Open Access Article
Open Access ArticleThe influence of global climate change on accumulation and toxicity of persistent organic pollutants and chemicals of emerging concern in Arctic food webs†
Katrine
Borgå‡
 *a,
Melissa A.
McKinney‡
*a,
Melissa A.
McKinney‡
 *b,
Heli
Routti
c,
Kim J.
Fernie
*b,
Heli
Routti
c,
Kim J.
Fernie
 d,
Julia
Giebichenstein
a,
Ingeborg
Hallanger
c and
Derek C. G.
Muir
e
d,
Julia
Giebichenstein
a,
Ingeborg
Hallanger
c and
Derek C. G.
Muir
e
aDepartment of Biosciences, University of Oslo, NO-0316 Oslo, Norway. E-mail: katrine.borga@ibv.uio.no
bDepartment of Natural Resource Sciences, McGill University, Sainte-Anne-de-Bellevue, QC H9X 3 V9, Canada. E-mail: melissa.mckinney@mcgill.ca
cNorwegian Polar Institute, Fram Centre, NO-9296 Tromsø, Norway
dEcotoxicology & Wildlife Health, Environment and Climate Change Canada, Burlington, ON L7S 1A1, Canada
eAquatic Contaminants Research Division, Environment and Climate Change Canada, Burlington, ON L7S 1A1, Canada
First published on 18th February 2022
Abstract
This review summarizes current understanding of how climate change-driven physical and ecological processes influence the levels of persistent organic pollutants (POPs) and contaminants of emerging Arctic concern (CEACs) in Arctic biota and food webs. The review also highlights how climate change may interact with other stressors to impact contaminant toxicity, and the utility of modeling and newer research tools in closing knowledge gaps on climate change-contaminant interactions. Permafrost thaw is influencing the concentrations of POPs in freshwater ecosystems. Physical climate parameters, including climate oscillation indices, precipitation, water salinity, sea ice age, and sea ice quality show statistical associations with POPs concentrations in multiple Arctic biota. Northward range-shifting species can act as biovectors for POPs and CEACs into Arctic marine food webs. Shifts in trophic position can alter POPs concentrations in populations of Arctic species. Reductions in body condition are associated with increases in levels of POPs in some biota. Although collectively understudied, multiple stressors, including contaminants and climate change, may act to cumulatively impact some populations of Arctic biota. Models are useful for predicting the net result of various contrasting climate-driven processes on POP and CEAC exposures; however, for some parameters, especially food web changes, insufficient data exists with which to populate such models. In addition to the impact of global regulations on POP levels in Arctic biota, this review demonstrates that there are various direct and indirect mechanisms by which climate change can influence contaminant exposure, accumulation, and effects; therefore, it is important to attribute POP variations to the actual contributing factors to inform future regulations and policies. To do so, a broad range of habitats, species, and processes must be considered for a thorough understanding and interpretation of the consequences to the distribution, accumulation, and effects of environmental contaminants. Given the complex interactions between climate change, contaminants, and ecosystems, it is important to plan for long-term, integrated pan-Arctic monitoring of key biota and ecosystems, and to collect ancillary data, including information on climate-related parameters, local meteorology, ecology, and physiology, and when possible, behavior, when carrying out research on POPs and CEACs in biota and food webs of the Arctic.
Environmental significanceAs it affects physical, biological, and ecological processes in the environment, global climate change has the potential to influence the uptake and fate of persistent organic pollutants (POPs) and contaminants of emerging Arctic concern (CEACs) in biota and food webs through multiple mechanisms. This review paper summarizes the current state of knowledge on the influence of both climate change-modulated physical environmental processes and ecological processes on the accumulation of POPs and CEACs in biota and food webs within Arctic marine, terrestrial, and freshwater ecosystems. It also covers climate change impacts on contaminant toxicity, and modeling and research tools to improve understanding of interactions between climate change and contaminants in biota and food webs. This study reveals that climate change is impacting the long-range transport of pollutants to the Arctic and within the Arctic by altering atmospheric, environmental, and ecological processes and, thus, is influencing the exposure and accumulation of POPs in Arctic wildlife. |
A Introduction
Global climate change affects physical, biological, and ecological processes in the environment, and thus has the potential to influence the uptake and fate of persistent organic pollutants (POPs) in biota and food webs through multiple mechanisms.1 Climate change is occurring most quickly and with the greatest amplitude in polar regions, and is predicted to continue to do so under future scenarios.2 Consequently, the effects of climate change on the bioaccumulation of POPs, as well as contaminants of emerging Arctic concern (CEACs; current-use chemicals with similar properties as POPs, including potential for transport to the Arctic), within food webs are best studied in these polar ecosystems.The potential effects of climate change on the food web accumulation of contaminants in polar regions was first identified just after the turn of the 21st century.1 As summarized in UNEP/AMAP (2011),3 the predicted effects of climate change on contaminant bioavailability and subsequent uptake and bioaccumulation were investigated based on various modeling studies constrained by Intergovernmental Panel on Climate Change (IPCC) forecasts. Prior to that, the focus was predominantly on understanding how the unique characteristics of polar ecosystems influence pollutant uptake and accumulation.4,5 In the decade that followed, national and international efforts, including the International Polar Year, obtained empirical data on the effects of climate change on POP food web accumulation in the Arctic, as summarized for marine ecosystems by McKinney et al.6
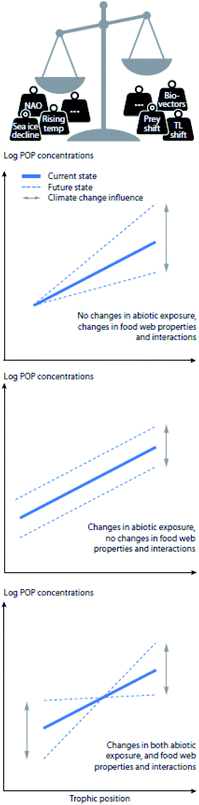
The bioavailability, uptake, bioaccumulation, and fate of POPs in Arctic ecosystems are affected by a multitude of complex and interacting factors, including environmental conditions, such as temperature, precipitation, and presence of sea ice; POP physicochemical properties, such as hydrophobicity and recalcitrance; and a number of biological factors, including physiological and ecological variables such as phenology, energy allocation and lipid dynamics, reproductive strategy, body size, age, sex, life-cycle stage, biotransformation capacity, habitat use, migration and feeding ecology (reviewed in Borgå et al.).7 POPs taken up by Arctic biota may therefore be directly impacted by physical changes in the environment, such as changes in sea ice extent, thickness, age (i.e., multi-year vs. first-year ice), length of the ice season in seasonal ice regions, presence of glaciers, snow cover and permafrost, as well as increased freshwater runoff and altered nutrient availability.8 Additionally, biological and ecological changes, including increased primary production, reduced population sizes of some ice-dependent species, northward range shifts of sub-Arctic and temperate marine and terrestrial species, and altered trophic structuring can influence POP dynamics in Arctic food webs.9–12 Climate change is projected to concurrently alter many of these processes, potentially resulting in decreased or increased contaminant exposure at the base of the food web and changes to food web accumulation (Fig. 1 and S1†).
Here, we review the current state of knowledge regarding climate change-driven physical and ecological factors impacting contaminant fate and accumulation in Arctic species and food webs (Table S1†), with a focus on the POPs and CEACs as defined in de Wit et al.13 We summarize findings from available studies published over the past decade since the previous UNEP/AMAP report3 to address two overarching questions: (1) how do climate change-driven physical processes influence POPs in Arctic wildlife? and (2) How do climate change-driven ecological processes influence the levels of POPs in Arctic biota and food webs? We also highlight how climate change may interact with other stressors to impact contaminant toxicity, as well as the utility of modeling and newer research tools in closing gaps in our understanding of climate change-contaminant interactions. The processes described herein are connected to those reviewed in the other papers in this issue on the abiotic environment14 and temporal trends in biota.15 Finally, we provide conclusions on our current understanding of climate change influences on POPs in Arctic species and food webs, identify knowledge gaps, and provide recommendations for future research and monitoring. Research findings for each topic are reviewed in order, from low- to high-trophic levels for marine, terrestrial, and freshwater ecosystems, when studies were available, and include results from empirical and model-based studies.
B How do physical environmental changes affect POP exposure in Arctic biota and food webs?
B.1 Temperature
Temperature is a central abiotic driver in the environment that regulates and limits biological processes (e.g., development, growth, reproduction), and plays a key role in contaminant dynamics, affecting the physical–chemical properties of a chemical, as well as the biological and physiological processes regulating xenobiotic exposure (i.e., uptake, metabolism, and elimination), especially in poikilothermic organisms. Surface air and ocean temperatures are also increasing due to climate change and are expected to continue to increase in the future. Under medium- to high-greenhouse gas emission scenarios, Arctic fall-winter temperatures of 4 to 5 °C above end of 20th century temperatures are predicted for mid-21st century.16To date, research on the direct relationship between temperature and POP uptake and elimination rates in Arctic biota is still scarce.17 Uptake and elimination are both likely to increase with warming temperatures due increases in metabolism (e.g., ref. 18); however, this relationship may be limited by species-specific biotransformation capacities related to physiological differences (inclusive of thermal tolerances), as well as variations in life history strategies (i.e., body size, longevity, age at maturity, and fecundity and age). In one study, the bioaccumulation potential of POP-like and hypothetical chemicals modeled in the food chain of a non-Arctic fish species, the round goby (Apollonia melanostomus), projected only a minimal impact of climate change on temperature-dependent uptake and elimination rates.19 Similarly, a modeling study of the Barents Sea food web concluded that annual temperature increases would elevate some processes and decrease others, to have only limited effects on net bioaccumulation.20
In addition to its effects on chemical uptake and elimination rates, temperature also affects the bioavailability of, and thus exposure to, contaminants in the Arctic. Warmer air temperatures can increase mobilization and long-range transport of POPs from primary emissions21,22 and re-mobilization from secondary sources, such as soil, melting permafrost, seawater, glacial ice, and sea ice.14,23,24 For example, sediment from Resolute Lake in the high Canadian Arctic might act as a net secondary source of polychlorinated biphenyls (PCBs) to the atmosphere.25 Additionally, long-term temporal trends of PCBs in Arctic air measured at Alert (Canada), Stórhöfði (Iceland), Zeppelin (Svalbard, Norway) and Pallas (Finland), between 1993 and 2012 showed a lower rate of decline in recent years and an increase of PCBs in air after the year 2000.26 This increase may be connected to re-emissions of PCBs from secondary sources due to higher ambient temperatures. The changing of environmental contaminant sinks to contaminant sources could have major effects on the exposure and thus bioaccumulation potential of POPs in wildlife. Nonetheless, it is important to note that both observed and predicted changes in the environmental fate of chemicals strongly depend on compound-specific physical–chemical properties, such as volatility and water solubility.27
B.2 Climate patterns
Large-scale climate patterns (i.e., atmospheric circulation, oceanic currents, wind and precipitation patterns), along with local and regional weather, influence the long-range transport (LRT) of POPs;22,24,28 and thus their concentrations in Arctic abiotic compartments,14 and their subsequent uptake and accumulation in Arctic wildlife. Yet, as climate changes extend across decadal time scales and geopolitical boundaries to impact ecological systems in complicated and interlinked ways, it is not necessarily easy, or even feasible, to identify individual meteorological variables influencing biotic POP levels. For this reason, metrics based on a combination of variables and reflecting broad-scale climate variations, such as climate oscillation indices, are often used to explore associations between climate and ecological changes, including POP temporal trends in biota.15 In particular, associations have been examined between the Arctic Oscillation (AO) and North Atlantic Oscillation (NAO) indices and changes in environmental contamination in Arctic studies.22Modeling and correlative studies strongly suggest that climate change affects the uptake and accumulation of POPs in Arctic wildlife through altered wind and precipitation impacts on contaminant transport or wet deposition.22 Correlations have been documented between the AO or NAO indices and contaminant trends in glaucous gulls (Larus hyperboreus) in the Norwegian Arctic and ringed seals (Pusa hispida) in the Greenlandic and Canadian Arctic.27,29,30 Glaucous gulls from Bjørnøya, Svalbard, showed positive relationships between POP concentrations and AO conditions in the preceding summer and preceding winter.29 Concentrations of POPs in ringed seals from West Greenland were also positively associated with winter AO conditions.31 Similarly, POP concentrations in ringed seals from the Canadian Arctic Archipelago and Hudson Bay were positively related to AO and NAO states, respectively, in the preceding year.30 These findings indicate that POP concentrations were higher in gulls and seals following years with greater transport of air masses from North America and Europe toward the Arctic. In glaucous gulls, negative relationships between concentrations of POPs and AO in the current winter were suggested to be related to changes in diet or overwintering areas.29 The cold winters associated with a negative AO phase (AO−) may decrease the availability of high trophic level prey during the following summer; alternatively, cold winters may force glaucous gulls to migrate further south, exposing them to higher levels of POPs.
Climate-related increases in precipitation were previously predicted to increase the deposition of POPs through scavenging.1 Consistent with this prediction, organochlorine contaminant concentrations in thick-billed murre (Uria lomvia) and northern fulmar (Fulmarus glacialis) eggs in the Canadian Arctic were related to the NAO and/or rainfall amounts between 1975 and 2014 after controlling for differences in chemical partitioning characteristics, feeding ecology, and chemical emissions, which explained the majority (70% in murres; 77% in fulmars) of variability in egg organochlorine contaminant concentrations.32 More specifically, years of higher rainfall were followed by years with thick-billed murre eggs having higher concentrations of chlorobenzenes, octachlorostyrene, dieldrin, DDE and most PCBs, and lower concentrations of heptachlor epoxide, oxychlordane, cis-nonachlor, PCB-170 and PCB-180. In fulmars, years experiencing positive NAO phase (NAO+) conditions, when industrial chemicals are transported to the Arctic from eastern North America and Eurasia, were followed by years when eggs contained higher concentrations of chlorobenzenes, trans- and cis-nonachlor, dieldrin, photomirex and mirex. In contrast, negative NAO phase (NAO−) conditions were associated with birds laying eggs with lower concentrations of these chemicals. A four- to nine-year time lag occurred between the occurrence of the climate pattern and the apparent influence on POP concentrations deposited in the bird eggs.32 POP concentrations in landlocked Arctic char (Salvelinus alpinus) from the Canadian Arctic were also positively correlated with interannual variations of the NAO.25
Changes in the movement of water masses (e.g., oceanic currents) can also influence contaminant transport to, and within, the Arctic, and thus affect exposure to biota. From 1994 until 2010, some POPs, including PCB-52, PCB-153, p,p′-dichlorodiphenyldichloroethylene (p,p′-DDE), hexachlorobenzene (HCB), and α- and β-HCHs decreased in the blubber of ringed seals from central West Greenland.31 β-HCH, a chemical known to undergo long-range oceanic transport, showed the slowest decline, which was correlated to changes in salinity resulting from the dominance of the certain currents around Greenland. That is, years dominated by warm, saline water experienced a greater influx of the Atlantic water branch, around West Greenland, and potentially with it, a higher influx of ocean-transported POPs. Similarly, an increased flux of warm, saline Atlantic water into the European Arctic33 will likely increase oceanic contaminant transport into the high Arctic. Passive samplers deployed at different depths in the Fram Strait, an important deep-water channel to the Arctic Ocean, revealed a net influx of 0.16 megagrams of PCBs per year from the Atlantic into the Arctic, and a net export of HCB and HCH from the Arctic into the Atlantic Ocean, highlighting the continuous omnipresence of these chemicals.34
B.3 Sea ice
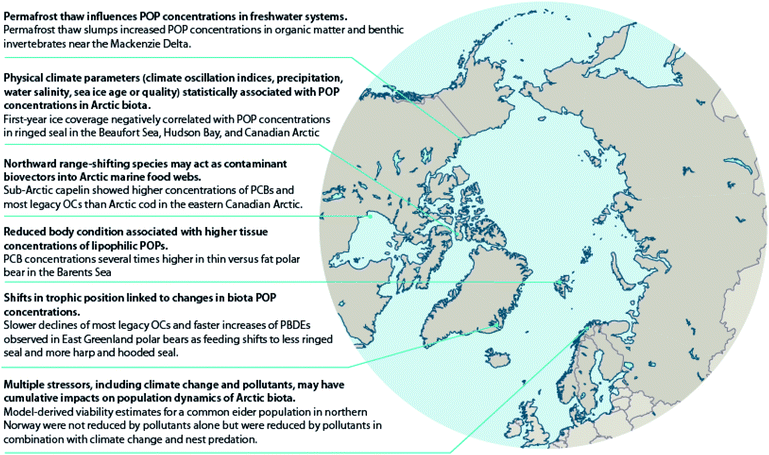
Early modeling efforts forecasted that less sea ice and more open water would increase the exchange of chemicals from the atmosphere to surface waters, especially for POPs still undergoing net loading to the Arctic Ocean, like PCBs and toxaphene.28 Yet, a comparison of pesticide concentrations in zooplankton (Calanus spp.) found higher chlordane (CHL) concentrations, but lower HCB concentrations, in zooplankton from ice-covered Arctic water masses than in those from Atlantic water masses without ice cover.35Several studies have shown relationships between sea ice parameters and POP concentrations in marine mammals (Table S1†), which may be related to various processes. Studies on ringed seals from several areas of the Canadian Arctic have reported significant relationships between concentrations of PCBs and/or chlorinated pesticides and sea ice coverage.30 The results mostly suggest that contaminants accumulate in ringed seals to a higher extent in years with greater total sea ice coverage and/or years with more multi-year sea ice (MYI). This may be related to the ability of sea ice to facilitate the delivery of organic contaminants to Arctic marine food webs,36 as sea ice, along with snowpack and glaciers, are reservoirs for organic contaminants. In addition, re-mobilization of contaminants from sea ice and water reservoirs is increasing due to increasing temperatures and decline of sea ice.23 However, changes in sea ice phenology also affect the prey composition of ringed seals.30 In contrast to the positive correlations between contaminants and total and MYI sea ice coverage, first-year sea ice (FYI) coverage was negatively correlated with POP levels in ringed seal blubber; therefore, the authors suggested that accumulation of contaminants in the cryosphere may also depend on the age of sea ice.30 Higher levels of POPs have also been associated with shorter sea ice seasons in ringed seals from the Beaufort Sea and Greenland, possibly related to variations in feeding opportunities.31,37 Although changes in prey composition were not evaluated in these studies, in both cases, the authors suggested POP changes could be related to prey type.
Studies from the Barents Sea have reported higher levels of lipophilic POPs and per- and polyfluoroalkyl substances (PFASs) in polar bears (Ursus maritimus) that use high-quality sea ice habitats (i.e., the marginal ice zone) in eastern Svalbard/Barents Sea, compared to bears using habitats with less or no sea ice in western Svalbard.38–41 These differences may be influenced by the presence/absence of sea ice,14 in addition to other biotic factors, which are discussed in Section C. The presence of sea ice is likely to increase uptake of atmospherically-deposited POPs into the marine food web. For example, PFASs deposited on surface snow are released and concentrated into the meltwater at the base of the snowpack by the end of the melting period,42 which is followed by phytoplankton bloom and subsequently large increases of zooplankton biomass.43 In contrast, PFASs deposited on open water surfaces are diluted by sea water and found at lower concentrations than in meltwater.44 Concentrations of lipophilic POPs in Barents Sea polar bears were also related to variations in the extent of sea ice on a seasonal scale.45 Plasma and adipose tissue concentrations of POPs in these polar bears were higher during seasons and in areas where sea ice conditions were poor. These relationships were, however, mainly related to changes in body fat, and are further discussed in Sections C.3 and C.4.
For Arctic foxes (Vulpes lagopus) in Svalbard, Norway, concentrations of β-HCH and several PFASs increased with increasing sea ice availability.38,46 However, these relationships were related to an increased availability of seals as further discussed in Section C.2.
