regime 3 (high 24 h average PM2.5 and high MDA8 O3) where OOMs are mainly sourced from ozone-related factors, followed by the Aliph-OOM factors (Fig. 7b). The role of VOC in PM2.5 and O3 pollution is usually interpreted based on traditional VOC oxidation mechanisms,81,85 our analysis indicates that the perturbations introduced by the yet-to-be-resolved part of VOC oxidation (i.e., formation of OOMs and organic nitrate) need to be addressed.
4 Summary and conclusions
We investigated the formation of gas-phase OOMs observed using a nitrate CI-APi-TOF in a megacity of eastern China during the winter. We performed binPMF analysis on the raw spectra. A high-quality OOM dataset containing over 1500 OOM molecules was established using binPMF to separate overlapping peaks. After excluding nitrated phenols, these OOMs have a mean total concentration of 4.7 × 107 molecules per cm3, which predominantly comprised organic nitrate (∼81% of the total), revealing the intense impact of NOx on the OOM formation. In addition, most OOMs contain 3 to 7 effective oxygen atoms owing to multigenerational oxidation and autoxidation, and their low-volatility feature favors SOA formation.The binPMF analysis also identified 7 factors characterizing anthropogenic OOMs (excluding NPs). All factors mainly comprise homologous compounds because their precursors, anthropogenic VOCs, are usually co-emitted. Specifically, the Arom-OOM factor (peaking at 10:00–14:00 LT) is characterized by aromatic-derived unsaturated mononitrates, such as CxH2x−5O6−8N (x = [6, 11]). The Aliph-OOM-I factor (peaking at 13:00 to 18:00 LT) is dominated by aliphatic-derived nearly and fully saturated organic nitrates (mononitrates are the most abundant), such as CxH2x−1O5,6N (x = [4, 12]), CxH2xO7N2 (x = [4, 14]) and CxH2x−2O7,8N2 (x = [6, 13]). The Aliph-OOM-II factor, markedly affected by transport and associated with haze accumulation, comprises aliphatic-derived saturated and near-saturated organic nitrates (dinitrates are the most abundant), such as CxH2xO7N2 (x = [4, 13]), CxH2x−2O7,8N2 (x = [5, 13]), and CxH2x−1O9,10N3 (x = [5, 10]). The O3-related-I factor (peaking around 13:00 LT), whose fingerprint molecules are CxH2x−3O6N (x = [4, 12]), is correlated with ozone and PAN; hence, it is suggested to be an important co-products with ozone from atmospheric photochemistry. The O3-related-II factor (peaking around 13:00 LT) also correlates with ozone. In addition to sharing the same fingerprint molecules with the O3-related-I factor, it contains the most abundant non-nitrates, such as CxH2x−4O4,5 (x = [5, 10]), possibly derived from RO2 terminations influenced by lower NO and higher temperature. The MT-mixed-OOM factor and the Mixed-OOM factor are derived from mixed precursors undergoing unknown processes. Notably, these characteristic profiles of OOMs have not been found in the available laboratory results and cannot be well explained by existing explicit chemical mechanisms. These observational findings can inform and inspire future laboratory and mechanistic studies of OOMs.
Finally, we analyzed the relationship of OOMs with PM2.5 and O3. As the condensation sink increases during the buildup of haze, the concentration of OOMs from ELVOC to SVOC shows an unexpected upward trend. This phenomenon illustrates that the VOC to SOA process is accelerated through OOM condensation and indicates the enhanced photochemical production of OOMs, which is powered by the accumulated precursors owing to limited air diffusion and sustained oxidants by rapid HOx (OH + HO2) cycling and heterogeneous reactions. Moreover, the highest OOM concentrations occurred in the case of the co-enhancement of PM2.5 and O3, which is generally due to active photochemistry on haze days. In detail, this is presumably ascribed to the chemical processes characterized by ozone-related factors that boost both O3 and low-volatility OOMs, as well as those represented by the Aliph-OOM factors that are tightly related to PM2.5. More studies should be conducted to unravel the formation of OOMs and to complement the understanding of the role of VOC oxidation in PM2.5 and ozone pollution from this perspective.
Author contributions
AD, WN and Yuliang Liu designed the study. Yuliang Liu, CL, WN and CY analyzed the data. YL, CL and WN wrote the manuscript. Yuanyuan Li, DG, LC, CZ, LW, YZ, TL, XQ, JW and XC collected other research materials. All authors participated in the relevant scientific discussion and commented on the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the tofTools team for providing programs for data analysis of mass spectrometry. This work was supported by the National Natural Science Foundation of China (NSFC) project (92044301, 42220104006, 41875175, 42075101 and 41975154), the Jiangsu Provincial Collaborative Innovation Center of Climate Change and the Fundamental Research Funds for the Central Universities.References
- J. L. Jimenez, M. R. Canagaratna, N. M. Donahue, A. S. H. Prevot, Q. Zhang, J. H. Kroll, P. F. DeCarlo, J. D. Allan, H. Coe, N. L. Ng, A. C. Aiken, K. S. Docherty, I. M. Ulbrich, A. P. Grieshop, A. L. Robinson, J. Duplissy, J. D. Smith, K. R. Wilson, V. A. Lanz, C. Hueglin, Y. L. Sun, J. Tian, A. Laaksonen, T. Raatikainen, J. Rautiainen, P. Vaattovaara, M. Ehn, M. Kulmala, J. M. Tomlinson, D. R. Collins, M. J. Cubison, E. J. Dunlea, J. A. Huffman, T. B. Onasch, M. R. Alfarra, P. I. Williams, K. Bower, Y. Kondo, J. Schneider, F. Drewnick, S. Borrmann, S. Weimer, K. Demerjian, D. Salcedo, L. Cottrell, R. Griffin, A. Takami, T. Miyoshi, S. Hatakeyama, A. Shimono, J. Y. Sun, Y. M. Zhang, K. Dzepina, J. R. Kimmel, D. Sueper, J. T. Jayne, S. C. Herndon, A. M. Trimborn, L. R. Williams, E. C. Wood, A. M. Middlebrook, C. E. Kolb, U. Baltensperger and D. R. Worsnop, Evolution of Organic Aerosols in the Atmosphere, Science, 2009, 326, 1525–1529 CrossRef CAS PubMed.
