Open Access Article
Open Access ArticleMass balance study of brominated flame retardants in female captive peregrine falcons†
Cynthia A.
de Wit
 *a,
Anna-Karin
Johansson‡
a,
Ulla
Sellström
a and
Peter
Lindberg
b
*a,
Anna-Karin
Johansson‡
a,
Ulla
Sellström
a and
Peter
Lindberg
b
aDepartment of Environmental Science and Analytical Chemistry, Stockholm University, SE-106 91, Stockholm, Sweden. E-mail: cynthia.dewit@aces.su.se; Fax: +46 8 674 7637; Tel: +46 8 674 71 80
bDepartment of Biological and Environmental Sciences, University of Gothenburg, SE-405 30 Göteborg, Sweden
First published on 19th June 2019
Abstract
Little is known about brominated flame retardant (BFR) dynamics in birds, especially large molecules such as decabromodiphenyl ether (BDE-209). In particular, bioaccumulation from food and transfer dynamics to eggs are poorly understood. Therefore, an input–output mass balance study of tri–decaBDEs, DBDPE and HBCDD was performed in three female peregrine falcons from a captive breeding program by analyzing their naturally contaminated food (quail, chicken (cockerels)), plasma, feces and eggs. Predominant BFRs in cockerels and quail were BDE-209 and DBDPE, as well as HBCDD in quail. The predominant BFRs found in falcon plasma were BDE-209, -153 and -183, in eggs, HBCDD, BDE-209 and -153 and in feces, BDE-209. Mean absorption efficiencies (AE) for the tetra-octabrominated BDEs ranged from 84–100% and 70% for HBCDD. The AEs for BDE-206, -207, -208 and -209 varied due to the large variability seen for feces fluxes. All egg/plasma ratios for BDEs were similar and greater than one (range 1.1–2.7), including for BDE-209, indicating efficient transfer from females to the eggs. Excretion via egg-laying was approximately 6.0–29% of the initial, pre-breeding body burden of individual penta–decaBDE congeners, (15–45% for BDE-206). HBCDD was not detected in plasma but was found in eggs, also indicating efficient transfer and excretion via eggs. Input fluxes from food exceeded the output fluxes (feces, eggs) indicating considerable metabolism for tetra–octaBDEs, possibly also for the nona–decaBDEs and HBCDD. Bioaccumulation factors calculated from lipid weight concentrations in plasma and food (BAFp) were highest for BDE-208 (31), -153 (23), -209 (19) and -207 (16) and from eggs and food (BAFe), were highest for HBCDD (140), BDE-153 (41), -208 (42), BDE-207 (24) and BDE-209 (21). BAFe and BAFp values were below 10 for BDE-47, -99 and -100. For one falcon, egg results were available from three different years and estimated half-lives were 65 d (BDE-99), 624 d (BDE-153), 31 d (BDE-154), 349 d (BDE-183), 77 d (BDE-196) and 89 d (BDE-197).
Environmental significanceResults from mass balance studies help in understanding the dynamics of organic contaminants in living organisms. Very few mass balance studies of brominated flame retardants have been carried out, particularly in birds. High concentrations of penta- and hexaBDEs, as well as decabromodiphenyl ether (BDE-209) and hexabromocyclododecane (HBCDD) have been found in wild peregrine falcon eggs. It is not clear if concentrations of higher brominated BFRs in eggs reflect body burden and it is therefore important to understand the extent that BFRs bioaccumulate from food, metabolize, are excreted in feces and transfered to eggs, particularly in high trophic level birds of prey. Such understanding of how dietary exposure translates to body burdens and egg concentrations helps in interpreting monitoring data from wild birds, which are often based on data from eggs. Using captive peregrine falcons as a surrogate for wild peregrine falcons also provides such data for a species that has high exposure to BFRs, where correlations have been seen between BDE concentrations and reproductive effects and that has previously been endangered due to biomagnification of other organic contaminants. |
Introduction
The polybrominated diphenyl ether (PBDE) technical products pentaBDE, octaBDE, decaBDE, hexabromocyclododecane (HBCDD) and decabromodiphenyl ethane (DBDPE) are additive brominated flame retardants (BFRs) used in textiles and polymers. PBDEs and HBCDD cause effects on neurobehavioral development, reproduction and the thyroid system in laboratory animals and associations with PBDEs have been seen for similar effects in humans.1–20 Due to the increasing concerns about the effects of PBDEs on the environment and human health, the penta- and octaBDE technical products, containing tri–octaBDEs, were banned within the EU in 2004![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21 and globally within the Stockholm Convention on Persistent Organic Pollutants (POPs) in 2009.22 In 2008, the decaBDE mixture, containing nona–decaBDEs was banned for use in electronic and electrical equipment in the EU,23 major producers in the US discontinued decaBDE mixture production and use at the end of 2013
21 and globally within the Stockholm Convention on Persistent Organic Pollutants (POPs) in 2009.22 In 2008, the decaBDE mixture, containing nona–decaBDEs was banned for use in electronic and electrical equipment in the EU,23 major producers in the US discontinued decaBDE mixture production and use at the end of 2013![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 and it was listed on the Stockholm Convention in 2017 (http://chm.pops.int/). HBCDD was added to the Stockholm Convention on POPs in 2014. DBDPE is currently not regulated and is marketed as a replacement for the decaBDE mixture.
24 and it was listed on the Stockholm Convention in 2017 (http://chm.pops.int/). HBCDD was added to the Stockholm Convention on POPs in 2014. DBDPE is currently not regulated and is marketed as a replacement for the decaBDE mixture.
In Sweden, tri–decaBDE congeners and HBCDD have been found in wild peregrine falcon (Falco peregrinus) eggs as well as in a captive breeding population, although in lower concentrations.25,26 Environmentally relevant concentrations of PBDE and HBCDD are associated with immunotoxic, thyroid, reproductive effects and eggshell thinning in laboratory studies of American kestrels (Falco spaverius)27–35 and zebra finches (Taeniopygia guttata).36–38 A significant negative correlation between BFR concentrations in eggs and reproduction has been seen in the wild Swedish peregrine falcons.26 A recent review of numerous studies of toxicological endpoints in many different bird species found raptors to be the most sensitive to flame retardant exposure.39
The presence of tri–decaBDE congeners and HBCDD in wild and captive Swedish falcon eggs implies exposure of adults via their diet, uptake from the gastrointestinal tract, accumulation and transfer to the eggs. DBDPE has not been studied in Swedish falcon eggs previously. Due to the differences in their hydrophobicity and molecular size, different BDE congeners have different uptake, excretion and accumulation rates, as seen in mass balance studies of rats and lactating cows.40–42 In the closely-related American kestrel, the half-lives of BDE-47, -99, -100 and -153 have been determined to be 72, 175, 178 and 572 days, respectively,43 which were comparable to half-lives of 100 days for BDE-99, -100 and 153 estimated for herring gulls (Larus argentatus).44 The long half-lives of these BDE congeners in birds indicate that they could be taken up and accumulated in peregrine falcons as well. However, in starlings and kestrels, the half-life of BDE-209 was determined to be only 13–14 days,45,46 possibly due to a higher rate of metabolism for this congener. A similar short half-life for BDE-209 has been seen in humans as well.47 In a recent study on kestrels, the half-life for HBCDD was found to be 14 days.48 For BDE-209 in particular, but for other BFRs as well, there are very few studies in birds of bioaccumulation from food or of transfer dynamics to eggs and none have studied the entire process of intake, accumulation, and excretion. To address this, an input–output mass balance study of tri–decaBDEs, HBCDD and DBDPE in captive female peregrine falcons was carried out to determine uptake and accumulation via the gastrointestinal tract, distribution to blood and excretion via eggs and feces.
Experimental section
Sampling
A captive breeding population of peregrine falcons was maintained at Nordens Ark (Hunnebostrand, Sweden) within the auspices of a Swedish Society for Nature Conservation falcon re-introduction project, with permission of the Swedish Environmental Protection Agency. The three females sampled in this study were hatched in captivity and information about them and the eggs collected are given in Table S1 in the ESI.† All experimental work including sampling of the peregrine falcons was performed in compliance with relevant Swedish Regulations and Guidelines on Laboratory Animals (Djurskyddsmyndighetens föreskrifter och allmänna råd (DFS 2004:4) om djurförsök m.m.) and was approved by the Swedish Animal Ethics Agency (Djurskyddsmyndigheten Permit Dnr 13-2005). Paired males and females were housed in separate outdoor cages with perches. The pairs were fed a diet predominated by commercially obtained one-day-old cockerels (Gallus gallus) (75% of diet) and quail (Coturnix coturnix) (16%), with a small proportion of white mice (9%) given sporadically. Blood samples were collected from the female peregrine falcons by a veterinarian after light carbon dioxide anesthesia during an ordinary health check on 16 February 2006, before the start of the breeding season. Blood was collected from the wing vein using a 2 mL syringe coated with liquid heparin. Immediately after drawing the blood it was transferred to a 5 mL Vacutainer tube to prevent clotting. A field blank of 5 mL NaCl (0.9%) was also prepared and treated identically with the plasma samples. The blood samples were centrifuged, the plasma removed and stored frozen at −20 °C until analysis. Six dead cockerels (mean weight 13.9 g each) and five dead quail (mean weight 91.8 g each) were collected at the same time as the blood samples were taken and stored frozen at −20 °C until homogenization. Feces samples were scraped off the metal plating on the cage walls and placed in brown tinted glass jars previously washed and burned at 450 °C and originated from both the male and the female sharing a cage. A small sample of the metal plating was taken as well. The females laid eggs in mid-May 2006 and unfertilised eggs were collected on June 6, 2006. Egg contents were removed immediately after collection and stored in tinted glass jars at −20 °C.Falcons at Nordens Ark swallow day-old cockerels whole but are fed quail where the legs have been removed. The falcons often remove the quail gastrointestinal tract before feeding. For chemical analysis, cockerels were thawed, plucked and the legs removed. The quail were thawed, plucked, and the legs, wings and gastrointestinal tract removed. The six cockerels were ground together in a stainless steel meat grinder and then homogenized with an ultra turrax homogenizer. The five quail were likewise combined, ground and homogenized. Three subsamples of each homogenate were removed and stored frozen at −20 °C until analysis.
Chemicals
Dichloromethane (DCM), n-hexane (both LiChrosolv), iso-propanol (LiChrosolv), acetone (Suprasolv), hydrochloric acid (HCl, 37% fuming, pro analysis), phosphoric acid (pro analysis) and silica gel 60 (0.063–0.200 mm) were obtained from Merck (Darmstadt, Germany). Diethyl ether (DEE) and iso-octane (both HPLC grade) were from LabScan (Gliwice, Poland). Potassium chloride (KCl, reagent grade) was from Scharlau Chemie S.A. (Barcelona, Spain), sodium chloride (NaCl, Anala R Normapur) from VWR Prolabo (Haasrode, Belgium), and sulfuric acid (H2SO4, 95–97%, pro analysis) from Fluka Chemie, Buchs, Switzerland.Single congeners of BDEs -28, -47, -99, -100, -153, -154, -183, and -209 (99% purity, Cambridge Isotope Laboratories (CIL), Andover, MA), BDE-203, -205 (97–100% purity, AccuStandard, New Haven, CT, USA), BDE-184, -191, -196, -197, -206, -207 and decabromodiphenyl ethane (DBDPE) (>98% purity, Wellington Laboratories, Guelph, ON, Canada) and technical HBCDD (95.5 ± 0.5% purity, Dr Ehrenstorfer, Augsburg, Germany) were used as reference standards. As surrogate standards for the PBDEs, BDE-138 (tetra- to heptaBDEs), and 13C12-BDE-209 (octa- to decaBDEs and DBDPE, both 99%, from CIL) were used. For HBCDD, Dechlorane 603 (Occidental Chemicals, previously Hooker Chemical, Dallas, TX, USA) was used as surrogate standard. 13C12-heptachlorobiphenyl (CB)-180 (>98% from CIL) was used as recovery standard. Reference standards were prepared in iso-octane at 5–11 levels covering the concentration ranges of interest.