B.4 Terrestrial runoff to surface waters
In many regions of the Arctic, climate change is expected to increase the amount of precipitation received in the form of rain. This change will increase the runoff of terrestrial-derived organic material from land to surface waters, leading to the browning of Arctic and sub-Arctic lakes and rivers, and darkening of coastal waters.28,47,48 Terrestrial runoff can carry organic contaminants49 and alter the bioavailability of contaminants by increasing loads of particulate organic matter, and thus influence contaminant exposure in freshwater biota. In addition, elevated terrestrial inputs may alter coastal food web dynamics by presenting zooplankton with lower-quality organic matter.50 If fed upon by zooplankton, the terrestrial energy input can change food web structure and contaminant trophic transfer into the food web.51 Darkened coastal waters due to terrestrial input might further alter predator-prey relationships by decreasing the hunting efficiency of optical predators, such as fish, in favor of tactile predators, such as jellyfish.52 Such changes in trophic interactions will ultimately alter the transfer of energy and contaminants. Thus, increased terrestrial runoff will likely increase exposure to POPs and alter trophic transfer in the food web (Fig. 1).In polar regions, runoff from terrestrial ecosystems includes meltwater from snow and glaciers, which can result in seasonal pulses of freshwater with contaminants to terrestrial, freshwater, and coastal ecosystems. Snow melt releases contaminants recently deposited from the atmosphere,53 as found for polycyclic aromatic hydrocarbons (PAHs) in Antarctica,54,55 whereas glacial melt transfers contaminants stored over the long-term to the terrestrial system, coastal waters, and lake sediments.56–58 However, in the Adventfjorden on Svalbard (Norway), which receives riverine inputs containing runoff from both a large tundra valley and glacier, benthic marine amphipods (Gammarus setosus) showed decreasing total PCB concentrations seasonally from April to August.59 The decrease in total PCB concentrations in amphipods coincided with an increase in terrestrial organic matter from riverine runoff, which may have both diluted and lowered the bioavailable fraction of contaminants at the base of the food web. Nonetheless, less hydrophobic PCBs peaked in amphipods in May/June, coinciding with snow melt. May and June dietary data (i.e., fatty acids) suggested pelagic diatoms made up a high proportion of the amphipod diets, and thus may be the link between the PCBs in snow melt and those detected in the amphipods.59 The net result of terrestrial runoff on contaminant accumulation in Arctic ecosystems is not known, and requires further investigation.60
B.5 Freshwater hydrology and permafrost thaw
As the climate warms, Arctic lakes and surrounding terrestrial environments are undergoing numerous changes that may impact related food webs and fish populations. These changes will ultimately affect POP inputs to lake surface waters and catchments, as well as bioaccumulation and food web biomagnification. Detailed overviews of the impacts of climate change on Arctic terrestrial hydrology and lake ecosystems have been published.61–64 Several factors are of particular relevance to POP bioaccumulation in Arctic lake food webs: changes in primary productivity, biogeochemical cycles and chemical transport pathways, seasonal phenology, and species compositions, which are driven by declining lake ice cover, thawing permafrost, increased early- and late-season precipitation in the form of rain, and warming average annual temperatures and summer air temperatures.61 These changes are briefly discussed below in the context of impacts on freshwater food webs, and ultimately on bioaccumulation of POPs. Note that such changes and processes are likely highly relevant for coastal marine waters as well.A general decline in the duration of ice cover has been observed in Arctic lakes greater than 1 km2 in area. Satellite imagery surveyed for 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 lakes across the circumpolar Arctic from 2000–2013 showed significant trends towards earlier ice break-up, ranging from −0.6 days per year in northern Alaska, to −0.1 days per year in northern Scandinavia.65 Lakes of this size generally have fish populations, with salmonids, and especially Arctic char, and are found largely above the treeline.66–68 These changes are likely to affect water temperatures and within-lake biogeochemical cycles, leading to increases in primary productivity and, consequently, changes in lake trophic relationships. Shifts in the diatom community structures of high Arctic lakes have been rationalized primarily by changing climatic factors such as shorter durations of ice coverage, longer growing seasons, and more favorable thermal stratification patterns.69
300 lakes across the circumpolar Arctic from 2000–2013 showed significant trends towards earlier ice break-up, ranging from −0.6 days per year in northern Alaska, to −0.1 days per year in northern Scandinavia.65 Lakes of this size generally have fish populations, with salmonids, and especially Arctic char, and are found largely above the treeline.66–68 These changes are likely to affect water temperatures and within-lake biogeochemical cycles, leading to increases in primary productivity and, consequently, changes in lake trophic relationships. Shifts in the diatom community structures of high Arctic lakes have been rationalized primarily by changing climatic factors such as shorter durations of ice coverage, longer growing seasons, and more favorable thermal stratification patterns.69
Climate warming is also thawing permafrost in lake catchments and shorelines resulting in changes to lake and river water chemistry. The thawing of permafrost results in inputs of dissolved organic carbon (DOC), inorganic solutes, including nutrients such as ammonium, nitrate, and phosphate and major ions such as Ca2+, Mg2+ and SO42−, and suspended solids to streams and lakes. Nonetheless, limnological properties are highly influenced by regional surroundings.64 Paleolimnological and water chemistry studies of retrogressive thaw slump-(RTS70) affected lakes located east of the Mackenzie River delta area of the Canadian Arctic have shown increases in the abundance and diversity of periphytic diatoms consistent with increased water clarity and subsequent development of aquatic macrophyte communities.71 There is broad agreement from several studies of lakes in this region that higher concentrations of Ca2+, Mg2+, SO42−, and other ions, lower DOC, and increased water clarity occur in RTS-impacted lakes compared to unimpacted lakes.72–74 The decline in DOC was attributed to the sedimentation of organics and particulates from the water column.74 In related work, reduced DOC in slump-affected lake water resulted in higher concentrations of POPs in sedimentary organic matter.75 Although the impacts of these changes on the bioaccumulation of POPs in these lake food webs have not been investigated in detail, amphipods (Gammarus sp.) from slump-affected lakes had higher mean ΣPCBs and ΣDDTs (27.5 ng g−1 lipid weight (lw) and 18.5 ng g−1 lw, respectively) than those from unaffected lakes (17.0 ng g−1 lw and 10.9 ng g−1 lw, respectively).76 Additionally, amphipod POP concentrations increased with the percentage of the catchment slumped.76 Mean ΣHCHs also differed between amphipods from slump-affected lakes (7.0 ng g−1 lw) and unaffected lakes (5.1 ng g−1 lw), although less so than for the more hydrophobic POPs. These water chemistry changes resulting from permafrost thawing and runoff might also affect the physiological condition of freshwater fish (Case study S1†). Other studies in northern Alaska (USA), northern Quebec (Canada), Siberia (Russia) and northern Sweden focused on tundra streams have shown elevated suspended sediments and DOC downstream of thermokarst activity.64,77
B.6 Seasonality
The large seasonal variation of solar irradiance in the Arctic not only guides day length and temperature, but also wind and precipitation patterns. Seasonality therefore has strong effects on both physical and biological processes during the year. Although climate change does not affect irradiance, the annual light cycle, and day length per se, the increase in temperature due to climate change will lead to alterations in the seasonality of physical drivers, such as terrestrial snow melt and runoff, and sea ice thickness, distribution, and break-up, which in turn, will affect the timing of ecosystem responses, such as phytoplankton blooms and availability of breeding grounds. Thus, the window of the productive season, to which Arctic species have adapted their life histories and trade-offs, will be altered, with implications for growth, reproduction, energy allocation, and migration.78Climate change might affect exposure, accumulation, and effects of POPs on wildlife through shifts in the seasonal timing of their biological responses (e.g., migration, availability of prey, reproduction). In multiple seabird species in the European Arctic, plasma or body POP levels measured in parents and their offspring at summer Arctic breeding grounds reflected their exposures to higher contaminant concentrations on the overwintering areas in industrialized and agricultural regions.79–81 Changes in migration timing and the relative time spent at winter and breeding grounds could therefore affect POP exposure. Although links to POPs have not been examined, climate-associated changes in the timing of migrations by Pacific Arctic beluga and bowhead whales have also been documented.82,83 Due to climate change, the Arctic will experience increasingly earlier springs, with earlier availability of breeding grounds and food.9 If migrating birds do not respond to this earlier onset of spring, they may experience an increasing mismatch between the timing of resource availability and their arrival at breeding grounds, eventually resulting in poorer condition and reduced fitness (e.g., ref. 84). Birds may respond to this shift in phenology by migrating north earlier, which could also affect their migration route, timing, and energy used for reproduction at breeding sites (i.e., income vs. capital breeders), and thus, the POPs accumulated and transferred to offspring. Animals that rely mostly on stored reserves for breeding purposes (e.g., egg formation) are considered “capital breeders”, and species relying mostly on concurrent intake for reproduction are “income breeders”; there is a continuum across species in using stored nutrients for breeding.85 In the terrestrial barnacle goose (Branta leucopsis), PCB levels were slightly increased in later-hatching eggs, suggesting females arriving later to Arctic breeding grounds spend more time consuming vegetation at more-contaminated overwintering grounds, compared to earlier-arriving females that predominantly rely on less-contaminated vegetation at the breeding grounds to accumulate the energy reserves for reproduction and egg-laying.86 Based on this example, animals arriving at breeding grounds earlier and relying on the resources consumed there for reproduction, might be expected to have offspring with lower contaminant concentrations.
In the marine ecosystem, the seasonal melting of marginal ice zones influences the timing of algal blooms. The earlier reduction in sea ice extent and volume can lead to a mismatch in the timing of energy availability for secondary producers such as copepods, a key species in the Arctic marine food web.43 This may in turn alter exposures to POPs in these consumers and their predators. Seasonal changes in a pelagic food web can offer insight into climate change impacts in this regard. In Svalbard (Norway), an effort to elucidate the effects of climate change on contaminant bioaccumulation and biomagnification in a pelagic food web was conducted between 2007–2008 using a ‘space-for-time’ approach, in which two or more contrasting sites embodying characteristics representative of present-day and expected future conditions are used to model potential changes over time. Contaminant transfer in the pelagic food web of two fjord systems, Kongsfjorden (Atlantic fjord, representative of future Arctic climate conditions) and Liefdefjorden (Arctic fjord, representative of present-day Arctic climate conditions), were studied with regards to seasonal changes in POP food web bioaccumulation (only Kongsfjorden) and differences between POP bioaccumulation in the food webs of the two fjord systems.87–89 At Kongsfjorden, seasonal differences in POP levels were observed in zooplankton (decrease from May to October), various fishes (different trends depending on species), little auks (Alle alle; similar trends as for zooplankton), and kittiwakes (Rissa tridactyla; different trends depending on contaminant).88,89 Given the diversity of seasonal changes in POPs experienced by different trophic levels and species of the fjord food web, knowledge of seasonal variation will be essential for differentiating climate change-induced alterations from seasonal changes in POP bioaccumulation and biomagnification.
In those studies, differences between the Atlantic- and Arctic-influenced fjords were observed both with regards to POP transfer and fjord-specific characteristics. Higher POP concentrations and bioaccumulation factors (BAFs) were generally observed in zooplankton species from Kongsfjorden. However, there were compound-specific differences, with Liefdefjorden having higher concentrations and proportions of the more volatile compounds, such as α-HCH and chlordanes.87 These differences were attributed to different water masses, ice cover, and freshwater input: all factors which have clear climate relevance but may represent confounding factors associated with the fjords. The seasonal input of freshwater due to the melting of glaciers and snow was earlier in southern Kongsfjorden than northern Liefdefjorden. Further, Kongsfjorden did not have sea ice during winter, while the sea ice in Liefdefjorden broke up shortly before sampling. These differences made the two fjords out of phase with each other with regard to the release of volatile POPs from sea to air. Delayed volatilization of compounds in Liefdefjorden due to the later melting of snow, glacial ice, and sea ice resulted in higher concentrations/exposure to volatile POPs in seawater in Liefdefjorden compared to Kongsfjorden.88,90,91 Therefore, the differences observed in zooplankton and the food webs of the two fjords could be due to seasonal variation and not climate change.88 Overall, there seemed to be a seasonal lag in the peak concentrations detected in biota beginning with an early season peak in zooplankton and progressing to mid- and late-season peaks in fish and birds. Environmental changes in contaminant exposure appear to be reflected faster in zooplankton than in longer-lived, higher trophic-level organisms.92 Thus, extrapolating the results of this study to approximate future climate change effects should be done with caution. Space-for-time approaches require two contrasting sites that are comparable with regards to other potential confounding factors within- or between-sites. This was not achieved within these two fjord systems. Additional investigations with sampling taking place over several years, and/or using Arctic and Atlantic locations outside the fjords that are not influenced by sea ice and meltwater, could be more suitable for elucidating climate change influenced alterations in bioaccumulation and biomagnification.
C How do ecological changes affect POP exposures in Arctic biota and food webs?
C.1 Primary production
Climate change is expected to affect both the seasonal timing (phenology) and the amount of terrestrial and aquatic primary production. The abundance of primary production will still vary seasonally, however, the overall biomass is predicted to change.93 This has implications for the bioavailability of POPs entering the base of the food web, as increased primary production leads to more particulate matter and particle-bound POPs, and conversely, less freely-dissolved POPs bioavailable for direct uptake from water.20 Although dietary uptake of POPs occurs via the particle phase (i.e., algae surface), the average POP concentration in the algae (i.e., on particulate matter) should be lower due to dilution in a larger overall pool of biomass. How, and if, these effects will counteract each other is unknown. In addition, the proportion of POPs subjected to sedimentation into the benthic systems could increase, which all else being equal, would result in higher POP exposure in benthic ecosystems through benthopelagic coupling.94,95In consumers at lower trophic levels of marine and freshwater food webs, POP exposure occurs both through direct uptake from water and through dietary exposure.87,96–98 Concentrations of POPs in Arctic marine zooplankton from Lancaster Sound in the Canadian Arctic decreased in the open water period, being lowest in August–September.96 This was due in part to reduced water and algae organochlorine contaminant concentrations, but may also have been related to increasing zooplankton lipid content during this time. Similar trends were observed in Arctic zooplankton from Kongsfjorden, Svalbard, with overall POP levels decreasing from May to October.87 In the western Canadian Arctic, burbot (Lota lota) from the McKenzie River showed increasing PCB concentrations from 1985–2009, despite decreasing or stable atmospheric concentrations over the same time period.99 The authors showed evidence that the rising PCB concentrations in these freshwater fish were likely related to increases in primary production and ‘algal contaminant scavenging’ into the food web.99
C.2 Species interactions
Climate is a major factor constraining the distribution of species on Earth, and consequently, climate change is shifting the composition of the planet's ecological communities. Species are undergoing global redistribution towards cooler regions, including the poles, higher altitudes, and greater aquatic depths.12 Movements into these areas are being undertaken to maintain thermal tolerances and in response to other species' movements, such as predators following range-shifting prey.100,101 The intensity of these climate-driven species invasions, as well as local extinctions, are predicted to be greatest in the Arctic and Antarctic.102 Thus, the formation of ‘no-analog’ communities, i.e., those with compositions not found under current conditions,103,104 may be especially pronounced in polar regions and possibly other cold regions such as the Tibetan Plateau.Increased temperature and ocean current velocities due to climate change are leading to changes in the northern boundary of various species, with Arctic species generally following the receding marginal ice zone northward, and boreal species increasing their presence and abundance in the Arctic, a phenomenon referred to as ‘borealization’.11,105 Borealization will lead to changes in the species composition of Arctic food webs, and thus changes in trophic interactions, and ultimately alterations in biomagnification. This concept is referred to as ‘Atlantification’ in the marine environment of the European Arctic and ‘Pacification’ in the western North American Arctic. Altered community composition gives rise to novel interactions among species, such as new competitive and predatory interactions. Disturbed predator-prey interactions, in particular, are expected to have outsized consequences for ecological communities through trophic cascades.106 These changing vertical interactions can be anticipated to impact POP burdens in biota, since most exposures in consumers occur largely via diet.5,87,107 Moreover, given that the greatest rates of species invasions, at least for marine ecosystems, are projected to occur in polar regions,102 and rapid Arctic borealization has already been documented (e.g., in the Barents Sea11), we can speculate that polar marine wildlife may be more at risk from climate-driven ecological changes and POP exposures, than other regions.108
With the current lack of understanding of how climate affects food web structure and the strengths of interspecific interactions for most species, the ability to forecast shifting biotic interactions under climate change remains limited.109 Hence, models exploring the future impacts of shifting food webs on POPs are non-existent. However, in the past decade, several studies have empirically examined the role of changing trophic relationships on tissue POP concentrations in Arctic species and food webs. The current state of knowledge is based on research from Canadian, Alaskan (USA), Greenlandic (Denmark), and Norwegian Arctic ecosystems, however, data from the Russian Arctic are lacking. Thus far, most published research on the impacts of shifting species interactions on POPs has focused on marine environments, with marine mammal and seabird studies predominating, but a few studies on marine fishes and a terrestrial mammal (Arctic foxes) have also been performed; findings from these studies are summarized below.
In three regions of varying latitudes within the eastern Canadian Arctic, concentrations of PCBs, organochlorine pesticides (OCPs), and various flame retardants were compared between marine prey fish communities, including native and redistributing non-native species.110 The comparisons particularly focused on the keystone species, Arctic cod (Boreogadus saida), and the sub-Arctic ‘replacement’ species, capelin (Mallotus villosus) and sand lance (Ammodytes sp.). Although not all species were collected in all regions, native benthic species, specifically sculpin (Cottoidea sp.) and northern shrimp (Pandalus borealis), showed the highest concentrations of ∑PCBs and ∑OCPs of all species. Nonetheless, capelin, and to a lesser extent, sand lance, showed higher lipid-normalized concentrations of many POPs than Arctic cod (Fig. 2). Furthermore, neither feeding habitat (based on δ13C values), trophic position (based on δ15N values), or fish length accounted for this difference. This finding, along with congener/compound pattern differences among the species (e.g., higher proportion of DDT vs. DDE in capelin relative to Arctic cod), suggested that the higher POP concentrations in invading sub-Arctic capelin may, at least in part, be a consequence of the fishes acquiring temperate contaminant signatures during seasonal migrations. Thus, these species may act as climate-driven contaminant biovectors into Arctic marine food webs.
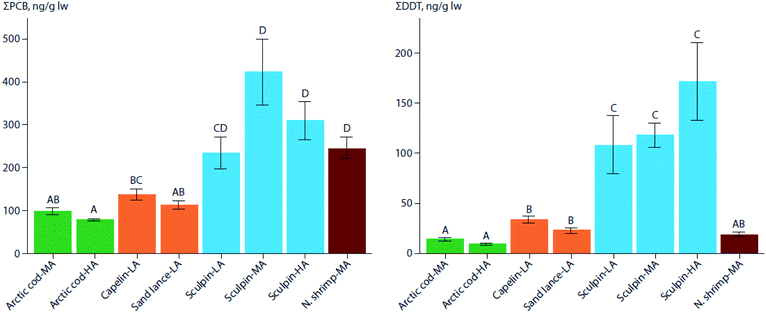 | ||
| Fig. 2 Mean (± SE) lipid weight (lw) concentrations of PCBs (left) and DDTs (right) in muscle of marine forage fish and invertebrates of the low-(LA), mid-(MA), and high-(HA) Canadian Arctic from 2012–2014. Arctic cod (green), the two sub-Arctic species, capelin, and sand lance (orange), sculpin (blue), and northern shrimp (red) are shown. Significant differences in contaminant concentrations among species are indicated by different letters above each bar. Note that the y-axes scales differ. Adapted from Pedro et al. (2017).110 | ||
Similarly, both transient marine fish and mammals showed higher POP concentrations than similar trophic-position Arctic-resident species in a study of the food web of Cumberland Sound, Canada.111 Elevated concentrations of several current-use pesticides (chlorothalonil, chlorpyrifos, dacthal, and endosulfan) were also found in the capelin of Cumberland Sound relative to other species at a similar trophic position.112 Nonetheless, the differences in POP concentrations between these native and non-native replacement fishes were small (two-fold or less), suggesting changes in these forage fish communities may have a limited impact on POP concentrations in piscivorous predators (i.e., predator fish, marine mammals, and seabirds). Direct impacts of these changes in prey fish on predator POP concentrations have been investigated for a few seabird species (as discussed further below), but not for any piscivorous fish or marine mammals to date.
Killer whales (Orcinus orca), perhaps the most contaminated species on Earth with respect to POPs,113 are undergoing a northward range-shift into Arctic and sub-Arctic waters in multiple circumpolar locations.114–116 Within the eastern Canadian Arctic and around southeast Greenland, killer whales appear to be targeting marine mammals for food, which may differ from their presumably more typical diet of fish in the North Atlantic.80,114,117–119 However, recent studies have suggested that some North Atlantic killer whale populations may also eat prey other than fish. Killer whales sampled between 2012–2014 around southeast Greenland showed mean blubber concentrations of PCBs (40 mg kg−1 lw) and OCPs (70 mg kg−1 lw) which were an order of magnitude higher than those found in killer whales sampled over similar years in the Faroe Islands.120 This substantial difference in contaminant burdens was suggested to be due, at least in part, to North Atlantic whales increasingly feeding on marine mammals in new Arctic habitats (Fig. 3).