- R. Zhang, G. Wang, S. Guo, M. L. Zamora, Q. Ying, Y. Lin, W. Wang, M. Hu and Y. Wang, Formation of Urban Fine Particulate Matter, Chem. Rev., 2015, 115, 3803–3855 CrossRef CAS PubMed.
- R. J. Huang, Y. L. Zhang, C. Bozzetti, K. F. Ho, J. J. Cao, Y. M. Han, K. R. Daellenbach, J. G. Slowik, S. M. Platt, F. Canonaco, P. Zotter, R. Wolf, S. M. Pieber, E. A. Bruns, M. Crippa, G. Ciarelli, A. Piazzalunga, M. Schwikowski, G. Abbaszade, J. Schnelle-Kreis, R. Zimmermann, Z. S. An, S. Szidat, U. Baltensperger, I. El Haddad and A. S. H. Prevot, High secondary aerosol contribution to particulate pollution during haze events in China, Nature, 2014, 514, 218–222 CrossRef CAS PubMed.
- J. Lelieveld, J. S. Evans, M. Fnais, D. Giannadaki and A. Pozzer, The contribution of outdoor air pollution sources to premature mortality on a global scale, Nature, 2015, 525, 367–371 CrossRef CAS PubMed.
- S. Szopa, V. Naik, B. Adhikary, P. Artaxo, T. Berntsen, W. D. Collins, S. Fuzzi, L. Gallardo, A. Kiendler-Scharr, Z. Klimont, H. Liao, N. Unger and P. Zanis, in Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, ed. V. Masson-Delmotte, P. Zhai, A. Pirani, S. L. Connors, C. Péan, S. Berger, N. Caud, Y. Chen, L. Goldfarb, M. I. Gomis, M. Huang, K. Leitzell, E. Lonnoy, J. B. R. Matthews, T. K. Maycock, T. Waterfield, O. Yelekçi, R. Yu and B. Zhou, Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, 2021, pp. 817–922, DOI:10.1017/9781009157896.008.
- M. Hallquist, J. C. Wenger, U. Baltensperger, Y. Rudich, D. Simpson, M. Claeys, J. Dommen, N. M. Donahue, C. George, A. H. Goldstein, J. F. Hamilton, H. Herrmann, T. Hoffmann, Y. Iinuma, M. Jang, M. E. Jenkin, J. L. Jimenez, A. Kiendler-Scharr, W. Maenhaut, G. McFiggans, T. F. Mentel, A. Monod, A. S. H. Prevot, J. H. Seinfeld, J. D. Surratt, R. Szmigielski and J. Wildt, The formation, properties and impact of secondary organic aerosol: current and emerging issues, Atmos. Chem. Phys., 2009, 9, 5155–5236 CrossRef CAS.
- P. J. Ziemann and R. Atkinson, Kinetics, products, and mechanisms of secondary organic aerosol formation, Chem. Soc. Rev., 2012, 41, 6582–6605 RSC.
- M. Ehn, J. A. Thornton, E. Kleist, M. Sipila, H. Junninen, I. Pullinen, M. Springer, F. Rubach, R. Tillmann, B. Lee, F. Lopez-Hilfiker, S. Andres, I. H. Acir, M. Rissanen, T. Jokinen, S. Schobesberger, J. Kangasluoma, J. Kontkanen, T. Nieminen, T. Kurten, L. B. Nielsen, S. Jorgensen, H. G. Kjaergaard, M. Canagaratna, M. D. Maso, T. Berndt, T. Petaja, A. Wahner, V. M. Kerminen, M. Kulmala, D. R. Worsnop, J. Wildt and T. F. Mentel, A large source of low-volatility secondary organic aerosol, Nature, 2014, 506, 476–479 CrossRef CAS PubMed.
- F. Bianchi, T. Kurten, M. Riva, C. Mohr, M. P. Rissanen, P. Roldin, T. Berndt, J. D. Crounse, P. O. Wennberg, T. F. Mentel, J. Wildt, H. Junninen, T. Jokinen, M. Kulmala, D. R. Worsnop, J. A. Thornton, N. Donahue, H. G. Kjaergaard and M. Ehn, Highly oxygenated organic molecules (HOM) from gas-phase autoxidation involving peroxy radicals: a key contributor to atmospheric aerosol, Chem. Rev., 2019, 119, 3472–3509 CrossRef CAS PubMed.
- W. Nie, C. Yan, D. D. Huang, Z. Wang, Y. Liu, X. Qiao, Y. Guo, L. Tian, P. Zheng, Z. Xu, Y. Li, Z. Xu, X. Qi, P. Sun, J. Wang, F. Zheng, X. Li, R. Yin, K. R. Dallenbach, F. Bianchi, T. Petäjä, Y. Zhang, M. Wang, M. Schervish, S. Wang, L. Qiao, Q. Wang, M. Zhou, H. Wang, C. Yu, D. Yao, H. Guo, P. Ye, S. Lee, Y. J. Li, Y. Liu, X. Chi, V.-M. Kerminen, M. Ehn, N. M. Donahue, T. Wang, C. Huang, M. Kulmala, D. Worsnop, J. Jiang and A. Ding, Secondary organic aerosol formed by condensing anthropogenic vapours over China’s megacities, Nat. Geosci., 2022, 15, 255–261 CrossRef CAS.
- H. Junninen, M. Ehn, T. Petaja, L. Luosujarvi, T. Kotiaho, R. Kostiainen, U. Rohner, M. Gonin, K. Fuhrer, M. Kulmala and D. R. Worsnop, A high-resolution mass spectrometer to measure atmospheric ion composition, Atmos. Meas. Tech., 2010, 3, 1039–1053 CrossRef CAS.
- T. Jokinen, M. Sipila, H. Junninen, M. Ehn, G. Lonn, J. Hakala, T. Petaja, R. L. Mauldin III, M. Kulmala and D. R. Worsnop, Atmospheric sulphuric acid and neutral cluster measurements using CI-APi-TOF, Atmos. Chem. Phys., 2012, 12, 4117–4125 CrossRef CAS.
- B. H. Lee, F. D. Lopez-Hilfiker, C. Mohr, T. Kurtén, D. R. Worsnop and J. A. Thornton, An iodide-adduct high-resolution time-of-flight chemical-ionization mass spectrometer: application to atmospheric inorganic and organic compounds, Environ. Sci. Technol., 2014, 48, 6309–6317 CrossRef CAS PubMed.