Extraction and cleanup
Surrogate standards (100 μL in iso-octane) were added to the samples which were allowed to equilibrate overnight. Peregrine falcon egg homogenates, peregrine falcon feces, cockerel and quail homogenates (approximately 5 g wet weight) were liquid–liquid extracted according to Jensen et al.49 The extraction was done in 50 mL centrifuge tubes, using centrifugation instead of filtering for separation. Plasma samples were extracted according to the method described by Hovander et al.50 Solvent amounts were reduced to half of the volumes described in that method due to the smaller amount of sample in this study. Lipid determination was done gravimetrically and extracted lipids were then re-dissolved in iso-octane.All the samples were treated first with approximately 10 mL of concentrated sulfuric acid and then centrifuged for 10 min, at 200 rpm. The sample volume was reduced to 0.5 mL and then cleaned-up on an acidified silica column (0.5 g, SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2SO4, 2
H2SO4, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The column was washed with 10 mL n-hexane and the sample then eluted with 10 mL of n-hexane. This clean-up was sufficient for the egg samples but not for the cockerel, quail, feces and plasma samples so these were subjected to further clean-up on a silica column (1 g, deactivated with 2% water). After a 4 mL pre-fraction (n-hexane, discarded), the analytes were eluted with 13 mL of DCM (collected). A recovery standard was added prior to the analysis and the sample volumes adjusted to 100 μL.
1). The column was washed with 10 mL n-hexane and the sample then eluted with 10 mL of n-hexane. This clean-up was sufficient for the egg samples but not for the cockerel, quail, feces and plasma samples so these were subjected to further clean-up on a silica column (1 g, deactivated with 2% water). After a 4 mL pre-fraction (n-hexane, discarded), the analytes were eluted with 13 mL of DCM (collected). A recovery standard was added prior to the analysis and the sample volumes adjusted to 100 μL.
GC-MS analysis
Analysis was done by gas chromatography – mass spectrometry (GC-MS). The system used was a Trace GC Ultra coupled to a DSQ II MS (both Thermo Scientific, Waltham, USA). Initially a split–splitless (SSL) injector (held at 280 °C, constant flow, closed for 1.5 min after injection) and later a programmable temperature vaporiser (PTV) injector was used. For PTV conditions, see Sahlström et al.51The GC columns were DB-5MS fused-silica (J&W Scientific, Folsom, CA, USA) with helium (purity 4.6, Aga, Lidingö, Sweden) as the carrier gas. For octa- to decaBDEs and DBDPE a shorter column (15 m, 0.25 mm i.d., 0.1 μm film thickness) was used in order to minimize thermal degradation of the higher brominated compounds. The oven temperature program started at 80 °C (held for 2 min), then increased by 20 °C min−1 to 200 °C followed by 6 °C min−1 to 315 °C, which was held for 5 min. For tetra–heptaBDEs and HBCDD, a longer column (30 m, 0.25 mm i.d., 0.25 μm film thickness) was used to achieve better chromatographic separation. The oven temperature program started at 80 °C (held for 2 min), then increased with 25 °C min−1 to 200 °C, followed by 4 °C min−1 to 315 °C, which was held for 40 min.
With this method the HBCDDs elute in one peak and only the total HBCDD could be determined. Previous analyses with LC-MS showed that only the α-HBCDD congener was present in Swedish peregrine falcon eggs.52
The MS was run in electron capture ionisation mode measuring the negative ions formed (ECNI). Ammonia (purity 5.0, Aga, Sweden) was used as moderating gas. The electron energy was 70 eV and ion source temperature 180 °C. Detailed information on instrumental settings can be found in Johansson et al.26 The MS was operated in selected ion monitoring (SIM) mode recording the bromide ions (m/z −78.9 and −80.9) for BDE-28, -35, -47, -49, -66, -77, -85, -99, -100, -138, -153, -154,-173, -183, -184, -191, -196, -197, -203, -205, HBCDD and DBDPE. Phenoxide fragment ions with 4 and 5 bromine atoms were recorded for octa- to decaBDEs: m/z −409 for native BDE-196 and -197, m/z −484.6 and 486.6 for native BDE-206, -207, -208 and -209. For 13C-labelled octa- to decaBDEs, the m/z −415, −494.6 and −496.6 ions were recorded, and Dechlorane 603 was measured using the m/z −236.7 and −238.7 ions. The quantification was performed with XCalibur 2.0.7 (Thermo Finnigan, San Jose, CA, USA).
Quality assurance
UV-light protection was mounted on all light fixtures and windows in the laboratory to minimize degradation of BDE-209. Brown glassware was used when possible; otherwise glassware was covered with aluminum foil. Glassware was heated to 450 °C overnight before use. On every extraction occasion, one laboratory blank and one in-house lab reference material (LRM) sample were extracted in parallel. The LRM samples were aliquots of a large salmon muscle homogenate used as a QA/QC sample for analyses within the national environmental monitoring program performed at Stockholm University. The average lipid percent in the LRM samples (n = 3) was 13.5% with a relative standard deviation of 1%. Only BDE-28, -47, -49, -66, -99, -100, -153, -154 and -183, as well as DBDPE and HBCDD were detected in the LRM samples in concentrations above the limit of detection (LOD)/limit of quantitation (LOQ) (relative standard deviations 4–15% for all except BDE-183 (32%)).A chromatographic peak was considered quantifiable when the signal-to-noise ratio was ≥5. For compounds present in the blanks, the LOD and LOQ were set as the average blank value +3 and +5 times the standard deviation, respectively.
GC-MS analysis was performed, mixing samples and calibration standards randomly. Samples were quantified using ≥5 point calibration curves. Compounds were positively identified if the mass isotope ratio was correct and the relative retention time (versus the surrogate standard) differed no more than 0.005 compared to the calibration standards.
Small amounts of BDE-209 are degraded to octa- and nonaBDEs during sample processing/analysis. These amounts are often so small that they do not notably influence the quantification of BDE-209. The quantification of octa- and nonaBDEs can however be affected since these most often/always are present in the samples at much lower concentrations than BDE-209. The extent of the degradation was determined by measuring the amounts of 13C-octa- and nonaBDEs that were formed from the 13C-BDE-209 that was added to all samples as a surrogate standard, assuming that the native and the 13C-labelled BDE-209 are degraded equally.
In this study, corrections for degradation of BDE-209 were necessary for BDE-197, -206, -207 and -208. The corrections were performed for each sample individually with the actual degradation percentage of BDE-209 in that sample using relative peak areas. The degradation of BDE-209 into octa- and nonaBDEs was on average 0.71% (BDE-197), 0.37% (BDE-206), 1.6% (BDE-207) and 0.88% (BDE-208). When the peregrine falcon egg samples were analysed, the phenoxide ions for BDE-197 were not monitored and because of this, the average degradation of BDE-197 in the other samples (0.71%) was used for correction.
13C12-CB-180 was added to the samples before the analysis in order to evaluate the absolute recoveries of the surrogate standards. The average ± SD recoveries were 69 ± 7% (BDE-138), 77 ± 7% (13C12-BDE-209), and 65 ± 5% (dechlorane) (Table S2, ESI†). The higher absolute recoveries in the blanks were due to the absence of a sample matrix and do not affect the relative recoveries. The relative recoveries of the analytes versus the surrogate standards were determined as described in the ESI† and were 88–109% for the PBDEs and 121% for HBCDD (Table S3, ESI†). No corrections for recoveries were made in the final results.
Calculations for the mass balance
In the following calculations, if analyte values were below the LOD in a matrix, the value was replaced by zero. Values that were above the LOD but below the LOQ, were replaced by the LOQ divided by the square root of 2.53The falcons were assumed to be in steady state based on their constant, long-term diet with low PBDE contamination. Concentrations of analytes in plasma lipids were also assumed to be in equilibrium with other body lipids. Total body lipid content was not available for peregrine falcons, but has been quantified in the closely-related American kestrel to be 10.8% so this value was used in the calculations.27 The total body burden in each falcon before breeding was calculated using the lipid weight concentrations of each analyte found in plasma multiplied by the estimated total body lipid content per kg body weight. The body burdens should thus be considered best estimates.
Input fluxes were calculated as the amount (g) of food ingested per day multiplied by the mean analyte concentrations found in the triplicate cockerel and quail homogenates weighted for the average number and body weight of each food type eaten daily over the three month period prior to blood sampling (see ESI† for details of calculations). As no analytical data were available for mice, a weighted average concentration based on the proportions of cockerels and quail ingested was used. These data were obtained from feeding diaries kept on the individual falcon pairs at Nordens Ark. As food was given to the breeding pair simultaneously, the female was assumed to eat half of each food type given.
Fecal excretion fluxes were calculated using the fecal output per day multiplied by the concentration of each analyte found in feces. The BFR concentrations in feces were assumed to be representative for the female, as the males were also hatched in captivity and fed the same diet. Excretion of BFRs via pellet egestion (consisting of feathers, bones) was considered negligible. As fecal output was not possible to measure in this study, it was estimated using data from the literature (6.5 g dw per kg body weight and day) as described in the ESI.† Egg-laying is also an excretion route for contaminants, and for the falcons, egg-laying occurred three months after the initial sampling was performed. Previous studies in peregrine falcons showed no statistically significant differences in organochlorine concentrations54,55 or in PBDE concentrations56,57 between eggs in the same clutch. Therefore, excretion via egg-laying was calculated from the weight of the egg contents multiplied by the concentrations found in the egg and the number of eggs produced by each female in 2006 (three each for two females, two clutches of four for one female). The amounts excreted were converted to fluxes (ng day−1) by dividing the total amount excreted in all eggs for each female by 365 days. When a specific analyte was quantifiable for all matrices, an input–output mass balance for the pre-breeding time point was calculated for each female peregrine falcon using the input fluxes from diet (cockerels, quail, mice), calculated body burdens and the output fluxes from feces.
The absorption efficiency (AE) from the gut for each falcon was calculated as the fraction of each analyte ingested that was not excreted in feces:
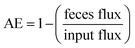 | (1) |
Bioaccumulation factors (BAFs) for the different analytes were calculated as the ratios between lipid weight concentrations in plasma and food (BAFp) or eggs and food (BAFe). The lipid weight concentrations of each analyte in food were weighted averages calculated based on the weighted average dietary intake of cockerels, quail and mice. Transfer to eggs was calculated as the ratio between egg concentrations and plasma concentrations (E/P) on a lipid-weight basis. Metabolic rate constants (MRCs) for individual analytes were estimated as the metabolism flux (total input flux minus feces flux) divided by the estimated body burden for each falcon.