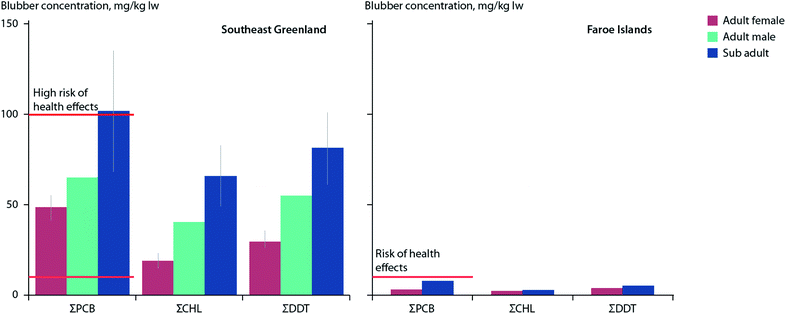 | ||
| Fig. 3 Mean concentrations (±SE) of ΣPCBs, ΣCHLs, and ΣDDTs (mg kg−1 lw) in blubber of eighteen killer whales sampled in 2012–2014 in southeast Greenland (left) and two killer whales sampled in 2008 in the Faroe Islands (right). Red horizontal lines indicate thresholds for risk (10 μg g−1 lw), and high risk (100 μg g−1 lw) of health effects associated with PCB exposure as reported by Dietz et al.190 Adapted from Pedro et al.120 | ||
Recent studies using stable isotopes as dietary markers in a killer whale population off the Norwegian coast also indicate that some whales may feed on marine mammals,121 resulting in elevated contaminant levels.122 Analysis of PCBs indicates 100% of the seal-eating killer whales were above the threshold for a risk of effects on immune and hormonal systems, compared to 43% of fish-eating killer whales (Fig. 4).123
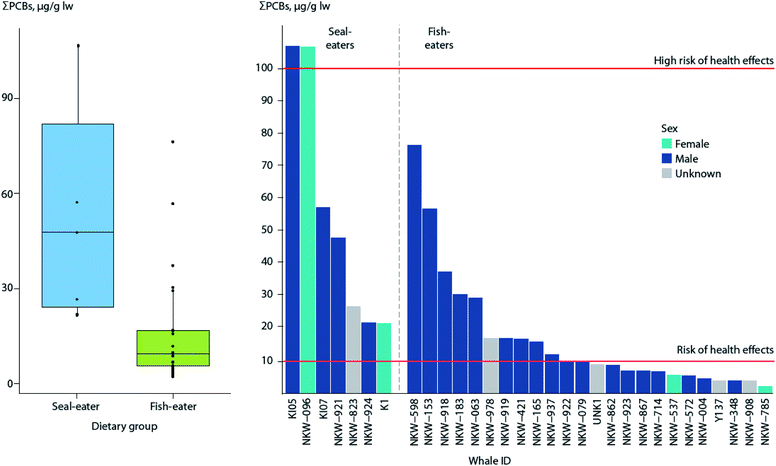 | ||
| Fig. 4 ΣPCB levels in blubber of seal-eating (n = 7) and fish-eating (n = 24) killer whales from Norway. (Left) Box plot of ΣPCB levels in killer whales by dietary group. Horizontal lines represent the median concentration, whiskers represent the lower (first) and upper (fourth) quartiles, and dots represent individual whales, with points outside the box and whiskers being outliers. (Right) ΣPCB levels of individual whales, in decreasing order by dietary group. Red horizontal lines indicate thresholds for risk (10 μg g−1 lw), and high risk (100 μg g−1 lw) of health effects associated with PCB exposure as reported by Dietz et al.190 Adapted from Andvik et al.122 | ||
With climate change has come a shift in the dietary composition of seabirds in the Canadian Arctic124 and in East Greenland,125 that in turn, has likely contributed to changes in their exposure and accumulation of POPs. In the Canadian Arctic, and particularly the Hudson Bay-Hudson Strait region, the diet of thick-billed murres has been shifting since the 1970s due to changing sea ice conditions. The diets of these low-Arctic breeding murres have changed to contain less ice-associated, cold-water Arctic cod and more of the sub-Arctic species, capelin, and sand lance.126–128 Conversely, high-Arctic breeding murres have continued to consume predominantly Arctic cod.129,130 These dietary patterns may explain inter- and intra-species differences in POPs of Canadian Arctic thick-billed murres and northern fulmars measured in 2008.124 Similarly, temporal increases in hepatic organochlorine concentrations in murres from the high Arctic measured in 1998, 2003 and 2008, may be partially related to concurrent changes in diet and trophic position.124 In East Greenland between 2004 and 2015, the diet of little auks (Alle alle) contained less lipid-rich, ice-associated prey when sea ice coverage decreased, with adult birds consuming zooplankton at a higher trophic position, as reflected by an increase in blood δ15N values.125 Concurrently, feather mercury (Hg) concentrations of these auks increased by 3.4% each year, notably occurring during the summer and not when overwintering.125,131 Surprisingly, additional research suggests that interannual variation in little auk feather Hg concentrations reflect changes in food contaminant levels, notably increased Hg levels in zooplankton during summertime, and not the reorganization of the food web or modification of seabird trophic ecology, based on δ15N and δ13C values in little auk whole blood.131 Similar patterns may occur for lipid-soluble POPs. For further studies of Hg, we refer the reader to the recent AMAP report on Hg in the Arctic in relation to climate change.132
Climate change is altering the structure of some Arctic food webs and influencing trophic positions of predatory seabirds and potentially influencing long-term trends in environmental contaminants. After adjusting for the reduced trophic position of thick-bil led murres in Hudson Bay, Canada (based on egg δ15N values), declining trends of organochlorine concentrations (HCB, heptachlor epoxide, oxychlordane, dieldrin, p,p′-DDE and ∑PCB69) measured in eggs between 1993–2003 were more moderate than originally thought.133 In contrast, adjusting for the increased trophic position of high-Arctic breeding murres, resulted in increased rates of organochlorine concentration declines, although p,p′-DDE and ∑PCB69 remained relatively unchanged. A direct link between climate change and contaminant trends was not investigated in this study.
Also, in the Canadian sub-Arctic region of western Hudson Bay, dietary and/or food web shifts were linked to changes in POP trends in polar bears. The diet change was associated with faster increases in adipose concentrations of total polybrominated diphenyl ethers (∑PBDEs) over time, and with a switch from slowly decreasing to slowly increasing concentrations of ∑PCBs from 1991–2007.134 Based on the decline of δ13C values and particular shift in fatty acid profiles observed, the authors speculated that the change in contaminant trends may have been related to changes in the proportion of bearded seal (Erignathus barbatus), sub-Arctic harbor seal (Phoca vitulina) and harp seal (Pagophilus groenlandicus) in the bears' diet, although other food web changes could also have contributed. A similar change was observed in East Greenland polar bears between 1984–2011; a shift towards feeding on sub-Arctic hooded seal (Cystophora cristata) and/or harp seal was directly estimated based on quantitative fatty acid signature analysis, and was associated with NAO conditions.135 However, at least over the time period studied, the shift did not significantly alter trends in adipose concentrations of any of the POPs examined (PCBs, OCPs, and PBDEs) (Fig. 5).135 A re-evaluation of this relationship including recent years through 2016 in East Greenland polar bears, suggested that hooded and/or harp seals now represent the majority of the polar bears' diet, and that a concurrent rise in concentrations of certain POPs, including PCBs, occurred at least until 2013.136 The effect of this ecological change on POPs concentrations may be due to harp seals, and particularly, hooded seals occupying a higher trophic position than the bears' historical main prey, ringed seals, and/or due to these migratory sub-Arctic seals acting as biovectors transporting contaminants from further south into Arctic marine food webs.111
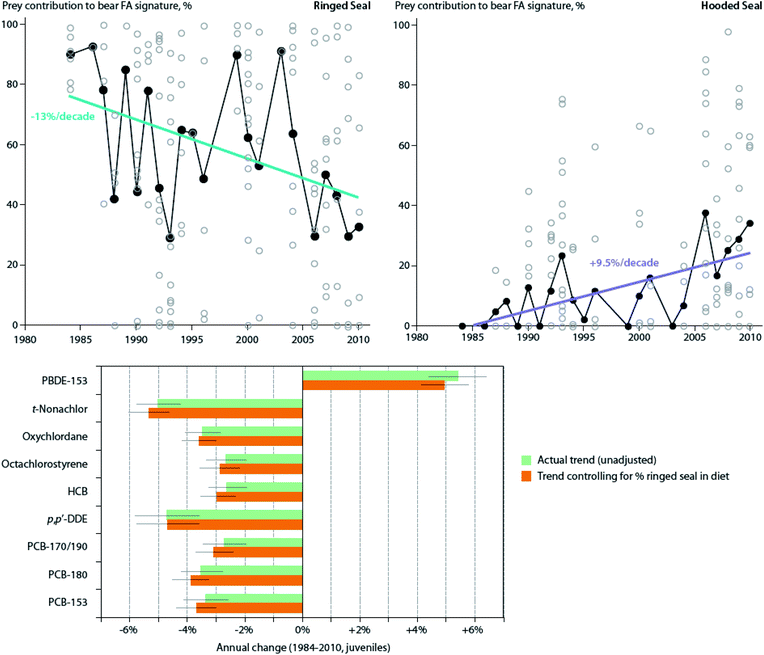 | ||
| Fig. 5 Dietary trends and POP concentrations in East Greenland polar bears. (Top) Contribution of ringed seal and hooded seal to polar bear diets in spring-summer from 1984–2011, based on estimates from quantitative fatty acid signature analysis. Open circles represent individual bears, while black circles represent annual means. Non-significant trends for bearded seal, harp seal, narwhal, and walrus not shown. (Bottom) Annual change of POP concentrations (mean ± SE) in these polar bears, both unadjusted and adjusted (controlled) for changes in percentage of ringed seal in the diet over this same time period. Note that controlling for dietary shift did not significantly alter trends Adapted from McKinney et al.135 | ||
In the southern Beaufort Sea, some individual polar bears are spending extended periods of time onshore during the reduced ice season and consuming onshore foods, namely the remains of subsistence-harvested bowhead whales (Balaena mysticetus).137,138 These onshore polar bears have shown lower concentrations of ∑CHLs, but not other legacy POPs, compared to individuals remaining on the sea ice year-round, likely because bowhead whales occupy a lower trophic position than the bears' main ice-associated prey, ringed seals.139 Recent observations of polar bears foraging on terrestrial species, including colonial nesting seabirds, have also been reported in the Beaufort Sea and for other subpopulations of polar bears,140–142 which all else being equal, would serve to lower their POP exposures.
Temporal trends of lipophilic POPs and PFASs in relation to changes in feeding habits and body condition have also been investigated in adult female polar bears sampled in spring from the Barents Sea.38,143 Temporal changes in δ15N and δ13C values indicate that the winter diet of Barents Sea female polar bears has shifted towards less marine, less ice-associated and lower trophic level prey items,38,143 which is likely related to the rapid decline of sea ice in the area.144 Although not statistically significant, the same polar bears also tended to get thinner over the period 1997–2006, whereas from 2006–2017 they got fatter.143 Contaminant concentrations were strongly related to polar bear diet (lipophilic POPs and PFASs) and body condition (lipophilic POPs only), however, temporal changes in diet and body condition did not significantly affect trends of the measured pollutants.38,143
Climate change responses also affect POPs in Arctic terrestrial communities. The Arctic fox is an opportunistic predator and scavenger that is among the most contaminated of Arctic mammals. Although a terrestrial species, it also feeds on marine foods at coastal tundra areas, such as Svalbard (Norway). Studies investigating the influence of feeding habits and food availability on contaminant temporal trends in Arctic foxes from Svalbard reported that concentrations of all measured lipophilic POPs and PFASs increased with a higher intake of marine food items. Additionally, concentrations of β-HCH, perfluorooctane sulfonic acid (PFOS), perfluoroheptanesulfonic acid (PFHpS) and perfluorotridecanoic acid (PFTrDA) were positively associated with sea ice availability.38,46 A similar, but non-significant tendency was also observed for p,p′-DDE, HCB, CHLs, PBDEs and for perfluorohexane sulfonic acid (PFHxS) and the C9–C11 perfluorocarboxylates (PFCAs). Arctic foxes use sea ice to scavenge the remains of seals killed by polar bears and to hunt newborn ringed seal pups.145 The increased availability of sea ice thus increased Arctic foxes' access to seals, which have higher levels of pollutants than terrestrial prey. Additionally, decreasing concentrations of HCB and PFASs in fox tissues were related to the increasing mortality of reindeer (Rangifer tarandus platyrhynchus).38,46 Reindeer mortality has been connected to rain-on-snow events,146 which entirely encapsulate short-growing vegetation in ice across large areas of high Arctic tundra.147 The frequency of these extreme weather events, such as warm spells and rain-on-snow events, has been increasing in Arctic terrestrial regions.148 Icing of the tundra prevents winter foraging opportunities for Arctic tundra herbivores, which affects their vital rates and has further consequences for the abundance of Arctic carnivores.146 In conclusion, climate change may decrease POP exposures in Arctic foxes as (1) decreases in sea ice will reduce opportunities for Arctic foxes to eat more-contaminated seals and (2) increased numbers of reindeer carcasses become available due to more frequent rain-on-snow events, providing foxes with less contaminated reindeer as a food source.
Behavioral changes and alternative uses of landscapes and food resources may, however, buffer the impacts of environmental change in some species.149,150 For example, the proportion of Svalbard reindeer feeding along the shoreline has increased along with icier winters, and stable isotope values indicate that these reindeer are likely feeding on washed-ashore kelp.150 Although not yet investigated, this may have further implications for contaminant intake of reindeer and their predators. In addition to increasing reindeer mortality, rain-on-snow events also promote the expansion of boreal predators into Arctic tundra. In Yamal, Russia, a high mortality event of domestic reindeer due to icing of the snow layer was followed by the increased presence of red foxes (Vulpes vulpes) and hooded crows (Corvus cornix).151 Other changes, such as the ‘shrubification’ of the Arctic tundra, are also underway,152,153 and like their marine counterparts, terrestrial fauna are also undergoing northward range shifts.9 These shifts in terrestrial plant and animal communities have the potential to alter both contaminant movement and fate within terrestrial Arctic food webs.
C.3 Lipid dynamics and energy allocation
Organisms store energy as lipids for mobilization in times of food shortage. This adaptation, i.e., a trade-off to store energy rather than use it for faster growth and reproduction, is favored in polar regions due to the strong seasonality in primary productivity and food availability in cold climates. In addition to influencing energy transfer within the food web, lipid dynamics directly affect the distribution of lipophilic POPs within the ecosystem.Neutral storage lipids represent the main organic phase that non-ionic chemicals, such as most POPs, partition into.154 The relationship between lipid content and POPs has rarely been studied over time in organisms with very high seasonal variation in lipid content. Arctic marine zooplankton adapted to fasting during winter hibernation, such as Calanoid copepods, store energy as energy-dense wax esters,155–157 and not only as triacylglycerols, which act as the dominant neutral storage lipid class in vertebrates and winter-active zooplankton. As POPs partition into wax esters more than into triacylglycerols,158 a change in the overwintering strategy of Arctic zooplankton to include more active feeding, combined with increased abundances of winter-active southern zooplankton in the Arctic, will change (and likely decrease) POP accumulation at the base of the aquatic food web.
Borealization is also expected to contribute to changes in lipid-dynamics within food webs by altering low- and mid-trophic species compositions. As the climate warms, the Arctic will likely experience an increasing abundance of species with lower lipid content, smaller size, faster growth, and an adaptation for income breeding rather than capital reproduction based on stored energy (Fig. 6).11,159,160 As a result, the lipid mass at the base of the Arctic food web is predicted to decrease with a greater influx of southern secondary producers such as Calanus finmarchicus, and reduced influence of large, lipid-rich Arctic species, such as Calanus glacialis and Calanus hyperboreus.160,161 However, at an ecosystem level, the overall lipid mass is expected to increase due to a more rapid turnover of copepods and a higher general biomass of zooplankton.160
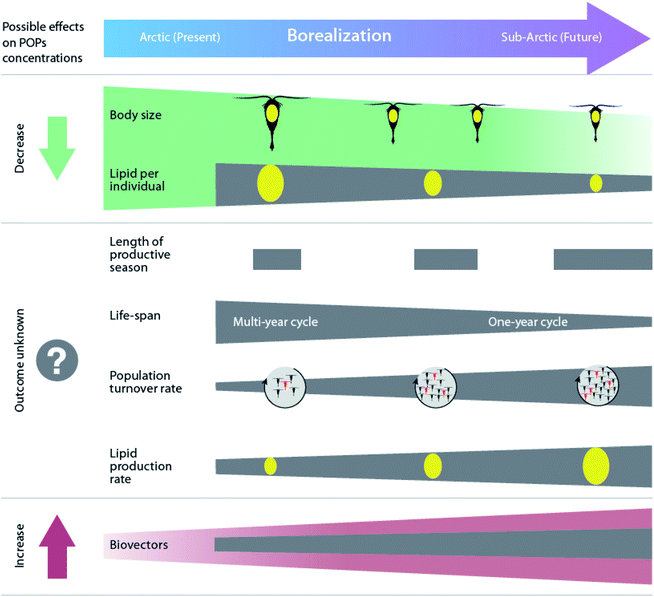 | ||
| Fig. 6 Projected effects of borealization on Arctic marine zooplankton and associated changes in organism POP concentrations. Modified from Renaud et al.160 | ||
A shift from capital to income breeding (i.e., being less dependent on stored lipid reserves) may in turn result in lower maternal transfer of POPs to offspring due to growth dilution, and lower POP storage capacity due to lower lipid content (see Section B.6). However, a meta-analysis of maternal transfer in marine mammals and seabirds did not show any relationship between reproductive strategy and maternal transfer of POPs to offspring for existing mother-pup data in pinnipeds162 and maternal investment in birds.86 These studies also identified significant knowledge gaps as most results to date come from capital breeders and not income breeders (Fig. 7).
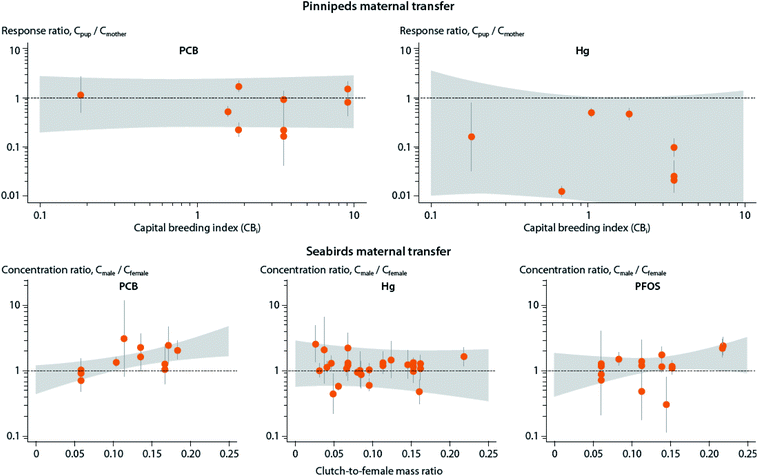 | ||
| Fig. 7 Relationship between reproductive strategy and maternal transfer of mercury (Hg) and POPs, including lipid-soluble PCBs and protein-associated PFOS, in marine mammals (upper panel) and seabirds (lower panel). Upper panel: Association of the estimated capital breeding index value and mother-to-pup contaminant transfer for various pinniped species. Response ratios indicate the ratio between concentration in offspring (Cpup) and mother (Cmother), with values above one indicating higher concentrations in offspring, and values below one indicating higher levels in mother. Gray areas depict confidence intervals. Capital breeding index values indicate the species’ relative reproductive strategy (i.e., the degree to which the pup is dependent on income (newly acquired) or capital (stored body lipids)) energy from the mother, with higher values indicating greater reliance on capital breeding strategy. Lower panel: Association of the seabirds’ estimated clutch-to-female mass ratio and the ratio between concentration in adult males and females, with values above one indicating higher concentrations in males, and values below one indicating higher levels in females (mothers). Clutch-to-female mass ratio represents the energy invested by the mother into the offspring. Figure from Hitchcock et al.86,162 | ||
The common eider (Somateria mollissima) is a large-bodied marine duck which undergoes large seasonal changes in lipid mass. Prior to breeding, females accumulate large lipid reserves and may subsequently lose up to 45% of their body mass during the egg laying and incubation fasting period due to lipid metabolism.163 One study investigated blood concentrations of PCB-153, p,p′-DDE and HCB in incubating common eiders in Svalbard and the sub-Arctic northern Norwegian mainland.164 The authors found that as a result of their higher metabolism, Arctic birds experienced higher blood-level exposures to POPs during the incubation period than sub-Arctic birds, as their use of energy reserves led to a greater re-mobilization of lipid stores, and thus movement of associated lipophilic POPs from fat into the blood. Moreover, increases in blood POP levels were more pronounced in females with low body condition compared to females with high body condition. Based on these findings, increasing temperature in the Arctic can be expected to lead to reduced metabolism and mobilization of lipids in breeding birds and consequently lower blood-level POP exposure during breeding.164
Arctic marine mammals also undergo seasonal changes in body fatness. For example, polar bears generally feed extensively in spring and early summer when ringed seals are pupping and molting on the sea ice.165 During sea ice-free periods, polar bears have reduced, if any, access to food, and therefore largely fast. Pregnant females spend winters in dens, fasting up to eight months in a row.165 The dynamics of lipophilic pollutants in polar bears are tightly connected to annual changes in lipid accumulation, as well as overall body condition, which has been documented to have decreased over time in several polar bear management areas.166–169 For example, studies from the Barents Sea have shown that concentrations of PCBs are several times higher in both plasma and adipose tissue of thin female polar bears compared to fat ones, and that body condition is a more important predictor for lipophilic POP concentrations than diet as inferred from stable isotopes.45 In seasons and areas with reduced sea ice extent, polar bears were thinner, and consequently had higher tissue concentrations of lipophilic contaminants. Although inverse relationships between sea ice and body condition with levels of pollutants have been shown at seasonal and inter-annual scales, long-term changes in springtime polar bear body condition, which are like ly related to sea ice conditions, did not seem to affect POP trends in polar bears.143 Plasma concentrations of proteinophilic POPs (i.e., PFASs) were not related to changes in body fatness of polar bears from the Barents Sea.38,39 However, PFAS concentrations were higher in fasting polar bears than in non-fasting bears, which may be related to higher protein concentrations or reduced metabolic rates, and thus lower contaminant excretion in fasting animals.39 Concentrations of the proteinophilic Hg (measured as total Hg) were inversely related to the body mass index (BMI) of polar bears from the southern Beaufort Sea.170
In the freshwater environment, wet weight PCB concentrations and lipid content in Arctic char from a lake in southwest Greenland decreased from 1994–2008 and were negatively correlated with summer air temperatures; however, the relationship between lipid-normalized PCB concentrations and air temperatures was less clear and did not show a significant trend.171 As there was no indication of changes in trophic morph or trophic level, air temperature was suggested as the influential factor impacting PCB levels in the fish by indirectly altering processes that resulted in reduced condition (i.e., lower lipid content) of the fish.