- F. Riccobono, S. Schobesberger, C. E. Scott, J. Dommen, I. K. Ortega, L. Rondo, J. Almeida, A. Amorim, F. Bianchi, M. Breitenlechner, A. David, A. Downard, E. M. Dunne, J. Duplissy, S. Ehrhart, R. C. Flagan, A. Franchin, A. Hansel, H. Junninen, M. Kajos, H. Keskinen, A. Kupc, A. Kuerten, A. N. Kvashin, A. Laaksonen, K. Lehtipalo, V. Makhmutov, S. Mathot, T. Nieminen, A. Onnela, T. Petaja, A. P. Praplan, F. D. Santos, S. Schallhart, J. H. Seinfeld, M. Sipila, D. V. Spracklen, Y. Stozhkov, F. Stratmann, A. Tome, G. Tsagkogeorgas, P. Vaattovaara, Y. Viisanen, A. Vrtala, P. E. Wagner, E. Weingartner, H. Wex, D. Wimmer, K. S. Carslaw, J. Curtius, N. M. Donahue, J. Kirkby, M. Kulmala, D. R. Worsnop and U. Baltensperger, Oxidation products of biogenic emissions contribute to nucleation of atmospheric particles, Science, 2014, 344, 717–721 CrossRef CAS PubMed.
- J. Kirkby, J. Duplissy, K. Sengupta, C. Frege, H. Gordon, C. Williamson, M. Heinritzi, M. Simon, C. Yan, J. Almeida, J. Trostl, T. Nieminen, I. K. Ortega, R. Wagner, A. Adamov, A. Amorim, A. K. Bernhammer, F. Bianchi, M. Breitenlechner, S. Brilke, X. M. Chen, J. Craven, A. Dias, S. Ehrhart, R. C. Flagan, A. Franchin, C. Fuchs, R. Guida, J. Hakala, C. R. Hoyle, T. Jokinen, H. Junninen, J. Kangasluoma, J. Kim, M. Krapf, A. Kurten, A. Laaksonen, K. Lehtipalo, V. Makhmutov, S. Mathot, U. Molteni, A. Onnela, O. Perakyla, F. Piel, T. Petaja, A. P. Praplan, K. Pringle, A. Rap, N. A. D. Richards, I. Riipinen, M. P. Rissanen, L. Rondo, N. Sarnela, S. Schobesberger, C. E. Scott, J. H. Seinfeld, M. Sipila, G. Steiner, Y. Stozhkov, F. Stratmann, A. Tome, A. Virtanen, A. L. Vogel, A. C. Wagner, P. E. Wagner, E. Weingartner, D. Wimmer, P. M. Winkler, P. L. Ye, X. Zhang, A. Hansel, J. Dommen, N. M. Donahue, D. R. Worsnop, U. Baltensperger, M. Kulmala, K. S. Carslaw and J. Curtius, Ion-induced nucleation of pure biogenic particles, Nature, 2016, 533, 521–526 CrossRef CAS PubMed.
- J. Trostl, W. K. Chuang, H. Gordon, M. Heinritzi, C. Yan, U. Molteni, L. Ahlm, C. Frege, F. Bianchi, R. Wagner, M. Simon, K. Lehtipalo, C. Williamson, J. S. Craven, J. Duplissy, A. Adamov, J. Almeida, A. K. Bernhammer, M. Breitenlechner, S. Brilke, A. Dias, S. Ehrhart, R. C. Flagan, A. Franchin, C. Fuchs, R. Guida, M. Gysel, A. Hansel, C. R. Hoyle, T. Jokinen, H. Junninen, J. Kangasluoma, H. Keskinen, J. Kim, M. Krapf, A. Kurten, A. Laaksonen, M. Lawler, M. Leiminger, S. Mathot, O. Mohler, T. Nieminen, A. Onnela, T. Petaja, F. M. Piel, P. Miettinen, M. P. Rissanen, L. Rondo, N. Sarnela, S. Schobesberger, K. Sengupta, M. Sipila, J. N. Smith, G. Steiner, A. Tome, A. Virtanen, A. C. Wagner, E. Weingartner, D. Wimmer, P. M. Winkler, P. Ye, K. S. Carslaw, J. Curtius, J. Dommen, J. Kirkby, M. Kulmala, I. Riipinen, D. R. Worsnop, N. M. Donahue and U. Baltensperger, The role of low-volatility organic compounds in initial particle growth in the atmosphere, Nature, 2016, 533, 527–531 CrossRef CAS PubMed.
- I. Riipinen, T. Yli-Juuti, J. R. Pierce, T. Petaja, D. R. Worsnop, M. Kulmala and N. M. Donahue, The contribution of organics to atmospheric nanoparticle growth, Nat. Geosci., 2012, 5, 453–458 CrossRef CAS.
- M. Kulmala, R. Cai, D. Stolzenburg, Y. Zhou, L. Dada, Y. Guo, C. Yan, T. Petäjä, J. Jiang and V.-M. Kerminen, The contribution of new particle formation and subsequent growth to haze formation, Environ. Sci.: Atmos., 2022, 2, 352–361 CAS.
- M. E. Jenkin, J. C. Young and A. R. Rickard, The MCM v3.3.1 degradation scheme for isoprene, Atmos. Chem. Phys., 2015, 15, 11433–11459 CrossRef CAS.
- J. D. Crounse, L. B. Nielsen, S. Jørgensen, H. G. Kjaergaard and P. O. Wennberg, Autoxidation of organic compounds in the atmosphere, J. Phys. Chem. Lett., 2013, 4, 3513–3520 CrossRef CAS.
- T. Jokinen, M. Sipila, S. Richters, V. M. Kerminen, P. Paasonen, F. Stratmann, D. Worsnop, M. Kulmala, M. Ehn, H. Herrmann and T. Berndt, Rapid autoxidation forms highly oxidized RO2 radicals in the atmosphere, Angew. Chem., Int. Ed., 2014, 53, 14596–14600 CrossRef CAS PubMed.
- J. J. Orlando and G. S. Tyndall, Laboratory studies of organic peroxy radical chemistry: an overview with emphasis on recent issues of atmospheric significance, Chem. Soc. Rev., 2012, 41, 6294–6317 RSC.