Results
Concentrations of BFRs
Lipid weight (lw) concentrations of individual BDE congeners, HBCDD and DBDPE in the different matrices from each of the three peregrine falcons and in the triplicate homogenates of cockerels and quail are given in Table 1. Fresh weight concentrations are given in Table S4 (ESI†). The predominant BFRs in cockerels and quail were BDE-209 (means of 0.28 and 0.32 ng g–1 lw, respectively) and DBDPE (means of 2.8 and 0.72 ng g–1 lw, respectively) as well as HBCDD in quail (0.2 ng g–1 lw) (Table 1 and Fig. S1–S3 (ESI†)). The quail also contained low concentrations of BDE-47, -49, -99, -100, -183, -197, -206, -207 and -208. The cockerels contained low concentrations of BDE-47, -99, -100, -153, -183, -196, -197, -206, -207 and -208, but HBCDD was not detected, and the concentrations for each BDE congener were 5–10 times higher than in quail.| BDE congener group | Sample type | Lipid % | Tetra | Tetra | Penta | Penta | Hexa | Hexa | Hepta | Octa | Octa | Octa | Nona | Nona | Nona | Deca | DBDPE | HBCDD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BDE-47 | BDE-49 | BDE-99 | BDE-100 | BDE-153 | BDE-154a | BDE-183 | BDE-196 | BDE-197 | BDE-203 | BDE-206 | BDE-207 | BDE-208b | BDE-209 | |||||
| a Quantified on peak height. Not baseline separated from other compound. b Estimated with BDE-206 and relative response factors. c Not detected due to high background concentrations from sampling trays. | ||||||||||||||||||
| Chicken (mean, n = 3) | Food | 8.3 | 0.071 | 0.0034 | 0.16 | 0.045 | 0.12 | <0.005 | 0.17 | 0.026 | 0.062 | <0.04 | 0.014 | 0.029 | 0.011 | 0.28 | 2.6 | <0.08 |
| Range | 7.45–9.79 | 0.055–0.082 | <0.002–0.0034 | 0.13–0.18 | 0.037–0.049 | 0.10–0.13 | <0.004–<0.006 | 0.14–0.18 | 0.024–0.028 | 0.052–0.072 | <0.03–<0.04 | <0.003–0.015 | 0.019–0.036 | 0.0064–0.012 | 0.18–0.31 | 2.1–3.7 | <0.07–<0.08 | |
| Quail (mean, n = 3) | Food | 10.4 | 0.014 | 0.0041 | 0.026 | 0.005 | <0.01 | <0.004 | 0.0087 | <0.006 | 0.002 | <0.03 | 0.004 | 0.015 | 0.0042 | 0.32 | 0.57 | 0.20 |
| Range | 10.3–10.5 | 0.013–0.016 | 0.0035–0.0046 | 0.025–0.027 | 0.0039–0.0056 | <0.01–<0.01 | <0.004–<0.005 | <0.006–0.013 | <0.004–<0.007 | 0.0015–0.0037 | <0.03–<0.03 | <0.003–0.0066 | 0.013–0.016 | <0.003–0.0051 | 0.14–0.66 | <0.3–1.2 | 0.20–0.20 | |
| Falcon identification code | ||||||||||||||||||
| 223 | Plasma | 1.01 | <0.7 | <0.1 | 0.2–0.7 | <2 | 3.0 | 0.80 | 1.1 | 0.2–0.5 | 0.30 | <2 | 0.02–0.05 | 0.55 | 0.38 | 6.2 | <2 | <1 |
| 398 | Plasma | 1.17 | <0.5 | <0.09 | <0.4 | <2 | 2.4 | 0.53 | 1.1 | 0.1–0.4 | 0.28 | <1 | 0.007–0.02 | 0.32 | 0.24 | 4.1 | <1 | <1 |
| 466 | Plasma | 1.41 | <0.5 | <0.08 | 0.70 | <2 | 1.3 | 0.41 | 0.71 | 0.1–0.4 | 0.23 | <1 | 0.01–0.03 | 0.41 | 0.29 | 5.7 | <1 | <1 |
| 223 | Egg | 5.64 | 0.091 | <0.006 | 1.0 | 0.40 | 6.2 | 1.4 | 1.7 | 0.47 | 0.56 | 0.22 | 0.073 | 0.70 | 0.46 | 6.2 | <0.8 | 15 |
| 398 | Egg | 4.95 | 0.03–0.09 | <0.007 | 0.43 | 0.16 | 3.6 | 0.71 | 1.5 | 0.43 | 0.48 | 0.17 | 0.046 | 0.54 | 0.40 | 5.2 | 0.3–1 | 5.9 |
| 466 | Egg | 5.59 | 0.14 | <0.006 | 1.0 | 0.35 | 2.3 | 0.65 | 0.84 | 0.32 | 0.36 | 0.07–0.2 | 0.055 | 0.67 | 0.38 | 6.3 | <0.8 | 12 |
| 223 | Feces | 0.983 | 0.1–0.4 | <0.04 | 0.1–0.3 | 0.071 | 0.44 | 0.03–0.1 | 0.21 | 0.14 | 0.047 | <0.5 | 0.18 | 0.25 | 0.13 | 5.4 | ndc | <0.9 |
| 398 | Feces | 5.64 | 0.07–0.2 | <0.02 | 0.22 | 0.079 | 0.36 | 0.02–0.07 | 0.21 | 0.32 | <0.02 | <0.3 | 0.88 | 1.2 | 0.92 | 21 | ndc | <0.6 |
| 466 | Feces | 4.27 | <0.4 | 0.11 | <0.4 | 0.070 | 0.32 | 0.07–0.2 | 0.34 | 0.07–0.2 | <0.04 | <0.8 | 0.71 | 0.93 | 0.57 | 19 | 2–7 | 3.2 |
| 466 | Feces | 2.19 | <0.3 | <0.04 | 0.1–0.3 | 0.072 | 0.32 | 0.03–0.1 | 0.15 | 0.16 | 0.043 | <0.5 | 0.14 | 0.27 | 0.18 | 4.7 | ndc | <0.9 |
Concentrations were below the detection limit (LOD) in all falcon samples for BDE-28, -66, -77, -85, -173, -184, -191 and -205. BDE-35 was detected in one falcon egg sample (0.019 ng g–1 lw). BDE-154 (which co-elutes with BB-153) was found in falcon plasma and eggs and BDE-203 in two falcon eggs. BDE-99, -153, -183, -197, -207, -208, and -209 were detected in most sample types (Table 1). Other BFRs that were detected in some of the sample types were BDE-47, -100, -196, -206, and HBCDD. DBDPE was below the detection limits in all falcon samples.
The predominant BFRs in falcon plasma were BDE-209 (4.1–6.2 ng g−1 lw), followed by BDE-153 (1.3–3.0 ng g–1 lw) and −183 (0.71–1.1 ng g–1 lw) (Table 1 and Fig. S1–S3 (ESI†)). In eggs, the predominant BFRs were HBCDD (5.9–15 ng g–1 lw) followed by BDE-209 (5.2–6.3 ng g–1 lw) and −153 (2.3–6.2 ng g–1 lw). BDE-209 was the predominant BFR in feces (4.7–21 ng g–1 lw). Indications of DBDPE were found in one falcon feces sample but due to high background concentrations from the metal plating itself, it was not possible to quantify DBDPE in the other feces samples. When present, the concentration of each individual BDE congener, as well as HBCDD, was found to be similar in each of the three peregrine falcon's plasma as well as in eggs, usually within a factor of 2–3 (Table 1). However, for feces this was only true for tetra–octaBDEs. For BDE-206, -207, -208 and -209, the feces concentrations varied 20- to 40-fold (dry weight basis) or 4- to 7-fold (lipid weight basis) between the individual falcons, or in the case of falcon 466, between two separate feces samples (Tables 1 and S4†). HBCDD was only detected in one of the four feces samples.
The congener profiles for major components of tetra-hexa, octa- and nonaBDE congeners found in falcon food, plasma, eggs and feces are compared to profiles of these congeners in the penta- (Bromkal 70-5DE), octa- (Bromkal 79-8DE) and decaBDE (Bromkal 82-0DE, Saytex 102E) technical mixtures59 in Fig. 1. For pentaBDE congeners, proportions of BDE-153 increase and BDE-47 and -99 decrease going from food to plasma and eggs (Fig. 1a), and when compared to the technical product. For octaBDE congeners, the proportion of BDE-196 increases and BDE-197 decreases going from technical product to food and plasma (Fig. 1b). Feces have the highest proportion of BDE-196. For the nonaBDEs, the proportion of BDE-206 decreases and BDE-208 increases from food to plasma and eggs, and this is quite pronounced compared to the technical decaBDE products (Fig. 1c). In general, food and feces patterns were more similar to each other, and plasma and egg patterns were more similar to each other.
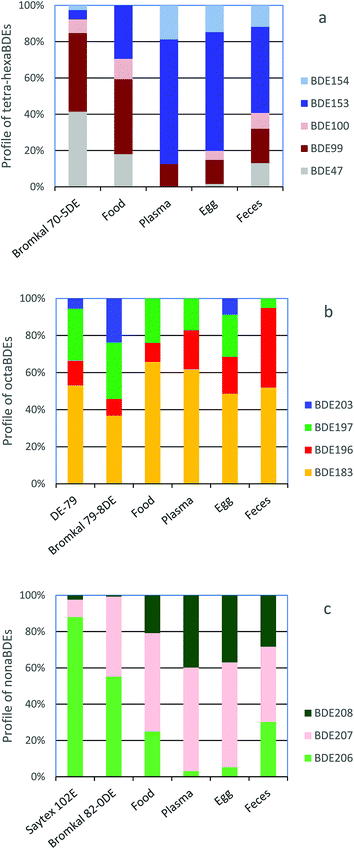 | ||
| Fig. 1 Comparison of (a) tetra–hexaBDE, (b) octaBDE and (c) nonaBDEcongener profiles in peregrine falcon eggs with those of commercial penta-, octa- and decaBDE mixtures.59 | ||
Mass balance results
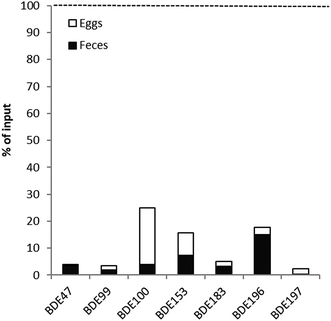
Calculated input–output fluxes and body burdens for individual analytes in each peregrine falcon as well as the means are presented in Table 2. Due to the large differences in fecal concentrations of BDE-206, -207, -208 and -209, the fecal excretion fluxes were more variable, and in some cases (falcon 398, falcon 466, feces 1) were larger than the input fluxes. Excretion via egg-laying was approximately 6.0–29% of the initial, pre-breeding body burden of individual penta–decaBDE congeners, (15–45% for BDE-206). As the concentrations of HBCDD in plasma samples were below the LOD of 1 ng g–1 lw, it was not possible to estimate excretion via egg-laying. When compared to mean excretion via feces in ng d−1 (Table 2), mean excretion in eggs was more important than fecal excretion for BDE-197, -154, -153 and HBCDD (feces flux/egg flux ratios below 1). Both fluxes were similar for BDE-99. Fecal excretion was more important for BDE-47, -100, -183, and -196 (1.8–7.6 times higher feces flux/egg flux ratios). Fecal excretion may play a role for nona–decaBDEs (15–100 times higher feces flux/egg flux ratios, Table 2), but due to the high variability in concentrations found for these BDEs, this should be viewed with some caution.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
Units | BDE-47 | BDE-99 | BDE-100 | BDE-153 | BDE-154 | BDE-183 | BDE-196 | BDE-197 | BDE-206 | BDE-207 | BDE-208 | BDE-209 | HBCDD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Falcon 223 | ||||||||||||||
| Chicken | ng day–1 | 0.57 | 1.3 | 0.36 | 0.95 | nd | 1.4 | 0.21 | 0.50 | 0.11 | 0.22 | 0.086 | 2.1 | 0.59 |
| Quail | ng day–1 | 0.031 | 0.055 | 0.010 | nd | nd | 0.018 | 0.10 | 0.0051 | 0.0092 | 0.031 | 0.0090 | 0.68 | 0.43 |
| Mice | ng day–1 | 0.054 | 0.12 | 0.033 | 0.085 | nd | 0.13 | 0.028 | 0.045 | 0.011 | 0.023 | 0.0085 | 0.25 | 0.092 |
| Sum intake | ng day–1 | 0.65 | 1.4 | 0.41 | 1.0 | nd | 1.5 | 0.34 | 0.55 | 0.13 | 0.28 | 0.10 | 3.1 | 1.1 |
| Body burden | ng | nd | 38 | nd | 230 | 60 | 83 | 26 | 23 | 2.6 | 42 | 29 | 470 | nd |
| Eggs | ng per egg | 0.17 | 2.0 | 0.77 | 12 | 2.7 | 3.2 | 0.87 | 1.1 | 0.14 | 1.3 | 0.87 | 12 | 28 |
| Eggs (n = 3) | Total ng excreted | 0.51 | 5.9 | 2.3 | 35 | 8.1 | 10 | 2.6 | 3.2 | 0.41 | 4.0 | 2.6 | 35 | 84 |
| Eggs | ng day–1 | 0.0014 | 0.016 | 0.0063 | 0.096 | 0.022 | 0.027 | 0.0071 | 0.0088 | 0.0011 | 0.011 | 0.0071 | 0.096 | 0.23 |
| Feces | ng day–1 | 0.013 | 0.0091 | 0.0032 | 0.020 | 0.0032 | 0.010 | 0.0059 | 0.0021 | 0.0082 | 0.011 | 0.0059 | 0.24 | nd |
| Ratio feces flux/egg flux | 9.3 | 0.6 | 0.5 | 0.2 | 0.1 | 0.4 | 0.8 | 0.2 | 7.3 | 1.0 | 0.8 | 2.5 | — | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||||||||
| Falcon 398 | ||||||||||||||
| Chicken | ng day–1 | 0.57 | 1.3 | 0.37 | 0.96 | nd | 1.4 | 0.21 | 0.50 | 0.11 | 0.23 | 0.087 | 2.2 | 0.59 |
| Quail | ng day–1 | 0.030 | 0.054 | 0.010 | nd | nd | 0.018 | 0.10 | 0.005 | 0.009 | 0.030 | 0.0088 | 0.66 | 0.42 |
| Mice | ng day–1 | 0.052 | 0.11 | 0.032 | 0.082 | nd | 0.12 | 0.026 | 0.043 | 0.010 | 0.022 | 0.0082 | 0.24 | 0.087 |
| Sum intake | ng day–1 | 0.66 | 1.5 | 0.41 | 1.0 | nd | 1.5 | 0.33 | 0.55 | 0.13 | 0.28 | 0.10 | 3.1 | 1.1 |
| Body burden | ng | nd | 32 | nd | 270 | 60 | 120 | 32 | 32 | 1.6 | 37 | 27 | 470 | nd |
| Eggs | ng per egg | 0.10 | 0.71 | 0.27 | 6.1 | 1.2 | 2.5 | 0.71 | 0.82 | 0.078 | 0.92 | 0.68 | 8.8 | 9.9 |
| Eggs (n = 3) | Total ng excreted | 0.31 | 2.1 | 0.82 | 18 | 3.6 | 7.4 | 2.1 | 2.4 | 0.23 | 2.8 | 2.0 | 26 | 30 |