C.4 Behavioral changes related to sea ice cover
Climate change alters the behavior of wildlife reliant on ice, including Arctic marine mammals and seabirds, which is connected to their diet, physiology, endocrine function, and ultimately exposures to, and effects of, contaminants. Altered foraging behaviors of little auks in East Greenland were observed between 2004–2015 as sea ice percent cover decreased and feather Hg concentrations increased; the birds spent more time flying, less time underwater, and took deeper and longer dives.125,131 In thick-billed murres breeding in Hudson Bay, Canada, circulating Hg levels were associated with levels of the thyroid hormone, triiodothyronine, and triiodothyronine was associated with underwater foraging time in years (i.e., 2016, 2017) when sea ice broke up earlier than the mean date over a 50 year period (1971–2021).172 Behavioral changes have also been observed in other marine mammal species,173–175 (Hamilton et al., 2015; Vacquié-Garcia et al., 2018; Lone et al., 2019), but their potential links with contaminants remain to be investigated.Substantial changes in the sea ice habitat of polar bears have forced them to spend longer portions of the year in lower-quality habitats with reduced access to high-quality prey176–178 or to move longer distances with greater energy expenditure.177,179,180 A comparison of selected polar bears with similar body conditions in the Barents Sea, showed that offshore polar bears were exposed to higher concentrations of pollutants than coastal bears and that this was related to differences in feeding habits, energy expenditure, and geographical distribution.41 Nonetheless, since offshore bears were, on average, fatter than coastal ones,181,182 plasma concentrations of lipophilic POPs were overall similar in bears with different strategies, and only proteinophilic PFASs were higher in pelagic bears.181 Compound-specific and bulk stable isotopes (δ15N and δ13C), home range, field metabolic rates (based on telemetry), as well as contaminant levels in harp seal prey from different locations, cumulatively indicated that higher POP concentrations in offshore Barents Sea polar bears were related to a combination of factors, including the consumption of greater proportions of high-trophic level and marine-based prey, higher levels of POPs in prey species, larger energy requirements, and distribution in marginal ice zones (Fig. 8).181,183 Conclusively, these studies indicate that the fate of pollutants in polar bears from the Barents Sea is the net effect of multiple factors that are driven by climatic conditions. If the costs of migration override the benefits of the energy-rich, high trophic-level prey in the future, offshore bears may exhibit higher concentrations of both lipophilic POPs and PFASs than coastal bears. Also, any change in movement strategies is likely to affect the fate of pollutants in polar bears.
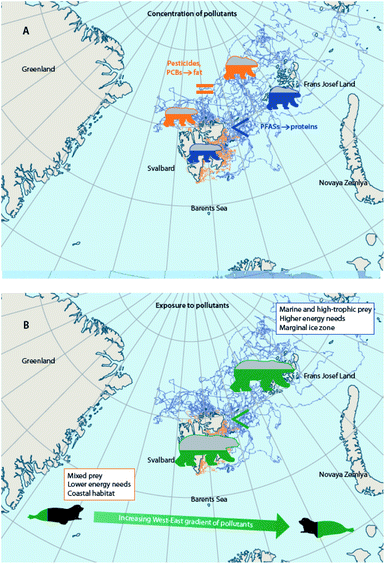 | ||
| Fig. 8 Combination of factors influencing differences in contaminant exposure and tissue concentrations of coastal and offshore polar bears from the Barents Sea. (A) Concentrations of lipid-soluble POPs are similar in fatter, offshore polar bears and in thinner, coastal bears from the Barents Sea, whereas PFAS concentrations are more elevated in the offshore bears.181 (B) When comparing offshore and coastal bears of similar condition, however, offshore polar bears are exposed to higher levels of POPs than coastal polar bears due to differences in feeding habits, energy expenditure, and geographical distribution.183 Tracks of offshore polar bears shown in blue, and tracks of coastal polar bears shown in orange. | ||
C.5 Ecological food web changes due to increased temperature
As water temperatures increase, the metabolism and food demands of fish and other ectotherms also increase, leading to an overgrazing of lower trophic levels.184 Moreover, the elevated metabolic demands associated with higher water temperatures can lead to reduced prey fish quality and quantity, with cascading effects for fish, seabirds, and marine mammal predators. This may be of particular significance for seabirds with high energy demands, such as the thick-billed murre that eats more than 50% of its body mass in fish per day. A decrease in prey quality and quantity, in addition to increased competition for available prey, has previously led to mass die offs of as many as one million murres based on estimates of those washed ashore.184 This increased mortality, combined with failed reproduction, leads to significant ecological changes in marine food webs of both pelagic and benthic communities due to the subsequent sinking of carcasses at sea. Previous studies have shown high levels of various contaminants and enantiomer-selective accumulation in Arctic benthic scavenging amphipods.185,186 Contaminant levels in these benthic amphipods were as high as those in marine mammals and seabirds.187 Thus, climate change and extreme events resulting in mass mortalities may lead to a shift in energy and contaminants within and between food webs, including from pelagic to benthic food webs as a result of the sinking of carcasses at sea, and from marine to terrestrial food webs in the case of seabird mortalities occurring at or near land-based nesting colonies.188,189D How will climate change, in combination with other stressors, affect contaminant toxicity?
Measuring and predicting the effects of climate change on contaminant toxicity in Arctic ecosystems is challenging because such interactions do not occur in isolation; climate change and environmental pollutants may interact with numerous other environmental and health stressors that may lessen, enhance, or produce unexpected impacts. Concurrent environmental stressors including ocean acidification, the presence of litter and plastics, increased fishing and hunting pressure, changes in food availability, oxygen depletion, harmful algal blooms, competition from invasive species, habitat destruction and biodiversity loss, along with a range of social, economic, and political factors, such as increasing human migration, resource exploration and extraction, local development, ship traffic, and recreation and tourism activities, may interact with the stress of climate change and contamination, resulting in both direct and indirect responses in ecosystems and organisms.Novel infectious diseases, changes in pathogen distribution, and an increasing presence of zoonotic pathogens are likely to occur with climate change.190 The physiological stress induced by increased temperature might impair the immune system in cold-blooded organisms such as fish, rendering them more susceptible to infection and disease.191 The rate of phocine distemper virus (PDV) infection in pinnipeds from the North Pacific Ocean was recently found to be significantly higher in years following the presence of an open water route along the northern coast of Russia.192 The authors concluded that reduced sea ice removed a barrier to pinniped movement, thus allowing viral transmission between the North Pacific and eastern North Atlantic Oceans. Although the role of contaminants on disease transmission or animal health was not investigated in this study, some POPs have been shown to suppress immune responses, specifically in marine mammals.193 Taken together, it is conceivable that the combined effects of altered environmental conditions (i.e., temperature-stress), contaminant exposure (i.e., immunosuppression) and ecological changes (e.g., ice loss leading to greater disease transmission), could have greater impacts to animal health than any one factor alone.
Adding another layer of complexity, organisms are simultaneously exposed to multiple types of environmental contaminants with varying toxicities, including but not limited to, the POPs, CEACs, Hg and other potentially unrecognized chemicals or substances. More recently, plastics, including microplastics and nanoplastics, have been identified as substances that may interact with contaminants, or regardless, affect organisms directly.194 Contaminants have been shown to bind to plastic particles with high affinity, and thus plastics may act as vectors transporting chemicals to the Arctic, within the Arctic, and facilitate their uptake by organisms. Moreover, plastics contain chemical additives, and therefore may serve a source of new contaminants to Arctic ecosystems. Plastics are also substrates for microbiota which may play a role in ecosystem functioning. However, as there are currently no studies addressing how climate change directly or indirectly interacts with microplastics to affect contaminants within Arctic food webs, this topic is not addressed further here. See Halsall et al.14 for additional details on microplastics in the abiotic environment.
The effects of climate change may also affect an individual's sensitivity to toxicants (i.e., climate-induced toxicant sensitivity)18,195,196 by altering the toxicokinetics (i.e., uptake, distribution, metabolism, and elimination of toxicants) and toxicodynamics (i.e., toxicant interactions with biological receptors and enzymes) of contaminants (see Section B.1). Such changes may also alter a species' sensitivity and thresholds for effects, including lowering effect thresholds such that impairments are experienced with lower levels of contaminant exposure, as seen in developing children.197
Most studies investigating environmental pollution in combination with other stressors usually focus on the impacts of climate-related environmental parameters (e.g., temperature increase or drought). Most, if not all species have an optimal range of temperatures for which they can maintain homeostasis, outside of which, their ability to function may be impaired. However, the effects of environmental temperature changes are especially pronounced in ectothermic organisms which heavily rely on external sources of heat to control body temperature. Ectotherms display a reaction norm (general performance curve) in response to temperature, whereby they have an optimal temperature window, as well as upper- and lower-critical limits. The response variable (i.e., performance) reflects important determinants of fitness, such as life history traits (e.g., age at maturity), that directly affect the vital rates of a life table (e.g., survival, fecundity, growth). The ability of an organism to cope with other stressors, such as toxicants, depends on where within this temperature range they are.198
Understanding the effects of multiple stressors, compared to a single stressor such as temperature, can be more challenging. Usually, exposures to multiple stressors are predicted to have synergistic outcomes, in other words, a combined effect that is greater than the sum of the two. However, most laboratory-based studies are designed such that synergistic effects are also the most likely outcome; for example, many studies investigate the effects resulting from two stressors under continuous exposure at the same time,199 when real exposure regimes most likely differ from this scenario. Organisms do not always experience constant and simultaneous exposure to all stressors; rather, exposures can be pulsed, or experienced out of phase with one another, depending on habitat, season, or other variables. Exposure to stressors such as climate change and toxicants may be on a small or large spatiotemporal scale, static or dynamic, and the timing of responses and their duration might differ depending on the stressor. Taking these more realistic exposure scenarios into account by recreating pulsed and/or staggered exposures and including an organisms' time for a compensatory physiological response to maintain homeostasis, might result in combined effects that are additive or antagonistic, rather than synergistic, and provide more ecologically-relevant outcomes. For example, recent non-Arctic studies suggest that pesticide toxicity increases with daily temperature fluctuations as illustrated by the increased mortality and growth rate of aquatic damselfly (Ischnura elegans) larvae after chlorpyrifos exposure under conditions of fluctuating daily temperatures, but not under constant temperatures.200 Chlorpyrifos exposure under fluctuating daily temperatures also resulted in reduced energy storage (fat content) and increased enzyme activity compared to exposures under constant temperature conditions. These findings demonstrate that natural variations and fluctuations can influence responses to multiple stressors, and therefore are important considerations for both laboratory- and field-based studies.
Correspondingly, the health effects of multiple stressors, including those related to climate change, are increasingly being identified as knowledge gaps for the Arctic.3,190 Multi-stressor laboratory experiments can aid in understanding the combined effects of climate change and pollution, however, experiments specific to Arctic biota are still scarce, as organisms can be difficult to obtain due to the inaccessibility, harsh conditions, and high costs of research in the region. Although relevant POP multi-stressor laboratory studies are currently lacking, studies on PAHs can provide insight, as these petroleum-related substances share some properties of POPs and therefore behave similarly in some species.201–203
Modeling was used to evaluate the effect of multiple stressors, including climate change, pollution, and egg predation, on population dynamics and viability in the sub-Arctic common eider duck.204 Eider ducks feed low in the food web, and generally have low contaminant levels, apart from periods in the breeding season, such as late in the egg incubation period, when the female has fasted for approximately 20 days. During incubation the use of lipids for energy results in the re-mobilization of contaminants from lipid stores and a corresponding increase of contaminants in blood.205 Thus, when body condition is poor (i.e., low lipid stores), circulating contaminant levels are high. Projections of eider duck population growth and abundances using the Leslie matrix population model showed that egg predation alone was a strong enough factor to cause population extinctions, whereas an increase in sea surface temperature and reduced clutch size due to elevated contaminant exposure alone did not.204 However, any combination of the three stressors reduced the population growth rate, and the effect of pollution on clutch size was increasingly negative when co-occurring with a warming climate and increased egg predation. Population viability was lowest when all stressors occurred simultaneously. For pollution alone to be a significant stressor on the population of eider ducks through effects on clutch size, the level of exposure needed to be unrealistically high, however, when co-occurring with increased predation pressure, the threshold level needed for pollution to cause extinctions was lower.204
Field-collected data on wildlife populations have also been used to investigate the impacts of multiple stressors. In the great skua (Stercorarius skua), a seabird that breeds in the North Atlantic from Iceland and the Shetland Islands, Scotland to Svalbard, Norway, organochlorine and organobromine contaminants had negative effects on their return rate (i.e., survival) and different sensitivities to contaminant exposure were observed among breeding colonies.206 Effects occurred at lower contaminant exposure levels in breeding colonies with birds in poorer body condition, probably because of food limitations. Complex correlations among circulating PFAS congeners, total triiodothyronine, adult body mass and hatch dates, were evident in thick-billed murres breeding in Hudson Bay (Canada), and the authors concluded that the interaction of PFAAs on thyroid activity, in conjunction with several indirect effects of climate change, may cause additional stress to the murres.207 The impact of multiple stressors on contaminant levels in free-ranging wildlife has also been shown for polar bears in the marginal ice zone of the Barents Sea.183
Several studies suggest that POP exposure has adverse effects on lipid metabolism, immune function, circulating thyroid hormones and neurochemistry of polar bears,208 and climate change is likely to affect the sensitivity of polar bears to these adverse effects of pollutants. A wide range of biomarkers for energy metabolism, specifically gene transcript levels in fat, physiological parameters, and metabolomic and lipidomic markers in plasma, were investigated for associations with pollutant exposure in female polar bears from Svalbard, Norway.209 Several biomarkers involved in lipid metabolism were related to POP exposure. Furthermore, the differences between the biomarker responses of low- and highly-polluted bears were more contrasted during a period with poor sea ice conditions (Fig. 9), suggesting that contaminant exposure and sea ice decline may have compound adverse effects on polar bears.
 | ||
| Fig. 9 Transcript levels of a key regulator of lipid metabolism, peroxisome proliferator-activated receptor gamma (PPARG), in adipose tissue of low- and highly-polluted female polar bears from the Barents Sea are more contrasted during a period with poor sea ice conditions. Data from Tartu et al.209 | ||
The decline of sea ice and increased pollutant exposure may also have additive or synergistic effects on polar bear susceptibility to disease. In vitro and correlative field studies indicate that the polar bear immune system is compromised by pollutant exposure, as well as by climate change. For example, polar bears from the southern Beaufort Sea staying for extended time periods on shore had heightened immune responses and were more exposed to pathogens than bears remaining on the sea ice year-round.139,210 Also, concentrations of a stress hormone, cortisol, were higher in polar bears with low body condition, indicating that thinner polar bears were more stressed, and thus potentially more prone to adverse effects of pollutants, than fatter bears.211
The many overlapping changes occurring in the Arctic will challenge the ability of ecological communities to adapt and maintain resilience.212–214 The interaction between toxicants, climate change and other environmental changes is complex and adds to the difficulty in predicting effects in wildlife; however, the impacts observed in the Arctic environment may be able to function as early warning signals for other regions.108
E What can we learn from modeling climate change effects on food web accumulation?
Mechanistic process-oriented models can add to the understanding of how the changing contributions and variations of different parameters such as primary and secondary emission sources, transport processes, temperature, lipid dynamics, and food web structure, both alone and in combination, could affect the uptake, elimination, and overall bioaccumulation of contaminants.215 The use of mechanistic models to understand Arctic environmental distribution, food web accumulation, and human exposure to contaminants is summarized in Wania et al.216 Various models focused on predicting exposure to Arctic indigenous populations are reviewed, including those that estimate human exposure directly stemming from consumption of specific traditional food items, as well as those that include the bioaccumulation of contaminants through the Arctic food web in calculations of human exposure. Further, the authors show how models can be used to identify the range of the physicochemical properties typical of chemicals that undergo LRT to the Arctic and accumulate in the food web. Models were also used to identify drivers of the contaminant temporal trends measured in indigenous populations and to investigate the link between estimated contaminant exposure in early life stages and adult human health parameters.216The published work on mechanistic modeling of food web POP accumulation in response to climate change over the past decade is scarce and does not always apply to Arctic or polar regions. Nevertheless, the task of evaluating how climate change will affect food web POP bioaccumulation can be divided into the efforts of (i) combining abiotic contaminant transport models with biotic energetics and bioaccumulation models, and (ii) isolating the climate dimension and its contribution to bioaccumulation. The combined environmental fate and transport model, CoZMo-POP,217 and the bioaccumulation model, ACC-Human to CoZMoMAN,218,219 are good examples of models combining abiotic transport, biotic energetics, and bioaccumulation models that are built on similar fugacity-based frameworks. To identify the influence of climate change on POPs using these or similar models, a climatic mode is included in the abiotic transport model to estimate changes to contaminant transport and exposure at the base of the food web (e.g., ref. 220). This modeling framework has been used to assess the potential implications of various climate change scenarios for long-term human exposure to POPs in the Arctic.221 When modeling the most important processes contributing to future Arctic human exposure to POPs (i.e., emissions, transport, abiotic vs. biotic changes), Armitage et al.221 concluded that climate-induced changes to food web biomagnification would be most influential. Details on climate-related impacts to the physical environment that would influence contaminant exposure are found in Halsall et al.14
The AQUAWEB bioaccumulation food web model222 was used to explore how future climate scenarios of increased ambient temperatures (+2 °C and +4 °C), increased primary production (+50% and +100%), and decreased organism lipid content (−10%), would affect contaminant levels through the Arctic marine food web from copepods to seabirds.223 Due to higher predicted levels of primary production, and therefore an increase in organic particles in the water, the net overall bioaccumulation in the food web was reduced. This reduction was greatest for the most hydrophobic compounds subjected to the largest temperature increase used in the model. The reduced bioavailability of POPs predicted by the model resulted from the projected increases in primary production. However, the actual effect of climate change on primary production is uncertain, and the potential for both increased and decreased levels of primary production under future climate change scenarios have been reported.
To approximate a more realistic climate scenario, one study modeled how seasonal changes in abiotic exposure, water temperature, lipid content, and food web structure affected contaminant biomagnification within an Arctic marine food web (Fig. 10).215 The annual cycle of temperature and baseline water contaminant exposure was estimated using the Danish Eulierian Hemispheric Model (DEHM) in climate mode to compare two years a century apart (2007 and 2107).215 The AQUAWEB bioaccumulation food web model,222 parameterized for an Arctic marine food web,223 was used to model seasonally-dynamic, temperature-sensitive parameters affecting contaminant uptake and elimination, seasonal lipid content, and food web structure. The best fit between present day (2007) empirical and modeled seasonal food web biomagnification, as assessed by trophic magnification factors (TMFs), resulted primarily from the inclusion of seasonal variations in lipid content; seasonal changes in temperature, abiotic exposure, and dietary relations had only minor effects on food web biomagnification. Since food web structure did not contribute to explaining variation in seasonal food web biomagnification, it was not altered in the model scenario of future Arctic climate. Modeled food web biomagnification for the year 2107 differed only marginally from that of 2007, with magnitude and direction depending on the contaminant modeled; the mean change from 2007 to 2107 was negative for PCB-52 (−3.1%) and PCB-153 (−1.7%), and positive for γ-HCH (0.4%). Thus, the seasonal variation in food web biomagnification observed over a single year is greater than the change expected in response to a predicted future climate state, at least for the three POPs modeled. These results also emphasize the importance of understanding the different dimensions of environmental and physiological variation for interpreting the future impacts of climate change. Additionally, the model's best-fit seasonal scenario was that which included annual variations in lipid content, indicating this physiological factor is also crucial for making sound predictions of contaminant food web bioaccumulation for the future. Changes in abiotic drivers alone are not sufficient to explain food web bioaccumulation on a temporal scale. As the focus of this study was the effect of climate change on food web accumulation, the DEHM model scenarios for each year were run using the same contaminant emission levels, however, it is expected that changes in contaminant emissions will occur in the future and will have a greater influence than climate change on exposure.14,22
Models have also been used to identify the effect of climate change on food web accumulation by comparing the outcomes of different ecosystem conditions under IPCC-based future climate scenarios. In the Laurentian Great Lakes of Lake Erie and Lake Superior, four climate scenarios were contrasted by combining model parameters on bioenergetics (i.e., temperature and body-size effects on consumption and respiration) and bioaccumulation of PCB-153 for various fish species with different temperature regimes.222,224,225 The authors predicted that future climate conditions would lead to higher growth and feeding rates, and thus increased bioaccumulation. However, the specific conditions leading to higher growth differed by species; high temperatures hampered growth in cold-water species such as the native forage fish, mottled sculpin (Cottus bairdii) and the predator species, lake trout (Salvelinus namaycush), whereas low temperatures lowered the growth of warm-water species, such as the invasive forage fish, the round goby.224 If we speculate on how this translates to Arctic ecosystems, expected temperature increases would result in reduced growth and lower bioaccumulation in Arctic-resident, cold-water species, and increased growth and higher bioaccumulation in warm-water species moving north due to borealization. Note that changes in diet due to climate change-induced alterations in predator-prey interactions were not considered, and thus the effects of feeding and dietary uptake on growth rate and accumulation due to climate change are not known.
F What research tools can be used to assess the effects of multiple stressors on wildlife and ecosystem health?