- O. Garmash, M. P. Rissanen, I. Pullinen, S. Schmitt, O. Kausiala, R. Tillmann, D. Zhao, C. Percival, T. J. Bannan, M. Priestley, Å. M. Hallquist, E. Kleist, A. Kiendler-Scharr, M. Hallquist, T. Berndt, G. McFiggans, J. Wildt, T. F. Mentel and M. Ehn, Multi-generation OH oxidation as a source for highly oxygenated organic molecules from aromatics, Atmos. Chem. Phys., 2020, 20, 515–537 CrossRef CAS.
- M. Wang, D. Chen, M. Xiao, Q. Ye, D. Stolzenburg, V. Hofbauer, P. Ye, A. L. Vogel, R. L. Mauldin, A. Amorim, A. Baccarini, B. Baumgartner, S. Brilke, L. Dada, A. Dias, J. Duplissy, H. Finkenzeller, O. Garmash, X.-C. He, C. R. Hoyle, C. Kim, A. Kvashnin, K. Lehtipalo, L. Fischer, U. Molteni, T. Petäjä, V. Pospisilova, L. L. J. Quéléver, M. Rissanen, M. Simon, C. Tauber, A. Tomé, A. C. Wagner, L. Weitz, R. Volkamer, P. M. Winkler, J. Kirkby, D. R. Worsnop, M. Kulmala, U. Baltensperger, J. Dommen, I. El-Haddad and N. M. Donahue, Photo-oxidation of aromatic hydrocarbons produces low-volatility organic compounds, Environ. Sci. Technol., 2020, 54, 7911–7921 CrossRef CAS PubMed.
- Y. Guo, C. Yan, Y. Liu, X. Qiao, F. Zheng, Y. Zhang, Y. Zhou, C. Li, X. Fan, Z. Lin, Z. Feng, Y. Zhang, P. Zheng, L. Tian, W. Nie, Z. Wang, D. Huang, K. R. Daellenbach, L. Yao, L. Dada, F. Bianchi, J. Jiang, Y. Liu, V. M. Kerminen and M. Kulmala, Seasonal variation in oxygenated organic molecules in urban Beijing and their contribution to secondary organic aerosol, Atmos. Chem. Phys., 2022, 22, 10077–10097 CrossRef CAS.
- C. Mohr, J. A. Thornton, A. Heitto, F. D. Lopez-Hilfiker, A. Lutz, I. Riipinen, J. Hong, N. M. Donahue, M. Hallquist, T. Petäjä, M. Kulmala and T. Yli-Juuti, Molecular identification of organic vapors driving atmospheric nanoparticle growth, Nat. Commun., 2019, 10, 4442 CrossRef CAS PubMed.
- Q. Zha, C. Yan, H. Junninen, M. Riva, N. Sarnela, J. Aalto, L. Quéléver, S. Schallhart, L. Dada, L. Heikkinen, O. Peräkylä, J. Zou, C. Rose, Y. Wang, I. Mammarella, G. Katul, T. Vesala, D. R. Worsnop, M. Kulmala, T. Petäjä, F. Bianchi and M. Ehn, Vertical characterization of highly oxygenated molecules (HOMs) below and above a boreal forest canopy, Atmos. Chem. Phys., 2018, 18, 17437–17450 CrossRef CAS.
- F. Bianchi, J. Tröstl, H. Junninen, C. Frege, S. Henne, C. R. Hoyle, U. Molteni, E. Herrmann, A. Adamov, N. Bukowiecki, X. Chen, J. Duplissy, M. Gysel, M. Hutterli, J. Kangasluoma, J. Kontkanen, A. Kürten, H. E. Manninen, S. Münch, O. Peräkylä, T. Petäjä, L. Rondo, C. Williamson, E. Weingartner, J. Curtius, D. R. Worsnop, M. Kulmala, J. Dommen and U. Baltensperger, New particle formation in the free troposphere: A question of chemistry and timing, Science, 2016, 352, 1109–1112 CrossRef CAS PubMed.
- F. Bianchi, H. Junninen, A. Bigi, V. A. Sinclair, L. Dada, C. R. Hoyle, Q. Zha, L. Yao, L. R. Ahonen, P. Bonasoni, S. Buenrostro Mazon, M. Hutterli, P. Laj, K. Lehtipalo, J. Kangasluoma, V. M. Kerminen, J. Kontkanen, A. Marinoni, S. Mirme, U. Molteni, T. Petäjä, M. Riva, C. Rose, K. Sellegri, C. Yan, D. R. Worsnop, M. Kulmala, U. Baltensperger and J. Dommen, Biogenic particles formed in the Himalaya as an important source of free tropospheric aerosols, Nat. Geosci., 2021, 14, 4–9 CrossRef CAS.
- X. Qiao, C. Yan, X. Li, Y. Guo, R. Yin, C. Deng, C. Li, W. Nie, M. Wang, R. Cai, D. Huang, Z. Wang, L. Yao, D. R. Worsnop, F. Bianchi, Y. Liu, N. M. Donahue, M. Kulmala and J. Jiang, Contribution of Atmospheric Oxygenated Organic Compounds to Particle Growth in an Urban Environment, Environ. Sci. Technol., 2021, 55(2), 13646–13656 CrossRef CAS PubMed.
- C. Ye, B. Yuan, Y. Lin, Z. Wang, W. Hu, T. Li, W. Chen, C. Wu, C. Wang, S. Huang, J. Qi, B. Wang, C. Wang, W. Song, X. Wang, E. Zheng, J. E. Krechmer, P. Ye, Z. Zhang, X. Wang, D. R. Worsnop and M. Shao, Chemical characterization of oxygenated organic compounds in the gas phase and particle phase using iodide CIMS with FIGAERO in urban air, Atmos. Chem. Phys., 2021, 21, 8455–8478 CrossRef CAS.
- Y. Wang, P. Clusius, C. Yan, K. Dällenbach, R. Yin, M. Wang, X.-C. He, B. Chu, Y. Lu, L. Dada, J. Kangasluoma, P. Rantala, C. Deng, Z. Lin, W. Wang, L. Yao, X. Fan, W. Du, J. Cai, L. Heikkinen, Y. J. Tham, Q. Zha, Z. Ling, H. Junninen, T. Petäjä, M. Ge, Y. Wang, H. He, D. R. Worsnop, V.-M. Kerminen, F. Bianchi, L. Wang, J. Jiang, Y. Liu, M. Boy, M. Ehn, N. M. Donahue and M. Kulmala, Molecular Composition of Oxygenated Organic Molecules and Their Contributions to Organic Aerosol in Beijing, Environ. Sci. Technol., 2022, 56, 770–778 CrossRef CAS PubMed.