| Eggs | ng day–1 | 0.00085 | 0.0058 | 0.0022 | 0.049 | 0.0099 | 0.020 | 0.0058 | 0.0066 | 0.00063 | 0.0077 | 0.0055 | 0.071 | 0.082 |
| Feces | ng day−1 | 0.055 | 0.089 | 0.030 | 0.14 | 0.021 | 0.082 | 0.12 | nd | 0.34 | 0.47 | 0.36 | 8.2 | nd |
| Ratio feces flux/egg flux | 65 | 15 | 13 | 2.8 | 2.1 | 4.0 | 21 | — | 540 | 61 | 66 | 115 | — | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||||||||
| Falcon 466 | ||||||||||||||
| Chicken | ng day–1 | 0.57 | 1.3 | 0.36 | 0.95 | nd | 1.4 | 0.21 | 0.50 | 0.11 | 0.23 | 0.086 | 2.2 | 0.59 |
| Quail | ng day–1 | 0.023 | 0.042 | 0.0080 | nd | nd | 0.014 | 0.080 | 0.0039 | 0.007 | 0.023 | 0.0068 | 0.51 | 0.33 |
| Mice | ng day–1 | 0.052 | 0.12 | 0.033 | 0.083 | nd | 0.12 | 0.025 | 0.044 | 0.010 | 0.022 | 0.0083 | 0.23 | 0.08 |
| Sum intake | ng day–1 | 0.64 | 1.4 | 0.40 | 1.0 | nd | 1.5 | 0.31 | 0.55 | 0.12 | 0.27 | 0.10 | 2.9 | 1.0 |
| Body burden | ng | nd | 74 | nd | 140 | 43 | 74 | 29 | 24 | 2.1 | 43 | 30 | 600 | nd |
| Eggs | ng per egg | 0.29 | 2.2 | 0.75 | 4.9 | 1.4 | 1.8 | 0.68 | 0.75 | 0.12 | 1.4 | 0.79 | 14 | 25 |
| Eggs (n = 8) | Total ng excreted | 2.4 | 17 | 6.0 | 39 | 11 | 14 | 5.4 | 6.0 | 0.94 | 11 | 6.3 | 110 | 200 |
| Eggs | ng day–1 | 0.0066 | 0.047 | 0.016 | 0.11 | 0.030 | 0.038 | 0.015 | 0.016 | 0.0026 | 0.030 | 0.017 | 0.30 | 0.55 |
| Feces 1 | ng day−1 | nd | nd | 0.019 | 0.088 | 0.036 | 0.088 | 0.04 | nd | 0.19 | 0.25 | 0.15 | 5.0 | 0.88 |
| Feces 2 | ng day–1 | nd | 0.0032 | 0.01 | 0.044 | 0.0088 | 0.020 | 0.022 | 0.0060 | 0.02 | 0.037 | 0.025 | 0.63 | nd |
| Ratio feces 1 flux/egg flux | — | — | 1.2 | 0.82 | 1.2 | 2.3 | 2.7 | — | 74 | 8.3 | 8.7 | 17 | 1.6 | |
| Ratio feces 2 flux/egg flux | — | 0.069 | 0.61 | 0.41 | 0.29 | 0.52 | 1.5 | 0.365 | 7.8 | 1.2 | 1.4 | 2.1 | — | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||||||||
| Mean for falcons (n = 3) | ||||||||||||||
| Chicken | ng day–1 | 0.57 | 1.3 | 0.36 | 0.95 | nd | 1.4 | 0.21 | 0.50 | 0.11 | 0.23 | 0.086 | 2.2 | 0.59 |
| Quail | ng day–1 | 0.028 | 0.050 | 0.0093 | nd | nd | 0.017 | 0.093 | 0.0047 | 0.0084 | 0.028 | 0.0082 | 0.62 | 0.39 |
| Mice | ng day–1 | 0.052 | 0.12 | 0.033 | 0.084 | nd | 0.12 | 0.026 | 0.044 | 0.010 | 0.022 | 0.0083 | 0.24 | 0.086 |
| Sum intake | ng day–1 | 0.65 | 1.4 | 0.41 | 1.0 | nd | 1.5 | 0.33 | 0.55 | 0.13 | 0.28 | 0.10 | 3 | 1.1 |
| Body burden | ng | nd | 48 | nd | 210 | 54 | 92 | 29 | 26 | 2.1 | 40 | 29 | 510 | nd |
| Eggs | ng per egg | 0.19 | 1.6 | 0.60 | 7.7 | 1.8 | 2.5 | 0.75 | 0.89 | 0.11 | 1.2 | 0.78 | 11 | 21 |
| Eggs | Total ng excreted | 1.1 | 8.4 | 3.1 | 31 | 7.6 | 10 | 3.4 | 3.9 | 0.53 | 6.1 | 3.7 | 57 | 100 |
| Eggs | ng day–1 | 0.0030 | 0.023 | 0.0085 | 0.085 | 0.021 | 0.027 | 0.0093 | 0.011 | 0.0015 | 0.017 | 0.010 | 0.16 | 0.27 |
| Feces | ng day–1 | 0.023 | 0.025 | 0.016 | 0.072 | 0.017 | 0.05 | 0.048 | 0.0020 | 0.14 | 0.19 | 0.13 | 3.5 | 0.22 |
| Ratio feces flux/egg flux | 7.6 | 1.1 | 1.9 | 0.85 | 0.82 | 1.8 | 5.2 | 0.19 | 96 | 11 | 13 | 22 | 0.4 | |
The full mass balance could be calculated for BDE-99, -153 (only cockerel intake), -183, -196, -197, -206, -207, -208 and -209. Partial mass balances could be calculated for BDE-47, -100, -154/BB-153 and HBCDD. For BDE-47, -99, -100, -153, -183, -196 and -197, the input fluxes exceeded the output fluxes, but for BDE-206, -207, -208 and -209, this varied. For falcon 223 and 466 (feces sample 2), input exceeded output, but for falcon 398 and 466 (feces sample 1), output exceeded input for these congeners.
The mean results for AEs for the three falcons are given in Table 3. The tetra–octabrominated BDEs have high mean AEs ranging from 84–100%. HBCDD has a somewhat lower mean AE of 70%. The AEs for BDE-206, -207, -208 and -209 varied due to the large variability seen for feces fluxes. When using the two lower feces fluxes, the AEs were 84 and 94% for BDE-206, 87 and 96% for BDE-207, 76 and 94% for BDE-208 and 79 and 92% for BDE-209. However, when the two higher feces fluxes were used, these were −52 and −170%, 7 and −69%, −50 and −240% and −74 and −170% for BDE-206, BDE-207, BDE-208 and BDE-209, respectively.
| Analyte (n) | AE (%) | E/P | MRC (d−1) |
|---|---|---|---|
| a Based on low/high fecal output fluxes (n = 2 for each value). | |||
| BDE-47 (2) | 97 | — | — |
| BDE-99 (3) | 97 | 1.7 | 0.03 |
| BDE-100 (3) | 96 | — | — |
| BDE-153 (3) | 93 | 1.8 | 0.004 |
| BDE-154 (3) | — | 1.6 | — |
| BDE-183 (3) | 97 | 1.4 | 0.02 |
| BDE-196 (3) | 84 | 1.3 | 0.009 |
| BDE-197 (3) | 100 | 1.7 | 1.0 |
| BDE-206 (3) | 90/−110a | 2.7 | 0.05/−0.07a |
| BDE-207 (3) | 92/−31a | 1.6 | 0.006/−0.005a |
| BDE-208 (3) | 85/−150a | 1.4 | 0.003/−0.005a |
| BDE-209 (3) | 86/−120a | 1.1 | 0.006/−0.007a |
| HBCDD (3) | 70 | — | |
The highest BAFp values were found for BDE-208 (31), BDE-153 (23), BDE-209 (19) and BDE-207 (16) and highest BAFe values were found for HBCDD (140), BDE-153 (41), BDE-208 (42), BDE-207 (24) and BDE-209 (21) (Table 4). Both BAFe and BAFp values were below 10 for BDE-47, -99 and -100. Where both BAFe and BAFp values were available, the BAFe values were somewhat higher (up to 2 times) but generally the two values were similar (Table 4).
| BDE | BAFp | BAFe | Sparrowhawk-passerine | Buzzard-rodent | Osprey-fish | Guillemot-herring | Kingfisher-fish |
|---|---|---|---|---|---|---|---|
| BDE-47 | — | 1.6 | 10 | 12 | 29 | 19 | 5.7 |
| BDE-99 | 3.7 | 6.1 | 20 | 14 | 32 | 17 | 3.6 |
| BDE-100 | — | 8.1 | 25 | 17 | 19 | 7.1 | 1.5 |
| BDE-153 | 23 | 41 | 21 | 22 | 46 | 6.2 | |
| BDE-154 | — | — | 24 | 20 | 2.1 | ||
| BDE-183 | 6.8 | 10 | 29 | 12 | 17 | ||
| BDE-196 | 14 | 18 | 15 | ||||
| BDE-197 | 5.3 | 9.0 | 16 | ||||
| BDE-206 | 1.9 | 4.8 | 1.0 | ||||
| BDE-207 | 16 | 24 | 4.0 | ||||
| BDE-208 | 31 | 42 | 4.4 | ||||
| BDE-209 | 19 | 21 | 1.7 | ||||
| HBCDD | — | 140 | |||||
| Reference | This study | This study | Voorspoels et al.63 | Voorspoels et al.63 | Chen et al.64,65 | de Wit et al.66 | Mo et al.62 |
All egg/plasma ratios were similar and all were greater than one (range 1.1–2.7), including for BDE-209, indicating efficient transfer from females to the eggs (Table 3).
The mean MRC values for those analytes that could be calculated are presented in Table 3. As for AEs, the MRCs for BDE-206, -207, -208 and -209 showed large variability depending on the feces fluxes. When using the two lower feces fluxes, both MRC values were 0.05 d−1 for BDE-206, 0.006 d−1 for BDE-207, 0.003 d−1 for BDE-208, and 0.006 and 0.005 d−1 for BDE-209. However, when the two higher feces fluxes were used, these were −0.1 and −0.03 d−1, −0.004 and −0.005 d−1, −0.002 and −0.009 d−1 and −0.004 and −0.01 d−1 for BDE-206, -207, -208 and -209, respectively.
Discussion
When present, the concentrations of individual BDE congeners, as well as HBCDD, were similar in each of the three peregrine falcon's plasma and in the eggs (Table 1) reflecting similar exposure due to being fed the same diet. The most likely explanation for finding low concentrations of PBDEs and HBCDD in the cockerel and quail diet fed to the falcons is probably due to low levels present in the feed given to the hens and subsequent transfer from hens to eggs. The source of these contaminants into feed may possibly be from air deposition to plants41,42 and the use of fish meal.58Lower brominated PBDEs
The peregrine falcon's main exposure to tetra–hexaBDEs was from ingestion of cockerels (Table 2). The mean AEs for BDE-47, -99, -100 and -153 (93–97%) in the peregrine falcons in this study (Table 3) were similar to AEs (termed bioavailabilities) found in a study in rats that were dosed with a commercial pentaBDE mixture in food (88–95% for BDE-47 to BDE-154)60 or fed tri–decaBDEs via diet contaminated with a high dose oil (90–94% for BDE-47 to -154).40 These were also similar to net absorption rates of 95–99% for tri–hexaBDEs found in grey seals fed a diet of herring for three months.61 In a mass balance study of tri–hexaBDEs in milk cows, somewhat lower AEs were seen, from 72% for BDE-47 to 35% for BDE-154.42 Thus, the AEs determined in falcons agreed reasonably well with what has been seen in mammalian studies.Table 4 presents biomagnification factors (BMFs) of BDE congeners from published bird studies calculated as the ratios between lipid weight concentrations in predator tissues (muscle, liver, eggs) and lipid weight concentrations in prey tissues (fat, liver, muscle), depending on the study. As for the peregrine falcon BAFs, Mo et al.62 found biomagnification factors (BMFs) for tetra–pentaBDEs for the kingfisher-fish food chain to be below 10. These results for the tetra–pentaBDEs are in contrast to BMFs determined in several other bird species. For terrestrial bird species, Voorspoels et al.63 found BMFs for BDE-47, -99 and -100 in a sparrowhawk-passerine food chain and a buzzard-rodent food chain to be greater than 10 (Table 4). These BMFs are 2–3 times higher than BAFs found for the falcons. In an osprey egg-fish food chain, BMFs were also higher for BDE-47, -99 and -100 compared to the falcons.64,65 For a guillemot egg-herring food chain, BMFs for BDE-47 and -99 were higher than in the falcons, but BDE-100 had a BMF of 7.1, which is similar to that of the falcons.66
For BDE-153, the BAFs found in the peregrine falcons (Table 4) were similar to the BMFs found for sparrowhawks and buzzards,63 and osprey,64,65 but were higher than found in kingfishers62 (Table 4). Voorspoels et al.63 found that BMFs from the sparrowhawk-passerine food chain increased with increasing log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOW for BDE-28 to BDE-183, whereas for the buzzard-rodent food chain, the BMFs increased from BDE-28 to BDE-153, and then dropped for BDE-183. For the peregrine falcons in the current study, the BAFe also increased with increasing log
KOW for BDE-28 to BDE-183, whereas for the buzzard-rodent food chain, the BMFs increased from BDE-28 to BDE-153, and then dropped for BDE-183. For the peregrine falcons in the current study, the BAFe also increased with increasing log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOW from BDE-47 to BDE-153, with a decrease for BDE-183.