The studies covered in this review have been examples of research reporting on the changes in exposure, bioaccumulation, and toxicity of single chemical or chemical groups associated with global climate change, although the exposure to multiple contaminants, in addition to multiple stressors, is a more realistic scenario (see Section D). Assessing the potential effects from these complex exposures, however, is challenging. A recent review of the biological effects of contaminants in Arctic wildlife and fish discussed different toolboxes that could potentially be used to assess the effects of toxicant mixtures and additional environmental factors on wildlife health.226 For example, risk quotient analyses, which assess links between contaminant exposures and various endocrine, reproductive or immune system effects could be used.226More commonly used indicators of risk, such as biomarkers of exposure or effects, are usually tied to a specific toxicant or specific modes and mechanisms of action. There has been a shift, however, towards realizing the need for a broader perspective to address the combined effect of multiple stressors, where integrated responses at a higher level of biological organization are addressed.227 One such response is the overall allocation and use of energy by an organism, which can be assessed using dynamic energy budgets.228–230 Typically, such integrated responses can be linked to the fitness of organisms and are easier to extrapolate to population-level effects using, for example, individual-based population models, as shown for eider ducks in Section D.204 Ideally, climate change and exposure models (see Section E) should be combined with effect models that assess the combined effects of multiple stressors on population levels. These may include models investigating changes in vital rates in response to pollutants and environmental parameters, as suggested for polar bears.208 Still, empirical studies of climate change-contaminant effects on population relevant parameters, such as survival and reproduction, are needed to support and ground-truth model projections.
With the large and growing number of contaminants present in the Arctic, there is still a need to investigate their interrelationships and the underlying structure in their variance. Although not new, statistical approaches (such as principal components analysis) are still very useful tools for condensing, and understanding the multidimensional space represented by contaminants and their drivers in the ecosystem.
Various omics methods are increasingly being used to identify changes in physiological and cellular processes due to contaminant exposure and other factors affected by climate change (e.g., sea ice extent, body condition). Combined with adverse outcome pathways that identify the sequence of cellular events leading to an adverse organism-level effect (e.g., ref. 231), these molecular-based methods hold promise for a more holistic understanding of individual and ecosystem health under multiple stressors by reflecting approaches used in human toxicology and having an integrated ‘one health’ view as goals on the horizon.
G Research conclusions, recommendations, and future perspectives
The main findings of this review article are summarized in Fig. 11 and in Table S1.† Current research suggests that climate change will impact the LRT of pollutants to the Arctic and within the Arctic by altering atmospheric, environmental, and ecological processes, and thus will influence the exposure and accumulation of POPs in Arctic wildlife. Without further action on climate change, alterations in the Arctic ecosystem are expected to continue and could impact the effectiveness of policies seeking to reduce POP exposures in Arctic people and wildlife through primary source reductions alone.G.1 Conclusions and recommendations
Temperature increases can affect the LRT of POPs to the Arctic and re-mobilization of POPs from secondary sources within the Arctic,14,22 thus potentially increasing exposure of Arctic biota to POPs. Therefore, knowledge of how sources and emissions are changing will be important for interpreting contaminant changes in Arctic wildlife. In combination with compound-specific physical–chemical properties, and species-specific biotransformation capacities, changes in environmental temperatures could affect the bioavailability of POPs for direct uptake, as well as temperature-sensitive uptake and elimination processes, especially for cold-blooded organisms. However, current findings from models suggest that climate-related changes in temperature may have minimal effects on bioaccumulation, however, empirical data is needed demonstrating the effect of temperature change on the uptake and elimination rates of POPs and CEACs with various physiochemical properties and recalcitrance depending on species-specific temperature performance curves.In terms of the influence of large-scale climate patterns on POPs in Arctic biota, currently, only a small fraction of the vast collections of climate data and POPs monitoring data that are available have been studied collectively. Of those that have, findings show that the exposure and accumulation of POPs is higher in Arctic biota following time periods with enhanced transport of air and water masses from North America and Europe toward the Arctic, but other underlying mechanisms may also be involved. Additional studies that include more species, time points, and locations (including freshwater and terrestrial ecosystems), are required for examining the relationship of meteorological parameters (i.e., wind and precipitation patterns) to biota POP concentrations; however, the use of climate indices to investigate the effects of climate change on pollutant exposure in Arctic ecosystems is not straightforward.58,232,233 The use of meta-analysis in ecotoxicology is still in its infancy, but could be applied in these studies if both monitoring programmes and individual research studies make raw data publicly available, or at least report metadata beyond its specific use in a particular study.234,235 For future studies, high-resolution spatial and temporal data and variability (e.g., local sea ice conditions, daily and seasonal temperature variations) should be considered, instead of climate variables averaged over large spatial and temporal scales, if possible. Considering the potential effects of intermittent and/or extreme weather events (e.g., rain-on-snow events) is also important as these anomalies are predicted to increase in response to a warming climate.
The melting of multi-year sea ice, just like the melting of permafrost and glaciers, may release stored contaminants into the Arctic marine food web. Although this could cause short-term increases in POP levels in biota, the eventual complete replacement of multi-year ice with annual ice, which may be less contaminated due to more frequent freeze–thaw cycles, should lead to reduced POP exposures via this pathway. Research findings available to date show generally, but not consistently, associations between sea ice condition and contaminant levels in biota, likely resulting from the influences of other species-, food web-, and ecosystem-specific factors. Currently, it is difficult to separate the direct effects of declining sea ice cover on POP levels in biota from the indirect effects resulting from changes in food web structure and function. As making robust conclusions by comparing single climate- and ecological-parameters (e.g., sea ice extent, primary productivity, dietary composition) to POP concentrations in biota can be challenging or impossible, on-going studies should strive to measure both abiotic and biotic parameters to simultaneously compare the strength of their associations with POP levels in Arctic biota. Studies should include ancillary biological and ecological data (e.g., body condition, lipid content, fatty acid profiles, stable carbon, nitrogen, and sulphur isotopes, and compound-specific isotopes (e.g., amino acid nitrogen isotopes)) to disentangle the impacts of climate-driven physical changes from those resulting from ecological or physiological effects on exposures in biota. Use of models and other research tools that account for multiple environmental and biotic variables could be useful in determining the contribution of single variables to changes in contaminant levels observed in wildlife.
Increased terrestrial runoff is predicted to affect both exposure and food web biomagnification of POPs, but there are few data to test or support this prediction. Terrestrial runoff affects nutrients, organic matter quality and quantity, and contaminants, in a complex process that influences both food web ecology and the availability and uptake of POPs and CEACs in biota. Additional experimental evidence for effects of terrestrial runoff on biota POP levels in aquatic and coastal systems is required.
Recent studies suggest that permafrost degradation and thaw can increase the amount of POPs bound to sedimentary organic matter and increase levels in amphipods in small lakes. There are knowledge gaps requiring further research to better understand the impacts of changing water quality (i.e., increasing nutrient concentrations and turbidity) on contaminant dynamics in freshwater food webs. These gaps include impacts on fish physiological conditions in lakes and rivers impacted by permafrost RTS, links between water quality and POP trends in fish from Arctic freshwater environments, and changes in water quality due to permafrost degradation and RTS. Additional studies are also needed from a wider range of lakes across the circumpolar Arctic.
Changes in seasonal timing and species phenology, e.g., migration, reproduction, and food availability, may have potential impacts on levels of POPs in Arctic biota. Many of these changes are dependent on species- and ecosystem-specific characteristics and may result in specific windows of elevated vulnerability during the year and during the organisms' life span. As the effects of phenological changes on contaminant bioaccumulation are not known, future empirical studies should assess whether further investigation of these factors is warranted. The cascading effects of changes in seasonal timing and potential mismatches in Arctic communities are unknown, warranting interdisciplinary collaborations with biologists/ecologists in future contaminant studies. At the local- and regional-levels, indigenous knowledge from hunters and fishers may provide useful data in this regard.
The net effect of increasing primary production on contaminant dynamics in Arctic food webs is unknown as predictions of future primary production and downstream impacts on dissolved versus particulate POP concentrations are uncertain. Yet, increased primary production in combination with temporal mismatches with grazers could lead to increased sedimentation and contaminant delivery to the benthic ecosystem, although changes in benthic-pelagic coupling due to the northward-range shifts of pelagic fishes may influence this. Given the potentially counteracting effects of increased primary production on contaminant dynamics (i.e., lower food web exposures via biodilution versus higher food web exposures via increased dietary intake of particulate-bound POPs), additional research on these processes should be undertaken.
With many unknowns and variable findings from different species and ecosystems, the net effect of climate change-driven alterations in species interactions on contaminant accumulation in food webs is not known. Sea ice decline may reduce the dietary importance of marine foods leading to lower intakes of POPs for some species (e.g., polar bears). Conversely, a foraging switch from Arctic- to boreal-prey species may lead to higher POP concentrations in predators if their new prey are seasonal migrants that act as contaminant biovectors into Arctic marine food webs, although the importance of this phenomenon is not fully understood. Dietary tracers and other ancillary biological and ecological data should be included in national biomonitoring programs to track changes and interpret ongoing contaminant datasets. Such data may assist in evaluating the role of changing species interactions on contaminant levels if/when trends deviate from expected patterns. Adjusting for some of these parameters will aid the interpretation of trend data. Modeling future scenarios of shifting food webs and their impacts on POP exposures in top predators should be pursued in subsequent studies once a better understanding of how climate change affects food web structure and function is developed.
Lipid amount, quality, and dynamics within organisms and ecosystems are expected to change with climate change, and thus have potential consequences for contaminants, especially lipophilic POPs. Decreased body condition can lead to increased blood levels of POPs in biota. When feasible, lipid content and body condition metrics should be routinely measured in contaminant trend studies and incorporated into data interpretations as changing lipid dynamics could introduce variability into POP temporal trends and cross-comparisons between individuals or populations. Investigation of the behavior of proteinophilic contaminants (e.g., PFASs and Hg) with changes in lean body mass may also be warranted.
Few studies have examined the consequences of behavioral changes resulting from climate change on exposure and accumulation of POPs in biota. Additional research examining the impact of behavioral changes on POP exposure is warranted. This should be done by studying relationships between pollutant exposure, movement ecology, diet, food web structure and energetics, and would benefit from multidisciplinary research teams and approaches to data collection. As behavioral and dietary changes may affect contaminant levels in biota, they may also introduce variation into long-term temporal trends that should be identified and accounted for.
Few studies have examined how climate change may interact with other stressors to affect POP toxicity in biota in the Arctic or elsewhere. An improved understanding of the effects of multiple stressors, inclusive of climate change impacts, on contaminant toxicity in Arctic biota is crucial. Knowledge of the effects of contaminants on the abundance, development, health, and behavior of wildlife populations, and the mechanisms and processes underlying these effects, is needed for toxicants individually and in combination with other stressors (i.e., climate change, pathogen and parasite exposure, etc.), as effect thresholds under these more realistic scenarios may be different from those based on exposure to a single contaminant or stressor in isolation. Protecting Arctic biota and people from the effects of POPs requires that contaminant researchers expand their scope of research and collaboration to consider POP exposures and effects within the true context of many co-occurring and potentially interacting anthropogenic and environmental stressors facing populations, communities, and ecosystems in the Arctic.
Models are important tools for evaluating the net result of contrasting processes, as climate change can lead to both increased and decreased exposures and enhanced or reduced bioaccumulation processes, depending on the processes, areas, chemicals, and species interactions affected. Mechanistic process-oriented models combining physicochemical transport models with bioenergetic and food web models, can help to understand how the variation in different parameters, such emission sources, transport processes, temperature, lipid dynamics, and food web structure, affect exposure, uptake, elimination, and overall bioaccumulation of contaminants. Nonetheless, for some of these parameters (e.g., food web changes), there remains insufficient understanding or empirical data to populate models. Models combining climate change impacts on abiotic and biotic processes affecting POPs and CEACs should be generated, as possible, and evaluated with measured data. In some cases, and related to other recommendations, additional research is required to constrain model parameters. In addition to including processes and physicochemical parameters tied to chemical emissions, transport and partitioning, more ecological realism, inclusive of variation, is needed for parameters related to ecosystem structure and functioning that may affect bioaccumulation and magnification.
G.2 Perspectives on future research and monitoring
Temporal trends of contaminants are used by the Global Monitoring Plan as a measure of the effectiveness of the Stockholm Convention on Persistent Organic Pollutants. In addition to the impact of global regulations, variations in climate parameters and trophic interactions affect these contaminant temporal trends; therefore, it is important to attribute temporal variations to the actual contributing factors to inform future regulations and policies. This review has demonstrated that there is vast diversity of direct and indirect mechanisms by which climate change can influence contaminant exposure, accumulation, and effects in ecosystems, and that ecosystem responses might vary substantially as well. This means that a broad range of habitats, species, and processes must be considered for a thorough understanding and interpretation of the consequences to the distribution, accumulation, and effects of environmental contaminants. Nonetheless, although biological and ecological diversity should be reflected in research, it is essential that studies are designed in a way to ensure they also possess adequate statistical power and data richness. Given the complex interactions between climate change, contaminants, and ecosystems, it is important to plan for long-term, integrated pan-Arctic monitoring of key biota and ecosystems, and to collect ancillary data, including information on climate-related parameters, local meteorology, ecology, and physiology, and when possible, behavior, in addition to contaminant data. To be operative, this requires a stable institutional background and the ability to integrate short-term, local efforts and resources into a coherent service.We recommend that future research identifies and characterizes the direct linkages between climate-induced changes in LRT of contaminants to the Arctic, and the exposure, accumulation, and toxicity of POPs and metals to wildlife. It will be challenging to separately identify the effects of climate change and LRT on Arctic wildlife that are also forced to cope with many other stressors that also influence the accumulation and toxicity of these chemicals and metals. However, forward progress may be made with comprehensive monitoring of Arctic ecosystems and wildlife in combination with lab-based mechanistic studies, new research methods and models that reflect the complexity of natural variation and species interactions, and risk assessments that holistically consider the effects of multiple exposures and stressors on individual and population health. As changes in the structure and function of Arctic ecosystems are not currently predictable, to project the effects of climate-driven changes in food webs on POPs in Arctic wildlife, we recommend that the contaminants community continue to work closely with other research disciplines.
Author contributions
KB and MM led the review. HR, KF, DM contributed to its initial structure, provided ongoing feedback, and wrote text sections. JG wrote text sections and contributed to figure creation. IH wrote text sections. All authors critically reviewed the final manuscript and are responsible for the contents.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This review is a result of efforts led by the Arctic Monitoring and Assessment Programme (AMAP) to produce the AMAP Assessment 2020: POPs and Chemicals of Emerging Arctic Concern; Influence of Climate Change. Studies reported in this review were supported by funding from the Norwegian Research Council, the DANCEA (Danish Cooperation for Environment in the Arctic) program, the Northern Contaminants Program (Department of Aboriginal Affairs and Northern Development Canada), and the Natural Sciences and Engineering Research Council of Canada. Additional support for the review came from the Research Council of Norway through the project ‘The Nansen Legacy’ (RCN#276730), the Canada Research Chairs Program (950-232183) and the Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grants Program (RGPIN-2019−05330). Thank you to the reviewers, including those that peer-reviewed the chapter from the AMAP report, which formed the basis of this journal article. Thanks also to the many Indigenous hunters, Arctic organizations, and colleagues that contributed to sample collections, data, and analysis for the studies reviewed in the present article.References
- AMAP, AMAP Assessment 2002: the Influence of Global Change on Contaminant Pathways to, within, and from the Arctic, Arctic Monitoring and Assessment Programme (AMAP), 2003 Search PubMed.
- IPCC, Technical Summary: IPCC Special Report on the Ocean and Cryosphere in a Changing Climate, 2019 Search PubMed.
- U. AMAP, Climate Change and POPs: Predicting the Impacts, 2011 Search PubMed.
- AMAP, AMAP Assessment Report: Arctic Pollution Issues, Arctic Monitoring and Assessment Programme (AMAP), 1998 Search PubMed.
- K. Borgå, A. T. Fisk, P. F. Hoekstra and D. C. Muir, Biological and chemical factors of importance in the bioaccumulation and trophic transfer of persistent organochlorine contaminants in arctic marine food webs, Environ. Toxicol. Chem., 2004, 23, 2367–2385 CrossRef.
- M. A. McKinney, S. Pedro, R. Dietz, C. Sonne, A. T. Fisk, D. Roy, B. M. Jenssen and R. J. Letcher, A review of ecological impacts of global climate change on persistent organic pollutant and mercury pathways and exposures in arctic marine ecosystems, Curr. Zool., 2015, 61, 617–628 CrossRef.
- K. Borgå, A. T. Fisk, P. F. Hoekstra and D. C. G. Muir, Biological and chemical factors of importance in the bioaccumulation and trophic transfer of persistent organochlorine contaminants in arctic marine food webs, Environ. Toxicol. Chem., 2004, 23, 2367–2385 CrossRef PubMed.
- D. K. Perovich and J. A. Richter-Menge, Loss of sea ice in the Arctic, Ann. Rev. Mar. Sci, 2009, 1, 417–441 CrossRef PubMed.
- E. Post, M. C. Forchhammer, M. S. Bret-Harte, T. V. Callaghan, T. R. Christensen, B. Elberling, A. D. Fox, O. Gilg, D. S. Hik and T. T. Høye, Ecological dynamics across the Arctic associated with recent climate change, science, 2009, 325, 1355–1358 CrossRef CAS PubMed.
- E. Post, U. S. Bhatt, C. M. Bitz, J. F. Brodie, T. L. Fulton, M. Hebblewhite, J. Kerby, S. J. Kutz, I. Stirling and D. A. Walker, Ecological consequences of sea-ice decline, Science, 2013, 341, 519–524 CrossRef CAS PubMed.
- M. Fossheim, R. Primicerio, E. Johannesen, R. B. Ingvaldsen, M. M. Aschan and A. V. Dolgov, Recent warming leads to a rapid borealization of fish communities in the Arctic, Nat. Clim. Change, 2015, 5, 673–677 CrossRef.
- G. T. Pecl, M. B. Araújo, J. D. Bell, J. Blanchard, T. C. Bonebrake, I.-C. Chen, T. D. Clark, R. K. Colwell, F. Danielsen and B. Evengård, Biodiversity redistribution under climate change: Impacts on ecosystems and human well-being, Science, 2017, 355, eaai9214 CrossRef.
- C. A. De Wit, D. C. G. Muir and K. Vorkamp, Influence of climate change on persistent organic pollutants and chemicals of emerging concern in the Arctic: State of knowledge and recommendations for future research, Environ. Sci.: Processes Impacts, 2022 10.1039/D1EM00531F.
- H. Hung, C. Halsall, H. Ball, T. Bidleman, J. Dachs, A. De Silva, M. Hermanson, R. Kallenborn, D. C. G. Muir, R. Sühring, X. Wang and S. Wilson, Climate change influence on the levels and trends of persistent organic pollutants (POPs) and chemicals of emerging Arctic concern (CEACs) in the Arctic physical environment – a review, Environ. Sci.: Processes Impacts, 2022 Search PubMed , in press.
- K. Vorkamp, P. Carlsson, S. Corsolini, C. A. de Wit, R. Dietz, M. O. Gribble, M. Houde, V. Kalia, R. J. Letcher, A. Morris, F. F. Rigét, H. Routti and D. C. G. Muir, Influences of climate change on long-term time series of persistent organic pollutants (POPs) in Arctic and Antarctic biota, Environ. Sci.: Processes Impacts, 2022 Search PubMed , in press.
- AMAP, Snow, Water, Ice and Permafrost in the Arctic (SWIPA) 2017, Arctic Monitoring and Asessment Programme (AMAP), 2017, p. 269 Search PubMed.
- J. J. Alava, W. W. Cheung, P. S. Ross and U. R. Sumaila, Climate change–contaminant interactions in marine food webs: Toward a conceptual framework, Global Change Biol., 2017, 23, 3984–4001 CrossRef PubMed.
- P. D. Noyes and S. C. Lema, Forecasting the impacts of chemical pollution and climate change interactions on the health of wildlife, Curr. Zool., 2015, 61, 669–689 CrossRef.
- T. Gouin, J. M. Armitage, I. T. Cousins, D. C. Muir, C. A. Ng, L. Reid and S. Tao, Influence of global climate change on chemical fate and bioaccumulation: The role of multimedia models, Environ. Toxicol. Chem., 2013, 32, 20–31 CrossRef CAS PubMed.
- K. Borgå, T. M. Saloranta and A. Ruus, Simulating climate change-induced alterations in bioaccumulation of organic contaminants in an Arctic marine food web, Environ. Toxicol. Chem., 2010, 29, 1349–1357 CrossRef PubMed.
- L. Lamon, H. Von Waldow, M. MacLeod, M. Scheringer, A. Marcomini and K. Hungerbühler, Modeling the global levels and distribution of polychlorinated biphenyls in air under a climate change scenario, Environ. Sci. Technol., 2009, 43, 5818–5824 CrossRef CAS PubMed.
- P. Bartlett, M. MacLeod, I. Cousins, C. Friedman, K. Mantzius Hansen, A. Gusev, G. Lammel, L. Li, Y. Lu, J. Ma and M. Muntean, Modeling emissions and long-range transport of POPs and CEACs under climate change, Environ. Sci.: Processes Impacts, 2022 Search PubMed , in press.
- J. Ma, H. Hung, C. Tian and R. Kallenborn, Revolatilization of persistent organic pollutants in the Arctic induced by climate change, Nat. Clim. Change, 2011, 1, 255–260 CrossRef CAS.
- J. Ma, H. Hung and R. W. Macdonald, The influence of global climate change on the environmental fate of persistent organic pollutants: A review with emphasis on the Northern Hemisphere and the Arctic as a receptor, Global Planet. Change, 2016, 146, 89–108 CrossRef.
- A. Cabrerizo, D. C. Muir, G. n. Köck, D. Iqaluk and X. Wang, Climatic influence on temporal trends of polychlorinated biphenyls and organochlorine pesticides in landlocked char from lakes in the Canadian High Arctic, Environ. Sci. Technol., 2018, 52, 10380–10390 CrossRef CAS PubMed.