- X. Li, Y. Li, R. Cai, C. Yan, X. Qiao, Y. Guo, C. Deng, R. Yin, Y. Chen, Y. Li, L. Yao, N. Sarnela, Y. Zhang, T. Petäjä, F. Bianchi, Y. Liu, M. Kulmala, J. Hao, J. N. Smith and J. Jiang, Insufficient Condensable Organic Vapors Lead to Slow Growth of New Particles in an Urban Environment, Environ. Sci. Technol., 2022, 56, 9936–9946 CrossRef CAS PubMed.
- C. Yan, W. Nie, M. Äijälä, M. P. Rissanen, M. R. Canagaratna, P. Massoli, H. Junninen, T. Jokinen, N. Sarnela, S. A. K. Häme, S. Schobesberger, F. Canonaco, L. Yao, A. S. H. Prévôt, T. Petäjä, M. Kulmala, M. Sipilä, D. R. Worsnop and M. Ehn, Source characterization of highly oxidized multifunctional compounds in a boreal forest environment using positive matrix factorization, Atmos. Chem. Phys., 2016, 16, 12715–12731 CrossRef CAS.
- H. Y. Li, M. R. Canagaratna, M. Riva, P. Rantala, Y. J. Zhang, S. Thomas, L. Heikkinen, P. M. Flaud, E. Villenave, E. Perraudin, D. Worsnop, M. Kulmala, M. Ehn and F. Bianchi, Atmospheric organic vapors in two European pine forests measured by a Vocus PTR-TOF: insights into monoterpene and sesquiterpene oxidation processes, Atmos. Chem. Phys., 2021, 21, 4123–4147 CrossRef CAS.
- P. Massoli, H. Stark, M. R. Canagaratna, J. E. Krechmer, L. Xu, N. L. Ng, R. L. Mauldin III, C. Yan, J. Kimmel, P. K. Misztal, J. L. Jimenez, J. T. Jayne and D. R. Worsnop, Ambient Measurements of Highly Oxidized Gas-Phase Molecules during the Southern Oxidant and Aerosol Study (SOAS) 2013, ACS Earth Space Chem., 2018, 2, 653–672 CrossRef CAS.
- Y. Liu, W. Nie, Y. Li, D. Ge, C. Liu, Z. Xu, L. Chen, T. Wang, L. Wang, P. Sun, X. Qi, J. Wang, Z. Xu, J. Yuan, C. Yan, Y. Zhang, D. Huang, Z. Wang, N. M. Donahue, D. Worsnop, X. Chi, M. Ehn and A. Ding, Formation of condensable organic vapors from anthropogenic and biogenic volatile organic compounds (VOCs) is strongly perturbed by NOx in eastern China, Atmos. Chem. Phys., 2021, 21, 14789–14814 CrossRef CAS.
- Y. Zhang, D. Li, Y. Ma, C. Dubois, X. Wang, S. Perrier, H. Chen, H. Wang, S. a. Jing, Y. Lu, S. Lou, C. Yan, W. Nie, J. Chen, C. Huang, C. George and M. Riva, Field Detection of Highly Oxygenated Organic Molecules in Shanghai by Chemical Ionization–Orbitrap, Environ. Sci. Technol., 2022, 56, 7608–7617 CrossRef CAS PubMed.
- J. L. Hu, Y. G. Wang, Q. Ying and H. L. Zhang, Spatial and temporal variability of PM2.5 and PM10 over the North China Plain and the Yangtze River Delta, China, Atmos. Environ., 2014, 95, 598–609 CrossRef CAS.
- A. J. Ding, C. B. Fu, X. Q. Yang, J. N. Sun, L. F. Zheng, Y. N. Xie, E. Herrmann, W. Nie, T. Petäjä, V. M. Kerminen and M. Kulmala, Ozone and fine particle in the western Yangtze River Delta: an overview of 1 yr data at the SORPES station, Atmos. Chem. Phys., 2013, 13, 5813–5830 CrossRef.
- Q. Zhang, Y. Zheng, D. Tong, M. Shao, S. Wang, Y. Zhang, X. Xu, J. Wang, H. He, W. Liu, Y. Ding, Y. Lei, J. Li, Z. Wang, X. Zhang, Y. Wang, J. Cheng, Y. Liu, Q. Shi, L. Yan, G. Geng, C. Hong, M. Li, F. Liu, B. Zheng, J. Cao, A. Ding, J. Gao, Q. Fu, J. Huo, B. Liu, Z. Liu, F. Yang, K. He and J. Hao, Drivers of improved PM2.5 air quality in China from 2013 to 2017, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 24463–24469 CrossRef CAS PubMed.
- A. Ding, X. Huang, W. Nie, X. Chi, Z. Xu, L. Zheng, Z. Xu, Y. Xie, X. Qi, Y. Shen, P. Sun, J. Wang, L. Wang, J. Sun, X.-Q. Yang, W. Qin, X. Zhang, W. Cheng, W. Liu, L. Pan and C. Fu, Significant reduction of PM2.5 in eastern China due to regional-scale emission control: evidence from SORPES in 2011–2018, Atmos. Chem. Phys., 2019, 19, 11791–11801 CrossRef CAS.
- K. Li, D. J. Jacob, H. Liao, J. Zhu, V. Shah, L. Shen, K. H. Bates, Q. Zhang and S. Zhai, A two-pollutant strategy for improving ozone and particulate air quality in China, Nat. Geosci., 2019, 12, 906–910 CrossRef CAS.
- H. Dai, J. Zhu, H. Liao, J. Li, M. Liang, Y. Yang and X. Yue, Co-occurrence of ozone and PM2.5 pollution in the Yangtze River Delta over 2013–2019: Spatiotemporal distribution and meteorological conditions, Atmos. Res., 2021, 249, 105363 CrossRef CAS.