KOW from BDE-47 to BDE-153, with a decrease for BDE-183.
Metabolic rate constants of 0.03 d−1 for BDE-99 and 0.004 d−1 for BDE-153 found for the peregrine falcons are quite similar to MRCs of 0.01 and 0.03 d−1 for BDE-99 and 0.001 and 0.007 d−1 for BDE-153 from a mass balance study of tri–hexaBDEs in two lactating cows.42 The lower MRC for BDE-153 indicates slower metabolism for this congener, which is supported by its higher BAF. This is also supported by the BDE congener profiles in Fig. 1a, which show a shift to higher proportions of BDE-153 and lower proportions of BDE-99 from food to plasma and egg.
The AEs, BAFs, and MRCs found for tetra–hexaBDEs in the peregrine falcons were within ranges reported for other studies and species discussed above.40,42,60–66 These results thus support the assumptions that have been used in the input–output calculations of the tetra–hexaBDEs. These include the assumptions used for calculating the estimated food intakes, fecal excretion rates, and body burdens, as well as the assumptions that the falcons were in steady state and that concentrations were in equilibrium between plasma and body lipids. Additional support comes from a study of wild birds (buzzard, sparrow hawk, owls) where equilibrium between body fat and serum/plasma lipids was also seen for tri–heptaBDEs.67
In steady state, the input flux from dietary ingestion in pre-breeding birds should equal the output flux via fecal excretion as long as no metabolism occurs. Fig. 2 shows the mean results of the input/output mass balance in the peregrine falcons for tetra–hexaBDEs. The large discrepancies seen in the peregrine falcons indicate substantial metabolism of tetra–hexaBDEs (more than 90%). Even with the added excretion via eggs, total excretion of parent compounds was still low, but was somewhat higher for BDE-100, -153 and -196, reflecting the lower MRCs for BDE-153 and -196 (BDE-100 could not be calculated).
Several studies of PBDEs in rats also found discrepancies between the amounts administered and those recovered in tissues and feces, indicating that 40–60% of these congeners were metabolized.40,60 In support of this, hydroxylated metabolites of several lower brominated PBDEs were also found in rat carcass and feces in one of these rat studies.60 Likewise, in a mass balance study of tri–hexaBDEs in milk cows, metabolism was inferred for several tetraBDE congeners.42 In an input–output study in grey seals, the input fluxes were also found to be much higher than output fluxes for tetra–hexaBDEs.61
BDE-47 and -99 were found to be metabolized to several OH–BDEs in chicken liver microsomes.68 In a previous study, rapid clearance of BDE-47 was seen in dosed kestrels.43 Several OH–BDEs originating from lower brominated BDEs were also found in plasma in wild peregrine falcon nestlings in Canada.69 Thus, these results indicate that chickens, kestrels and peregrine falcons are able to metabolize lower brominated BDEs, including to hydroxylated metabolites in chickens and falcons. However, no OH–BDE metabolites were seen in starlings dosed with a pentaBDE mixture via silastic implants.70 This may indicate differences in metabolic capacity and/or different metabolic pathways between different bird taxa. This has been shown in a few studies. For example, hepatic cytochrome P450 activity was found to be higher in omnivorous birds and lower in fish-eating and predatory birds such as kestrels and sparrowhawks.71,72 For PCBs, northern fulmar had higher hepatic phase I and II metabolic activity, and higher concentrations of OH–PCB metabolites than kittiwakes, indicating differences in metabolic capacity between these two bird species.73 Thus, the congener profile changes seen in Fig. 1a going from food to plasma, egg and feces were probably due to metabolism of some congeners.
Hepta–decaBDEs
Exposure to hepta–decaBDEs was primarily from ingestion of cockerels (Table 2). The variable concentrations found in feces seem to indicate that exposure to and excretion of BDE-206, -207, -208, -209 were more variable. This led to both positive and negative estimates of AEs and MRCs for these congeners. The positive values found for the low fecal output could possibly be considered reasonable estimates of longer term exposure, whereas the negative values obtained from the high fecal output may be more reflective of sporadic short term exposure (Table 3). These differences could be due to a source of technical decaBDE contamination in the breeding pen that the birds ingest episodically or due to fluctuations in concentrations in the food. In support of the latter, Moser and McLachlan74 found that short term ingestion of food contaminated with high concentrations of polychlorinated furans led to higher concentrations in feces and higher feces flux in humans, particularly for the highly chlorinated and hydrophobic octachlorinated dibenzofuran (OCDF).The congener profile for the nonaBDEs seen in the four feces samples was quite similar and did not match any technical decaBDE products despite the large differences in concentrations (Fig. 1c). The congener profile for nonaBDEs was also quite similar in food as in feces (Fig. 1c). These results would seem to rule out direct ingestion of a technical decaBDE, in favor of the hypothesis that food with sporadically higher contamination levels was a more likely source of the high feces concentrations. A possible explanation for sporadic high concentrations in food could be that decaBDE-related congeners vary in concentration in cockerels and quail, but since individuals were pooled and homogenized, only a mean concentration for intake was available for each. Another possible explanation could be that the mice that the falcons were fed were more contaminated with decaBDE-related congeners than cockerels and quails. Mice were fed sporadically and only occasionally to the falcons and this could have led to short-term ingestion and excretion of a high decaBDE-related congener dose on these occasions. This could also explain why both low and high concentrations of nona–decaBDEs were found in feces samples from the same falcon. However, since no mice were analyzed, it was not possible to confirm this. As the most likely explanation for the high feces concentrations and fluxes was due to short-term sporadic ingestion of nona–decaBDEs from food, we assumed that the lower feces concentrations and fluxes were more representative of the long term exposure in the three captive peregrine falcons. We have therefore used the positive values found for AEs and MRCs in the following discussion.
The AEs in falcons for BDE-183, -196 and -197 ranged from 84–100%. For BDE-206, -207, -208 and -209, based on the lower feces fluxes, the AEs were 85–92% (Table 3). Rats fed with tri–decaBDEs via diet contaminated with a high dose oil, had AEs of 71–78% for BDE-183 and -197, which were lower than for tetra–hexaBDEs. The lowest AEs in the rats were for BDE-196 (46%), BDE-206 (32%), BDE-207 (45%) and BDE-209 (37%),40 which were considerably lower than found for the falcons. However, using radioactively-labelled BDE-209 in rats, Mörck et al.75 found 10% of the single oral dose to be absorbed, 90% of the dose to be excreted in feces, and 65% of the fecal excretion to be metabolites, indicating that uptake could actually be much higher. Similarly, Sandholm et al.76 found BDE-209 uptake in rats to be 26%, but the presence of numerous hydroxylated metabolites indicated even higher uptake. In support of the high AEs found in falcons, in particular for BDE-209, grey seals fed a supplement of BDE-209 in cod liver oil for one month had a mean net absorption of 89%.61
For BDE-183, BAFs/BMFs were similar for peregrine falcons, sparrowhawk, buzzard and kingfisher (Table 4). For other higher brominated BDEs, the only BMF data to compare to come from the kingfisher study. The BAF/BMF for BDE-196 was similar in both bird species, the BAF for BDE-197 was somewhat lower in the falcons than in kingfisher, and the BAFs for BDE-206, -207, -208 and -209 were from 2–10 times higher in the falcons than the corresponding BMFs in kingfisher (Table 4). These differences for the nona–decaBDEs could be due to differences in ability to metabolically debrominate BDE-209 or differences in diet.
BDE-197 had a high MRC (1.0 d−1), indicating more efficient metabolism of this congener. The MRC of BDE-183 was intermediate (0.02 d−1) and BDE-196 had a relatively low MRC (0.009 d−1), which was similar to that of the more recalcitrant BDE-153, indicating that this congener may be more slowly metabolized (Table 3). The congener profiles from food to plasma and egg (Fig. 1b) support this, as there was a shift to somewhat higher proportions of BDE-196 and lower proportions of BDE-197. The positive values for MRCs for BDE-206, -207, -208 and -209 were low and in the same ranges as most of the other BDE congeners, indicating that these congeners were also probably metabolized. This is supported by comparison of the BDE congener profiles from food to plasma and egg (Fig. 1c), where there is a shift to higher proportions of BDE-208 and lower proportions of BDE-206.
If the falcons were in steady state, the input flux from dietary ingestion in pre-breeding birds should equal the output flux via fecal excretion as long as no metabolism occurs. Fig. 2 shows the mean results of the input/output mass balance in the three peregrine falcons for hepta–octaBDEs. The large discrepancies seen in the peregrine falcons for BDE-183, -196 and -197 indicate substantial metabolism of these BDEs. The results for all analyzed BDEs including BDE-206, -207, -208 and -209 for each individual falcon and feces sample are shown in Fig. S4.† Substantial metabolism is indicated for the nona–decaBDEs when using the lower feces outputs, but when the higher feces outputs were used, these result in higher output than input.
Huwe et al.40 also found discrepancies between the amounts of several octa–decaBDEs dosed via dust or oil in rats and those recovered in tissues and feces, indicating that some of these were also metabolized. As stated previously, Mörck et al.75 and Sandholm et al.76 also found evidence of BDE-209 metabolism to numerous hydroxylated metabolites containing five to nine bromines, as well as traces of nonaBDEs.
Reductive debromination of BDE-209 in mammals and birds has also been shown to occur. In rats dosed with commercial decaBDE containing predominantly BDE-209 over 21 days and then allowed to depurate, only 5% of the parent BDE-209 was found in the body and only 4% was found in feces, indicating substantial metabolism.77 BDE-197 and -207 were also recovered in higher amounts than in the dose given, indicating reductive debromination of BDE-209. Similarly, in dairy cattle, metabolic debromination of BDE-209 to BDE-196, -197 and -207 after absorption from feed was also indicated.41
Previous laboratory studies in starlings and kestrels have shown debromination of BDE-209 to lower brominated BDEs. Van den Steen et al.45 found BDE-196, -197, -206, -207, and -208 as debromination products of BDE-209 in serum from starlings with BDE-209 silastic implants. Letcher et al.46 estimated that 80% of BDE-183, -196, -197, -206, -207, and -208 amounts later found in kestrel tissues and plasma originated from the metabolic debromination of BDE-209 given via diet. The formation of OH–BDEs was not included in these studies. Debromination of BDE-209 to lower brominated BDEs was also indicated by Holden et al.78 as wild peregrine falcon eggs had different octa–nonaBDE congener profiles than technical octa- and decaBDE products. The octa- and nonaBDE congener profiles seen in the captive peregrine falcon eggs (Fig. 1b and c) were very similar to those seen by Holden et al.,78 and thus metabolic debromination is also indicated in the captive falcons.
Reductive debromination of BDE-209 would therefore lead to endogenous production of some of these octa–nonaBDE congeners in the falcons. This would mean that the plasma concentrations measured were a combination of accumulation from food intake and debromination of BDE-209, and this would also lead to higher concentrations in eggs. This might lead to overestimation of true BAFs based on plasma and egg concentrations, which might explain why these were fairly high for BDE-183, -196, and -197, and particularly for nonaBDEs (BDE-207 and -208) in the Swedish falcons but does not explain the high BAF for BDE-209 (Table 4).