- H. Hung, A. A. Katsoyiannis, E. Brorström-Lundén, K. Olafsdottir, W. Aas, K. Breivik, P. Bohlin-Nizzetto, A. Sigurdsson, H. Hakola and R. Bossi, Temporal trends of Persistent Organic Pollutants (POPs) in arctic air: 20 years of monitoring under the Arctic Monitoring and Assessment Programme (AMAP), Environ. Pollut., 2016, 217, 52–61 CrossRef CAS PubMed.
- F. Rigét, A. Bignert, B. Braune, M. Dam, R. Dietz, M. Evans, N. Green, H. Gunnlaugsdóttir, K. S. Hoydal and J. Kucklick, Temporal trends of persistent organic pollutants in Arctic marine and freshwater biota, Sci. Total Environ., 2019, 649, 99–110 CrossRef PubMed.
- R. Macdonald, T. Harner and J. Fyfe, Recent climate change in the Arctic and its impact on contaminant pathways and interpretation of temporal trend data, Sci. Total Environ., 2005, 342, 5–86 CrossRef CAS.
- J. O. Bustnes, G. W. Gabrielsen and J. Verreault, Climate variability and temporal trends of persistent organic pollutants in the Arctic: a study of glaucous gulls, Environ. Sci. Technol., 2010, 44, 3155–3161 CrossRef CAS PubMed.
- M. Houde, X. Wang, T.-L. Colson, P. Gagnon, S. Ferguson, M. Ikonomou, C. Dubetz, R. Addison and D. Muir, Trends of persistent organic pollutants in ringed seals (Phoca hispida) from the Canadian Arctic, Sci. Total Environ., 2019, 665, 1135–1146 CrossRef CAS PubMed.
- F. Rigét, K. Vorkamp, K. A. Hobson, D. C. Muir and R. Dietz, Temporal trends of selected POPs and the potential influence of climate variability in a Greenland ringed seal population, Environ. Sci.: Processes Impacts, 2013, 15, 1706–1716 RSC.
- K. L. Foster, B. M. Braune, A. J. Gaston and M. L. Mallory, Climate influence on legacy organochlorine pollutants in Arctic seabirds, Environ. Sci. Technol., 2019, 53, 2518–2528 CrossRef CAS PubMed.
- I. V. Polyakov, A. V. Pnyushkov, M. B. Alkire, I. M. Ashik, T. M. Baumann, E. C. Carmack, I. Goszczko, J. Guthrie, V. V. Ivanov and T. Kanzow, Greater role for Atlantic inflows on sea-ice loss in the Eurasian Basin of the Arctic Ocean, Science, 2017, 356, 285–291 CrossRef CAS PubMed.
- Y. Ma, D. A. Adelman, E. Bauerfeind, A. Cabrerizo, C. A. McDonough, D. Muir, T. Soltwedel, C. Sun, C. C. Wagner, E. M. Sunderland and R. Lohmann, Concentrations and water mass transport of legacy POPs in the Arctic Ocean, Geophys. Res. Lett., 2018, 45, 12972–12981 CAS.
- P. Carlsson, N. A. Warner, I. G. Hallanger, D. Herzke and R. Kallenborn, Spatial and temporal distribution of chiral pesticides in Calanus spp. from three Arctic fjords, Environ. Pollut., 2014, 192, 154–161 CrossRef CAS PubMed.
- M. Pućko, G. A. Stern, R. W. Macdonald, L. M. Jantunen, T. F. Bidleman, F. Wong, D. G. Barber and S. Rysgaard, The delivery of organic contaminants to the Arctic food web: Why sea ice matters, Sci. Total Environ., 2015, 506, 444–452 CrossRef PubMed.
- A. Gaden, S. H. Ferguson, L. Harwood, H. Melling, J. Alikamik and G. Stern, Western Canadian Arctic ringed seal organic contaminant trends in relation to sea ice break-up, Environ. Sci. Technol., 2012, 46, 4427–4433 CrossRef CAS PubMed.
- H. Routti, J. Aars, E. Fuglei, L. Hanssen, K. Lone, A. Polder, Å. Ø. Pedersen, S. Tartu, J. M. Welker and N. G. Yoccoz, Emission changes dwarf the influence of feeding habits on temporal trends of per-and polyfluoroalkyl substances in two Arctic top predators, Environ. Sci. Technol., 2017, 51, 11996–12006 CrossRef CAS PubMed.
- S. Tartu, S. Bourgeon, J. Aars, M. Andersen, K. Lone, B. M. Jenssen, A. Polder, G. W. Thiemann, V. Torget and J. M. Welker, Diet and metabolic state are the main factors determining concentrations of perfluoroalkyl substances in female polar bears from Svalbard, Environ. Pollut., 2017, 229, 146–158 CrossRef CAS PubMed.
- S. Tartu, J. Aars, M. Andersen, A. Polder, S. Bourgeon, B. Merkel, A. D. Lowther, J. Bytingsvik, J. M. Welker and A. E. Derocher, Choose your poison—space-use strategy influences pollutant exposure in Barents Sea polar bears, Environ. Sci. Technol., 2018, 52, 3211–3221 CrossRef CAS PubMed.
- P. Blévin, J. Aars, M. Andersen, M.-A. Blanchet, L. Hanssen, D. Herzke, R. M. Jeffreys, E. S. Nordøy, M. Pinzone and C. de la Vega, Pelagic vs Coastal—Key Drivers of Pollutant Levels in Barents Sea Polar Bears with Contrasted Space-Use Strategies, Environ. Sci. Technol., 2019, 54, 985–995 CrossRef.
- G. Codling, C. Halsall, L. Ahrens, S. Del Vento, K. Wiberg, M. Bergknut, H. Laudon and R. Ebinghaus, The fate of per-and polyfluoroalkyl substances within a melting snowpack of a boreal forest, Environ. Pollut., 2014, 191, 190–198 CrossRef CAS.
- J. E. Søreide, E. V. Leu, J. Berge, M. Graeve and S. Falk-Petersen, Timing of blooms, algal food quality and Calanus glacialis reproduction and growth in a changing Arctic, Global Change Biol., 2010, 16, 3154–3163 Search PubMed.
- K. Y. Kwok, E. Yamazaki, N. Yamashita, S. Taniyasu, M. B. Murphy, Y. Horii, G. Petrick, R. Kallerborn, K. Kannan and K. Murano, Transport of perfluoroalkyl substances (PFAS) from an arctic glacier to downstream locations: implications for sources, Sci. Total Environ., 2013, 447, 46–55 CrossRef CAS.
- S. Tartu, S. Bourgeon, J. Aars, M. Andersen, A. Polder, G. W. Thiemann, J. M. Welker and H. Routti, Sea ice-associated decline in body condition leads to increased concentrations of lipophilic pollutants in polar bears (Ursus maritimus) from Svalbard, Norway, Sci. Total Environ., 2017, 576, 409–419 CrossRef CAS.
- M. S. Andersen, E. Fuglei, M. König, I. Lipasti, Å. Ø. Pedersen, A. Polder, N. G. Yoccoz and H. Routti, Levels and temporal trends of persistent organic pollutants (POPs) in arctic foxes (Vulpes lagopus) from Svalbard in relation to dietary habits and food availability, Sci. Total Environ., 2015, 511, 112–122 CrossRef CAS PubMed.
- N. Dupont and D. L. Aksnes, Centennial changes in water clarity of the Baltic Sea and the North Sea, Estuarine, Coastal Shelf Sci., 2013, 131, 282–289 CrossRef CAS.
- M. Wauthy, M. Rautio, K. S. Christoffersen, L. Forsström, I. Laurion, H. L. Mariash, S. Peura and W. F. Vincent, Increasing dominance of terrigenous organic matter in circumpolar freshwaters due to permafrost thaw, Limnol. Oceanogr. Lett., 2018, 3, 186–198 CrossRef CAS.
- M. Ripszam, J. Paczkowska, J. Figueira, C. Veenaas and P. Haglund, Dissolved organic carbon quality and sorption of organic pollutants in the Baltic Sea in light of future climate change, Environ. Sci. Technol., 2015, 49, 1445–1452 CrossRef CAS PubMed.
- A. Andersson, S. Brugel, J. Paczkowska, O. F. Rowe, D. Figueroa, S. Kratzer and C. Legrand, Influence of allochthonous dissolved organic matter on pelagic basal production in a northerly estuary, Estuarine, Coastal Shelf Sci., 2018, 204, 225–235 CrossRef CAS.
- S. Jonsson, A. Andersson, M. B. Nilsson, U. Skyllberg, E. Lundberg, J. K. Schaefer, S. Åkerblom and E. Björn, Terrestrial discharges mediate trophic shifts and enhance methylmercury accumulation in estuarine biota, Sci. Adv., 2017, 3, e1601239 CrossRef PubMed.
- D. L. Aksnes, N. Dupont, A. Staby, Ø. Fiksen, S. Kaartvedt and J. Aure, Coastal water darkening and implications for mesopelagic regime shifts in Norwegian fjords, Mar. Ecol.: Prog. Ser., 2009, 387, 39–49 CrossRef CAS.
- F. Wania, J. T. Hoff, C. Q. Jia and D. Mackay, The effects of snow and ice on the environmental behaviour of hydrophobic organic chemicals, Environ. Pollut., 1998, 102, 25–41 CrossRef CAS.
- P. Casal, A. Cabrerizo, M. Vila-Costa, M. Pizarro, B. a. Jiménez and J. Dachs, Pivotal role of snow deposition and melting driving fluxes of polycyclic aromatic hydrocarbons at Coastal Livingston Island (Antarctica), Environ. Sci. Technol., 2018, 52, 12327–12337 CrossRef CAS PubMed.
- P. Casal, G. Casas, M. Vila-Costa, A. Cabrerizo, M. Pizarro, B. Jiménez and J. Dachs, Snow Amplification of Persistent Organic Pollutants at Coastal Antarctica, Environ. Sci. Technol., 2019, 53, 8872–8882 CrossRef CAS PubMed.
- O. Garmash, M. H. Hermanson, E. Isaksson, M. Schwikowski, D. Divine, C. Teixeira and D. C. Muir, Deposition history of polychlorinated biphenyls to the Lomonosovfonna glacier, Svalbard: a 209 congener analysis, Environ. Sci. Technol., 2013, 47, 12064–12072 CrossRef CAS.
- I. Lehnherr, V. L. St. Louis, M. Sharp, A. S. Gardner, J. P. Smol, S. L. Schiff, D. C. G. Muir, C. A. Mortimer, N. Michelutti, C. Tarnocai, K. A. St. Pierre, C. A. Emmerton, J. A. Wiklund, G. Köck, S. F. Lamoureux and C. H. Talbot, The world's largest High Arctic lake responds rapidly to climate warming, Nat. Commun., 2018, 9, 1290 CrossRef.
- M. H. Hermanson, E. Isaksson, S. Forsström, C. Teixeira, D. C. G. Muir, V. A. Pohjola and R. S. V. van de Wal, Deposition History of Brominated Flame Retardant Compounds in an Ice Core from Holtedahlfonna, Svalbard, Norway, Environ. Sci. Technol., 2010, 44, 7405–7410 CrossRef CAS PubMed.
- E. Skogsberg, Master thesis, University of Oslo 2019.
- M. McGovern, A. Evenset, K. Borgå, H. A. de Wit, H. F. V. Braaten, D. O. Hessen, S. Schultze, A. Ruus and A. Poste, Implications of Coastal Darkening for Contaminant Transport, Bioavailability, and Trophic Transfer in Northern Coastal Waters, Environ. Sci. Technol., 2019, 53, 7180–7182 CrossRef CAS PubMed.
- F. J. Wrona, J. D. Reist, P.-A. Amundsen, P. A. Chambers, K. Christoffersen, J. M. Culp, P. D. di Cenzo, L. Forsström, J. Hammar, R. K. Heikkinen, J. Heino, K. K. Kahilainen, H. Lehtonen, J. Lento, L. Lesack, M. Luoto, D. J. Marcogliese, P. Marsh, P. A. Moquin, T. Mustonen, M. Power, T. D. Prowse, M. Rautio, H. K. Swanson, M. Thompson, H. Toivonen, V. Vasiliev, R. Virkkala and S. Zavalko, Freshwater ecosystems, Arctic Biodiversity Assessment: Status and Trends in Arctic Biodiversity, Conservation of Arctic Flora and Fauna, Akureyri, 2013, pp. 444–485 Search PubMed.
- A. Bring, I. Fedorova, Y. Dibike, L. Hinzman, J. Mård, S. Mernild, T. Prowse, O. Semenova, S. L. Stuefer and M. K. Woo, Arctic terrestrial hydrology: A synthesis of processes, regional effects, and research challenges, J. Geophys. Res.: Biogeosci., 2016, 121, 621–649 CrossRef.
- T. Prowse, A. Bring, J. Mård and E. Carmack, Arctic freshwater synthesis: Introduction, J. Geophys. Res.: Biogeosci., 2015, 120, 2121–2131 CrossRef.
- J. E. Vonk, S. E. Tank, W. B. Bowden, I. Laurion, W. F. Vincent, P. Alekseychik, M. Amyot, M. Billet, J. Canário and R. M. Cory, Reviews and syntheses: Effects of permafrost thaw on Arctic aquatic ecosystems, Biogeosciences, 2015, 12, 7129–7167 CrossRef CAS.
- T. Šmejkalová, M. E. Edwards and J. Dash, Arctic lakes show strong decadal trend in earlier spring ice-out, Sci. Rep., 2016, 6, 1–8 CrossRef PubMed.
- F. Riget, E. Jeppesen, F. Landkildehus, T. Lauridsen, P. Geertz-Hansen, K. Christoffersen and H. Sparholt, Landlocked Arctic charr (Salvelinus alpinus) population structure and lake morphometry in Greenland–is there a connection?, Polar Biol., 2000, 23, 550–558 CrossRef.
- A. E. Hershey, S. Beaty, K. Fortino, M. Keyse, P. Mou, W. O'brien, A. Ulseth, G. Gettel, P. Lienesch and C. Luecke, Effect of landscape factors on fish distribution in arctic Alaskan lakes, Freshwater Biol., 2006, 51, 39–55 CrossRef.
- A. E. Hershey, G. M. Gettel, M. E. McDonald, M. C. Miller, H. Mooers, W. J. O'Brien, J. Pastor, C. Richards and J. A. Schuldt, A geomorphic–trophic model for landscape control of Arctic lake food webs, BioScience, 1999, 49, 887–897 CrossRef.
- ACIA, Arctic Climate Impact Assessment (ACIA), Cambridge University Press Cambridge, 2005 Search PubMed.
- C. R. Burn and A. Lewkowicz, Canadian landform examples-17 retrogressive thaw slumps, Canadian Geographer/Le Géographe canadien, 1990, 34, 273–276 CrossRef.
- J. R. Thienpont, K. M. Ruehland, M. F. Pisaric, S. V. Kokelj, L. E. Kimpe, J. M. Blais and J. P. Smol, Biological responses to permafrost thaw slumping in Canadian Arctic lakes, Freshwater Biol., 2013, 58, 337–353 CrossRef.
- S. Kokelj, R. Jenkins, D. Milburn, C. R. Burn and N. Snow, The influence of thermokarst disturbance on the water quality of small upland lakes, Mackenzie Delta region, Northwest Territories, Canada, Permafr. Periglac. Process., 2005, 16, 343–353 CrossRef.
- M. S. Thompson, F. J. Wrona and T. D. Prowse, Shifts in plankton, nutrient and light relationships in small tundra lakes caused by localized permafrost thaw, Arctic, 2012, 367–376 Search PubMed.
- A. J. Houben, T. D. French, S. V. Kokelj, X. Wang, J. P. Smol and J. M. Blais, The impacts of permafrost thaw slump events on limnological variables in upland tundra lakes, Mackenzie Delta region, Fundam. Appl. Limnol., 2016, 189, 11–35 CrossRef.
- D. C. Eickmeyer, L. E. Kimpe, S. V. Kokelj, M. F. Pisaric, J. P. Smol, H. Sanei, J. R. Thienpont and J. M. Blais, Interactions of polychlorinated biphenyls and organochlorine pesticides with sedimentary organic matter of retrogressive thaw slump-affected lakes in the tundra uplands adjacent to the Mackenzie Delta, NT, Canada, J. Geophys. Res.: Biogeosci., 2016, 121, 411–421 CrossRef CAS.
- R. D'Onofrio, Effects of permafrost thaw slumps on benthic invertebrates and on concentrations of persistent organic pollutants in lakes of the Mackenzie Delta Uplands, N.T., MSc thesis, Dept. of Biology, University of Ottawa, Ontario, 2014.
- B. W. Abbott, J. B. Jones, S. E. Godsey, J. R. Larouche and W. B. Bowden, Patterns and persistence of hydrologic carbon and nutrient export from collapsing upland permafrost, Biogeosciences, 2015, 12, 3725–3740 CrossRef.
- Ø. Varpe, Life history adaptations to seasonality, Integr. Comp. Biol., 2017, 57, 943–960 CrossRef PubMed.
- J. Baert, C. Janssen, K. Borga and F. De Laender, Migration and opportunistic feeding increase PCB accumulation in Arctic seabirds, Environ. Sci. Technol., 2013, 47, 11793–11801 CrossRef CAS PubMed.
- S. Bourgeon, E. K. Leat, R. W. Furness, K. Borgå, S. A. Hanssen and J. O. Bustnes, Dietary versus maternal sources of organochlorines in top predator seabird chicks: an experimental approach, Environ. Sci. Technol., 2013, 47, 5963–5970 CrossRef CAS PubMed.
- E. H. Leat, S. Bourgeon, E. Magnusdottir, G. W. Gabrielsen, W. J. Grecian, S. A. Hanssen, K. Olafsdottir, A. Petersen, R. A. Phillips and H. Strøm, Influence of wintering area on persistent organic pollutants in a breeding migratory seabird, Mar. Ecol.: Prog. Ser., 2013, 491, 277–293 CrossRef CAS.
- D. D. Hauser, K. L. Laidre, K. M. Stafford, H. L. Stern, R. S. Suydam and P. R. Richard, Decadal shifts in autumn migration timing by Pacific Arctic beluga whales are related to delayed annual sea ice formation, Global Change Biol., 2017, 23, 2206–2217 CrossRef PubMed.
- M. L. Druckenmiller, J. J. Citta, M. C. Ferguson, J. T. Clarke, J. C. George and L. Quakenbush, Trends in sea-ice cover within bowhead whale habitats in the Pacific Arctic, Deep Sea Res., Part II, 2018, 152, 95–107 CrossRef.
- N. Saino, R. Ambrosini, D. Rubolini, J. von Hardenberg, A. Provenzale, K. Hüppop, O. Hüppop, A. Lehikoinen, E. Lehikoinen and K. Rainio, Climate warming, ecological mismatch at arrival and population decline in migratory birds, Proc. R. Soc. B, 2011, 278, 835–842 CrossRef PubMed.
- K. Jaatinen, M. Öst and K. A. Hobson, State-dependent capital and income breeding: a novel approach to evaluating individual strategies with stable isotopes, Front. Zool., 2016, 13, 1–8 CrossRef PubMed.
- D. J. Hitchcock, T. Andersen, Ø. Varpe, M. J. Loonen, N. A. Warner, D. Herzke, I. M. Tombre, L. R. Griffin, P. Shimmings and K. Borgå, Potential effect of migration strategy on pollutant occurrence in eggs of Arctic breeding barnacle geese (Branta leucopsis), Environ. Sci. Technol., 2019, 53, 5427–5435 CrossRef CAS PubMed.
- I. G. Hallanger, A. Ruus, D. Herzke, N. A. Warner, A. Evenset, E. S. Heimstad, G. W. Gabrielsen and K. Borgå, Influence of season, location, and feeding strategy on bioaccumulation of halogenated organic contaminants in Arctic marine zooplankton, Environ. Toxicol. Chem., 2011, 30, 77–87 CrossRef CAS PubMed.
- I. G. Hallanger, A. Ruus, N. A. Warner, D. Herzke, A. Evenset, M. Schøyen, G. W. Gabrielsen and K. Borgå, Differences between Arctic and Atlantic fjord systems on bioaccumulation of persistent organic pollutants in zooplankton from Svalbard, Sci. Total Environ., 2011, 409, 2783–2795 CrossRef CAS PubMed.
- I. G. Hallanger, N. A. Warner, A. Ruus, A. Evenset, G. Christensen, D. Herzke, G. W. Gabrielsen and K. Borgå, Seasonality in contaminant accumulation in Arctic marine pelagic food webs using trophic magnification factor as a measure of bioaccumulation, Environ. Toxicol. Chem., 2011, 30, 1026–1035 CrossRef CAS PubMed.
- B. T. Hargrave, L. A. Barrie, T. F. Bidleman and H. E. Welch, Seasonality in exchange of organochlorines between Arctic air and seawater, Environ. Sci. Technol., 1997, 31, 3258–3266 CrossRef CAS.
- R. Lohmann, R. Gioia, K. C. Jones, L. Nizzetto, C. Temme, Z. Xie, D. Schulz-Bull, I. Hand, E. Morgan and L. Jantunen, Organochlorine pesticides and PAHs in the surface water and atmosphere of the North Atlantic and Arctic Ocean, Environ. Sci. Technol., 2009, 43, 5633–5639 CrossRef CAS PubMed.
- K. Borgå, G. W. Gabrielsen, J. U. Skaare, L. Kleivane, R. J. Norstrom and A. T. Fisk, Why do organochlorine differences between arctic regions vary among trophic levels?, Environ. Sci. Technol., 2005, 39, 4343–4352 CrossRef PubMed.