- N. Wang, J. Xu, C. Pei, R. Tang, D. Zhou, Y. Chen, M. Li, X. Deng, T. Deng, X. Huang and A. Ding, Air Quality During COVID-19 Lockdown in the Yangtze River Delta and the Pearl River Delta: Two Different Responsive Mechanisms to Emission Reductions in China, Environ. Sci. Technol., 2021, 55, 5721–5730 CrossRef CAS PubMed.
- Y. Zhang, O. Peräkylä, C. Yan, L. Heikkinen, M. Äijälä, K. R. Daellenbach, Q. Zha, M. Riva, O. Garmash, H. Junninen, P. Paatero, D. Worsnop and M. Ehn, A novel approach for simple statistical analysis of high-resolution mass spectra, Atmos. Meas. Tech., 2019, 12, 3761–3776 CrossRef.
- Y. Zhang, O. Peräkylä, C. Yan, L. Heikkinen, M. Äijälä, K. R. Daellenbach, Q. Zha, M. Riva, O. Garmash, H. Junninen, P. Paatero, D. Worsnop and M. Ehn, Insights into atmospheric oxidation processes by performing factor analyses on subranges of mass spectra, Atmos. Chem. Phys., 2020, 20, 5945–5961 CrossRef CAS.
- A. Ding, W. Nie, X. Huang, X. Chi, J. Sun, V.-M. Kerminen, Z. Xu, W. Guo, T. Petäjä, X. Yang, M. Kulmala and C. Fu, Long-term observation of air pollution-weather/climate interactions at the SORPES station: a review and outlook, Front. Environ. Sci. Eng., 2016, 10, 15 CrossRef.
- Y. Liu, W. Nie, Z. Xu, T. Wang, R. Wang, Y. Li, L. Wang, X. Chi and A. Ding, Semi-quantitative understanding of source contribution to nitrous acid (HONO) based on 1 year of continuous observation at the SORPES station in eastern China, Atmos. Chem. Phys., 2019, 19, 13289–13308 CrossRef CAS.
- X. M. Qi, A. J. Ding, W. Nie, T. Petäjä, V. M. Kerminen, E. Herrmann, Y. N. Xie, L. F. Zheng, H. Manninen, P. Aalto, J. N. Sun, Z. N. Xu, X. G. Chi, X. Huang, M. Boy, A. Virkkula, X. Q. Yang, C. B. Fu and M. Kulmala, Aerosol size distribution and new particle formation in the western Yangtze River Delta of China: 2 years of measurements at the SORPES station, Atmos. Chem. Phys., 2015, 15, 12445–12464 CrossRef CAS.
- J. Wang, W. Nie, Y. Cheng, Y. Shen, X. Chi, J. Wang, X. Huang, Y. Xie, P. Sun, Z. Xu, X. Qi, H. Su and A. Ding, Light absorption of brown carbon in eastern China based on 3-year multi-wavelength aerosol optical property observations and an improved absorption Ångström exponent segregation method, Atmos. Chem. Phys., 2018, 18, 9061–9074 CrossRef CAS.
- P. Sun, W. Nie, X. Chi, Y. Xie, X. Huang, Z. Xu, X. Qi, Z. Xu, L. Wang, T. Wang, Q. Zhang and A. Ding, Two years of online measurement of fine particulate nitrate in the western Yangtze River Delta: influences of thermodynamics and N2O5 hydrolysis, Atmos. Chem. Phys., 2018, 18, 17177–17190 CrossRef CAS.
- Y. Shen, A. Virkkula, A. Ding, J. Wang, X. Chi, W. Nie, X. Qi, X. Huang, Q. Liu, L. Zheng, Z. Xu, T. Petäjä, P. P. Aalto, C. Fu and M. Kulmala, Aerosol optical properties at SORPES in Nanjing, east China, Atmos. Chem. Phys., 2018, 18, 5265–5292 CrossRef CAS.
- L. Chen, X. Qi, W. Nie, J. Wang, Z. Xu, T. Wang, Y. Liu, Y. Shen, Z. Xu, T. V. Kokkonen, X. Chi, P. P. Aalto, P. Paasonen, V.-M. Kerminen, T. Petäjä, M. Kulmala and A. Ding, Cluster Analysis of Submicron Particle Number Size Distributions at the SORPES Station in the Yangtze River Delta of East China, J. Geophys. Res.: Atmos., 2021, 126, e2020JD034004 Search PubMed.
- Z. N. Xu, W. Nie, Y. L. Liu, P. Sun, D. D. Huang, C. Yan, J. Krechmer, P. L. Ye, Z. Xu, X. M. Qi, C. J. Zhu, Y. Y. Li, T. Y. Wang, L. Wang, X. Huang, R. Z. Tang, S. Guo, G. L. Xiu, Q. Y. Fu, D. Worsnop, X. G. Chi and A. J. Ding, Multifunctional products of isoprene oxidation in polluted atmosphere and their contribution to SOA, Geophys. Res. Lett., 2021, 48, e2020GL089276 CAS.
- D. Stolzenburg, L. Fischer, A. L. Vogel, M. Heinritzi, M. Schervish, M. Simon, A. C. Wagner, L. Dada, L. R. Ahonen, A. Amorim, A. Baccarini, P. S. Bauer, B. Baumgartner, A. Bergen, F. Bianchi, M. Breitenlechner, S. Brilke, S. B. Mazon, D. Chen, A. Dias, D. C. Draper, J. Duplissy, I. El Haddad, H. Finkenzeller, C. Frege, C. Fuchs, O. Garmash, H. Gordon, X. He, J. Helm, V. Hofbauer, C. R. Hoyle, C. Kim, J. Kirkby, J. Kontkanen, A. Kuerten, J. Lampilahti, M. Lawler, K. Lehtipalo, M. Leiminger, H. Mai, S. Mathot, B. Mentler, U. Molteni, W. Nie, T. Nieminen, J. B. Nowak, A. Ojdanic, A. Onnela, M. Passananti, T. Petaja, L. L. J. Quelever, M. P. Rissanen, N. Sarnela, S. Schallhart, C. Tauber, A. Tome, R. Wagner, M. Wang, L. Weitz, D. Wimmer, M. Xiao, C. Yan, P. Ye, Q. Zha, U. Baltensperger, J. Curtius, J. Dommen, R. C. Flagan, M. Kulmala, J. N. Smith, D. R. Worsnop, A. Hansel, N. M. Donahue and P. M. Winkler, Rapid growth of organic aerosol nanoparticles over a wide tropospheric temperature range, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 9122–9127 CrossRef CAS PubMed.