HBCDD
Technical HBCDD contains mainly γ-HBCDD (75–89%) with small proportions of α- (10–13%) and β-HBCDD (1–12%),79 but falcons have only α-HBCDD in eggs.52,80 The main exposure to HBCDD was from ingestion of quail but as only total HBCDD was quantified, we do not know which stereoisomers were present in quail. HBCDD concentrations were below the detection limits in plasma (<1 ng g–1 lw, <0.01 ng g–1 ww) but it was found in all eggs and one of four feces samples, indicating that uptake from diet occurs and it is excreted in both feces and eggs.The AE was 70% which is similar to absorption seen for α-, β- and γ-HBCDD stereoisomers in rats (73–83%).81 The BAFe was high, 140 (Table 4). The BAFe was probably an overestimation of actual bioaccumulation by at least a factor of six and possibly more, as indicated by the high egg/plasma partitioning ratios (6.1–18) found in the toxicokinetic study of HBCDD stereoisomers in American kestrels by Letcher et al.48 and from estimated minimum egg/plasma ratios calculated (5.9–15) using the falcon plasma LOD of 1 ng g–1 lw. If the LOD of 1 ng g–1 lw were used, the BAFp (plasma LOD/weighted food concentration on a lipid weight basis) for the peregrine falcons would be estimated to be a minimum of 12–13. It was not possible to calculate MRC values for HBCDD. For the one falcon with detectable amounts of HBCDD in feces, Fig. S4† indicates that some metabolism occurred. The lack of detection in plasma samples may also be a sign that HBCDD stereoisomers are metabolized, which has previously been seen for treated kestrels,48 chicken liver microsomes in vitro68 and in rats.81
Transfer to eggs
Synthesis of yolk precursor lipids occurs in the liver with subsequent secretion to plasma and deposition in egg follicles over a short period of time.82,83 The similar concentrations and congener profiles of tetra–decaBDEs in both plasma and eggs of the falcons indicate that the plasma and egg lipids are in reasonable equilibrium with each other. These results confirm that tetra–decaBDE concentrations in eggs reflect the female's body concentration, and eggs are thus a good monitoring matrix for these contaminants in peregrine falcons. Although the egg/plasma ratios decrease somewhat with increasing bromination degree (Table 3) and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow, no significant correlation was found. Van den Steen et al.70 also found similar congener profiles between serum and eggs of starlings for the tetra–hexaBDEs present in the pentaBDE technical mixture, indicating similar transfer for the different BDE congeners from maternal stores to eggs. In a dosing study of BDE-99 in zebra finches, Eng et al.83 determined egg/plasma ratios based on plasma samples collected at the time of laying the first egg. For the control and low-dose groups, the ratio was approximately 2, similar to the mean ratio of 1.7 (range 1.4–2.1) found for BDE-99 in the falcons.
Kow, no significant correlation was found. Van den Steen et al.70 also found similar congener profiles between serum and eggs of starlings for the tetra–hexaBDEs present in the pentaBDE technical mixture, indicating similar transfer for the different BDE congeners from maternal stores to eggs. In a dosing study of BDE-99 in zebra finches, Eng et al.83 determined egg/plasma ratios based on plasma samples collected at the time of laying the first egg. For the control and low-dose groups, the ratio was approximately 2, similar to the mean ratio of 1.7 (range 1.4–2.1) found for BDE-99 in the falcons.
The egg/plasma ratios for α-HBCDD seen in the kestrel study48 (6.1 and 18) and estimated from the current study (5.9–15) seem to indicate that transfer of HBCDD from the female to eggs is quite efficient during egg development in these two Falconid species, and that HBCDD transfer may be higher than for PBDEs in the falcons. Letcher et al.48 speculated that this may be due to protein-specific binding and transport to eggs. Another possible explanation could be that HBCDD is metabolized quickly in plasma so that no equilibrium is established between plasma and egg. In support of this, Letcher et al. recently found a half-life of 15 days for HBCDD in kestrels.48 Also HBCDD was not detected in serum from bald eagles84 and was found at very low concentrations (median 0.05 ng g–1 ww) in 7 of 15 bald eagles85 and in wild peregrine falcon nestlings (GM of 0.39 ng g–1 ww).69
Half-lives
Eggs from female 223 laid in 1998 and 1999 have previously been analysed for PBDEs and HBCDD.26 Comparison of these data with those from the egg laid in 2006 analysed with the same methods (Fig. S5†), indicates that concentrations of the lower brominated BDEs (BDE-99, -153, -154/BB-153, -196, -197) have declined with time. However, the higher brominated BDEs such as BDE-209, as well as HBCDD, were detected in the 2006 egg, but not in the earlier eggs. The appearance of BDE-209 and HBCDD is likely due to the lack of regulation of these technical products in 2006 and their increasing concentrations in the environment,86,87 whereas the declines in the lower brominated BDEs reflect the effect of previous restrictions and bans on environmental levels. Thus, these results reflect changes in use patterns of the different technical products and diffuse contamination into feed and food sources. These results are supported by temporal trend studies showing declines for BDEs present in technical penta- and octaBDE, but increases for BDEs present in technical decaBDE as well as HBCDD in wild peregrine falcons in Sweden.87By plotting the data from these three time points it was possible to roughly estimate the half-lives of several BDEs in this peregrine falcon using linear regression (Fig. S6†). The results are presented in Table 5 together with those for kestrels and herring gulls taken from the literature. Generally, the half-lives found in the peregrine falcons were similar to those found for both kestrels and herring gulls. The half-lives in falcon 223 were probably a reflection of being fed cleaner food over time (due to reduced environmental contamination) and thus depuration, as the values differ considerably for related congeners. In a previous study, a female peregrine falcon, originally wild but also kept in the captive breeding program, had eggs from two consecutive years analysed and the half-life for BDE-153 was multiyear but could not be quantified.25 No estimate of the half-life for BDE-209 in peregrine falcons could be made, but the half-life has been found to be 13 days in starlings,45 14 days in kestrels46 and 8.5–13 d in grey seals.61
Weaknesses of the study
A number of assumptions were used in calculating the input and output variables for the peregrine falcons. The study was a snapshot of the contaminant status and assumed that the falcons were in steady state and thus there was equilibrium between body lipids and plasma. However, Huwe et al.88 found more of the higher brominated BDEs in plasma than adipose in a 21 d dosing study of tri–decaBDEs in rats using dust or oil. Thus, the assumption of equilibrium may not be correct, which would lead to overestimations of the uptake and body burdens of higher brominated BDEs. Tri–heptaBDEs and HBCDD have been found in low concentrations in feathers of terrestrial raptors and concentrations generally correlate with internal concentrations.89–93 Thus, annual moulting is an additional excretion pathway for tri–heptaBDEs and HBCDD. This occurs in the autumn in peregrine falcons, and was not considered in the mass balance. However, due to the low concentrations that are found in feathers, this is probably not a significant excretion route. Other assumptions included using an estimated total body lipid content based on that of kestrels, that food intake for females was assumed to be 50% of what was offered and that missing data for contaminants in mice were estimated from data for cockerels and quail.Conclusions
The input–output mass balance study showed efficient dietary uptake, bioaccumulation and transfer to eggs of most BDE congeners, including BDE-209, as well as HBCDD. Egg-to-plasma ratios were between 1.1 and 2.7, confirming that egg concentrations reflect body burdens, even for higher brominated BDEs. The high concentrations of BDE-209 that have been found in wild peregrine falcon eggs are thus reflective of body burdens. The input fluxes from food exceeded the output fluxes from feces and eggs, indicating considerable metabolism of tetra–octaBDEs and possibly for nona–decaBDEs and HBCDD. Despite the weaknesses in the study, the input–output variables estimated for tetra–heptaBDEs were supported by results from other published studies. For the octa–decaBDEs, there is more uncertainty due to large fluctuations in feces concentrations. For HBCDD, results for some variables are also more tentative due to the lack of detection in plasma and most feces samples. There is a need for more studies of metabolism in birds, including the formation of OH–BDEs and reductive debromination products of BDE-209. In general, more studies of HBCDD in raptors are needed.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
We thank Ulla Eriksson for technical assistance. This study was funded by the Swedish Research Council FORMAS, Göran Gustafssons Stiftelse, Alvins Fond, The Swedish Society for Nature Conservation and the Swedish Environmental Protection Agency.References
- A. R. Zota, J. S. Park, Y. Wang, M. Petreas, R. T. Zoeller and T. J. Woodruff, Polybrominated Diphenyl Ethers, Hydroxylated Polybrominated Diphenyl Ethers, and Measures of Thyroid Function in Second Trimester Pregnant Women in California, Environ. Sci. Technol., 2011, 45, 7896–7905 CrossRef CAS PubMed.
- P. O. Darnerud, Toxic effects of brominated flame retardants in man and in wildlife, Environ. Int., 2003, 29, 841–853 CrossRef CAS PubMed.
- P. O. Darnerud, Brominated flame retardants as possible endocrine disrupters, Int. J. Androl., 2008, 31, 152–160 CrossRef CAS PubMed.
- L. S. Birnbaum and D. F. Staskal, Brominated flame retardants: Cause for concern?, Environ. Health Perspect., 2004, 112, 9–17 CrossRef CAS PubMed.
- L. G. Costa and G. Giordano, Developmental neurotoxicity of polybrominated diphenyl ether (PBDE) flame retardants, NeuroToxicology, 2007, 28, 1047–1067 CrossRef CAS PubMed.
- K. Akutsu, S. Takatori, S. Nozawa, M. Yoshiike, H. Nakazawa, K. Hayakawa, T. Makino and T. Iwamoto, Polybrominated diphenyl ethers in human serum and sperm quality, Bull. Environ. Contam. Toxicol., 2008, 80, 345–350 CrossRef CAS PubMed.
- M. E. Turyk, V. W. Persky, P. Imm, L. Knobeloch, R. Chatterton and H. A. Anderson, Hormone Disruption by PBDEs in Adult Male Sport Fish Consumers, Environ. Health Perspect., 2008, 116, 1635–1641 CrossRef CAS PubMed.
- J. B. Herbstman, A. Sjodin, M. Kurzon, S. A. Lederman, R. S. Jones, V. Rauh, L. L. Needham, D. Tang, M. Niedzwiecki, R. Y. Wang and F. Perera, Prenatal Exposure to PBDEs and Neurodevelopment, Environ. Health Perspect., 2010, 118, 712–719 CrossRef CAS PubMed.
- K. G. Harley, A. R. Marks, J. Chevrier, A. Bradman, A. Sjodin and B. Eskenazi, PBDE Concentrations in Women's Serum and Fecundability, Environ. Health Perspect., 2010, 118, 699–704 CrossRef CAS PubMed.
- L. T. M. van der Ven, A. Verhoef, T. van de Kuil, W. Slob, P. E. G. Leonards, T. J. Visser, T. Hamers, M. Herlin, H. Hakansson, H. Olausson, A. H. Piersma and J. G. Vos, A 28-day oral dose toxicity study enhanced to detect endocrine effects of hexabromocyclododecane in wistar rats, Toxicol. Sci., 2006, 94, 281–292 CrossRef CAS PubMed.
- L. T. M. van der Ven, T. van de Kuil, P. E. G. Leonards, W. Slob, H. Lilienthal, S. Litens, M. Herlin, H. Hakansson, R. F. Canton, M. van den Berg, T. J. Visser, H. van Loveren, J. G. Vos and A. H. Piersma, Endocrine effects of hexabromocyclododecane (HBCD) in a one-generation reproduction study in Wistar rats, Toxicol. Lett., 2009, 185, 51–62 CrossRef CAS PubMed.
- Y. Saegusa, H. Fujimoto, G. H. Woo, K. Inoue, M. Takahashi, K. Mitsumori, M. Hirose, A. Nishikawa and M. Shibutani, Developmental toxicity of brominated flame retardants, tetrabromobisphenol A and 1,2,5,6,9,10-hexabromocyclododecane, in rat offspring after maternal exposure from mid-gestation through lactation, Reprod. Toxicol., 2009, 28, 456–467 CrossRef CAS PubMed.
- M. Ema, S. Fujii, M. Hirata-Koizumi and M. Matsumoto, Two-generation reproductive toxicity study of the flame retardant hexabromocyclododecane in rats, Reprod. Toxicol., 2008, 25, 335–351 CrossRef CAS PubMed.
- P. Eriksson, C. Fischer, M. Wallin, E. Jakobsson and A. Fredriksson, Impaired behaviour, learning and memory, in adult mice neonatally exposed to hexabromocyclododecane (HBCDD), Environ. Toxicol. Pharmacol., 2006, 21, 317–322 CrossRef CAS PubMed.
- H. Lilienthal, L. van der Ven, A. Hack, A. Roth-Harer, A. Piersma and J. Vos, Neurobehavioral Effects in Relation to Endocrine Alterations Caused by Exposure to Brominated Flame Retardants in Rats-Comparison to Polychlorinated Biphenyls, Hum. Ecol. Risk Assess., 2009, 15, 76–86 CrossRef CAS.
- H. M. Stapleton, S. Eagle, R. Anthopolos, A. Wolkin and M. L. Miranda, Associations between Polybrominated Diphenyl Ether (PBDE) Flame Retardants, Phenolic Metabolites, and Thyroid Hormones during Pregnancy, Environ. Health Perspect., 2011, 119, 1454–1459 CrossRef CAS PubMed.