- IPCC, Climate change 2013: The physical science basis Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, ed. T. F. Stocker, D. Qin, G. Plattner, M. Tignor, S. Allen, J. Boschung, A. Nauels, Y. Xia, V. Bex and P. Midgley, Intergovernmental Panel on Climate Change (IPCC), Geneva, Switzerland, 2013, p. 1535 Search PubMed.
- N. W. van den Brink, M. J. Riddle, M. van den Heuvel-Greve and J. A. van Franeker, Contrasting time trends of organic contaminants in Antarctic pelagic and benthic food webs, Mar. Pollut. Bull., 2011, 62, 128–132 CrossRef CAS PubMed.
- C. C. Wagner, H. M. Amos, C. P. Thackray, Y. Zhang, E. W. Lundgren, G. Forget, C. L. Friedman, N. E. Selin, R. Lohmann and E. M. Sunderland, A global 3-D ocean model for PCBs: Benchmark compounds for understanding the impacts of global change on neutral persistent organic pollutants, Global Biogeochem. Cycles, 2019, 33, 469–481 CrossRef CAS.
- B. T. Hargrave, G. A. Phillips, W. P. Vass, P. Bruecker, H. E. Welch and T. D. Siferd, Seasonality in bioaccumulation of organochlorines in lower trophic level arctic marine biota, Environ. Sci. Technol., 2000, 34, 980–987 CrossRef CAS.
- A. T. Fisk, P. F. Hoekstra, J.-M. Gagnon, J. Duffe, R. J. Norstrom, K. A. Hobson, M. Kwan and D. C. Muir, Influence of habitat, trophic ecology and lipids on, and spatial trends of, organochlorine contaminants in Arctic marine invertebrates, Mar. Ecol.: Prog. Ser., 2003, 262, 201–214 CrossRef CAS.
- L. Nizzetto, M. Macleod, K. Borgå, A. Cabrerizo, J. Dachs, A. D. Guardo, D. Ghirardello, K. M. Hansen, A. Jarvis, A. Lindroth, B. Luwig, D. Monteith, J. A. Perlinger, M. Scheringer, L. Schwendenmann, K. T. Semple, L. Y. Wick, G. Zhang and K. C. Jones, Past, present, and future controls on levels of persistent organic pollutants in the global environment, Environ. Sci. Technol., 2010, 44, 6526–6531 CrossRef CAS PubMed.
- J. Carrie, F. Wang, H. Sanei, R. Macdonald, P. Outridge and G. Stern, Increasing contaminant burdens in an Arctic fish, burbot (Lota lota), in a warming climate, Environ. Sci. Technol., 2010, 44, 316–322 CrossRef CAS PubMed.
- I.-C. Chen, J. K. Hill, R. Ohlemüller, D. B. Roy and C. D. Thomas, Rapid range shifts of species associated with high levels of climate warming, Science, 2011, 333, 1024–1026 CrossRef CAS PubMed.
- E. S. Poloczanska, C. J. Brown, W. J. Sydeman, W. Kiessling, D. S. Schoeman, P. J. Moore, K. Brander, J. F. Bruno, L. B. Buckley and M. T. Burrows, Global imprint of climate change on marine life, Nat. Clim. Change, 2013, 3, 919–925 CrossRef.
- W. W. Cheung, V. W. Lam, J. L. Sarmiento, K. Kearney, R. Watson and D. Pauly, Projecting global marine biodiversity impacts under climate change scenarios, Fish Fish., 2009, 10, 235–251 CrossRef.
- J. W. Williams, S. T. Jackson and J. E. Kutzbach, Projected distributions of novel and disappearing climates by 2100 AD, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 5738–5742 CrossRef CAS PubMed.
- M. C. Urban, J. J. Tewksbury and K. S. Sheldon, On a collision course: competition and dispersal differences create no-analogue communities and cause extinctions during climate change, Proc. R. Soc. B, 2012, 279, 2072–2080 CrossRef PubMed.
- L. Oziel, A. Baudena, M. Ardyna, P. Massicotte, A. Randelhoff, J.-B. Sallée, R. B. Ingvaldsen, E. Devred and M. Babin, Faster Atlantic currents drive poleward expansion of temperate phytoplankton in the Arctic Ocean, Nat. Commun., 2020, 11, 1–8 Search PubMed.
- P. L. Zarnetske, D. K. Skelly and M. C. Urban, Biotic multipliers of climate change, Science, 2012, 336, 1516–1518 CrossRef CAS PubMed.
- M. A. McKinney, R. J. Letcher, J. Aars, E. W. Born, M. Branigan, R. Dietz, T. J. Evans, G. W. Gabrielsen, D. C. Muir and E. Peacock, Regional contamination versus regional dietary differences: understanding geographic variation in brominated and chlorinated contaminant levels in polar bears, Environ. Sci. Technol., 2011, 45, 896–902 CrossRef CAS PubMed.
- K. Borgå, The Arctic ecosystem: A canary in the coal mine for global multiple stressors, Environ. Toxicol. Chem., 2019, 38, 487–488 CrossRef PubMed.
- M. C. Urban, P. L. Zarnetske and D. K. Skelly, Searching for biotic multipliers of climate change, Integr. Comp. Biol., 2017, 57, 134–147 CrossRef PubMed.
- S. Pedro, A. T. Fisk, G. T. Tomy, S. H. Ferguson, N. E. Hussey, S. T. Kessel and M. A. McKinney, Mercury and persistent organic pollutants in native and invading forage species of the canadian arctic: Consequences for food web dynamics, Environ. Pollut., 2017, 229, 229–240 CrossRef CAS PubMed.
- M. A. McKinney, B. C. McMeans, G. T. Tomy, B. Rosenberg, S. H. Ferguson, A. Morris, D. C. Muir and A. T. Fisk, Trophic transfer of contaminants in a changing arctic marine food web: Cumberland Sound, Nunavut, Canada, Environ. Sci. Technol., 2012, 46, 9914–9922 CrossRef CAS PubMed.
- A. D. Morris, D. C. Muir, K. R. Solomon, R. J. Letcher, M. A. McKinney, A. T. Fisk, B. C. McMeans, G. T. Tomy, C. Teixeira and X. Wang, Current-use pesticides in seawater and their bioaccumulation in polar bear–ringed seal food chains of the Canadian Arctic, Environ. Toxicol. Chem., 2016, 35, 1695–1707 CrossRef CAS PubMed.
- P. S. Ross, G. M. Ellis, M. G. Ikonomou, L. G. Barrett-Lennard and R. F. Addison, High PCB concentrations in free-ranging Pacific killer whales, Orcinus orca: Effects of age, sex and dietary preference, Mar. Pollut. Bull., 2000, 40, 504–515 CrossRef CAS.
- J. W. Higdon, D. D. Hauser and S. H. Ferguson, Killer whales (Orcinus orca) in the Canadian Arctic: distribution, prey items, group sizes, and seasonality, Mar. Mammal Sci., 2012, 28, E93–E109 CrossRef.
- J. W. Higdon, K. H. Westdal and S. H. Ferguson, Distribution and abundance of killer whales (Orcinus orca) in Nunavut, Canada—an Inuit knowledge survey, J. Mar. Biol. Assoc. U. K., 2014, 94, 1293–1304 CrossRef.
- J. Clarke, K. Stafford, S. E. Moore, B. Rone, L. Aerts and J. Crance, Subarctic Cetaceans in the Southern Chukchi Sea, Oceanography, 2013, 26, 136–149 CrossRef.
- S. H. Ferguson, M. C. Kingsley and J. W. Higdon, Killer whale (Orcinus orca) predation in a multi-prey system, Popul. Ecol., 2012, 54, 31–41 CrossRef.
- N. Reinhart, S. Ferguson, W. Koski, J. Higdon, B. LeBlanc, O. Tervo and P. Jepson, Occurrence of killer whale Orcinus orca rake marks on Eastern Canada-West Greenland bowhead whales Balaena mysticetus, Polar Biol., 2013, 36, 1133–1146 CrossRef.
- J. Bourque, R. Dietz, C. Sonne, J. S. Leger, S. Iverson, A. Rosing-Asvid, M. Hansen and M. A. McKinney, Feeding habits of a new Arctic predator: insight from full-depth blubber fatty acid signatures of Greenland, Faroe Islands, Denmark, and managed-care killer whales Orcinus orca, Mar. Ecol.: Prog. Ser., 2018, 603, 1–12 CrossRef CAS.
- S. Pedro, C. Boba, R. Dietz, C. Sonne, A. Rosing-Asvid, M. Hansen, A. Provatas and M. A. McKinney, Blubber-depth distribution and bioaccumulation of PCBs and organochlorine pesticides in Arctic-invading killer whales, Sci. Total Environ., 2017, 601, 237–246 CrossRef PubMed.
- E. Jourdain, C. Andvik, R. Karoliussen, A. Ruus, D. Vongraven and K. Borgå, Isotopic niche differs between seal and fish-eating killer whales (Orcinus orca) in northern Norway, Ecol. Evol., 2020, 10, 4115–4127 CrossRef PubMed.
- C. Andvik, E. Jourdain, A. Ruus, J. L. Lyche, R. Karoliussen and K. Borgå, Preying on seals pushes killer whales from Norway above pollution effects thresholds, Sci. Rep., 2020, 10, 1–10 CrossRef PubMed.
- AMAP, AMAP Assessment 2018: Biological Effects of Contaminants on Arctic Wildlife and Fish, Report 8279711066, Arctic Monitoring and Assessment Programme (AMAP), 2018 Search PubMed.
- B. M. Braune, A. J. Gaston, R. J. Letcher, H. G. Gilchrist, M. L. Mallory and J. F. Provencher, A geographical comparison of chlorinated, brominated and fluorinated compounds in seabirds breeding in the eastern Canadian Arctic, Environ. Res., 2014, 134, 46–56 CrossRef CAS PubMed.
- F. Amélineau, D. Grémillet, A. M. Harding, W. Walkusz, R. Choquet and J. Fort, Arctic climate change and pollution impact little auk foraging and fitness across a decade, Sci. Rep., 2019, 9, 1–15 Search PubMed.
- A. J. Gaston and M. S. Bradstreet, Intercolony differences in the summer diet of thick-billed murres in the eastern Canadian Arctic, Can. J. Zool., 1993, 71, 1831–1840 CrossRef.
- A. J. Gaston, K. Woo and J. M. Hipfner, Trends in forage fish populations in northern Hudson Bay since 1981, as determined from the diet of nestling thick-billed murres Uria lomvia, Arctic, 2003, 227–233 Search PubMed.
- A. J. Gaston, P. A. Smith and J. F. Provencher, Discontinuous change in ice cover in Hudson Bay in the 1990s and some consequences for marine birds and their prey, ICES J. Mar. Sci., 2012, 69, 1218–1225 CrossRef.
- M. L. Mallory, N. J. Karnovsky, A. J. Gaston, K. A. Hobson, J. F. Provencher, M. R. Forbes, G. L. Hunt Jr, T. Byers and T. A. Dick, Temporal and spatial patterns in the diet of northern fulmars Fulmarus glacialis in the Canadian High Arctic, Aquat. Biol., 2010, 10, 181–191 CrossRef.
- J. F. Provencher, A. J. Gaston, P. D. O'Hara and H. G. Gilchrist, Seabird diet indicates changing Arctic marine communities in eastern Canada, Mar. Ecol.: Prog. Ser., 2012, 454, 171–182 CrossRef.
- J. Fort, D. Grémillet, G. Traisnel, F. Amélineau and P. Bustamante, Does temporal variation of mercury levels in Arctic seabirds reflect changes in global environmental contamination, or a modification of Arctic marine food web functioning?, Environ. Pollut., 2016, 211, 382–388 CrossRef CAS PubMed.
- AMAP, 2021 AMAP Mercury Assessment, Oslo, Norway, 2021 Search PubMed.
- B. M. Braune, A. J. Gaston, K. A. Hobson, H. G. Gilchrist and M. L. Mallory, Changes in trophic position affect rates of contaminant decline at two seabird colonies in the Canadian Arctic, Ecotoxicol. Environ. Saf., 2015, 115, 7–13 CrossRef CAS PubMed.
- M. A. McKinney, E. Peacock and R. J. Letcher, Sea ice-associated diet change increases the levels of chlorinated and brominated contaminants in polar bears, Environ. Sci. Technol., 2009, 43, 4334–4339 CrossRef CAS PubMed.
- M. A. McKinney, S. J. Iverson, A. T. Fisk, C. Sonne, F. F. Rigét, R. J. Letcher, M. T. Arts, E. W. Born, A. Rosing-Asvid and R. Dietz, Global change effects on the long-term feeding ecology and contaminant exposures of E ast G reenland polar bears, Global Change Biol., 2013, 19, 2360–2372 CrossRef PubMed.
- R. Dietz, F. F. Rigét, I. Eulaers, J.-P. Desforges, K. Vorkamp, R. Bossi, J. Søndergaard, P. Ambus, M. McKinney, R. L. Letcher and C. Sonne, Unexpected Increases of Persistent Organic Pollutant and Mercury Levels in East Greenland Polar Bears (UNEXPECTED), Aarhus University, DCE – Danish Centre for Environment and Energy, 2021 Search PubMed.
- M. A. McKinney, T. C. Atwood, S. J. Iverson and E. Peacock, Temporal complexity of southern B eaufort S ea polar bear diets during a period of increasing land use, Ecosphere, 2017, 8, e01633 CrossRef.
- T. C. Atwood, E. Peacock, M. A. McKinney, K. Lillie, R. Wilson, D. C. Douglas, S. Miller and P. Terletzky, Rapid environmental change drives increased land use by an Arctic marine predator, PLoS One, 2016, 11, e0155932 CrossRef PubMed.
- T. C. Atwood, C. Duncan, K. A. Patyk, P. Nol, J. Rhyan, M. McCollum, M. A. McKinney, A. M. Ramey, C. K. Cerqueira-Cézar and O. C. Kwok, Environmental and behavioral changes may influence the exposure of an Arctic apex predator to pathogens and contaminants, Sci. Rep., 2017, 7, 1–12 CrossRef CAS PubMed.
- S. A. Iverson, H. G. Gilchrist, P. A. Smith, A. J. Gaston and M. R. Forbes, Longer ice-free seasons increase the risk of nest depredation by polar bears for colonial breeding birds in the Canadian Arctic, Proc. R. Soc. B, 2014, 281, 20133128 CrossRef PubMed.
- G. J. Divoky, P. M. Lukacs and M. L. Druckenmiller, Effects of recent decreases in arctic sea ice on an ice-associated marine bird, Prog. Oceanogr., 2015, 136, 151–161 CrossRef.
- J. Bourque, T. C. Atwood, G. J. Divoky, C. Stewart and M. A. McKinney, Fatty acid-based diet estimates suggest ringed seal remain the main prey of southern Beaufort Sea polar bears despite recent use of onshore food resources, Ecol. Evol., 2020, 10, 2093–2103 CrossRef PubMed.
- A. Lippold, S. Bourgeon, J. Aars, M. Andersen, A. Polder, J. L. Lyche, J. Bytingsvik, B. M. Jenssen, A. E. Derocher and J. M. Welker, Temporal trends of persistent organic pollutants in Barents Sea polar bears (Ursus maritimus) in relation to changes in feeding habits and body condition, Environ. Sci. Technol., 2018, 53, 984–995 CrossRef PubMed.
- H. L. Stern and K. L. Laidre, Sea-ice indicators of polar bear habitat, Cryosphere, 2016, 10, 2027–2041 CrossRef.
- I. Gjertz and C. Lydersen, Polar bear predation on ringed seals in the fast-ice of Hornsund, Svalbard, Polar Res., 1986, 4, 65–68 CrossRef.
- B. B. Hansen, V. Grøtan, R. Aanes, B.-E. Sæther, A. Stien, E. Fuglei, R. A. Ims, N. G. Yoccoz and Å. Ø. Pedersen, Climate events synchronize the dynamics of a resident vertebrate community in the high Arctic, Science, 2013, 339, 313–315 CrossRef CAS PubMed.
- B. Peeters, Å. Ø. Pedersen, L. E. Loe, K. Isaksen, V. Veiberg, A. Stien, J. Kohler, J.-C. Gallet, R. Aanes and B. B. Hansen, Spatiotemporal patterns of rain-on-snow and basal ice in high Arctic Svalbard: detection of a climate-cryosphere regime shift, Environ. Res. Lett., 2019, 14, 015002 CrossRef.
- R. Bintanja and O. Andry, Towards a rain-dominated Arctic, Nat. Clim. Change, 2017, 7, 263–267 CrossRef.
- L. E. Loe, B. B. Hansen, A. Stien, S. D. Albon, R. Bischof, A. Carlsson, R. J. Irvine, M. Meland, I. M. Rivrud and E. Ropstad, Behavioral buffering of extreme weather events in a high-Arctic herbivore, Ecosphere, 2016, 7, e01374 CrossRef.
- B. B. Hansen, J. R. Lorentzen, J. M. Welker, Ø. Varpe, R. Aanes, L. T. Beumer and Å. Ø. Pedersen, Reindeer turning maritime: Ice-locked tundra triggers changes in dietary niche utilization, Ecosphere, 2019, 10, e02672 Search PubMed.
- A. A. Sokolov, N. A. Sokolova, R. A. Ims, L. Brucker and D. Ehrich, Emergent rainy winter warm spells may promote boreal predator expansion into the Arctic, Arctic, 2016, 121–129 Search PubMed.
- M. Sturm, C. Racine and K. Tape, Increasing shrub abundance in the Arctic, Nature, 2001, 411, 546–547 CrossRef CAS PubMed.
- K. Tape, M. Sturm and C. Racine, The evidence for shrub expansion in Northern Alaska and the Pan-Arctic, Global Change Biol., 2006, 12, 686–702 CrossRef.
- E. H. Jørgensen, S. J. S. Johansen and M. Jobling, Seasonal patterns of growth, lipid deposition and lipid depletion in anadromous Arctic charr, J. Fish Biol., 1997, 51, 312–326 CrossRef.
- W. Hagen and H. Auel, Seasonal adaptations and the role of lipids in oceanic zooplankton, Zoology, 2001, 104, 313–326 CrossRef CAS PubMed.
- C. L. Scott, S. Kwasniewski, S. Falk-Petersen and J. R. Sargent, Lipids and fatty acids in the copepod Jaschnovia brevis (Jaschnov) and in particulates from Arctic waters, Polar Biol., 2002, 25, 65–71 CrossRef.
- R. F. Lee, W. Hagen and G. Kattner, Lipid storage in marine zooplankton, Mar. Ecol.: Prog. Ser., 2006, 307, 273–306 CrossRef CAS.
- A. Ruus, I. J. Allan, K. Bæk and K. Borgå, Partitioning of persistent hydrophobic contaminants to different storage lipid classes, Chemosphere, 2021, 263, 127890 CrossRef CAS PubMed.
- Ø. Varpe, C. Jørgensen, G. A. Tarling and Ø. Fiksen, The adaptive value of energy storage and capital breeding in seasonal environments, Oikos, 2009, 118, 363–370 CrossRef.
- P. E. Renaud, M. Daase, N. S. Banas, T. M. Gabrielsen, J. E. Søreide, Ø. Varpe, F. Cottier, S. Falk-Petersen, C. Halsband and D. Vogedes, Pelagic food-webs in a changing Arctic: a trait-based perspective suggests a mode of resilience, ICES J. Mar. Sci., 2018, 75, 1871–1881 CrossRef.
- E. F. Møller and T. G. Nielsen, Borealization of Arctic zooplankton—smaller and less fat zooplankton species in Disko Bay, Western Greenland, Limnol. Oceanogr., 2020, 65, 1175–1188 CrossRef.
- D. J. Hitchcock, Ø. Varpe, T. Andersen and K. Borgå, Effects of reproductive strategies on pollutant concentrations in pinnipeds: a meta-analysis, Oikos, 2017, 126, 772–781 CrossRef CAS.
- H. Parker and H. Halvor, Patterns of Nutrient and Energy Expenditure in Female Common Eiders Nesting in the High Arctic, Auk, 1990, 107, 660–668 CrossRef.
- J. O. Bustnes, B. Moe, S. A. Hanssen, D. Herzke, A. A. Fenstad, T. Nordstad, K. Borgå and G. W. Gabrielsen, Temporal dynamics of circulating persistent organic pollutants in a fasting seabird under different environmental conditions, Environ. Sci. Technol., 2012, 46, 10287–10294 CrossRef CAS PubMed.
- S. Atkinson and M. Ramsay, The effects of prolonged fasting of the body composition and reproductive success of female polar bears (Ursus maritimus), Funct. Ecol., 1995, 559–567 CrossRef.
- K. D. Rode, E. Peacock, M. Taylor, I. Stirling, E. W. Born, K. L. Laidre and Ø. Wiig, A tale of two polar bear populations: ice habitat, harvest, and body condition, Popul. Ecol., 2012, 54, 3–18 CrossRef.
- K. D. Rode, R. R. Wilson, D. C. Douglas, V. Muhlenbruch, T. C. Atwood, E. V. Regehr, E. S. Richardson, N. W. Pilfold, A. E. Derocher and G. M. Durner, Spring fasting behavior in a marine apex predator provides an index of ecosystem productivity, Global Change Biol., 2018, 24, 410–423 CrossRef PubMed.
- L. Sciullo, G. Thiemann and N. Lunn, Comparative assessment of metrics for monitoring the body condition of polar bears in western Hudson Bay, J. Zool., 2016, 300, 45–58 CrossRef.