- A. Kuerten, L. Rondo, S. Ehrhart and J. Curtius, Calibration of a chemical ionization mass spectrometer for the measurement of gaseous sulfuric acid, J. Phys. Chem. A, 2012, 116, 6375–6386 CrossRef CAS PubMed.
- M. Heinritzi, M. Simon, G. Steiner, A. C. Wagner, A. Kuerten, A. Hansel and J. Curtius, Characterization of the mass-dependent transmission efficiency of a CIMS, Atmos. Meas. Tech., 2016, 9, 1449–1460 CrossRef CAS.
- P. Paatero and U. Tapper, Positive matrix factorization – a nonnegative factor model with optimal utilization of error-estimates of data values, Environmetrics, 1994, 5, 111–126 CrossRef.
- F. Canonaco, M. Crippa, J. G. Slowik, U. Baltensperger and A. S. H. Prévôt, SoFi, an IGOR-based interface for the efficient use of the generalized multilinear engine (ME-2) for the source apportionment: ME-2 application to aerosol mass spectrometer data, Atmos. Meas. Tech., 2013, 6, 3649–3661 CrossRef.
- X. Cheng, Q. Chen, Y. Li, G. Huang, Y. Liu, S. Lu, Y. Zheng, W. Qiu, K. Lu, X. Qiu, F. Bianchi, C. Yan, B. Yuan, M. Shao, Z. Wang, M. R. Canagaratna, T. Zhu, Y. Wu and L. Zeng, Secondary production of gaseous nitrated phenols in polluted urban environments, Environ. Sci. Technol., 2021, 55, 4410–4419 CrossRef CAS PubMed.
- K. Song, S. Guo, H. Wang, Y. Yu, H. Wang, R. Tang, S. Xia, Y. Gong, Z. Wan, D. Lv, R. Tan, W. Zhu, R. Shen, X. Li, X. Yu, S. Chen, L. Zeng and X. Huang, Measurement report: Online measurement of gas-phase nitrated phenols utilizing a CI-LToF-MS: primary sources and secondary formation, Atmos. Chem. Phys., 2021, 21, 7917–7932 CrossRef CAS.
- Y. Chen, P. Zheng, Z. Wang, W. Pu, Y. Tan, C. Yu, M. Xia, W. Wang, J. Guo, D. Huang, C. Yan, W. Nie, Z. Ling, Q. Chen, S. Lee and T. Wang, Secondary Formation and Impacts of Gaseous Nitro-Phenolic Compounds in the Continental Outflow Observed at a Background Site in South China, Environ. Sci. Technol., 2021, 56, 6933–6943 CrossRef PubMed.
- C. Lu, X. Wang, J. Zhang, Z. Liu, Y. Liang, S. Dong, M. Li, J. Chen, H. Chen, H. Xie, L. Xue and W. Wang, Substantial emissions of nitrated aromatic compounds in the particle and gas phases in the waste gases from eight industries, Environ. Pollut., 2021, 283, 117132 CrossRef CAS PubMed.
- D. Ge, W. Nie, P. Sun, Y. Liu, T. Wang, J. Wang, J. Wang, L. Wang, C. Zhu, R. Wang, T. Liu, X. Chi and A. Ding, Characterization of particulate organic nitrates in the Yangtze River Delta, East China, using the time-of-flight aerosol chemical speciation monitor, Atmos. Environ., 2022, 272, 118927 CrossRef CAS.
- C. Bloss, V. Wagner, M. E. Jenkin, R. Volkamer, W. J. Bloss, J. D. Lee, D. E. Heard, K. Wirtz, M. Martin-Reviejo, G. Rea, J. C. Wenger and M. J. Pilling, Development of a detailed chemical mechanism (MCMv3.1) for the atmospheric oxidation of aromatic hydrocarbons, Atmos. Chem. Phys., 2005, 5, 641–664 CrossRef CAS.
- S. Wang, R. Wu, T. Berndt, M. Ehn and L. Wang, Formation of Highly Oxidized Radicals and Multifunctional Products from the Atmospheric Oxidation of Alkylbenzenes, Environ. Sci. Technol., 2017, 51, 8442–8449 CrossRef CAS PubMed.
- Y. Wang, A. Mehra, J. E. Krechmer, G. Yang, X. Hu, Y. Lu, A. Lambe, M. Canagaratna, J. Chen, D. Worsnop, H. Coe and L. Wang, Oxygenated products formed from OH-initiated reactions of trimethylbenzene: autoxidation and accretion, Atmos. Chem. Phys., 2020, 20, 9563–9579 CrossRef CAS.
- X. Cheng, Q. Chen, Y. Jie Li, Y. Zheng, K. Liao and G. Huang, Highly oxygenated organic molecules produced by the oxidation of benzene and toluene in a wide range of OH exposure and NOx conditions, Atmos. Chem. Phys., 2021, 21, 12005–12019 CrossRef CAS.
- D. S. Wang and L. Hildebrandt Ruiz, Chlorine-initiated oxidation of n-alkanes under high-NOx conditions: insights into secondary organic aerosol composition and volatility using a FIGAERO–CIMS, Atmos. Chem. Phys., 2018, 18, 15535–15553 CrossRef CAS.
- L. G. Jahn, D. S. Wang, S. V. Dhulipala and L. H. Ruiz, Gas-Phase Chlorine Radical Oxidation of Alkanes: Effects of Structural Branching, NOx, and Relative Humidity Observed during Environmental Chamber Experiments, J. Phys. Chem. A, 2021, 125, 7303–7317 CrossRef CAS PubMed.
- A. E. Perring, S. E. Pusede and R. C. Cohen, An Observational Perspective on the Atmospheric Impacts of Alkyl and Multifunctional Nitrates on Ozone and Secondary Organic Aerosol, Chem. Rev., 2013, 113, 5848–5870 CrossRef CAS PubMed.
- V. F. McNeill, Aqueous Organic Chemistry in the Atmosphere: Sources and Chemical Processing of Organic Aerosols, Environ. Sci. Technol., 2015, 49, 1237–1244 CrossRef CAS PubMed.