- E. Roze, L. Meijer, A. Bakker, K. N. J. A. Van Braeckel, P. J. J. Sauer and A. F. Bos, Prenatal Exposure to Organohalogens, Including Brominated Flame Retardants, Influences Motor, Cognitive, and Behavioral Performance at School Age, Environ. Health Perspect., 2009, 117, 1953–1958 CrossRef CAS PubMed.
- B. Eskenazi, J. Chevrier, S. A. Rauch, K. Kogut, K. G. Harley, C. Johnson, C. Trujillo, A. Sjodin and A. Bradman, In Utero and Childhood Polybrominated Diphenyl Ether (PBDE) Exposures and Neurodevelopment in the CHAMACOS Study, Environ. Health Perspect., 2013, 121, 257–262 CrossRef PubMed.
- N. Abdelouahab, M. F. Langlois, L. Lavoie, F. Corbin, J. C. Pasquier and L. Takser, Maternal and Cord-Blood Thyroid Hormone Levels and Exposure to Polybrominated Diphenyl Ethers and Polychlorinated Biphenyls During Early Pregnancy, Am. J. Epidemiol., 2013, 178, 701–713 CrossRef PubMed.
- J. Lam, B. P. Lanphear, D. Bellinger, D. A. Axelrad, J. McPartland, P. Sutton, L. Davidson, N. Daniels, S. Sen and T. J. Woodruff, Developmental PBDE Exposure and IQ/ADHD in Childhood: A Systematic Review and Meta-analysis, Environ. Health Perspect., 2017, 125 CrossRef PubMed.
- P. Cox and P. Efthymiou, Directive 2003/11/EC of the European parliament and of the council of February 6, 2003 amending for the 24th time Council Directive 76/669/EEC relating to restrictions on the marketing and use of certain dangerous substances and preparations (pentabromodiphenyl ether, octabromodiphenyl ether), Off. J. European Union, 2003, OJ L, vol. 42, pp. 45–46 Search PubMed.
- UNEP, The Stockholm Convention On Persistent Organic Pollutants as amended in 2009, http://chm.pops.int/Convention/ConventionText/tabid/2232/Default.aspx, accessed May 2012, 2009 Search PubMed.
- E. C. o. Justice, Cases C-14/06 and C-295/06, Judgement of the Court, 1 April 2008, Directive 2002/95/EC and Commission Decision 2005/717/EC, http://curia.europa.eu., 2008 Search PubMed.
- G. Hess, Industry to phase-out decaBDE, Chem. Eng. News, 2009, http://pubs.acs.org/cen/news/87/i51/8751notw12.html, accessed May 2012 Search PubMed.
- P. Lindberg, U. Sellström, L. Häggberg and C. A. de Wit, Higher brominated diphenyl ethers and hexabromocyclododecane found in eggs of peregrine falcons (Falco peregrines) breeding in Sweden, Environ. Sci. Technol., 2004, 38, 93–96 CrossRef CAS PubMed.
- A. K. Johansson, U. Sellstrom, P. Lindberg, A. Bignert and C. A. de Wit, Polybrominated Diphenyl Ether Congener Patterns, Hexabromocyclododecane, and Brominated Biphenyl 153 in Eggs of Peregrine Falcons (Falco Peregrinus) Breeding in Sweden, Environ. Toxicol. Chem., 2009, 28, 9–17 CrossRef CAS PubMed.
- K. J. Fernie, J. L. Shutt, G. Mayne, D. Hoffman, R. J. Letcher, K. G. Drouillard and I. J. Ritchie, Exposure to polybrominated diphenyl ethers (PBDEs): Changes in thyroid, vitamin A, glutathione homeostasis, and oxidative stress in American kestrels (Falco sparverius), Toxicol. Sci., 2005, 88, 375–383 CrossRef CAS PubMed.
- K. J. Fernie, G. Mayne, J. L. Shutt, C. Pekarik, K. A. Grasman, R. J. Letcher and K. Drouillard, Evidence of immunomodulation in nestling American kestrels (Falco sparverius) exposed to environmentally relevant PBDEs, Environ. Pollut., 2005, 138, 485–493 CrossRef CAS PubMed.
- K. J. Fernie, J. L. Shutt, R. J. Letcher, J. I. Ritchie, K. Sullivan and D. M. Bird, Changes in reproductive courtship behaviors of adult American kestrels (Falco sparverius) exposed to environmentally relevant levels of the polybrominated diphenyl ether mixture, DE-71, Toxicol. Sci., 2008, 102, 171–178 CrossRef CAS PubMed.
- K. J. Fernie, J. L. Shutt, R. J. Letcher, I. J. Ritchie and D. M. Bird, Environmentally Relevant Concentrations of DE-71 and HBCD Alter Eggshell Thickness and Reproductive Success of American Kestrels, Environ. Sci. Technol., 2009, 43, 2124–2130 CrossRef CAS PubMed.
- K. J. Fernie, S. C. Marteinson, D. M. Bird, I. J. Ritchie and R. J. Letcher, Reproductive Changes in American Kestrels (Falco Sparverius) in Relation to Exposure to Technical Hexabromocyclododecane Flame Retardant, Environ. Toxicol. Chem., 2011, 30, 2570–2575 CrossRef CAS PubMed.
- S. C. Marteinson, D. M. Bird, J. L. Shutt, R. J. Letcher, I. J. Ritchie and K. J. Fernie, Multi-Generational Effects of Polybrominated Diphenylethers Exposure: Embryonic Exposure of Male American Kestrels (Falco Sparverius) to De-71 Alters Reproductive Success and Behaviors, Environ. Toxicol. Chem., 2010, 29, 1740–1747 CAS.
- S. C. Marteinson, S. Kimmins, R. J. Letcher, V. P. Palace, D. M. Bird, I. J. Ritchie and K. J. Fernie, Diet exposure to technical hexabromocyclododecane (HBCD) affects testes and circulating testosterone and thyroxine levels in American kestrels (Falco sparverius), Environ. Res., 2011, 111, 1116–1123 CrossRef CAS PubMed.
- S. C. Marteinson, D. M. Bird, R. J. Letcher, K. M. Sullivan, I. J. Ritchie and K. J. Fernie, Dietary exposure to technical hexabromocyclododecane (HBCD) alters courtship, incubation and parental behaviors in American kestrels (Falco sparverius), Chemosphere, 2012, 89, 1077–1083 CrossRef CAS PubMed.
- B. A. Rattner, R. S. Lazarus, G. H. Heinz, N. K. Karouna-Renier, S. L. Schultz and R. C. Hale, Comparative embryotoxicity of a pentabrominated diphenyl ether mixture to common terns (Sterna hirundo) and American kestrels (Falco sparverius), Chemosphere, 2013, 93, 441–447 CrossRef CAS PubMed.
- M. L. Eng, J. E. Elliott, S. A. Macdougall-Shackleton, R. J. Letcher and T. D. Williams, Early Exposure to 2,2′,4,4′,5-Pentabromodiphenyl Ether (BDE-99) Affects Mating Behavior of Zebra Finches, Toxicol. Sci., 2012, 127, 269–276 CrossRef CAS PubMed.
- M. L. Eng, T. D. Williams and J. E. Elliott, Developmental exposure to a brominated flame retardant: An assessment of effects on physiology, growth, and reproduction in a songbird, the zebra finch, Environ. Pollut., 2013, 178, 343–349 CrossRef CAS PubMed.
- V. Winter, T. D. Williams and J. E. Elliott, A three-generational study of In ovo exposure to PBDE-99 in the zebra finch, Environ. Toxicol. Chem., 2013, 32, 562–568 CrossRef CAS PubMed.
- M. F. Guigueno and K. J. Fernie, Birds and flame retardants: A review of the toxic effects on birds of historical and novel flame retardants, Environ. Res., 2017, 154, 398–424 CrossRef CAS PubMed.
- J. K. Huwe, H. Hakk, D. J. Smith, J. J. Diliberto, V. Richardson, H. M. Stapleton and L. S. Birnbaum, Comparative absorption and bioaccumulation of polybrominated diphenyl ethers following ingestion via dust and oil in male rats, Environ. Sci. Technol., 2008, 42, 2694–2700 CrossRef CAS PubMed.
- A. Kierkegaard, L. Asplund, C. A. de Wit, M. S. McLachlan, G. O. Thomas, A. J. Sweetman and K. C. Jones, Fate of higher brominated PBDEs in lactating cows, Environ. Sci. Technol., 2007, 41, 417–423 CrossRef CAS PubMed.
- A. Kierkegaard, C. A. de Wit, L. Asplund, M. S. McLachlan, G. O. Thomas, A. J. Sweetman and K. C. Jones, A Mass Balance of Tri-Hexabrominated Diphenyl Ethers in Lactating Cows, Environ. Sci. Technol., 2009, 43, 2602–2607 CrossRef CAS PubMed.
- K. G. Drouillard, K. J. Fernie, R. J. Letcher, L. J. Shutt, M. Whitehead, W. Gebink and D. A. Bird, Bioaccumulation and biotransformation of 61 polychlorinated biphenyl and four polybrominated diphenyl ether congeners in juvenile American kestrels (Falco sparverius), Environ. Toxicol. Chem., 2007, 26, 313–324 CrossRef CAS PubMed.
- R. J. Norstrom, M. Simon, J. Moisley, B. Wakeford and D. V. C. Weseloh, Geographical distribution (2000) and temporal trends (1981–2000) of brominated diphenyl ethers in Great Lakes herring gull eggs, Environ. Sci. Technol., 2002, 36, 4783–4789 CrossRef CAS PubMed.
- E. Van den Steen, A. Covaci, V. L. B. Jaspers, T. Dauwe, S. Voorspoels, M. Eens and R. Pinxten, Accumulation, tissue-specific distribution and debromination of decabromodiphenyl ether (BDE 209) in European starlings (Sturnus vulgaris), Environ. Pollut., 2007, 148, 648–653 CrossRef CAS PubMed.
- R. J. Letcher, S. C. Marteinson and K. J. Fernie, Dietary exposure of American kestrels (Falco sparverius) to decabromodiphenyl ether (BDE-209) flame retardant: Uptake, distribution, debromination and cytochrome P450 enzyme induction, Environ. Int., 2014, 63, 182–190 CrossRef CAS PubMed.
- K. Thuresson, P. Hoglund, L. Hagmar, A. Sjodin, A. Bergman and K. Jakobsson, Apparent half-lives of hepta- to decabrominated diphenyl ethers in human serum as determined in occupationally exposed workers, Environ. Health Perspect., 2006, 114, 176–181 CrossRef CAS PubMed.
- R. J. Letcher, L. C. Mattioli, S. C. Marteinson, D. A. Bird, I. J. Ritchie and K. J. Fernie, Uptake, distribution, depletion and in ovo transfer of isomers of hexabromocyclododecane flame retardant in diet-exposed American kestrels (Falco sparverius), Environ. Toxicol. Chem., 2015, 34, 1103–1112 CrossRef CAS PubMed.
- S. Jensen, L. Haggberg, H. Jorundsdottir and G. Odham, A quantitative lipid extraction method for residue analysis of fish involving nonhalogenated solvents, J. Agric. Food Chem., 2003, 51, 5607–5611 CrossRef CAS PubMed.
- L. Hovander, T. Malmberg, M. Athanasiadou, I. Athanassiadis, S. Rahm, Å. Bergman and E. Klasson Wehler, Identification of hydroxylated PCB metabolites and other phenolic halogenated pollutants in human blood plasma, Arch. Environ. Contam. Toxicol., 2002, 42, 105–117 CrossRef CAS PubMed.
- L. Sahlström, U. Sellstrom and C. A. de Wit, Clean-up method for determination of established and emerging brominated flame retardants in dust, Anal. Bioanal. Chem., 2012, 404, 459–466 CrossRef PubMed.
- K. Janak, U. Sellstrom, A. K. Johansson, G. Becher, C. A. de Wit, P. Lindberg and B. Helander, Enantiomer-specific accumulation of hexabromocyclododecanes in eggs of predatory birds, Chemosphere, 2008, 73, S193–S200 CrossRef CAS PubMed.
- A. Baccarelli, R. Pfeiffer, D. Consonni, A. C. Pesatori, M. Bonzini, D. G. Patterson, P. A. Bertazzi and M. T. Landi, Handling of dioxin measurement data in the presence of non-detectable values: Overview of available methods and their application in the Seveso chloracne study, Chemosphere, 2005, 60, 898–906 CrossRef CAS PubMed.
- I. Newton, J. A. Bogan and M. B. Haas, Organochlorines and mercury in the eggs of british peregrines falco-peregrinus, Ibis, 1989, 131, 355–376 CrossRef.