- M. E. Obbard, M. R. Cattet, E. J. Howe, K. R. Middel, E. J. Newton, G. B. Kolenosky, K. F. Abraham and C. J. Greenwood, Trends in body condition in polar bears (Ursus maritimus) from the Southern Hudson Bay subpopulation in relation to changes in sea ice, Arctic Science, 2016, 2, 15–32 CrossRef.
- M. A. McKinney, T. C. Atwood, S. Pedro and E. Peacock, Ecological change drives a decline in mercury concentrations in southern Beaufort Sea polar bears, Environ. Sci. Technol., 2017, 51, 7814–7822 CrossRef CAS PubMed.
- F. Rigét, K. Vorkamp and D. Muir, Temporal trends of contaminants in Arctic char (Salvelinus alpinus) from a small lake, southwest Greenland during a warming climate, J. Environ. Monit., 2010, 12, 2252–2258 RSC.
- K. H. Elliott and K. J. Fernie, Climate Change, Contaminants, Ecotoxicology: Interactions in Arctic Seabirds at Their Southern Range Limits, 2016-2018. Report to the Northern Contaminants Program, Crown-Indigenous Relations and Northern Affairs Canada., 2019 Search PubMed.
- C. D. Hamilton, C. Lydersen, R. A. Ims and K. M. Kovacs, Predictions replaced by facts: A keystone species’ behavioural responses to declining arctic sea-ice, Biol. Lett., 2015, 11, 20150803 CrossRef PubMed.
- J. Vacquié-Garcia, C. Lydersen, R. A. Ims and K. M. Kovacs, Habitats and movement patterns of white whales Delphinapterus leucas in Svalbard, Norway in a changing climate, Mov. Ecol., 2018, 6, 21 CrossRef PubMed.
- K. Lone, C. D. Hamilton, J. Aars, C. Lydersen and K. M. Kovacs, Summer habitat selection by ringed seals (Pusa hispida) in the drifting sea ice of the northern Barents sea, Polar Res., 2019, 38, 1–10 CrossRef.
- K. Lone, K. M. Kovacs, C. Lydersen, M. Fedak, M. Andersen, P. Lovell and J. Aars, Aquatic behaviour of polar bears (Ursus maritimus) in an increasingly ice-free Arctic, Sci. Rep., 2018, 8, 9677 CrossRef PubMed.
- J. V. Ware, K. D. Rode, J. F. Bromaghin, D. C. Douglas, R. R. Wilson, E. V. Regehr, S. C. Amstrup, G. M. Durner, A. M. Pagano, J. Olson, C. T. Robbins and H. T. Jansen, Habitat degradation affects the summer activity of polar bears, Oecologia, 2017, 184, 87–99 CrossRef PubMed.
- K. L. Laidre, H. Stern, E. W. Born, P. Heagerty, S. Atkinson, Ø. Wiig, N. J. Lunn, E. V. Regehr, R. McGovern and M. Dyck, Changes in winter and spring resource selection by polar bears Ursus maritimus in Baffin Bay over two decades of sea-ice loss, Endanger. Species Res., 2018, 36, 1–14 CrossRef.
- B. D. Griffen, Modeling the metabolic costs of swimming in polar bears (Ursus maritimus), Polar Biol., 2018, 41, 491–503 CrossRef.
- A. M. Pagano, G. M. Durner, K. D. Rode, T. C. Atwood, S. N. Atkinson, E. Peacock, D. P. Costa, M. A. Owen and T. M. Williams, High-energy, high-fat lifestyle challenges an Arctic apex predator, the polar bear, Science, 2018, 359, 568 CrossRef CAS PubMed.
- S. Tartu, J. Aars, M. Andersen, A. Polder, S. Bourgeon, B. Merkel, A. D. Lowther, J. Bytingsvik, J. M. Welker, A. E. Derocher, B. M. Jenssen and H. Routti, Choose your poison - Space-use strategy influences pollutant exposure in Barents Sea polar bears, Environ. Sci. Technol., 2018, 52, 3211–3221 CrossRef CAS PubMed.
- M. A. Blanchet, J. Aars, M. Andersen and H. Routti, Space-use strategy affects energy requirements in Barents Sea polar bears, Mar. Ecol.: Prog. Ser., 2020, 639, 1–19 CrossRef.
- P. Blévin, J. Aars, M. Andersen, M.-A. Blanchet, L. Hanssen, D. Herzke, R. M. Jeffreys, E. S. Nordøy, M. Pinzone, C. de la Vega and H. Routti, Pelagic vs Coastal—Key Drivers of Pollutant Levels in Barents Sea Polar Bears with Contrasted Space-Use Strategies, Environ. Sci. Technol., 2020, 54, 985–995 CrossRef PubMed.
- J. F. Piatt, J. K. Parrish, H. M. Renner, S. K. Schoen, T. T. Jones, M. L. Arimitsu, K. J. Kuletz, B. Bodenstein, M. García-Reyes, R. S. Duerr, R. M. Corcoran, R. S. A. Kaler, G. J. McChesney, R. T. Golightly, H. A. Coletti, R. M. Suryan, H. K. Burgess, J. Lindsey, K. Lindquist, P. M. Warzybok, J. Jahncke, J. Roletto and W. J. Sydeman, Extreme mortality and reproductive failure of common murres resulting from the northeast Pacific marine heatwave of 2014-2016, PLoS One, 2020, 15, e0226087 CrossRef CAS PubMed.
- K. Borgå and T. F. Bidleman, Enantiomer fractions of organic chlorinated pesticides in arctic marine ice fauna, zooplankton, and benthos, Environ. Sci. Technol., 2005, 39, 3464–3473 CrossRef PubMed.
- T. F. Bidleman, G. A. Stern, G. T. Tomy, B. T. Hargrave, L. M. Jantunen and R. W. MacDonald, Scavenging amphipods: Sentinels for penetration of mercury and persistent organic chemicals into food webs of the deep Arctic Ocean, Environ. Sci. Technol., 2013, 47, 5553–5561 CrossRef CAS PubMed.
- T. C. Svendsen, L. Camus, B. Hargrave, A. Fisk, D. C. G. Muir and K. Borgå, Polyaromatic hydrocarbons, chlorinated and brominated organic contaminants as tracers of feeding ecology in polar benthic amphipods, Mar. Ecol.: Prog. Ser., 2007, 337, 155–164 CrossRef CAS.
- A. Evenset, J. Carroll, G. N. Christensen, R. Kallenborn, D. Gregor and G. W. Gabrielsen, Seabird guano is an efficient conveyer of persistent organic pollutants (POPs) to Arctic lake ecosystems, Environ. Sci. Technol., 2007, 41, 1173–1179 CrossRef CAS PubMed.
- S. M. Kristiansen, H. P. Leinaas, D. Herzke, K. Hylland, G. W. Gabrielsen, M. Harju and K. Borgå, Seabird-Transported Contaminants Are Reflected in the Arctic Tundra, but Not in Its Soil-Dwelling Springtails (Collembola), Environ. Sci. Technol., 2019, 53, 12835–12845 CrossRef CAS PubMed.
- R. Dietz, R. J. Letcher, J.-P. Desforges, I. Eulaers, C. Sonne, S. Wilson, E. Andersen-Ranberg, N. Basu, B. D. Barst, J. O. Bustnes, J. Bytingsvik, T. M. Ciesielski, P. E. Drevnick, G. W. Gabrielsen, A. Haarr, K. Hylland, B. M. Jenssen, M. Levin, M. A. McKinney, R. D. Nørregaard, K. E. Pedersen, J. Provencher, B. Styrishave, S. Tartu, J. Aars, J. T. Ackerman, A. Rosing-Asvid, R. Barrett, A. Bignert, E. W. Born, M. Branigan, B. Braune, C. E. Bryan, M. Dam, C. A. Eagles-Smith, M. Evans, T. J. Evans, A. T. Fisk, M. Gamberg, K. Gustavson, C. A. Hartman, B. Helander, M. P. Herzog, P. F. Hoekstra, M. Houde, K. Hoydal, A. K. Jackson, J. Kucklick, E. Lie, L. Loseto, M. L. Mallory, C. Miljeteig, A. Mosbech, D. C. G. Muir, S. T. Nielsen, E. Peacock, S. Pedro, S. H. Peterson, A. Polder, F. F. Rigét, P. Roach, H. Saunes, M.-H. S. Sinding, J. U. Skaare, J. Søndergaard, G. Stenson, G. Stern, G. Treu, S. S. Schuur and G. Víkingsson, Current state of knowledge on biological effects from contaminants on arctic wildlife and fish, Sci. Total Environ., 2019, 696, 133792 CrossRef CAS.
- S. Mariana and G. Badr, Impact of heat stress on the immune response of fishes, Journal of Survey in Fisheries Sciences, 2019, 5, 149–159 CrossRef.
- E. VanWormer, J. A. K. Mazet, A. Hall, V. A. Gill, P. L. Boveng, J. M. London, T. Gelatt, B. S. Fadely, M. E. Lander, J. Sterling, V. N. Burkanov, R. R. Ream, P. M. Brock, L. D. Rea, B. R. Smith, A. Jeffers, M. Henstock, M. J. Rehberg, K. A. Burek-Huntington, S. L. Cosby, J. A. Hammond and T. Goldstein, Viral emergence in marine mammals in the North Pacific may be linked to Arctic sea ice reduction, Sci. Rep., 2019, 9, 15569 CrossRef CAS PubMed.
- AMAP, AMAP Assessment 2018: Biological Effects of Contaminants on Arctic Wildlife and Fish, Oslo, Norway, 2018 Search PubMed.
- AMAP, AMAP Assessment 2016: Chemicals of Emerging Arctic Concern, Oslo, Norway, 2017 Search PubMed.
- S. J. Moe, K. De Schamphelaere, W. H. Clements, M. T. Sorensen, P. J. Van den Brink and M. Liess, Combined and interactive effects of global climate change and toxicants on populations and communities, Environ. Toxicol. Chem., 2013, 32, 49–61 CrossRef CAS PubMed.
- M. J. Hooper, G. T. Ankley, D. A. Cristol, L. A. Maryoung, P. D. Noyes and K. E. Pinkerton, Interactions between chemical and climate stressors: A role for mechanistic toxicology in assessing climate change risks, Environ. Toxicol. Chem., 2013, 32, 32–48 CrossRef CAS PubMed.
- J. Stein, T. Schettler, D. Wallinga and M. Valenti, In harm's way: Toxic threats to child development, J. Dev. Behav. Pediatr., 2002, 23, S13–S22 CrossRef PubMed.
- B. J. Sinclair, K. E. Marshall, M. A. Sewell, D. L. Levesque, C. S. Willett, S. Slotsbo, Y. Dong, C. D. G. Harley, D. J. Marshall, B. S. Helmuth and R. B. Huey, Can we predict ectotherm responses to climate change using thermal performance curves and body temperatures?, Ecol. Lett., 2016, 19, 1372–1385 CrossRef PubMed.
- A. R. Gunderson, E. J. Armstrong and J. H. Stillman, Multiple Stressors in a Changing World: The Need for an Improved Perspective on Physiological Responses to the Dynamic Marine Environment, Ann. Rev. Mar. Sci, 2016, 8, 357–378 CrossRef PubMed.
- J. Verheyen and R. Stoks, Current and future daily temperature fluctuations make a pesticide more toxic: Contrasting effects on life history and physiology, Environ. Pollut., 2019, 248, 209–218 CrossRef CAS PubMed.
- J. C. Grenvald, T. G. Nielsen and M. Hjorth, Effects of pyrene exposure and temperature on early development of two co-existing Arctic copepods, Ecotoxicology, 2013, 22, 184–198 CrossRef CAS PubMed.
- M. Hjorth and T. G. Nielsen, Oil exposure in a warmer Arctic: Potential impacts on key zooplankton species, Mar. Biol., 2011, 158, 1339–1347 CrossRef.
- K. Van Dinh, M. W. Olsen, D. Altin, B. Vismann and T. G. Nielsen, Impact of temperature and pyrene exposure on the functional response of males and females of the copepod Calanus finmarchicus, Environ. Sci. Pollut. Res., 2019, 26, 29327–29333 CrossRef CAS PubMed.
- B. J. Bårdsen, S. A. Hanssen and J. O. Bustnes, Multiple stressors: Modeling the effect of pollution, climate, and predation on viability of a sub-arctic marine bird, Ecosphere, 2018, 9, e02342 CrossRef.
- J. O. Bustnes, G. W. Gabrielsen and J. Verreault, Climate variability and temporal trends of persistent organic pollutants in the Arctic: A study of glaucous gulls, Environ. Sci. Technol., 2010, 44, 3155–3161 CrossRef CAS PubMed.
- J. O. Bustnes, S. Bourgeon, E. H. K. Leat, E. Magnusdóttir, H. Strøm, S. A. Hanssen, A. Petersen, K. Olafsdóttir, K. Borgà, G. W. Gabrielsen and R. W. Furness, Multiple stressors in a top predator seabird: Potential ecological consequences of environmental contaminants, population health and breeding conditions, PLoS One, 2015, 10, e0131769 CrossRef PubMed.
- E. S. Choy, K. H. Elliott, I. Esparza, A. Patterson, R. J. Letcher and K. J. Fernie, Potential disruption of thyroid hormones by perfluoroalkyl acids in an Arctic seabird with associations to reproduction, Environ. Pollut., under revision Search PubMed.
- H. Routti, T. C. Atwood, T. Bechshoft, A. Boltunov, T. M. Ciesielski, J. P. Desforges, R. Dietz, G. W. Gabrielsen, B. M. Jenssen, R. J. Letcher, M. A. McKinney, A. D. Morris, F. F. Rigét, C. Sonne, B. Styrishave and S. Tartu, State of knowledge on current exposure, fate and potential health effects of contaminants in polar bears from the circumpolar Arctic, Sci. Total Environ., 2019, 664, 1063–1083 CrossRef CAS PubMed.
- S. Tartu, S. Bourgeon, J. Aars, M. Andersen, A. Polder, G. W. Thiemann, J. M. Welker and H. Routti, Sea ice-associated decline in body condition leads to increased concentrations of lipophilic pollutants in polar bears (Ursus maritimus) from Svalbard, Norway, Sci. Total Environ., 2017, 576, 409–419 CrossRef CAS PubMed.
- J. P. Whiteman, H. J. Harlow, G. M. Durner, E. V. Regehr, S. C. Amstrup and M. Ben-David, Heightened immune system function in polar bears using terrestrial habitats, Physiol. Biochem. Zool., 2019, 92, 1–11 CrossRef PubMed.
- P. Mislan, A. E. Derocher, V. L. S. Louis, E. Richardson, N. J. Lunn and D. M. Janz, Assessing stress in Western Hudson Bay polar bears using hair cortisol concentration as a biomarker, Ecol. Indic., 2016, 71, 47–54 CrossRef CAS.
- AMAP, Adaptation Actions for a Changing Arctic: Perspectives from the Barents Area, Oslo, Norway, 2017 Search PubMed.
- AMAP, Adaptation Actions for a Changing Arctic: Perspectives from the Bering-Chukchi-Beaufort Region, Oslo, Norway, 2017 Search PubMed.
- AMAP, Adaptation Actions for a Changing Arctic: Perspectives from the Baffin Bay/Davis Strait Region, Oslo, Norway, 2018 Search PubMed.
- AMAP, Influence of Climate Change on Transport, Levels, and Effects of Contaminants in Northern Areas – Part 2, ed. P. Carlsson, J.H. Christensen, K. Borgå, R. Kallenborn, K. Aspmo Pfaffhuber, Ø. Odland, L.O. Reiersen and J. F. Pawlak, Oslo, Norway, 2016 Search PubMed.
- F. Wania, M. J. Binnington and M. S. Curren, Mechanistic modeling of persistent organic pollutant exposure among indigenous Arctic populations: Motivations, challenges, and benefits, Environ. Rev., 2017, 25, 396–407 CrossRef CAS.
- F. Wania, K. Breivik, N. J. Persson and M. S. McLachlan, CoZMo-POP 2 - A fugacity-based dynamic multi-compartmental mass balance model of the fate of persistent organic pollutants, Environmental Modelling & Software, 2006, 21, 868–884 Search PubMed.
- G. Czub and M. S. McLachlan, A food chain model to predict the levels of lipophilic organic contaminants in humans, Environ. Toxicol. Chem., 2004, 23, 2356–2366 CrossRef CAS PubMed.
- K. Breivik, G. Czub, M. S. McLachlan and F. Wania, Towards an understanding of the link between environmental emissions and human body burdens of PCBs using CoZMoMAN, Environ. Int., 2010, 36, 85–91 CrossRef CAS PubMed.
- L. Lamon, H. von Waldow, M. Macleod, M. Scheringer, A. Marcomini and K. Hungerbuhler, Modeling the global levels and distribution of polychlorinated biphenyls in air under a climate change scenario, Environ. Sci. Technol., 2009, 43, 5818–5824 CrossRef CAS PubMed.
- J. M. Armitage, C. L. Quinn and F. Wania, Global climate change and contaminants-an overview of opportunities and priorities for modelling the potential implications for long-term human exposure to organic compounds in the Arctic, J. Environ. Monit., 2011, 13, 1532–1546 RSC.
- J. A. Arnot and F. A. P. C. Gobas, A food web bioaccumulation model for organic chemicals in aquatic ecosystems, Environ. Toxicol. Chem., 2004, 23, 2343–2355 CrossRef CAS PubMed.
- K. Borgå, T. M. Saloranta and A. Ruus, Simulating climate change-induced alterations in bioaccumulation of organic contaminants in an arctic marine food web, Environ. Toxicol. Chem., 2010, 29, 1349–1357 CrossRef PubMed.
- C. A. Ng and K. A. Gray, Forecasting the effects of global change scenarios on bioaccumulation patterns in great lakes species, Global Change Biol., 2011, 17, 720–733 CrossRef.
- C. A. Ng and K. A. Gray, Tracking bioaccumulation in aquatic organisms: A dynamic model integrating life history characteristics and environmental change, Ecol. Model., 2009, 220, 1266–1273 CrossRef CAS.
- R. Dietz, R. J. Letcher, J.-P. Desforges, I. Eulaers, C. Sonne, S. Wilson, E. Andersen-Ranberg, N. Basu, B. D. Barst and J. O. Bustnes, Current state of knowledge on biological effects from contaminants on arctic wildlife and fish, Sci. Total Environ., 2019, 696, 133792 CrossRef CAS.
- H. Segner, M. Schmitt-Jansen and S. Sabater, Assessing the impact of multiple stressors on aquatic biota: The receptor's side matters, Environ. Sci. Technol., 2014, 48, 7690–7696 CrossRef CAS PubMed.
- T. Jager, Making sense of chemical stress, Application of Dynamic Energy Budget Theory in Ecotoxicology and Stress Ecology. Version 2.0, Leanpub, 2019 Search PubMed.
- T. Jager, E. H. W. Heugens and S. A. L. M. Kooijman, Making sense of ecotoxicological test results: Towards application of process-based models, Ecotoxicology, 2006, 15, 305–314 CrossRef CAS PubMed.
- T. Jager and E. I. Zimmer, Simplified Dynamic Energy Budget model for analysing ecotoxicity data, Ecol. Model., 2012, 225, 74–81 CrossRef CAS.
- M. Leist, A. Ghallab, R. Graepel, R. Marchan, R. Hassan, S. H. Bennekou, A. Limonciel, M. Vinken, S. Schildknecht, T. Waldmann, E. Danen, B. van Ravenzwaay, H. Kamp, I. Gardner, P. Godoy, F. Y. Bois, A. Braeuning, R. Reif, F. Oesch, D. Drasdo, S. Höhme, M. Schwarz, T. Hartung, T. Braunbeck, J. Beltman, H. Vrieling, F. Sanz, A. Forsby, D. Gadaleta, C. Fisher, J. Kelm, D. Fluri, G. Ecker, B. Zdrazil, A. Terron, P. Jennings, B. van der Burg, S. Dooley, A. H. Meijer, E. Willighagen, M. Martens, C. Evelo, E. Mombelli, O. Taboureau, A. Mantovani, B. Hardy, B. Koch, S. Escher, C. van Thriel, C. Cadenas, D. Kroese, B. van de Water and J. G. Hengstler, Adverse outcome pathways: opportunities, limitations and open questions, Arch. Toxicol., 2017, 91, 3477–3505 CrossRef CAS PubMed.
- M. Stock, C. Ritter, V. Aaltonen, W. Aas, D. Handorff, A. Herber, R. Treffeisen and K. Dethloff, Where does the optically detectable aerosol in the European arctic come from?, Tellus, Ser. B: Chem. Phys. Meteorol., 2014, 66, 21450 CrossRef.
- M. H. Hermanson, E. Isaksson, C. Teixeira, D. C. G. Muir, K. M. Compher, Y. F. Li, M. Igarashi and K. Kamiyama, Current-use and legacy pesticide history in the Austfonna ice cap, Svalbard, Norway, Environ. Sci. Technol., 2005, 39, 8163–8169 CrossRef CAS PubMed.
- T. Ø. Bechshøft, A. E. Derocher, M. Viengkone, H. Routti, J. Aars, R. J. Letcher, R. Dietz, C. Sonne, B. M. Jenssen, E. Richardson and N. J. Lunn, On the integration of ecological and physiological variables in polar bear toxicology research: A systematic review, Environ. Rev., 2018, 26, 1–12 CrossRef.
- D. J. Hitchcock, T. Andersen, Ø. Varpe and K. Borgå, Improving Data Reporting in Ecotoxicological Studies, Environ. Sci. Technol., 2018, 52, 8061–8062 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1em00469g |
| ‡ These authors contributed equally to the work. |
| This journal is © The Royal Society of Chemistry 2022 |