- Y. Liu, H. Shen, J. Mu, H. Li, T. Chen, J. Yang, Y. Jiang, Y. Zhu, H. Meng, C. Dong, W. Wang and L. Xue, Formation of peroxyacetyl nitrate (PAN) and its impact on ozone production in the coastal atmosphere of Qingdao, North China, Sci. Total Environ., 2021, 778, 146265 CrossRef CAS PubMed.
- H. Wang, X. Ma, Z. Tan, H. Wang, X. Chen, S. Chen, Y. Gao, Y. Liu, Y. Liu, X. Yang, B. Yuan, L. Zeng, C. Huang, K. Lu and Y. Zhang, Anthropogenic monoterpenes aggravating ozone pollution, Natl. Sci. Rev., 2022, 9, nwac103 CrossRef PubMed.
- C. L. Heald and J. H. Kroll, The fuel of atmospheric chemistry: Toward a complete description of reactive organic carbon, Sci. Adv., 2020, 6, eaay8967 CrossRef CAS PubMed.
- J. Wang, J. Ye, Q. Zhang, J. Zhao, Y. Wu, J. Li, D. Liu, W. Li, Y. Zhang, C. Wu, C. Xie, Y. Qin, Y. Lei, X. Huang, J. Guo, P. Liu, P. Fu, Y. Li, H. C. Lee, H. Choi, J. Zhang, H. Liao, M. Chen, Y. Sun, X. Ge, S. T. Martin and D. J. Jacob, Aqueous production of secondary organic aerosol from fossil-fuel emissions in winter Beijing haze, Proc. Natl. Acad. Sci. U. S. A., 2021, 118, e2022179118 CrossRef CAS PubMed.
- T. Petäjä, L. Järvi, V. M. Kerminen, A. J. Ding, J. N. Sun, W. Nie, J. Kujansuu, A. Virkkula, X. Yang, C. B. Fu, S. Zilitinkevich and M. Kulmala, Enhanced air pollution via aerosol-boundary layer feedback in China, Sci. Rep., 2016, 6, 18998 CrossRef PubMed.
- A. J. Ding, X. Huang, W. Nie, J. N. Sun, V. M. Kerminen, T. Petäjä, H. Su, Y. F. Cheng, X. Q. Yang, M. H. Wang, X. G. Chi, J. P. Wang, A. Virkkula, W. D. Guo, J. Yuan, S. Y. Wang, R. J. Zhang, Y. F. Wu, Y. Song, T. Zhu, S. Zilitinkevich, M. Kulmala and C. B. Fu, Enhanced haze pollution by black carbon in megacities in China, Geophys. Res. Lett., 2016, 43, 2873–2879 CrossRef CAS.
- L. Yang, W. Nie, Y. Liu, Z. Xu, M. Xiao, X. Qi, Y. Li, R. Wang, J. Zou, P. Paasonen, C. Yan, Z. Xu, J. Wang, C. Zhou, J. Yuan, J. Sun, X. Chi, V.-M. Kerminen, M. Kulmala and A. Ding, Towards building a physical proxy for gas-phase sulfuric acid concentration based on its budget analysis in polluted Yangtze River Delta, east China, Environ. Sci. Technol., 2021, 55, 6665–6676 CrossRef CAS PubMed.
- K. Lu, H. Fuchs, A. Hofzumahaus, Z. Tan, H. Wang, L. Zhang, S. H. Schmitt, F. Rohrer, B. Bohn, S. Broch, H. Dong, G. I. Gkatzelis, T. Hohaus, F. Holland, X. Li, Y. Liu, Y. Liu, X. Ma, A. Novelli, P. Schlag, M. Shao, Y. Wu, Z. Wu, L. Zeng, M. Hu, A. Kiendler-Scharr, A. Wahner and Y. Zhang, Fast Photochemistry in Wintertime Haze: Consequences for Pollution Mitigation Strategies, Environ. Sci. Technol., 2019, 53, 10676–10684 CrossRef CAS PubMed.
- X. Peng, W. Wang, M. Xia, H. Chen, A. R. Ravishankara, Q. Li, A. Saiz-Lopez, P. Liu, F. Zhang, C. Zhang, L. Xue, X. Wang, C. George, J. Wang, Y. Mu, J. Chen and T. Wang, An unexpected large continental source of reactive bromine and chlorine with significant impact on wintertime air quality, Natl. Sci. Rev., 2021, 8, nwaa304 CrossRef CAS PubMed.
- G. He, J. Ma, B. Chu, R. Hu, H. Li, M. Gao, Y. Liu, Y. Wang, Q. Ma, P. Xie, G. Zhang, X. C. Zeng, J. S. Francisco and H. He, Inside Cover: Generation and Release of OH Radicals from the Reaction of H2O with O2 over Soot (Angew. Chem. Int. Ed. 21/2022), Angew. Chem., Int. Ed., 2022, 61, e202204829 Search PubMed.
- P. M. Edwards, S. S. Brown, J. M. Roberts, R. Ahmadov, R. M. Banta, J. A. deGouw, W. P. Dubé, R. A. Field, J. H. Flynn, J. B. Gilman, M. Graus, D. Helmig, A. Koss, A. O. Langford, B. L. Lefer, B. M. Lerner, R. Li, S.-M. Li, S. A. McKeen, S. M. Murphy, D. D. Parrish, C. J. Senff, J. Soltis, J. Stutz, C. Sweeney, C. R. Thompson, M. K. Trainer, C. Tsai, P. R. Veres, R. A. Washenfelder, C. Warneke, R. J. Wild, C. J. Young, B. Yuan and R. Zamora, High winter ozone pollution from carbonyl photolysis in an oil and gas basin, Nature, 2014, 514, 351–354 CrossRef CAS PubMed.
- K. Li, D. J. Jacob, H. Liao, Y. Qiu, L. Shen, S. Zhai, K. H. Bates, M. P. Sulprizio, S. Song, X. Lu, Q. Zhang, B. Zheng, Y. Zhang, J. Zhang, H. C. Lee and S. K. Kuk, Ozone pollution in the North China Plain spreading into the late-winter haze season, Proc. Natl. Acad. Sci. U. S. A., 2021, 118, e2015797118 CrossRef CAS PubMed.
- T. Wang, L. Xue, Z. Feng, J. Dai, Y. Zhang and Y. Tan, Ground-level ozone pollution in China: a synthesis of recent findings on influencing factors and impacts, Environ. Res. Lett., 2022, 17, 063003 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ea00143h |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2023 |