- P. Lindberg, Relations between the diet of Fennoscandian peregrines Falco peregrinus and organochlorines and mercury in their eggs and feathers, with a comparison to the gyrfalcon Falco rusticolus, PhD dissertation, University of Gothenburg, 1983.
- K. Vorkamp, M. Thomsen, K. Falk, H. Leslie, S. Moller and P. B. Sorensen, Temporal development of brominated flame retardants in peregrine falcon (Falco peregrinus) eggs from South Greenland (1986–2003), Environ. Sci. Technol., 2005, 39, 8199–8206 CrossRef CAS PubMed.
- K. E. Potter, B. D. Watts, M. J. La Guardia, E. P. Harvey and R. C. Hale, Polybrominated Diphenyl Ether Flame Retardants in Chesapeake Bay Region, Usa, Peregrine Falcon (Falco Peregrinus) Eggs: Urban/Rural Trends, Environ. Toxicol. Chem., 2009, 28, 973–981 CrossRef CAS PubMed.
- K. Suominen, A. Hallikainen, P. Ruokojarvi, R. Airaksinen, J. Koponen, R. Rannikko and H. Kiviranta, Occurrence of PCDD/F, PCB, PBDE, PFAS, and Organotin Compounds in Fish Meal, Fish Oil and Fish Feed, Chemosphere, 2011, 85, 300–306 CrossRef CAS PubMed.
- M. J. La Guardia, R. C. Hale and E. Harvey, Detailed polybrominated diphenyl ether (PBDE) congener composition of the widely used penta-, octa-, and deca-PBDE technical flame-retardant mixtures, Environ. Sci. Technol., 2006, 40, 6247–6254 CrossRef CAS.
- J. Huwe, H. Hakk and M. Lorentzsen, Bioavailability and mass balance studies of a commercial pentabromodiphenyl ether mixture in male Sprague-Dawley rats, Chemosphere, 2007, 66, 259–266 CrossRef CAS PubMed.
- G. O. Thomas, S. E. W. Moss, L. Asplund and A. J. Hall, Absorption of decabromodiphenyl ether and other organohalogen chemicals by grey seals (Halichoerus grypus), Environ. Pollut., 2005, 133, 581–586 CrossRef CAS PubMed.
- L. Mo, J. P. Wu, X. J. Luo, F. S. Zou and B. X. Mai, Bioaccumulation of polybrominated diphenyl ethers, decabromodiphenyl ethane, and 1,2-bis(2,4,6-tribromophenoxy) ethane flame retardants in kingfishers (Alcedo atthis) from an electronic waste-recycling site in South China, Environ. Toxicol. Chem., 2012, 31, 2153–2158 CrossRef CAS PubMed.
- S. Voorspoels, A. Covaci, V. L. B. Jaspers, H. Neels and P. Schepens, Biomagnification of PBDEs in three small terrestrial food chains, Environ. Sci. Technol., 2007, 41, 411–416 CrossRef CAS PubMed.
- D. Chen, R. C. Hale, B. D. Watts, M. J. La Guardia, E. Harvey and E. K. Mojica, Species-specific accumulation of polybrominated diphenyl ether flame retardants in birds of prey from the Chesapeake Bay region, USA, Environ. Pollut., 2010, 158, 1883–1889 CrossRef CAS PubMed.
- D. Chen and R. C. Hale, A global review of polybrominated diphenyl ether flame retardant contamination in birds, Environ. Int., 2010, 36, 800–811 CrossRef CAS PubMed.
- C. A. de Wit, An overview of brominated flame retardants in the environment, Chemosphere, 2002, 46, 583–624 CrossRef CAS PubMed.
- S. Voorspoels, A. Covaci, P. Lepom, V. L. B. Jaspers and P. Schepens, Levels and distribution of polybrominated diphenyl ethers in various tissues of birds of prey, Environ. Pollut., 2006, 144, 218–227 CrossRef CAS PubMed.
- X. B. Zheng, C. Erratico, M. A. E. Abdallah, N. Negreira, X. J. Luo, B. X. Mai and A. Covaci, In vitro metabolism of BDE-47, BDE-99, and alpha-, beta-, gamma-HBCD isomers by chicken liver microsomes, Environ. Res., 2015, 143, 221–228 CrossRef CAS PubMed.
- K. J. Fernie and R. J. Letcher, Historical Contaminants, Flame Retardants, and Halogenated Phenolic Compounds in Peregrine Falcon (Falco peregrinus) Nestlings in the Canadian Great Lakes Basin, Environ. Sci. Technol., 2010, 44, 3520–3526 CrossRef CAS PubMed.
- E. Van den Steen, M. Eens, A. Covaci, A. C. Dirtu, V. L. B. Jaspers, H. Neels and R. Pinxten, An exposure study with polybrominated diphenyl ethers (PBDEs) in female European starlings (Sturnus vulgaris): Toxicokinetics and reproductive effects, Environ. Pollut., 2009, 157, 430–436 CrossRef CAS PubMed.
- C. H. Walker, Avian forms of cytochrome P450, Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol., 1998, 121, 65–72 CrossRef CAS.
- C. H. Walker, I. Newton, S. D. Hallam and M. J. J. Ronis, Activities and toxicological significance of hepatic-microsomal enzymes of the kestrel (falco-tinnunculus) and sparrowhawk (accipiter-nisus), Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol., 1987, 86, 379–382 CrossRef CAS.
- L. B. Helgason, A. Arukwe, G. W. Gabrielsen, M. Harju, M. N. Hegseth, E. S. Heimstad, E. H. Jorgensen, A. S. Mortensen and J. Wolkers, Biotransformation of PCBs in Arctic seabirds: Characterization of phase I and II pathways at transcriptional, translational and activity levels, Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol., 2010, 152, 34–41 Search PubMed.
- G. A. Moser and M. S. McLachlan, The influence of dietary concentration on the absorption and excretion of persistent lipophilic organic pollutants in the human intestinal tract, Chemosphere, 2001, 45, 201–211 CrossRef CAS PubMed.
- A. Mörck, H. Hakk, U. Örn and E. Klasson Wehler, Decabromodiphenyl ether in the rat - absorption, distribution, metabolism and excretion, Drug Metab. Dispos., 2003, 31, 900–907 CrossRef PubMed.
- A. Sandholm, B. M. Emanuelsson and E. K. Wehler, Bioavailability and half-life of decabromodiphenyl ether (BDE-209) in rat, Xenobiotica, 2003, 33, 1149–1158 CrossRef CAS PubMed.
- J. K. Huwe and D. J. Smith, Accumulation, whole-body depletion, and debromination of decabromodiphenyl ether in male Sprague-Dawley rats following dietary exposure, Environ. Sci. Technol., 2007, 41, 2371–2377 CrossRef CAS.
- A. Holden, J. S. Park, V. Chu, M. Kim, G. Choi, Y. T. Shi, T. Chin, C. Chun, J. Linthicum, B. J. Walton and K. Hooper, Unusual Hepta- and Octabrominated Diphenyl Ethers and Nonabrominated Diphenyl Ether Profile in California, Usa, Peregrine Falcons (Falco Peregrinus): More Evidence for Brominated Diphenyl Ether-209 Debromination, Environ. Toxicol. Chem., 2009, 28, 1906–1911 CrossRef CAS PubMed.
- A. Covaci, A. C. Gerecke, R. J. Law, S. Voorspoels, M. Kohler, N. V. Heeb, H. Leslie, C. R. Allchin and J. de Boer, Hexabromocyclododecanes (HBCDs) in the environment and humans: A review, Environ. Sci. Technol., 2006, 40, 3679–3688 CrossRef CAS.
- K. Vorkamp, K. Falk, S. Moller, F. F. Riget and P. B. Sorensen, Regulated and Unregulated Halogenated Flame Retardants in Peregrine Falcon Eggs from Greenland, Environ. Sci. Technol., 2018, 52, 474–483 CrossRef CAS PubMed.
- H. Hakk, Comparative Metabolism Studies of Hexabromocyclododecane (HBCD) Diastereomers in Male Rats Following a Single Oral Dose, Environ. Sci. Technol., 2016, 50, 89–96 CrossRef CAS PubMed.
- K. G. Drouillard and R. J. Norstrom, Quantifying maternal and dietary sources of 2,2′,4,4′,5,5′-hexachlorobiphenyl deposited in eggs of the ring dove (Streptopelia risoria), Environ. Toxicol. Chem., 2001, 20, 561–567 CrossRef CAS PubMed.
- M. L. Eng, J. E. Elliott, R. J. Letcher and T. D. Williams, Individual variation in body burden, lipid status, and reproductive investment is related to maternal transfer of a brominated diphenyl ether (BDE-99) to eggs in the zebra finch, Environ. Toxicol. Chem., 2013, 32, 345–352 CrossRef CAS PubMed.
- M. A. Mckinney, L. S. Cesh, J. E. Elliott, T. D. Williams, D. K. Garcelon and R. J. Letcher, Brominated flame retardants and halogenated phenolic compounds in North American west coast bald eaglet (Haliaeetus leucocephalus) plasma, Environ. Sci. Technol., 2006, 40, 6275–6281 CrossRef CAS PubMed.
- M. Venier, M. Wierda, W. W. Bowerman and R. A. Hites, Flame retardants and organochlorine pollutants in bald eagle plasma from the Great Lakes region, Chemosphere, 2010, 80, 1234–1240 CrossRef CAS PubMed.
- R. J. Law, J. Barry, P. Bersuder, J. L. Barber, R. Deaville, R. J. Reid and P. D. Jepson, Levels and Trends of Brominated Diphenyl Ethers in Blubber of Harbor Porpoises (Phocoena phocoena) from the UK, 1992-2008, Environ. Sci. Technol., 2010, 44, 4447–4451 CrossRef CAS PubMed.
- A. K. Johansson, U. Sellstrom, P. Lindberg, A. Bignert and C. A. de Wit, Temporal trends of polybrominated diphenyl ethers and hexabromocyclododecane in Swedish Peregrine Falcon (Falco peregrinus peregrinus) eggs, Environ. Int., 2011, 37, 678–686 CrossRef CAS PubMed.
- J. K. Huwe, H. Hakk and L. S. Birnbaum, Tissue distribution of polybrominated diphenyl ethers in male rats and implications for biomonitoring, Environ. Sci. Technol., 2008, 42, 7018–7024 CrossRef CAS PubMed.
- V. L. B. Jaspers, A. Covaci, E. Van den Steen and M. Eens, Is external contamination with organic pollutants important for concentrations measured in bird feathers?, Environ. Int., 2007, 33, 766–772 CrossRef CAS PubMed.
- V. L. B. Jaspers, S. Voorspoels, A. Covaci, G. Lepoint and M. Eens, Evaluation of the usefulness of bird feathers as a non-destructive biomonitoring tool for organic pollutants: A comparative and meta-analytical approach, Environ. Int., 2007, 33, 328–337 CrossRef CAS PubMed.
- S. C. Marteinson, I. Eulaers, V. L. B. Jaspers, A. Covaci, M. Eens, R. J. Letcher and K. J. Fernie, Transfer of hexabromocyclododecane flame retardant isomers from captive American kestrel eggs to feathers and their association with thyroid hormones and growth, Environ. Pollut., 2017, 220, 441–451 CrossRef CAS PubMed.
- I. Eulaers, A. Covaci, D. Herzke, M. Eens, C. Sonne, T. Moum, L. Schnug, S. A. Hanssen, T. V. Johnsen, J. O. Bustnes and V. L. B. Jaspers, A first evaluation of the usefulness of feathers of nestling predatory birds for non-destructive biomonitoring of persistent organic pollutants, Environ. Int., 2011, 37, 622–630 CrossRef CAS PubMed.
- I. Eulaers, V. L. B. Jaspers, R. Pinxten, A. Covaci and M. Eens, Legacy and current-use brominated flame retardants in the Barn Owl, Sci. Total Environ., 2014, 472, 454–462 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Four tables with information on the three peregrine falcons, absolute recoveries of surrogate standards, relative recoveries from spiked eggs, wet weight concentrations in all matrices, text explaining determination of relative recoveries, food ingestion calculations and fecal output calculations, six figures (comparison of concentrations of all analytes in all matrices for each falcon, input–output balances including nona–decaBDE and HBCDD, time trends of analytes in eggs from falcon 223 in three different years, linear regressions and half-lives estimated for falcon 223). See DOI: 10.1039/c9em00177h |
| ‡ Current address: County Administrative Board of Gävleborg, Borgmästarplan, SE-801 70 Gävle, Sweden. |
| This journal is © The Royal Society of Chemistry 2019 |